Water Level Fluctuations and Air Temperatures Affect Common Reed Habitus and Productivity in an Intermittent Wetland Ecosystem
Abstract
1. Introduction
2. Materials and Methods
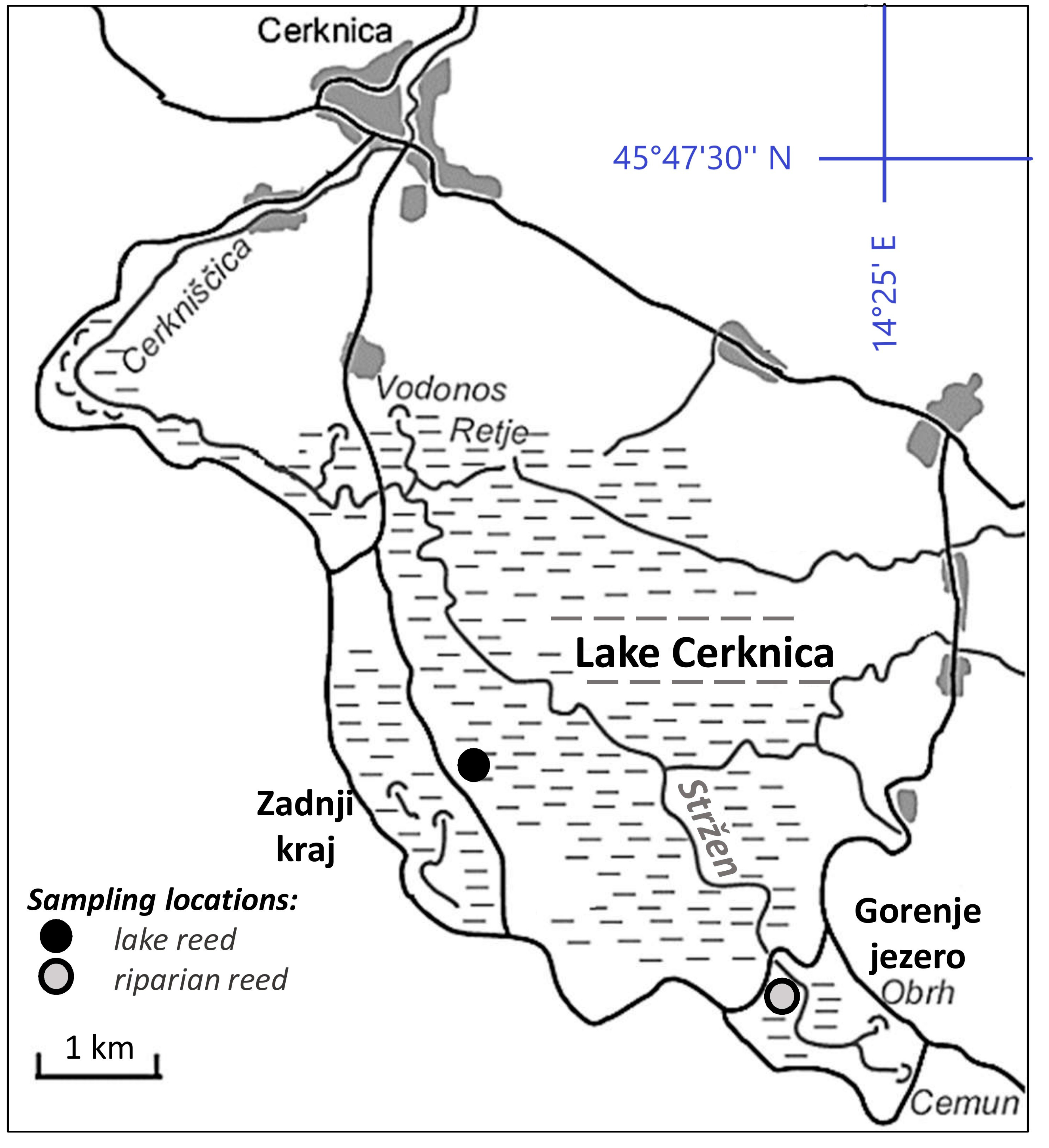
2.1. Site Description
2.2. Productivity Parameters
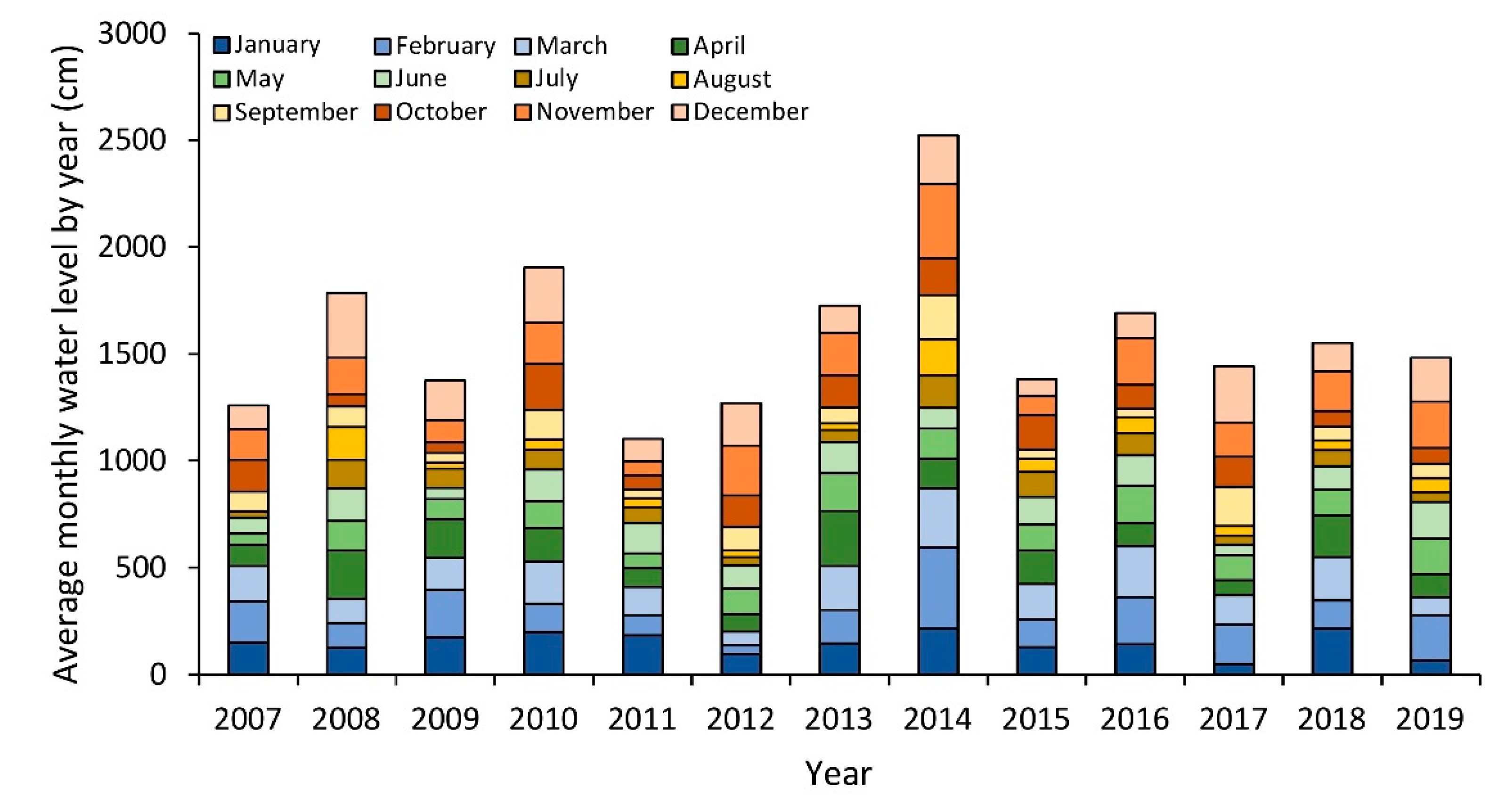
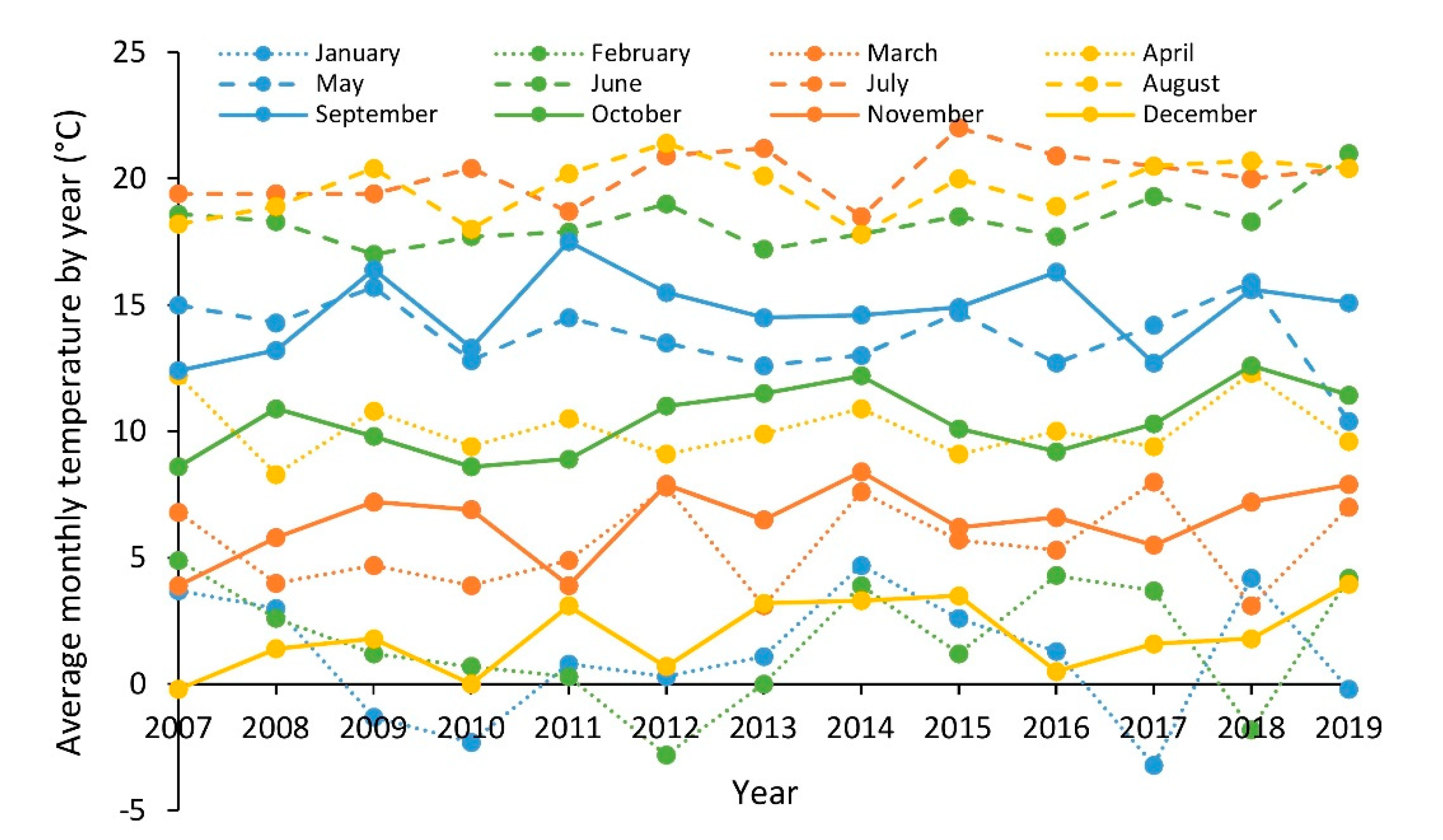
2.3. Environmental Parameters
2.4. Statistical Analysis
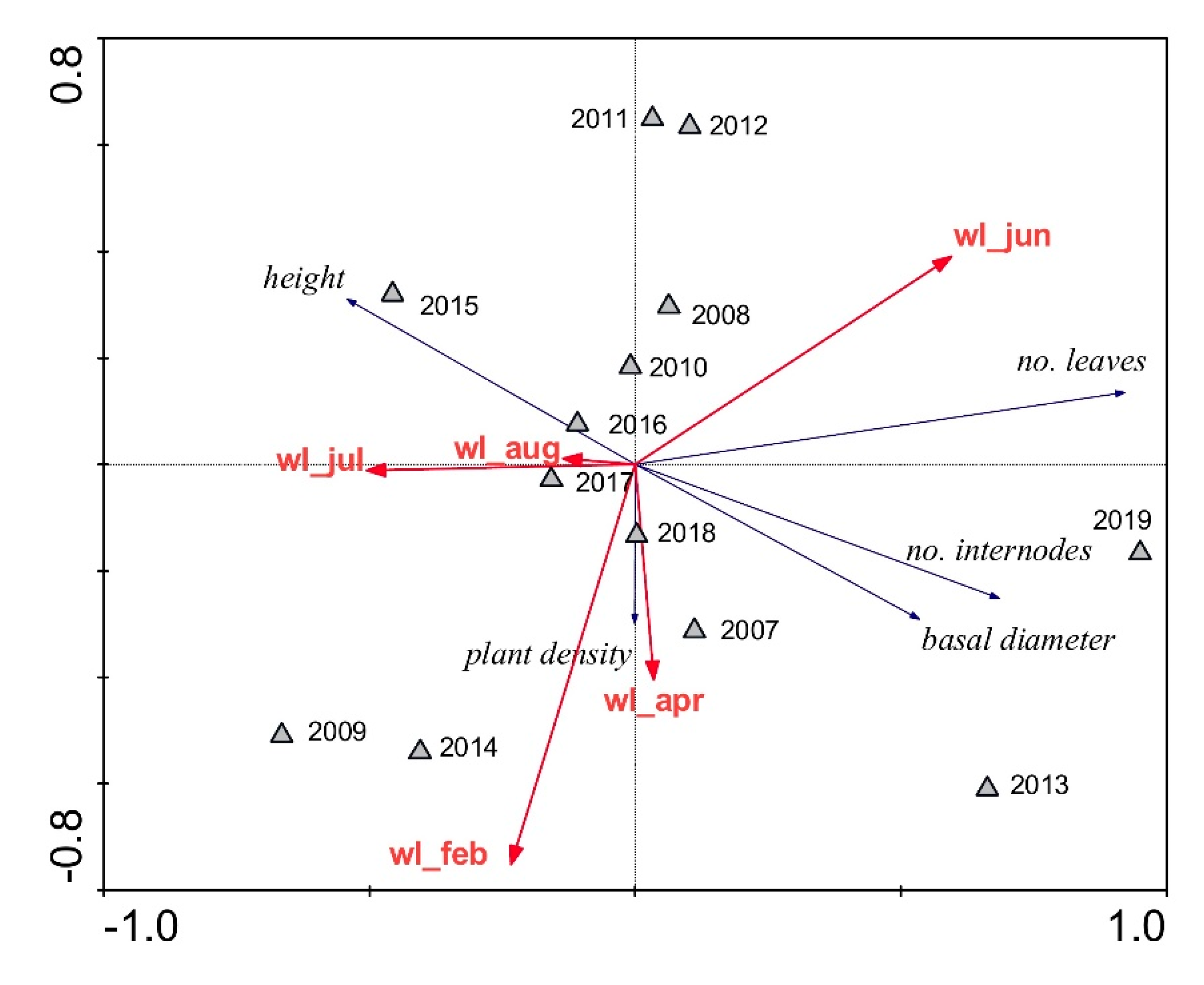
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yi, Y.; Xie, H.; Yang, Y.; Zhou, Y.; Yang, Z. Suitable habitat mathematical model of common reed (Phragmites australis) in shallow lakes with coupling cellular automaton and modified logistic function. Ecol. Model. 2020, 419, 108938. [Google Scholar] [CrossRef]
- Hill, N.M.; Keddy, P.A.; Wisheu, I.C. A Hydrological model for predicting the effects of dams on the shoreline vegetation of lakes and reservoirs. Environ. Manag. 1998, 22, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Clevering, O.A.; Brix, H.; Lukavská, J. Geographic variation in growth responses in Phragmites australis. Aquat. Bot. 2001, 69, 89–108. [Google Scholar] [CrossRef]
- Hocking, P.J.; Finlayson, C.M.; Chick, A.J. The biology of Australian weeds. 12. Phragmites australis (Cav.) Trin. ex Stuedel. J. Aust. Institute Agric. Sci. 1983, 40, 123–132. [Google Scholar]
- Dolinar, N.; Regvar, M.; Abram, D.; Gaberščik, A. Water-level fluctuations as a driver of Phragmites australis primary productivity, litter decomposition, and fungal root colonisation in an intermittent wetland. Hydrobiology 2015, 774, 69–80. [Google Scholar] [CrossRef]
- Eller, F.; Skálová, H.; Caplan, J.S.; Bhattarai, G.P.; Burger, M.K.; Cronin, J.T.; Guo, W.-Y.; Guo, X.; Hazelton, E.L.G.; Kettenring, K.M.; et al. Cosmopolitan species as models for ecophysiological responses to global change: The common reed Phragmites australis. Front. Plant. Sci. 2017, 8, 1833. [Google Scholar] [CrossRef]
- Clevering, O.A.; Lissner, J. Taxonomy, chromosome numbers, clonal diversity and population dynamics of Phragmites australis. Aquat. Bot. 1999, 64, 185–208. [Google Scholar] [CrossRef]
- Engloner, A.I.; Papp, M. Vertical differences in Phragmites australis culm anatomy along a water depth gradient. Aquat. Bot. 2006, 85, 137–146. [Google Scholar] [CrossRef]
