Abstract
As a species for ecological restoration in northern China, Tamarix ramosissima plays an important role in river protection, flood control, regional climate regulation, and landscape construction with vegetation. Two sampling sites were selected in the hillside and floodplain habitats along the Lanzhou City, and the xylems of T. ramosissima and potential water sources were collected, respectively. The Bayesian mixture model (MixSIAR) and soil water excess (SW-excess) were applied to analyze the relationship on different water pools and the utilization ratios of T. ramosissima to potential water sources in two habitats. The results showed that the slope and intercept of local meteoric water line (LMWL) in two habitats were smaller compared with the global meteoric water line (GMWL), which indicated the existence of drier climate and strong evaporation in the study area, especially in the hillside habitat. Except for the three months in hillside, the SW-excess of T. ramosissima were negative, which indicated that xylems of T. ramosissima are more depleted in δ2H than the soil water line. In growing seasons, the main water source in hillside habitat was deep soil water (80~150 cm) and the utilization ratio was 63 ± 17% for T. ramosissima, while the main water source in floodplain habitat was shallow soil water (0~30 cm), with a utilization ratio of 42.6 ± 19.2%, and the water sources were different in diverse months. T. ramosissima has a certain adaptation mechanism and water-use strategies in two habitats, and also an altered water uptake pattern in acquiring the more stable water. This study will provide a theoretical basis for plant water management in ecological environment protection in the Loess Plateau.
1. Introduction
As a necessary nutrient for plant growth, water affects the vegetation composition and community structure of the ecosystem [1,2], and it is the primarily limiting factor of vegetation growth in arid and semi-arid areas [3,4]. With the variations of global climatic conditions, the hydrological process in arid and semi-arid areas will change, resulting in the change of water use pattern of plants [5]. Under the background of climate change, it is thus essential to analyze the water sources of plants in arid and semi-arid regions to gain a comprehensive knowledge of ecohydrological process and ecological management [6,7].
At present, more and more achievements have been made in the research on plants water sources in arid and semi-arid regions [4,8,9,10,11,12,13,14,15], many studies have shown that plants water sources vary with plant species [8,9], groundwater level [10,11], the distance to the river bank [12,13], and the habitats [14,15]. In terms of different habitats, water plays indispensable roles in plant productivity and species diversity in terrestrial ecosystems and influences the distribution and ecological functions of vegetation [16]. The plants water uptake is not invariable, plants will respond to water stress as habitats change, thus reflecting the growth strategies of vegetation [17]. Plants have a strong ability of self-regulation and adaptation [4], which can adapt to changes in habitat by changing the main water source, especially in water functioning as the main limiting factor of ecological system. The water source conversion ability is conducive to improve its advantage in interspecies competition [18], which is very beneficial to plant survival, breeding and competition [19]. The self-regulation and adaptability of plants in arid and semi-arid regions can be analyzed by studying the variation of water sources in different habitats. So, it may help understand the effects of water conditions change in different habitats on plant water source, water-use efficiency and drought resistance.
As a tracer of water cycle, water stable isotopes (δ18O and δ2H) has been commonly used in many fields within the discipline of ecohydrology in recent years, mainly including plants water strategy [20,21,22], soil water movement process [23,24], precipitation and soil water mixing process [25,26,27] and other aspects. Stable hydrogen and oxygen isotopes provide a more effective, powerful and nondestructive approach to identify plant water sources compared with root system excavation means [22]. For most terrestrial plants, there is no isotopic discrimination in the water absorption of plant before transpiration [28], thus, plant water sources can be confirmed by comparing the isotopic compositions in xylem water and all potential water sources [29]. Many methods and models have been used to calculate the contribution ratios of potential water sources to plants, such as the graphical inference method, Isosource model, Bayesian isotope mixing model (MixSIR, SIAR and MixSIAR), etc. Wang et al. and Zhang et al. [30,31] found that the MixSIAR model has a better water source apportionment performance by comparing different methods. However, a study indicated that hydrogen isotopic fractionation does occur in xerophytes and halophytes when they absorb the water [32], so only oxygen isotopes are selected to calculate the utilization ratios of plant by MixSIAR model in this study.
Tamarix ramosissima is a shrub or small tree, with the advantages of drought tolerance, salt and alkali resistance, high survival rate, rapid growth and strong adaptability. It is the dominant plant in the mountains and floodplains of Lanzhou City, and acts as an afforestation and river protection species for the northern and southern mountains of Lanzhou City and Lanzhou section of the Yellow River. It not only has the functions of river protection and flood control, but can also regional climate regulation and beautify the environment. For Lanzhou City, existing researches were mainly focused on the water source of T. ramosissima in the floodplain habitat. It was found that shallow soil water was the main source of water for T. ramosissima, and in low soil moisture months, T. ramosissima increased the use of deep soil water and river water [33]. Other studies on the water sources of plants in Lanzhou City have mainly focused on the foothills and hillside habitats [31,34]. Nevertheless, there were fewer comparative studies on different habitats. To address this research gap, this study took T. ramosissima in the hillside and floodplain habitats of Lanzhou City as the research object, and collected samples of plant xylem and various potential water sources during the plant growth period (April to October), then determined the contribution ratios of potential water sources for T. ramosissima based on the MixSIAR model. Finally, water use strategies and the adaptation mechanism of T. ramosissima in two habitats were revealed, which provided a theoretical support for ecological construction in the Loess Plateau.