- Tóth, V.R. Reed stands during different water level periods: Physico-chemical properties of the sediment and growth of Phragmites australis of Lake Balaton. Hydrobiology 2016, 778, 193–207. [Google Scholar] [CrossRef]
- Grašič, M.; Dobravc, M.; Golob, A.; Vogel-Mikuš, K.; Gaberščik, A. Water shortage reduces silicon uptake in barley leaves. Agric. Water Manag. 2019, 217, 47–56. [Google Scholar] [CrossRef]
- Armstrong, W.; Justin, S.; Beckett, P.; Lythe, S. Root adaptation to soil waterlogging. Aquat. Bot. 1991, 39, 57–73. [Google Scholar] [CrossRef]
- Asaeda, T.; Karunaratne, S. Dynamic modeling of the growth of Phragmites australis: Model description. Aquat. Bot. 2000, 67, 301–318. [Google Scholar] [CrossRef]
- Li, C.; Ye, X.; Wu, M.; Shao, X. Effects of water depth and coexistence on growth characteristics of Phragmites australis and Typha domingensis. Soc. Wetl. Sci. Bull. 2015, 13, 609–615. [Google Scholar]
- Squires, L.; Van Der Valk, A.G. Water-depth tolerances of the dominant emergent macrophytes of the Delta Marsh, Manitoba. Can. J. Bot. 1992, 70, 1860–1867. [Google Scholar] [CrossRef]
- Tóth, V.R.; Szabó, K. Morphometric structural analysis of Phragmites australis stands in Lake Balaton. Ann. de Limnol. -Int. J. Limnol. 2012, 48, 241–251. [Google Scholar] [CrossRef]
- Armstrong, J.; Armstrong, W. Light-enhanced convective throughflow increases oxygenation in rhizomes and rhizosphere of Phragmites australis (Cav.). Trin. ex Steud. New Phytol. 1990, 114, 121–128. [Google Scholar] [CrossRef]
- Brändle, R.; Crawford, R.M.M. Rhizome anoxia tolerance and habitat specialization in wetland plants. In Plant Life in Aquatic and Amphibious Habitats; Crawford, R.M.M., Ed.; Blackwell Scientific Publications: Oxford, UK, 1987; pp. 397–410. [Google Scholar]
- Armstrong, J.; Afreen-Zobayed, F.; Blyth, S.; Armstrong, W. Phragmites australis: Effects of shoot submergence on seedling growth and survival and radial oxygen loss from roots. Aquat. Bot. 1999, 64, 275–289. [Google Scholar] [CrossRef]
- Schmieder, K.; Dienst, M.; Ostendorp, W.; Jöhnk, K. Effects of water level variations on the dynamics of the reed belts of Lake Constance. Ecohydrol. Hydrobiol. 2004, 4, 229–239. [Google Scholar]
- Martinčič, A.; Leskovar, I. Vegetation. In The Vanishing Lake—Monograph on Lake Cerknica; Gaberščik, A., Ed.; Slovenian ecological society: Ljubljana, Slovenia, 2003; pp. 80–96. [Google Scholar]
- Dolinar, N.; Šraj, N.; Gaberščik, A. Water regime changes and the function of an intermittent wetland. In Water and Nutrient Management in Natural and Constructed Wetlands; Vymazal, J., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 251–262. [Google Scholar]
- Kranjc, A. Hydrological characteristics. In The Vanishing Lake—Monograph on Lake Cerknica; Gaberščik, A., Ed.; Slovenian Ecological Society: Ljubljana, Slovenia, 2003; pp. 26–38. [Google Scholar]
- Gaberščik, A.; Urbanc-Bercic, O.; Kržič, N.; Kosi, G.; Brancelj, A. The intermittent Lake Cerknica: Various faces of the same ecosystem. Lakes Reserv. Res. Manag. 2003, 8, 159–168. [Google Scholar] [CrossRef]
- Eller, F.; Lambertini, C.; Nguyen, L.X.; Achenbach, L.; Brix, H. Interactive effects of elevated temperature and CO2 on two phylogeographically distinct clones of common reed (Phragmites australis). AoB PLANTS 2013, 5, pls051. [Google Scholar] [CrossRef]
- Dolinar, N.; Rudolf, M.; Šraj, N.; Gaberščik, A. Environmental changes affect ecosystem services of the intermittent Lake Cerknica. Ecol. Complex. 2010, 7, 403–409. [Google Scholar] [CrossRef]
- Květ, J.; Westlake, D.F.; Dykyjova, D.; Marshall, E.J.P.; Ondok, J. Primary production in wetlands. In The Production Ecology of Wetlands; Westlake, D.F., Květ, J., Szczepansky, A., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 78–168. [Google Scholar]
- Cronk, J.K.; Fennessy, M.S. Wetland Plants: Biology and Ecology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Packer, J.G.; Meyerson, L.A.; Skálová, H.; Pyšek, P.; Kueffer, C. Biological flora of the British isles: Phragmites australis. J. Ecol. 2017, 105, 1123–1162. [Google Scholar] [CrossRef]
- Hayball, N.; Pearce, M. Influences of simulated grazing and water-depth on the growth of juvenile Bolboschoenus caldwellii, Phragmites australis and Schoenoplectus validus plants. Aquat. Bot. 2004, 78, 233–242. [Google Scholar] [CrossRef]
- Onimaru, K.; Yabe, K. Comparisons of nutrient recovery and specific leaf area variation between Carex lasiocarpa var. occultans and Carex thunbergii var. appendiculata with reference to nutrient conditions and shading by Phragmites australis. Ecol. Res. 1996, 11, 139–147. [Google Scholar] [CrossRef]
- Vretare, V.; Weisner, S.E.B.; Strand, J.A.; Granéli, W. Phenotypic plasticity in Phragmites australis as a functional response to water depth. Aquat. Bot. 2001, 69, 127–145. [Google Scholar] [CrossRef]