2. Materials and Methods
2.1. Study Area
This study was conducted in Lanzhou City (35°34′~37°07′ N, 102°35′~104°34′ E), Gansu Province, China, which is located in the northwest of the Loess Plateau and the upper reaches of the Yellow River (Figure 1). It is the only provincial capital city where the Yellow River flows from southwest to northeast, traverses the whole city, cuts through mountains, and forms a beaded river valley landform between the canyon and the basin [35]. The overall topography is high in the southwest and low in the northeast, and is characterized by a long and narrow banded basin from east to west, with an average altitude of 1500 m. The mean annual temperature and precipitation are 10.3 °C and 324 mm, with the temperate continental climate of study area [36]. The soil is mainly sierozem, and the parent material is loess [37]. The annual average and minimum streamflow in Lanzhou section of the Yellow River are 1022 m3/s and 213 m3/s, and the average highest and lowest water level are 1513.63 m and 1511.30 m, respectively. The sediment is mainly concentrated in the flood season (July to October). The natural vegetation is mainly perennial grasses, xerophytic shrubs and small trees. The vegetation types in the South and North mountains of Lanzhou City are mainly ecological protection forests and scenic forests, which mainly includes Platycladus orientalis, Tamarix chinensis, Caragana sinica, Reaumuria soongarica, etc. [38]. Among them, riparian plants mainly include Salix matsudana, Tamarix ramosissima, Ulmus pumila, Phragmites australis, Typha orientalis, Chenopodium glaucum, etc. [39].
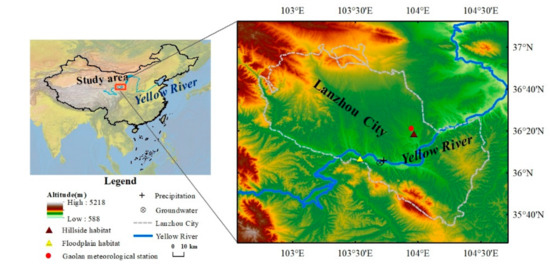
Figure 1.
Location of the study area and sampling sites, including the hillside and floodplain habitats in the Lanzhou City.
2.2. Experimental Design and Sample Collection
Considering the distribution of vegetation in the study area and the convenience of sampling, During the growing seasons of plants, from April to October 2019, the experiment was carried out in Lanzhou City and the T. ramosissima in the habitats of hillside and floodplain was selected as the research object (Figure 1). All samples were collected once a month. Each sampling should be completed between 08:00 and 11:00 (Beijing time) to avoid the influence of light and temperature on the experiment. When collecting plant samples, selected three non-green branches from different individuals with a diameter of 3–5 mm as the collection object of xylem of T. ramosissima, peeled off the outer bark and phloem of the plant quickly, and put the xylem in 10 mL glass bottles, because isotopic fractionation did occur in the outer bark and phloem [40,41].
A soil profile of 150 cm depth was dug around the sampling plants, and soil samples were collected at intervals of 10 cm. Due to the limitation of soil conditions and tools during sampling, the soil depth of hillside habitat was 110 cm in April. Two parts soil samples were collected from each layer, one in an aluminum box for the determination of soil water content (SWC) and the other in 10 mL glass bottles for isotopic analysis, with two parallel samples taken for each layer.
Before collecting the river water and groundwater, cleaned the sample bottles three times with river water or groundwater. The river water was collected at floodplain habitat sampling site and groundwater samples were obtained from the well of the Yellow River bank near the south gate of Gansu Agricultural University.
The precipitation samples in hillside and floodplain habitats were taken from the standard rainwater container placed in Gaolan meteorological station and the new campus of Northwest Normal University, respectively (Figure 1). In order to reduce the evaporation of samples, we put samples after each precipitation immediately into 50 mL plastic bottles.
The xylem, river water and groundwater samples were taken in parallel samples for three times during sampling. All the samples were immediately placed into glass bottles, sealed with parafilm, and kept freezing before analyzing.
2.3. Isotopic Analyses
The water contained in plant xylem and soil samples was extracted by the cryogenic vacuum distillation system (LI-2100, LICA, Beijing, China) [42]. In order to eliminate impurities and organic contamination, the extracted water, precipitation, river water and groundwater were filtered by 0.22-μm organic phase pin-type filters. The δ18O and δ2H of different water pools were measured using the liquid-water isotope analyzer T-LWIA-45-EP (ABB-Los Gatos Research, CA, USA) at the Stable Isotope Laboratory, College of geography and environmental science, Northwest Normal University. Each sample and isotopic standard were measured sequentially six times and the average of last four results was accepted as the final value with the first two injections being discarded. The measurement precision is ±0.3‰ for δ18O and ±1‰ for δ2H, respectively.
The ratio of stable isotopes can be expressed by the symbol “δ” and relative to Vienna Standard Mean Ocean Water (V-SMOW):
where Rsample is the ratio of 18O/16O or 2H/1H in the samples and Rstandard is the ratio of 18O/16O or 2H/1H in V-SMOW.
Extracted water using cryogenic vacuum distillation system can codistill organic compounds (e.g., methanol and ethanol) that may affect the spectrum and cause erroneous isotope values. It was found that organic compounds mainly existed in water extracted from plant xylem samples and less in soil water [29].
The values after spectral contamination correction were selected as the δ18O and δ2H of soil and xylem samples, and the spectral contamination correction was carried out by the ways provided by Los Gatos Research Company.
Soil water content (SWC) was measured by a loss-on-drying method. First measured the weight of wet soil with an electronic balance (0.0001 g), then baked the soil sample in an oven at (150 ± 2) °C for about 24 h until a constant weight, and finally measured the dry soil weight after being placed at normal temperature.
2.4. Data Analyses
2.4.1. Division of Plant Water Sources
The potential water sources of hillside habitat include soil water, precipitation and groundwater, while that of floodplain habitat includes soil water, river water, precipitation and groundwater. As each sampling was carried out in sunny days as far as possible and precipitation eventually infiltrated into soil water, thus precipitation in both habitats was not regarded a potential water source for plants. Due to the dry climate, strong evaporation and deeply buried groundwater (>120 m) in hillside habitat, thus it is difficult for vegetation to absorb and use the groundwater. Considering this, soil water at different depths is considered as a potential water source for hillside habitat [34]. The interaction between river water and groundwater in the floodplain habitat is relatively strong, leading to a similar isotopic value between river and groundwater, so they are regarded as the same water source in the floodplain habitat [29]. Therefore, soil water at different layers and river water are water sources for floodplain habitats.