- Thevs, N.; Zerbe, S.; Gahlert, F.; Mijit, M.; Succow, M. Productivity of reed (Phragmites australis Trin. ex Steud.) in continental-arid NW China in relation to soil, groundwater, and land-use. J. Appl. Bot. Food Qual. 2007, 81, 62–68. [Google Scholar]
- Haslam, S.M. The performance of Phragmites communis Trin. in relation to temperature. Ann. Bot. 1975, 39, 883–888. [Google Scholar] [CrossRef]
- White, S.D.; Ganf, G.G. A comparison of the morphology, gas space anatomy and potential for internal aeration in phragmites australis under variable and static water regimes. Aquat. Bot. 2002, 73, 115–127. [Google Scholar] [CrossRef]
- Dolinar, N.; Gaberščik, A. Mycorrhizal colonization and growth of Phragmites australis in an intermittent wetland. Aquat. Bot. 2010, 93, 93–98. [Google Scholar] [CrossRef]
- Dolinar, N.; Šraj, N.; Pongrac, P.; Regvar, M.; Gaberščik, A. The presence of mycorrhiza in different habitats of an intermittent aquatic ecosystem. In Water and Nutrient Management in Natural and Constructed Wetlands; Vymazal, J., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 299–308. [Google Scholar]
- Dinka, M.; Ágoston-Szabó, E.; Urbanc-Berčič, O.; Germ, M.; Šraj-Kržič, N.; Gaberščik, A. Reed stand conditions at selected wetlands in Slovenia and Hungary. In Wastewater Treatment, Plant Dynamics and Management in Constructed and Natural Wetlands; Vymazal, J., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1–12. [Google Scholar]
- Zhang, W.; Tong, S.; Wang, X.; Liu, B.; Lv, X.; Yang, X. The interaction of water, vegetation and soil in Zhalong wetland, China. J. Food Agric. Environ. 2013, 11, 1287–1294. [Google Scholar]
- Engloner, A.I. Structure, growth dynamics and biomass of reed (Phragmites australis)—GA review. Flora 2009, 204, 331–346. [Google Scholar] [CrossRef]
- Hong, M.G.; Nam, B.E.; Kim, J.G. Differences in functional traits of leaf blade and culm of common reed in four habitat types. J. Ecol. Environ. 2019, 43, 12. [Google Scholar] [CrossRef]
- Afreen, F.; Zobayed, S.; Armstrong, J.; Armstrong, W. Pressure gradients along whole culms and leaf sheaths, and other aspects of humidity-induced gas transport in Phragmites australis. J. Exp. Bot. 2007, 58, 1651–1662. [Google Scholar] [CrossRef]
- Pagter, M.; Bragato, C.; Brix, H. Tolerance and physiological responses of Phragmites australis to water deficit. Aquat. Bot. 2005, 81, 285–299. [Google Scholar] [CrossRef]
- Bodensteiner, L.R.; Gabriel, A.O. Response of mid-water common reed stands to water level variations and winter conditions in Lake Poygan, Wisconsin, USA. Aquat. Bot. 2003, 76, 49–64. [Google Scholar] [CrossRef]
- Tulbure, M.G.; Johnston, C.A. Environmental conditions promoting non-native Phragmites australis expansion in Great Lakes coastal wetlands. Wetlands 2010, 30, 577–587. [Google Scholar] [CrossRef]
- Brix, H.; Ye, S.; Laws, E.A.; Sun, D.; Li, G.; Ding, X.; Yuan, H.; Zhao, G.; Wang, J.; Pei, S. Large-scale management of common reed, Phragmites australis, for paper production: A case study from the Liaohe Delta, China. Ecol. Eng. 2014, 73, 760–769. [Google Scholar] [CrossRef]
- Gaberščik, A.; Murlis, J. The role of vegetation in the water cycle. Ecohydrol. Hydrobiol. 2011, 11, 175–181. [Google Scholar] [CrossRef]
- Kiviat, E. Ecosystem services of Phragmites in North America with emphasis on habitat functions. AoB PLANTS 2013, 5, plt008. [Google Scholar] [CrossRef]
- Köbbing, J.F.; Thevs, N.; Zerbe, S. The utilisation of reed (Phragmites australis): A review. Mires Peat 2013, 13. [Google Scholar]
- Gaglio, M.; Lanzoni, M.; Nobili, G.; Viviani, D.; Castaldelli, G.; Fano, E.A. Ecosystem services approach for sustainable governance in a brackish water lagoon used for aquaculture. J. Environ. Plan. Manag. 2019, 62, 1501–1524. [Google Scholar] [CrossRef]
- Baibagyssov, A.; Thevs, N.; Nurtazin, S.; Waldhardt, R.; Beckmann, V.; Salmurzauly, R. Biomass resources of Phragmites australis in Kazakhstan: Historical developments, utilization, and prospects. Resources 2020, 9, 74. [Google Scholar] [CrossRef]
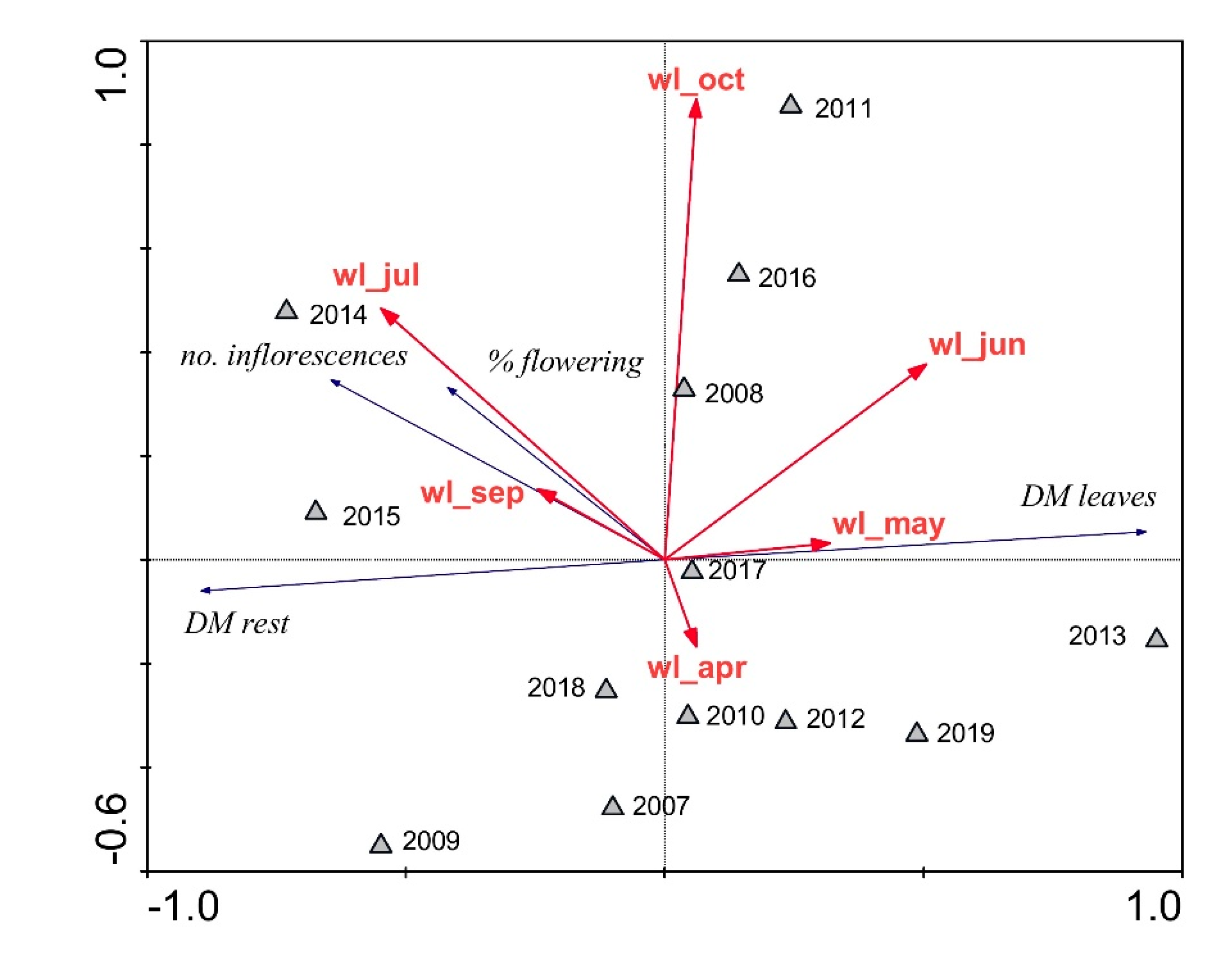
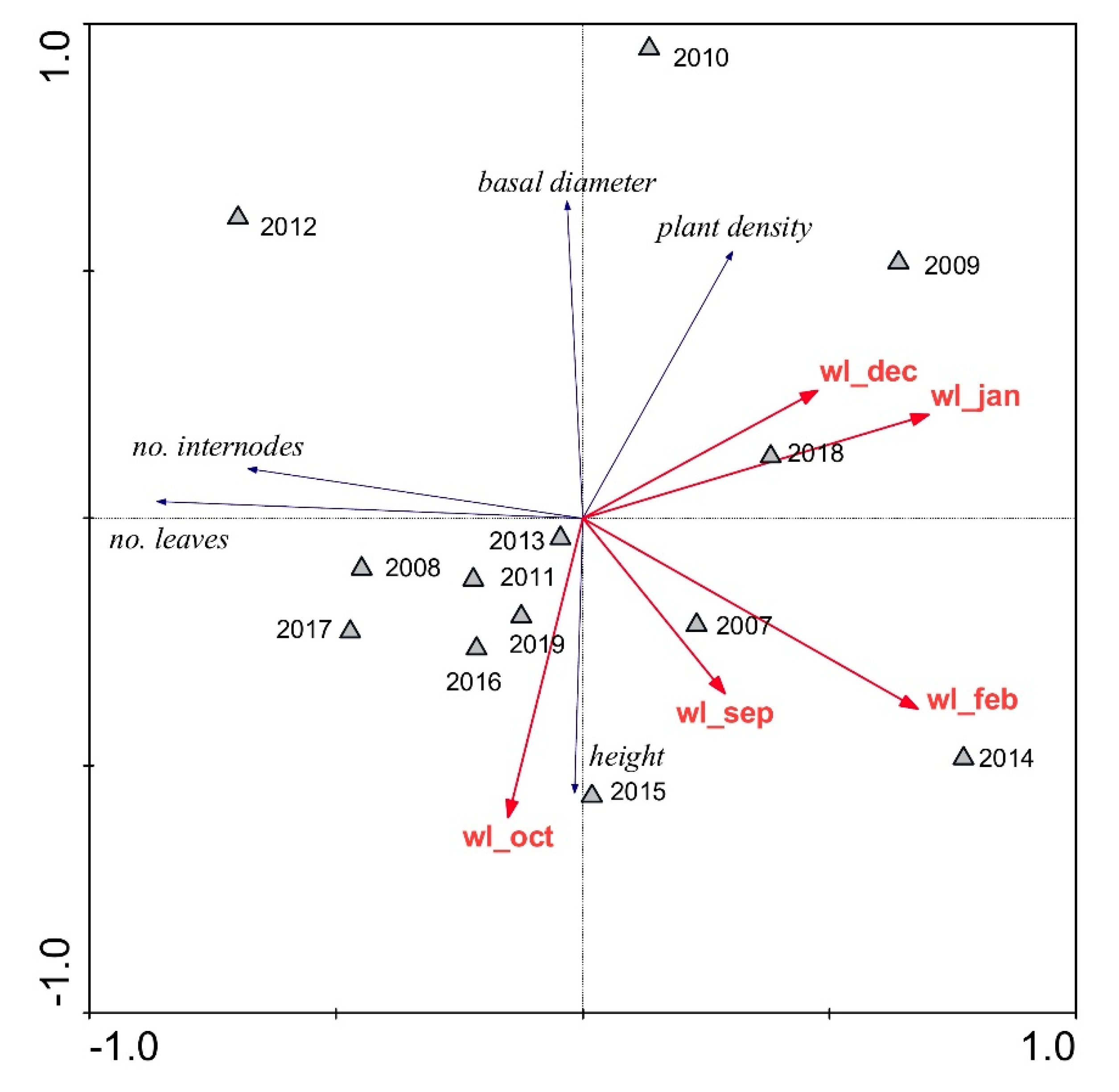
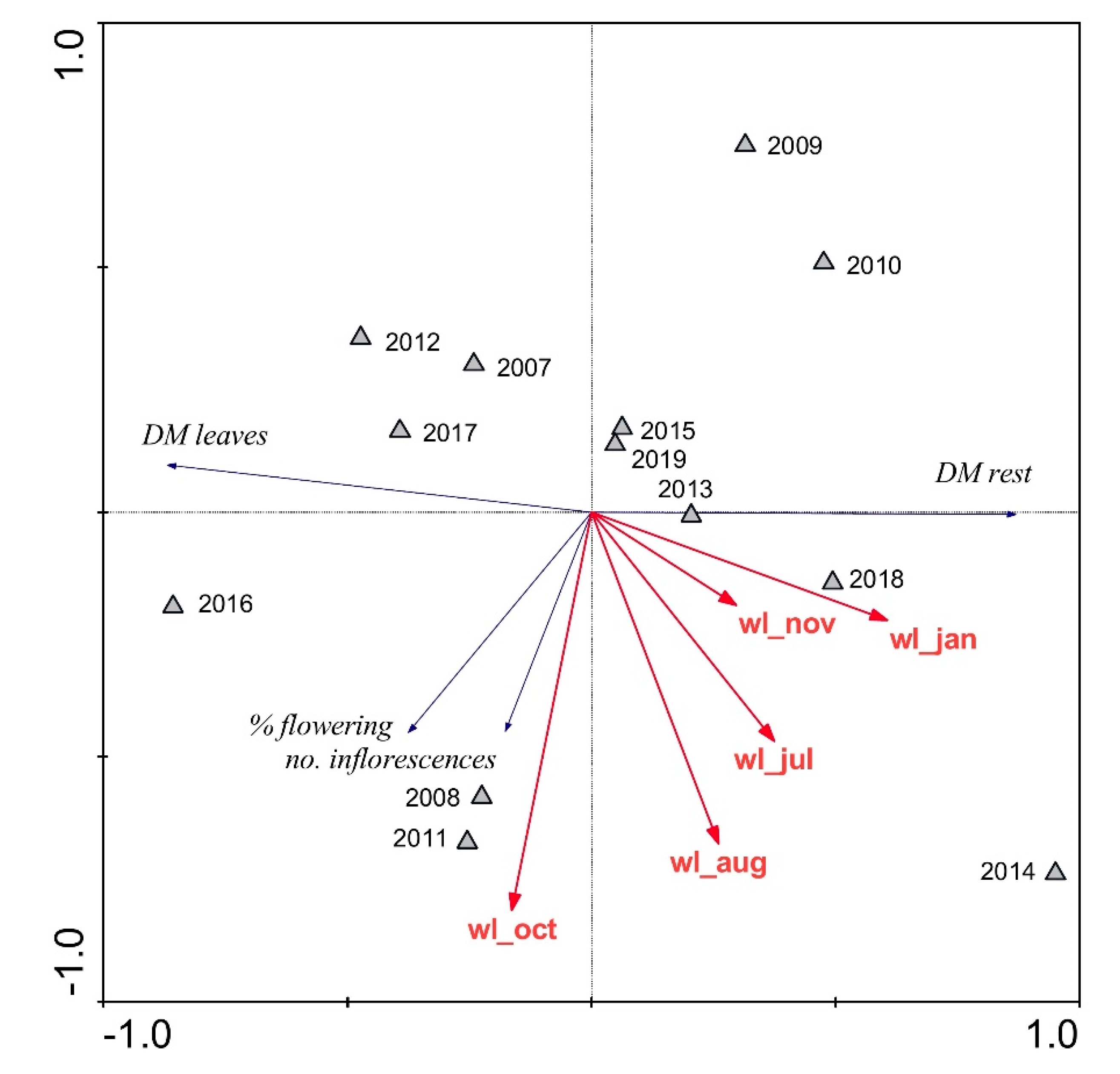
| Parameter | Units | Reed Stand | p | |
|---|---|---|---|---|
| Lake | Riparian | |||
| Mean ± S.D. | Mean ± S.D. | |||
| Height | cm | 148.05 ± 30.59 | 183.27 ± 26.49 | ≤0.001 |
| Basal diameter | mm | 5.25 ± 1.00 | 5.15 ± 0.78 | ns |
| Internodes/plant | n | 16.33 ± 1.70 | 16.01 ± 1.68 | ns |
| Leaves/plant | n | 9.46 ± 3.20 | 9.89 ± 2.40 | ns |
| Plants | n/m2 | 50.40 ± 20.89 | 64.86 ± 26.36 | ≤0.001 |
| Leaf dry mass | g/m2 | 105.28 ± 46.77 | 159.26 ± 72.37 | ≤0.001 |
| Rest dry mass | g/m2 | 338.67 ± 192.44 | 619.62 ± 270.72 | ≤0.001 |
| Flowering | n/m2 | 22.98 ± 15.23 | 36.36 ± 14.32 | ≤0.001 |