One-way ANOVA was used to calculate the difference of soil water δ18O and δ2H in every layer, and the least significant difference (LSD) method was used for conduct multiple comparisons of the data, and its significance was tested at the level of 0.05. All statistical analyses were conducted using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). The adjacent soil layers were classified, and average value was taken as the isotope value to divide the new soil layer [43]. According to the test results, soil profile was classified into three soil layers as follows: shallow soil layer (0~30 cm), middle soil layer (30~80 cm) and deep soil layer (80~150 cm). Therefore, water sources of the hillside habitat were divided into the shallow, middle and deep soil water, where those of floodplain habitat were restricted to the shallow, middle and deep soil water and river water.
2.4.2. MixSIAR Model
The contribution of potential water sources for plants can be estimated by the Bayesian mixing model MixSIAR of an installation package used in R [44]. Bayesian mixing models improve upon simpler linear mixing models by explicitly taking uncertainty in source values, categorical and continuous covariates, and prior information into account [45]. MixSIAR incorporates several years of advances in Bayesian mixing model theory since MixSIR and SIAR [46]. The MixSIAR include mixing data (the raw xylem δ18O), and source data (average and standard error of δ18O in each potential water source) and discrimination data were set to zero for δ18O. The running length of Markov Chain Monte Carlo (MCMC) was set to “long”, the error structure and specify prior were set to “Residual only” and “Uninformative/Generalist” in the MixSIAR model, respectively [7]. Gelman Rubin and Geweke diagnostic tests were used to determine whether the model was close to convergence.
2.4.3. SW-Excess
Landwehr and Coplen [47] proposed the line-conditioned excess (LC-excess) to estimate the isotopic offset between xylem water and its potential water sources:
where a and b are the slope and intercept of the local meteoric water line (LMWL), respectively.
LC-excess = δ2H − aδ18O − b
However, since plants are more likely to use soil water directly than precipitation. Barbeta et al. [29] proposed the deviation of plant xylem with respect to soil water line based on LC-excess equation, was described as SW-excess:
where as and bs represent the slope and intercept of the soil water line (SWL) from the same sampling site and date, respectively, and δ2H and δ18O are the isotopic composition of a xylem water collected on that site and date. The SW-excess of xylem water express the offsets of δ2H between xylem water and soil water lines. The SW-excess of positive values illustrate xylem water more enriched δ2H than the soil water line, while that of negative values illustrate xylem water more depleted δ2H than the soil water line.
SW-excess = δ2H − asδ18O − bs
3. Results
3.1. Environmental Conditions
The variation characteristics of the environmental conditions in the two habitats of the study area in 2019 are shown in Figure 2. From April to October, the mean temperature was 18.8 °C, with a mean highest temperature of 23 °C in July and a mean lowest temperature of −3 °C in January (Figure 2). The total precipitation under the habitats of hillside and floodplain were 1199.1 mm and 1170.9 mm and 93% occurred during the growing seasons. The average relative humidity was 47% in both habitats.
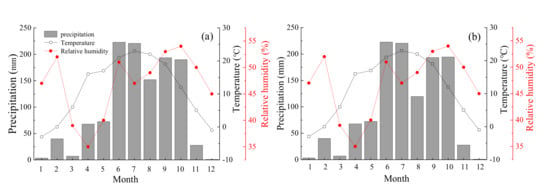
Figure 2.
Variations in temperature, precipitation and relative humidity at the hillside (a) and floodplain (b) habitats during the sampling periods. The meteorological data were downloaded the weather query website (https://tianqi.911cha.com/).
The vertical distribution characteristics of SWC are shown in the Figure 3, the SWC in hillside and floodplain changed with soil layers and months and the soil water content in the floodplain was higher than that in hillside as a whole. The SWC of hillside basically increased with soil depths and with a slight fluctuation (Figure 3a). However, the shallow soil water content in June was significantly greater than other months and reaching 11.38 ± 2.53%, which may be related to the rainfall before sampling. The SWC of floodplain presented more variation, which basically presented a trend of decreasing first, then increasing to a certain peak and finally stabilizing (Figure 3b). The highest SWC was found in August, and the lowest in May. The mean SWC in the shallow, middle and deep layers of the hillside were 6.03 ± 3.21%, 7.14 ± 2.23% and 8.97 ± 2.65%, and that of the floodplain were 15.86 ± 2.66%, 22.74 ± 2.51% and 25.11 ± 1.72%, respectively.
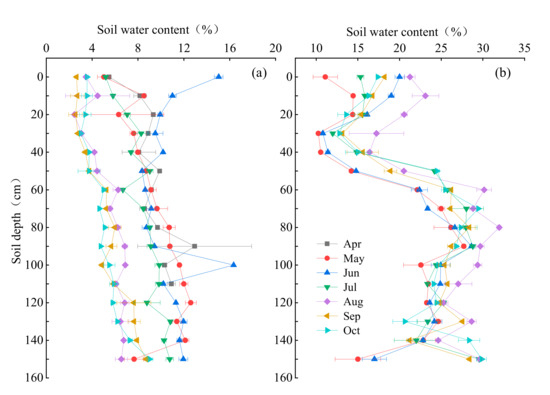
Figure 3.
Variations of soil profile water content in the hillside (a) and floodplain (b) habitats (Mean ± SD, n = 2).
3.2. Isotopic Characteristics of Various Water Pools
As the δ2H and δ18O of different water pools in two habitats are shown (Figure 4). The δ18O of precipitation in hillside and floodplain habitats ranged from −15.39 to 21.17‰ and −14.49 to 8.28‰, with an average of −4.53 ± 6.48‰ and −5.47 ± 4.82‰, respectively. The local meteoric water line (LMWL) was fitted based on the δ2H and δ18O of precipitation under hillside and floodplain habitats, which were δ2H = 4.98δ18O − 10.94 (R2 = 0.906, p < 0.0001) and δ2H = 7.05δ18O + 4.60 (R2 = 0.944, p < 0.0001), respectively. The slope and intercept of the LMWL in both habitats were less than those of the GMWL [48], indicating the existence of a relatively dry climate and strong evaporation in the sampling sites, especially in the hillside habitat.
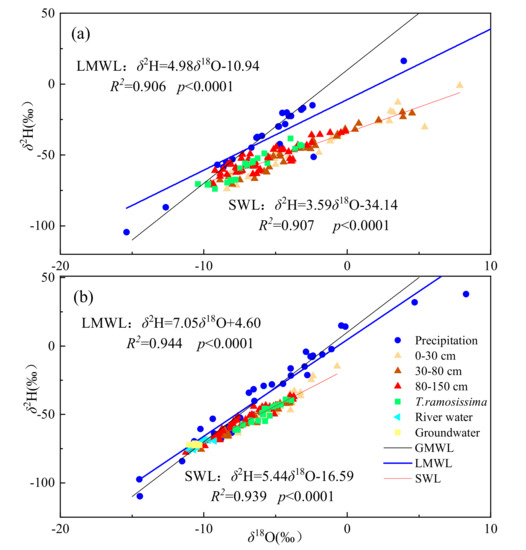
Figure 4.
The distribution characteristics of δ18O and δ2H of precipitation, xylem water, Yellow River water, groundwater, and different depth of soil water at the hillside (a) and floodplain (b) habitats during the sampling periods. SWL is the soil water line. LMWL is the local meteoric water line, GMWL represents the global meteoric water line (δ2H = 8δ18O + 10).
The isotopic compositions of the soil and xylem water in hillside showed a larger fluctuation with sampling dates compared with floodplain habitat. The δ18O variation of soil water in hillside and floodplain habitats were from −9.53 to 7.85‰ and −11.25 to −0.7‰, and mean values were −3.77 ± 4.05‰ and −6.48 ± 2.22‰, respectively (Figure 5). The soil water line (SWL) of the hillside and floodplain habitats were δ2H = 3.59δ18O − 34.14 (R2 = 0.907 p < 0.0001) and δ2H = 5.44δ18O − 16.59 (R2 = 0.939 p < 0.0001), which were similar to the LMWL, and the slope of SWL in hillside was also smaller than that of the floodplain.
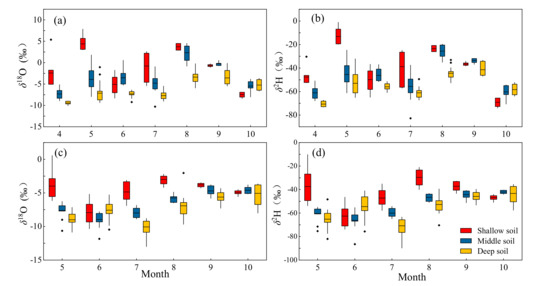
Figure 5.
Isotopic composition of three soil layers water in hillside (a,b) and floodplain (c,d) for each sampling campaign, where (a,c) are the values of δ18O, (b,d) are the values of δ2H.
The xylem water of T. ramosissima ranged from −9.94 to 3.36‰ for δ18O and −70.67 to −38.42‰ for δ2H in hillside, and ranged from −7.72 to −4.76‰ for δ18O and −61.02 to −42.25‰ for δ2H in floodplain, respectively. The δ2H and δ18O of river water and groundwater were not statistically different and relatively stable over time (Figure 6a,b). Almost all of the values of the xylem and soil water were plotted to the right of the LMWL (Figure 2), implying the existence of strong evaporation and isotopic enrichment compared with precipitation in the study area. Compared with xylem water, the isotopic values of the soil water, river water and groundwater were closer to the LMWL, which reflected the recharge of precipitation on soil water, Yellow river water and groundwater.
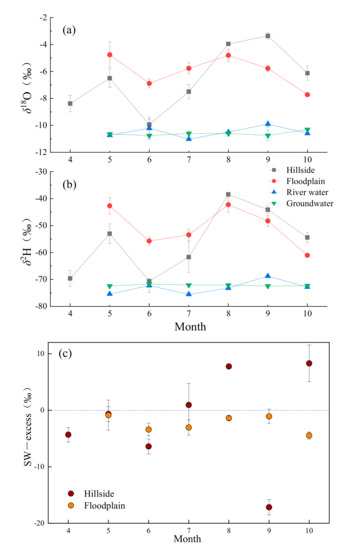
Figure 6.
Temporal variations of δ18O (a) and δ2H (b) xylem water, groundwater and Yellow river water in the hillside and floodplain habitats and SW-excess (c) of T. ramosissima during the sampling periods (Mean ± SD, N = 3).
3.3. Isotopic Composition of Water Sources
In total, the δ18O and δ2H of the soil water varied with the soil layers and seasons (Figure 5), and the isotopic values in shallow soil layer presented higher variation than that in middle and deep soil layers. The δ18O and δ2H of soil water were negative, except for some shallow and middle soil layers of the hillside habitat. In hillside, the values of δ18O for soil water in the shallow, middle and deep soil layers were −0.99 ± 4.64‰, −3.23 ± 3.68‰ and −5.92 ± 2.53‰, respectively. From shallow to deep layer, the δ18O of soil water in floodplain were −4.75 ± 1.98‰, −6.6 ± 1.83‰ and −7.38 ± 2.06‰, respectively. The isotopic fluctuation in shallow soil water was greater than that in middle and deep layers from April to July, while it exhibited an opposite trend from August to October. The isotopic compositions in 0–30 cm soil water are more enriched compared with that in deeper soil water, and those in hillside were greater than floodplain habitat. The δ18O and δ2H of the deep layer were relatively stable among the same month in hillside habitat (Figure 5a,b).
The isotopic values in river water and groundwater were lower than soil water, and the variation of groundwater was smaller (Figure 5 and Figure 6). Averages of δ18O and δ2H values for the river water were −10.5 ± 0.39‰ and −73 ± 2.48‰, and groundwater samples collected from the wells exhibited an average δ18O of −10.6 ± 0.18‰ and δ2H of −72.08 ± 0.41‰.
3.4. Isotopic Compositions in Xylem Water and SW-Excess
The δ18O and δ2H of xylem water varied among different habitats and sampling dates (Figure 6). The average isotopic compositions for T. ramosissima in hillside and floodplain habitats were −55.99 ± 12.21‰ and −50.53 ± 7.51‰ for δ2H, respectively, and −6.54 ± 2.34‰ and −5.95 ± 1.17‰ for δ18O, respectively. The highest δ18O of xylem of hillside and floodplain habitats were −3.36 ± 0.24‰ and −3.36 ± 0.24‰ in September and May, respectively, while the lowest δ18O of those were −9.94 ± 0.4‰ and −7.72 ± 0.09‰ in June and October, respectively. From May to July, the xylem value of T. ramosissima in floodplain was larger than hillside habitat, while the opposite happened from August to October. The δ2H and δ18O of xylem in hillside presented higher variation compared with floodplain habitat, and the trend of change was consistent with both sampling sites.