| Flowering | % | 47.59 ± 26.63 | 58.71 ± 18.09 | ≤0.05 |
| Parameter | Previous Year | Current Year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sept. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | Apr. | May. | Jun. | Jul. | Aug. | |
| Water Level | ||||||||||||
| Height | 0.13 | −0.30 | −0.29 | 0.15 | 0.10 | 0.01 | 0.07 | 0.09 | −0.47 | −0.28 | 0.07 | −0.17 |
| Basal diameter | −0.16 | −0.70 | −0.38 | 0.06 | −0.16 | 0.06 | −0.29 | 0.05 | −0.40 | −0.34 | −0.35 | −0.48 |
| Internodes/plant | 0.15 | −0.28 | −0.26 | 0.08 | −0.15 | 0.30 | −0.08 | −0.08 | −0.33 | −0.21 | −0.22 | −0.18 |
| Leaves/plant | −0.28 | −0.27 | −0.37 | −0.40 | −0.42 | −0.33 | −0.47 | −0.02 | 0.20 | 0.58 | −0.31 | 0.09 |
| Plants/m2 | 0.03 | −0.22 | −0.02 | 0.27 | 0.25 | 0.11 | 0.06 | 0.17 | −0.03 | −0.19 | 0.00 | −0.32 |
| Leaf dry matter/m2 | −0.16 | −0.55 | −0.48 | 0.01 | −0.00 | −0.29 | −0.18 | 0.02 | −0.22 | −0.06 | −0.51 | −0.61 |
| Rest dry matter/m2 | 0.00 | −0.54 | −0.38 | 0.23 | 0.23 | −0.04 | −0.01 | 0.08 | −0.48 | −0.47 | −0.12 | −0.51 |
| Flowering (n/m2) | 0.17 | −0.19 | −0.21 | 0.21 | 0.22 | 0.09 | 0.07 | 0.08 | −0.50 | −0.44 | 0.19 | −0.21 |
| Flowering (%) | 0.15 | −0.01 | −0.17 | −0.02 | 0.03 | −0.06 | 0.11 | 0.01 | −0.41 | −0.28 | 0.16 | −0.00 |
| Air Temperature | ||||||||||||
| Height | 0.00 | 0.05 | 0.03 | 0.22 | −0.03 | 0.22 | −0.13 | 0.00 | 0.56 | 0.00 | −0.02 | −0.20 |
| Basal diameter | 0.25 | 0.13 | −0.09 | 0.09 | −0.30 | 0.13 | 0.08 | −0.02 | 0.33 | 0.11 | −0.02 | 0.11 |
| Internodes/plant | 0.07 | 0.52 | 0.18 | 0.46 | 0.06 | 0.51 | 0.18 | 0.21 | 0.36 | 0.33 | 0.04 | 0.01 |
| Leaves/plant | 0.32 | −0.13 | −0.01 | −0.14 | −0.14 | 0.07 | −0.05 | −0.40 | −0.38 | 0.45 | 0.13 | 0.03 |
| Plants/m2 | −0.03 | 0.05 | −0.08 | 0.03 | −0.13 | −0.27 | −0.09 | 0.16 | 0.03 | −0.46 | −0.08 | 0.05 |
| Leaf dry matter/m2 | 0.41 | −0.21 | −0.05 | −0.05 | −0.29 | −0.29 | −0.23 | −0.03 | 0.06 | −0.09 | 0.11 | 0.11 |
| Rest dry matter/m2 | 0.16 | −0.02 | −0.14 | 0.20 | −0.17 | −0.16 | −0.05 | 0.09 | 0.49 | −0.22 | −0.07 | −0.01 |
| Flowering (n/m2) | −0.16 | 0.03 | −0.11 | 0.20 | 0.05 | 0.16 | −0.02 | 0.09 | 0.61 | −0.19 | −0.21 | −0.22 |
| Flowering (%) | −0.06 | −0.03 | 0.02 | 0.18 | 0.17 | 0.27 | −0.02 | −0.07 | 0.52 | 0.11 | −0.03 | −0.28 |
| Parameter | Previous Year | Current Year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sept. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | Apr. | May. | Jun. | Jul. | Aug. | |