As shown in Figure 6c, the SW-excess of T. ramosissima were negative, except for the three months in the hillside habitat. On average, xylem water samples in hillside and floodplain habitats had a SW-excess of −1.65 ± 8.81‰ and −2.37 ± 1.47‰. In terms of different months, the SW-excess fluctuation in floodplain was obviously less than that in hillside, and relatively stable. The SW-excess of T. ramosissima in hillside reached the maximum value in October (8.3 ± 3.25‰), while the minimum value in September (−17.16 ± 1.36‰). The SW-excess of T. ramosissima was the highest in May (−0.87 ± 2.67‰) and the lowest in October (−4.47 ± 0.64‰) in floodplain habitat.
3.5. Variations in the Proportion of Plant Water Uptake
Using MixSIAR model to calculate the utilization rate of potential water sources by plants and the results were shown in Figure 7. In the hillside habitat, the deep soil water was the main water source for T. ramosissima during growing seasons, and the utilization ratios was 63 ± 17%, the shallow soil showed the weakest contribution (15.3 ± 10.9%). While T. ramosissima in floodplain primarily absorbed water from shallow soil (42.6 ± 19.2%), groundwater had the lowest ratio (18 ± 15.8%), and the contribution ratio of middle and deep soil water was about 20%.
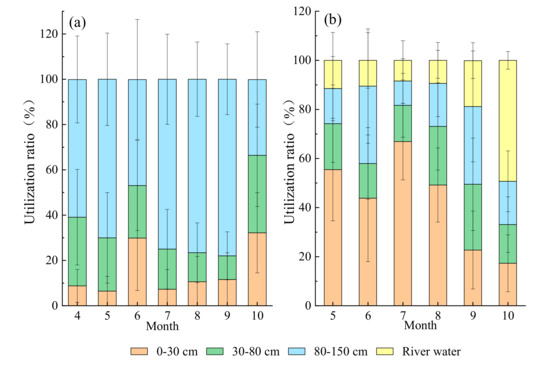
Figure 7.
Monthly variations in contribution ratios of potential water sources to T. ramosissima based on MixSIAR in hillside (a) and floodplain (b) habitats (Mean ± SD, N = 3).
The water uptake patten of T. ramosissima varied with habitats and seasons. At hillside, the fraction of water absorb from 80–150 cm soil layer was the greatest in September (78 ± 15.6%) and the lowest in October (33.5 ± 21.1%), and the utilization rate of T. ramosissima on shallow soil water was higher in June and October, which were 29.9 ± 23.2% and 32.2 ± 17.7%, respectively (Figure 7a). At floodplain, T. ramosissima derived the largest proportion (66.9 ± 15.6%) of water from the 0–30 cm soil layer in July, and the lowest in October (17.3 ± 11.6%). The utilization of shallow soil water by T. ramosissima decreased significantly, but that of river water increased in September and October (Figure 7b). The proportion of river water absorbed by T. ramosissima remained at about 8% from May to August, while in September and October it increased to 18.7 ± 7.3% and 49.3 ± 3.6%, respectively.
4. Discussion
Our results displayed that the δ2H and δ18O of xylem water in hillside and floodplain habitats approach those of soil water, and both of them were less than precipitation. The values of δ2H and δ18O for the Yellow River and groundwater were smaller compared with other water pools in floodplain habitat. Both of the above illustrated that soil water was the direct water source of T. ramosissima, while the Yellow river water, groundwater and precipitation were the indirect water source for T. ramosissima. Previous studies have also reached alike conclusions [25] that the river water, groundwater and precipitation need to infiltrate into the soil layer and eventually be absorbed and utilized by plants [49]. In this transformational process, the δ2H and δ18O of soil water would be enriched due to isotopic fractionation.
As water resources in environment change with time and space, plants will adopt different water use strategies to cope with water stress caused by this change [50]. Our results showed that T. ramosissima had different water use strategies between shallow and deep soil water along the two habitats, which because the differences in soil moisture across various habitats. In the floodplain habitat, the SWC of three soil layers were higher, and the water absorption layer of T. ramosissima was relatively shallow (Figure 7). This result is consistent with the results of a previous study in tropical monsoon region in which shallow soil was the main water source during the rainy season [51]. Li et al. [22] also observed that the soil water content was relatively high and the dominant depth of plant water uptake was relatively shallow in the riparian zone of arid regions. In the hillside habitat, due to the limitation of shallow SWC, T. ramosissima had a deeper water-absorbing layer and mainly used the deep soil water. Other studies have come to similar conclusions. In the northern Ningxia Plain of China, T. ramosissima was heavily dependent on deep soil water and groundwater [52]. Zhou et al. [43] also found deep soil water and groundwater were primary water sources of T. ramosissima, and even reached more than 90% in the southeastern Junggar Basin. The results of a study in the middle reaches of Heihe River have shown that T. ramosissima mainly absorbed soil water below the depth of 185 cm in July [53]. The various utilization ratios of T. ramosissima from potential water in the hillside and floodplain habitats, reflects the difference in water use strategies in diverse water conditions.
In arid and semi-arid areas, the roots of vegetation distributed in shallow soil mainly absorb soil water recharged by precipitation, and the deeper roots absorb soil water supplemented by precipitation in winter and spring or groundwater [54,55]. Because the roots in the shallow soil may be in an inactive state under drought conditions [56], when there is not enough precipitation, the plants can only depend on its roots to absorb water from the deep soil [57]. Where there is more precipitation to increase the shallow soil water content, the plants will raise the utilization of shallow soil water [58]. The previous research has found that the T. ramosissima was a deep-rooted plant that can absorb simultaneously water from both shallow and deep soil layers [59,60]. In the floodplain habitat, since the shallow SWC is larger and the shallow soil water is close to plants, which can shorten the time of absorbing water and make it easier to be absorbed and used by plants. The T. ramosissima has the highest utilization rate of shallow soil water on the whole [33]. In the hillside habitat, owing to the stress of soil water, the T. ramosissima turns to use the deep soil water with slightly higher soil water content. Yet, the shallow soil is infiltrated by the precipitation in June, and the utilization rate of shallow soil water will be increased, which indicated that the T. ramosissima can respond to the changes of water conditions in time.