| Water Level | ||||||||||||
| Height | −0.04 | 0.16 | −0.12 | −0.30 | −0.21 | 0.20 | 0.04 | 0.02 | 0.17 | 0.14 | 0.36 | 0.46 |
| Basal diameter | 0.32 | −0.11 | −0.13 | 0.26 | 0.31 | 0.02 | 0.07 | 0.45 | −0.10 | 0.22 | 0.44 | 0.06 |
| Internodes/plant | −0.23 | 0.02 | −0.00 | −0.35 | −0.34 | 0.36 | 0.04 | 0.03 | 0.35 | 0.01 | −0.11 | 0.20 |
| Leaves/plant | −0.25 | −0.03 | −0.06 | −0.30 | −0.27 | 0.18 | 0.08 | 0.10 | 0.24 | −0.19 | −0.19 | −0.09 |
| Plants/m2 | −0.07 | −0.26 | −0.19 | 0.09 | 0.13 | −0.06 | −0.12 | −0.10 | −0.19 | 0.03 | −0.12 | −0.14 |
| Leaf dry matter/m2 | −0.02 | −0.12 | −0.32 | −0.03 | 0.10 | 0.05 | 0.01 | 0.07 | −0.13 | −0.06 | 0.04 | −0.09 |
| Rest dry matter/m2 | 0.13 | −0.02 | −0.16 | 0.07 | 0.22 | 0.13 | 0.11 | 0.11 | −0.06 | 0.11 | 0.30 | 0.14 |
| Flowering (n/m2) | −0.01 | −0.05 | −0.07 | −0.06 | 0.06 | 0.13 | 0.07 | −0.02 | 0.09 | 0.07 | 0.12 | 0.16 |
| Flowering (%) | 0.05 | 0.30 | 0.11 | −0.22 | −0.09 | 0.12 | 0.13 | −0.03 | 0.25 | 0.05 | 0.22 | 0.35 |
| Air Temperature | ||||||||||||
| Height | −0.10 | 0.22 | 0.02 | 0.33 | 0.23 | 0.53 | 0.17 | −0.20 | −0.05 | 0.19 | 0.08 | −0.32 |
| Basal diameter | −0.31 | 0.11 | 0.10 | −0.03 | 0.16 | 0.15 | −0.37 | −0.02 | 0.20 | −0.36 | −0.34 | −0.56 |
| Internodes/plant | −0.02 | 0.16 | −0.10 | −0.03 | 0.10 | 0.40 | 0.13 | 0.03 | −0.23 | 0.21 | 0.10 | 0.08 |
| Leaves/plant | −0.05 | −0.15 | −0.31 | −0.21 | 0.02 | 0.05 | −0.05 | 0.03 | −0.08 | −0.03 | 0.18 | 0.25 |
| Plants/m2 | 0.15 | −0.05 | 0.05 | −0.01 | −0.18 | −0.01 | −0.01 | 0.06 | 0.02 | −0.00 | −0.21 | −0.08 |
| Leaf dry matter/m2 | −0.13 | −0.14 | −0.19 | −0.05 | 0.05 | 0.13 | −0.14 | 0.11 | 0.17 | −0.14 | −0.22 | −0.14 |
| Rest dry matter/m2 | −0.12 | 0.15 | 0.10 | 0.19 | 0.14 | 0.27 | −0.09 | 0.07 | 0.08 | −0.16 | −0.23 | −0.43 |
| Flowering (n/m2) | 0.14 | 0.30 | 0.17 | 0.35 | 0.10 | 0.20 | 0.15 | 0.00 | −0.13 | 0.07 | −0.00 | −0.24 |
| Flowering (%) | −0.01 | 0.29 | 0.09 | 0.36 | 0.34 | 0.21 | 0.24 | −0.08 | −0.14 | 0.18 | 0.17 | −0.14 |
| Parameter Class | Monthly Parameter | Month | Explained Variance (%) | p |
|---|---|---|---|---|
| Growth | Water level | June | 22 | 0.001 |
| July | 25 | 0.001 | ||
| August | 8 | 0.002 | ||
| April | 5 | 0.003 | ||
| February | 5 | 0.003 | ||
| Air temperature | May | 29 | 0.001 | |
| August | 12 | 0.001 | ||