T. ramosissima showed an optimal water absorption pattern, and changed the water source to obtain more stable moisture, which was beneficial to enhance the adaptability of T. ramosissima to the changing environment and improve water-use efficiency in ecosystems [18]. The study analyzed the preliminary water use strategies of T. ramosissima in different habitats in Lanzhou City, and it will provide a theoretical basis for in-depth understanding of water-use relationship of the Loess Plateau and the stability of the ecosystem. Since only one sampling point was selected in each habitat when sampling in the field, the current study only can compare the difference of these two sites. However, we selected the three T. ramosissima plants from different individuals, so they were also representative to some extent. If a comparative study of habitats is to be conducted in the future, we will select at least three sampling sites. This paper also only used the isotopic composition of xylem to reveal its water use relationship. However, plant water use is closely related to other eco-physiological processes (transpiration and photosynthesis), especially existing drought stress [61]. Thus, it is also the next to be considered in revealing plant water use strategies.
5. Conclusions
In this paper, stable isotopes and MixSIAR model were used to study the water uptake patterns of T. ramosissima, a dominant species of hillside and floodplain habitats in Lanzhou City. We found that the slope and intercept of LMWL were lesser in both habitats, indicating the existence of a drier climate and strong evaporation in the study area, and more intense evaporation under the hillside habitat. The SW-excess of the hillside habitat were more enriched and had more variation than that of floodplain habitat. The MixSIAR model predicted that hillside T. ramosissima exhibited different water use strategies compared with floodplain during the growing seasons. In hillside habitats, T. ramosissima mainly absorbed deep soil water and the utilization rate was 63 ± 17%, while the main water source in floodplain was shallow soil water, with a utilization ratio of 42.6 ± 19.2%. T. ramosissima can adapt to variable habitats by altering the water use patterns, which benefits its competition capability for water sources and shows a growth strategy under drought stresses. This study provides a theoretical basis for in-depth understanding of plant water use strategies and ecological restoration for vegetation in the Loess Plateau.
Author Contributions
Conceptualization, M.Z. and P.S.; methodology, P.S.; software, P.S.; investigation, P.S., Y.Z., J.W., X.Y., H.X., and D.Q.; writing—original draft preparation, P.S.; writing—review and editing, P.S.; project administration, M.Z.; funding acquisition, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (41771035), and the Scientific Research Program of Higher Education Institutions of Gansu Province (2018C-02).
Acknowledgments
We special thank the colleagues in the Northwest Normal University for their assistance with sample collection and experimental analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pausas, J.G.; Austin, M.P. Patterns of plant species richness in relation to different environments: An appraisal. J. Veg. Sci. 2001, 12, 153–166. [Google Scholar] [CrossRef]
- Zeng, H.H.; Liu, W.J.; Wu, J.E.; Zhu, X.A. Plant water use strategies in jungle rubber in Xishuangbanna, Southwest China. Chin. J. Ecol. 2019, 38, 394–403. (In Chinese) [Google Scholar]
- Dube, O.P.; Pickup, G. Effects of rainfall variability and communal and semi-commercial grazing on land cover in southern African rangelands. Clim. Res. 2001, 17, 195–208. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, W.Z.; He, Z.B. Water sources of Nitraria sibirica and response to precipitation in two desert habitats. Chin. J. Appl. Ecol. 2017, 28, 2083–2092. (In Chinese) [Google Scholar]
- Sun, W.Y.; Song, X.Y.; Mu, X.M.; Gao, P.; Wang, F.; Zhao, G.G. Spatiotemporal vegetation cover variations associated with climate change and ecological restoration in the Loess Plateau. Agric. For. Meteorol. 2015, 209, 87–99. [Google Scholar] [CrossRef]
- Wu, H.W.; Li, X.Y.; Jiang, Z.Y.; Chen, H.Y.; Zhang, C.C.; Xiao, X. Contrasting water use pattern of introduced and native plants in an alpine desert ecosystem, Northeast Qinghai-Tibet Plateau, China. Sci. Total Environ. 2016, 542, 182–191. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.J.; Lu, N.; Zhang, L. Seasonal variation in water uptake patterns of three plant species based on stable isotopes in the semi-arid Loess Plateau. Sci. Total Environ. 2017, 609, 27–37. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, Y.N.; Chen, Y.P. Relationship among water use of different plants in Heihe River riparian forests. Chin. J. EcoAgric. 2014, 22, 972–979. (In Chinese) [Google Scholar]
- Wang, Y.Y.; Chen, Y.P.; Li, W.H.; Wang, R.Z.; Zhou, Y.Y.; Zhang, J.P. Water sources of typical desert riparian plants in the lower reaches of Tarim River. J. Des. Res. 2017, 37, 1150–1157. (In Chinese) [Google Scholar]
- Pettit, N.E.; Froend, R.H. How important is groundwater availability and stream perenniality to riparian and floodplain tree growth? Hydrol. Process. 2018, 32, 1502–1514. [Google Scholar] [CrossRef]
- Li, E.G.; Tong, Y.Q.; Huang, Y.M.; Li, X.Y.; Wang, P.; Chen, H.Y.; Yang, C.Y. Responses of two desert riparian species to fluctuating groundwater depths in hyperarid areas of Northwest China. Ecohydrology 2019, 12, e2078. [Google Scholar] [CrossRef]
- Mensforth, L.J.; Thorburn, P.J.; Tyerman, S.D.; Walker, G.R. Sources of water used by riparian Eucalyptus camaldulensis overlying highly saline groundwater. Oecologia 1994, 100, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Q.; Li, X.Y.; Wu, H.W.; Zhang, S.Y.; Li, G.Y. Study on plant water use in Myricaria squamosa with stable hydrogen isotope tracer in Qinghai Lake basin. Chin. J. Plant Ecol 2013, 37, 1091–1100. (In Chinese) [Google Scholar]
- Zeng, Q.; Ma, J.Y. Plant water sources of different habitats and its environmental indication in Heihe River basin. J. Glaciol. Geocryol. 2013, 35, 148–155. (In Chinese) [Google Scholar]
- Si, J.H.; Feng, Q.; Cao, S.K.; Yu, T.F.; Zhao, C.Y. Water use sources of desert riparian Populus euphratica forests. Environ. Monit. Assess. 2014, 186, 5469–5477. [Google Scholar] [CrossRef]
- Porporato, A.; Daly, E.; Rodriguez-Iturbe, I. Soil water balance and ecosystem response to climate change. Am. Nat. 2004, 164, 625–632. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic plasticity in plants: A case study in ecological development. Evol. Dev. 2003, 5, 25–33. [Google Scholar] [CrossRef]
- Zhu, J.F.; Liu, J.T.; Sun, J.K.; Zhao, Y.Y.; Lu, Z.H.; Li, J.S. Water sources for Tamarix chinensis Lour. in different habitats on Chenier Island. Chin. J. Ecol. 2017, 36, 2367–2374. (In Chinese) [Google Scholar]
- Dawson, T.E.; Pate, J.S. Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: A stable isotope investigation. Oecologia 1996, 107, 13–20. [Google Scholar] [CrossRef]
- Bowling, D.R.; Schulze, E.S.; Hall, S.J. Revisiting streamside trees that do not use stream water: Can the two water worlds hypothesis and snowpack isotopic effects explain a missing water source? Ecohydrology 2017, 10, e1771. [Google Scholar] [CrossRef]
- Oerter, E.J.; Siebert, G.; Bowling, D.R.; Bowen, G. Soil water vapour isotopes identify missing water source for streamside trees. Ecohydrology 2019, 12, e2083. [Google Scholar] [CrossRef]
- Li, Y.F.; Yu, J.J.; Lu, K.; Wang, P.; Zhang, Y.C.; Du, C.Y. Water sources of Populus euphratica and Tamarix ramosissima in Ejina Delta, the lower reaches of the Heihe River, China. Chin. J. Plant Ecol. 2017, 41, 519–528. (In Chinese) [Google Scholar]
- Mathieu, R.; Bariac, T. An isotopic study (2H and 18O) of water movements in clayey soils under a semiarid climate. Water Resour. Res. 1996, 32, 779–789. [Google Scholar] [CrossRef]
- Jasechko, S.; Birks, S.J.; Gleeson, T.; Wada, Y.; Fawcett, P.J.; Sharp, Z.D.; McDonnell, J.J.; Welker, J.M. The pronounced seasonality of global groundwater recharge. Water Resour. Res. 2014, 50, 8845–8867. [Google Scholar] [CrossRef]
- Brooks, J.R.; Barnard, H.R.; Coulombe, R.; McDonnell, J.J. Ecohydrologic separation of water between trees and streams in a Mediterranean climate. Nat. Geosci. 2010, 3, 100–104. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, M.J.; Wang, S.J.; Evaristo, J.; Argiriou, A.A.; Guo, R.; Chen, R.; Meng, H.F.; Che, C.W. The test of the ecohydrological separation hypothesis in a dry zone of the northeastern Tibetan Plateau. Ecohydrology 2019, 12, e2077. [Google Scholar] [CrossRef]
- Lyu, S.D.; Song, X.W.; Wen, X.F. Ecohydrologic separation of the mixing process between precipitation and soil water: A review. Chin. J. Appl. Ecol. 2019, 30, 1797–1806. (In Chinese) [Google Scholar]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Barbeta, A.; Jones, S.P.; Clavé, L.; Wingate, L.; Gimeno, T.E.; Fréjaville, B.; Wohl, S.; Ogée, J. Unexplained hydrogen isotope offsets complicate the identification and quantification of tree water sources in a riparian forest. Hydrol. Earth Syst. Sci. 2019, 23, 2129–2146. [Google Scholar] [CrossRef]
- Wang, J.; Lu, N.; Fu, B.J. Inter-comparison of stable isotope mixing models for determining plant water source partitioning. Sci. Total Environ. 2019, 666, 685–693. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.J.; Wang, S.J.; Guo, R.; Che, C.W.; Du, Q.Q.; Ma, Z.Z.; Su, P.Y. Comparison of different methods for determining plant water sources based on stable oxygen isotope. Chin. J. Ecol. 2020, 39, 1356–1368. (In Chinese) [Google Scholar]
- Ellsworth, P.Z.; Williams, D.G. Hydrogen isotope fractionation during water uptake by woody xerophytes. Plant Soil. 2007, 291, 93–107. [Google Scholar] [CrossRef]
- Su, P.Y.; Zhang, M.J.; Wang, S.J.; Qiu, X.; Wang, J.X.; Du, Q.Q.; Guo, R.; Che, C.W. Water sources of riparian plants based on stable hydrogen and oxygen isotopes in Lanzhou section of the Yellow River, China. Chin. J. Appl. Ecol. 2020, 31, 1835–1843. (In Chinese) [Google Scholar]
- Zhang, Y.; Zhang, M.J.; Qu, D.Y.; Duan, W.G.; Wang, J.X.; Su, P.Y.; Guo, R. Water use strategies of dominant species (Caragana korshinskii and Reaumuria soongorica) in natural shrubs based on stable isotopes in the Loess Hill, China. Water 2020, 12, 1923. [Google Scholar] [CrossRef]
- Pan, B.T.; Su, H.; Hu, Z.B.; Gao, H.S.; Li, J.J.; Kirby, E. Evaluating the role of climate and tectonics during non-steady incision of the Yellow River: Evidence from a 1.24 Ma terrace record near Lanzhou, China. Quat. Sci. Rev. 2009, 28, 3281–3290. [Google Scholar] [CrossRef]