| July | 10 | 0.001 | ||
| January | 8 | 0.001 | ||
| March | 5 | ˂0.01 | ||
| December | 5 | ˂0.01 | ||
| September | 4 | ˂0.01 | ||
| October | 4 | ˂0.01 | ||
| April | 3 | ˂0.01 | ||
| July | 3 | ˂0.01 | ||
| February | 3 | ˂0.01 | ||
| Assimilate allocation | Water level | July | 23 | 0.001 |
| June | 29 | 0.001 | ||
| April | 10 | 0.001 | ||
| May | 6 | 0.004 | ||
| October | 4 | ˂0.01 | ||
| September | 4 | ˂0.01 | ||
| Air temperature | May | 19 | 0.001 | |
| December | 13 | 0.001 | ||
| October | 8 | 0.001 | ||
| January | 7 | 0.002 | ||
| March | 7 | 0.002 | ||
| April | 5 | 0.001 | ||
| February | 5 | 0.001 | ||
| September | 6 | ˂0.01 | ||
| August | 6 | ˂0.01 |
| Parameter Class | Monthly Parameter | Month | Explained Variance (%) | p |
|---|---|---|---|---|
| Growth | Water level | February | 19 | 0.001 |
| December | 15 | 0.001 | ||
| September | 9 | 0.002 | ||
| October | 5 | 0.002 | ||
| January | 7 | 0.001 | ||
| Air temperature | October | 15 | 0.001 | |
| June | 11 | 0.002 | ||
| January | 8 | ˂0.01 | ||
| July | 8 | ˂0.01 | ||
| Assimilate allocation | Water level | January | 22 | 0.001 |
| November | 19 | 0.001 | ||
| July | 9 | 0.001 | ||
| October | 9 | 0.007 | ||
| August | 6 | 0.006 | ||
| Air temperature | July | 17 | 0.001 | |
| February | 11 | 0.004 | ||
| October | 9 | 0.007 | ||
| April | 6 | 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaberščik, A.; Grašič, M.; Abram, D.; Zelnik, I. Water Level Fluctuations and Air Temperatures Affect Common Reed Habitus and Productivity in an Intermittent Wetland Ecosystem. Water 2020, 12, 2806. https://doi.org/10.3390/w12102806
Gaberščik A, Grašič M, Abram D, Zelnik I. Water Level Fluctuations and Air Temperatures Affect Common Reed Habitus and Productivity in an Intermittent Wetland Ecosystem. Water. 2020; 12(10):2806. https://doi.org/10.3390/w12102806
Chicago/Turabian StyleGaberščik, Alenka, Mateja Grašič, Dragan Abram, and Igor Zelnik. 2020. "Water Level Fluctuations and Air Temperatures Affect Common Reed Habitus and Productivity in an Intermittent Wetland Ecosystem" Water 12, no. 10: 2806. https://doi.org/10.3390/w12102806
APA StyleGaberščik, A., Grašič, M., Abram, D., & Zelnik, I. (2020). Water Level Fluctuations and Air Temperatures Affect Common Reed Habitus and Productivity in an Intermittent Wetland Ecosystem. Water, 12(10), 2806. https://doi.org/10.3390/w12102806