- Wang, Y.S.; Gao, W.D. Analysis on Characteristics and Rules of Precipitation and Temperature Change of Lanzhou City in Recent 55 Years. Yellow River 2015, 37, 18–20. (In Chinese) [Google Scholar]
- Wu, Y.H.; Zhong, F. Effects of utilization types on soil properties of forest-grassland in Lanzhou South Region. Pratacult. Sci 2014, 31, 803–810. [Google Scholar]
- Duan, Z.H.; Xiao, H.L.; Song, Y.X.; Cheng, G.D. Soil water variation in different terrains and influence on plant in lanzhou suburb on western loess plateau. J. Desert Res. 2006, 26, 522–526. (In Chinese) [Google Scholar]
- Zhang, Y.; Wu, Y.H.; Zhao, F. Study on species diversity of typical plant community in wetland along Yellow River in Lanzhou. Grassl. Turf. 2016, 36, 65–71. [Google Scholar]
- Dawson, T.E.; Ehleringer, J.R. Isotopic enrichment of water in the “woody” tissues of plants: Implications for plant water source, water uptake, and other studies which use the stable isotopic composition of cellulose. Geochim. Cosmochim. Acta 1993, 57, 3487–3492. [Google Scholar] [CrossRef]
- Martín-Gómez, P.; Serrano, L.; Ferrio, J.P. Short-term dynamics of evaporative enrichment of xylem water in woody stems: Implications for ecohydrology. Tree Physiol. 2017, 37, 511–522. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, M.J.; Wang, S.J.; Argiriou, A.A.; Chen, R.; Meng, H.F.; Guo, R. Water stable isotopes in an alpine setting of the Northeastern Tibetan Plateau. Water 2019, 11, 770. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, X.J.; Tang, L.S.; Li, Y. Differences and similarities between water sources of Tamarix ramosissima, Nitraria sibirica and Reaumuria soongorica in the southeastern Junggar Basin. Chin. J. Plant Ecol. 2013, 37, 665–673. (In Chinese) [Google Scholar] [CrossRef]
- Moore, J.W.; Semmens, B.X. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 2008, 11, 470–480. [Google Scholar] [CrossRef]
- Stock, B.; Semmens, B.X. Unifying error structures in commonly used biotracer mixing models. Ecology 2016, 97, 2562–2569. [Google Scholar] [CrossRef]
- Stock, B.C.; Semmens, B.X. MixSIAR GUI User Manual, Version 3.1. Available online: http://conserver.iugocafe.org/user/brice.semmens/MixSIAR (accessed on 10 March 2016).
- Landwehr, J.M.; Coplen, T.B. Line-conditioned excess: A new method for characterizing stable hydrogen and oxygen isotope ratios in hydrologic systems. In Proceedings of the International Conference on Isotopes in Environmental Studies; IAEA: Vienna, Austria, 2006; pp. 132–135. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:37043527 (accessed on 5 October 2020).
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Evaristo, J.; Jasechko, S.; Mcdonnell, J.J. Global separation of plant transpiration from groundwater and streamflow. Nature 2015, 525, 91–94. [Google Scholar] [CrossRef]
- Stratton, L.C.; Goldstein, G.; Meinzer, F.C. Temporal and spatial partitioning of water resources among eight woody species in a Hawaiian dry forest. Oecologia 2000, 124, 309–317. [Google Scholar] [CrossRef]
- Wang, P.Y.; Liu, W.J.; Zhang, J.L.; Yang, B.; Kumar, S.A.; Wu, J.E.; Jiang, X.J. Seasonal and spatial variations of water use among riparian vegetation in tropical monsoon region of SW China. Ecohydrology 2019, 12, e2085. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Jia, Z.Q.; Lu, Q.; Hao, Y.G.; Zhang, J.; Li, L.; Qi, Y.L. Water use strategy of five shrubs in Ulanbuh Desert. Sci. Silvae Sin. 2010, 46, 15–21. (In Chinese) [Google Scholar]
- Zhou, C.X.; Sun, Z.Y.; Yu, W.S. Using D and δ18O stable isotopes to determine the water sources of sand dune plants in Linze, middle reaches of Heihe River. Geol. Sci. Technol. Inf. 2011, 30, 107–113. [Google Scholar]
- Williams, D.G.; Ehleringer, J.R. Intra- and interspecific variation for summer precipitation use in pinyon-juniper woodlands. Ecol. Monogr. 2000, 70, 517–537. [Google Scholar]
- Chimner, R.A.; Cooper, D.J. Using stable oxygen isotopes to quantify the water source used for transpiration by native shrubs in the San Luis Valley, Colorado U.S.A. Plant Soil 2004, 260, 225–236. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Ehleringer, J.R.; Marshall, J.D. Differential uptake of summer precipitation among co-occurring trees and shrubs in a pinyon-juniper woodland. Plant Cell Environ. 1992, 15, 831–836. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Dawson, T.E. Water uptake by plants: Perspectives from stable isotope composition. Plant Cell Environ. 1992, 15, 1073–1082. [Google Scholar] [CrossRef]
- Duan, D.Y.; Ouyang, H.; Song, M.H.; Hu, Q.W. Water sources of dominant species in three alpine ecosystems on the Tibetan Plateau, China. J. Integr. Plant Biol. 2008, 50, 257–264. [Google Scholar] [CrossRef]
- Busch, D.; Smith, S.D. Mechanisms associated with decline of woody species in riparian ecosystems of the southwestern US. Ecol. Monogr. 1995, 65, 347–370. [Google Scholar] [CrossRef]
- Tiemuerbieke, B.; Min, X.J.; Zang, Y.X.; Xing, P.; Ma, J.Y.; Sun, W. Water use patterns of co-occurring C3 and C4 shrubs in the Gurbantonggut desert in northwestern China. Sci. Total. Environ. 2018, 634, 341–354. [Google Scholar] [CrossRef]
- Fang, J.; Wei, Y.F.; Liu, S.; Zhao, X.Y.; Li, S.G. Stable isotopic analysis on water utilization sources of Pinus sylvestris var. mongolica plantations in inter-dune lowland in Horqin Sandy Land. Chin. J. Ecol. 2011, 30, 1894–1900. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).