Urea Inputs Drive Picoplankton Blooms in Sarasota Bay, Florida, U.S.A.
Abstract
1. Introduction
2. Methods
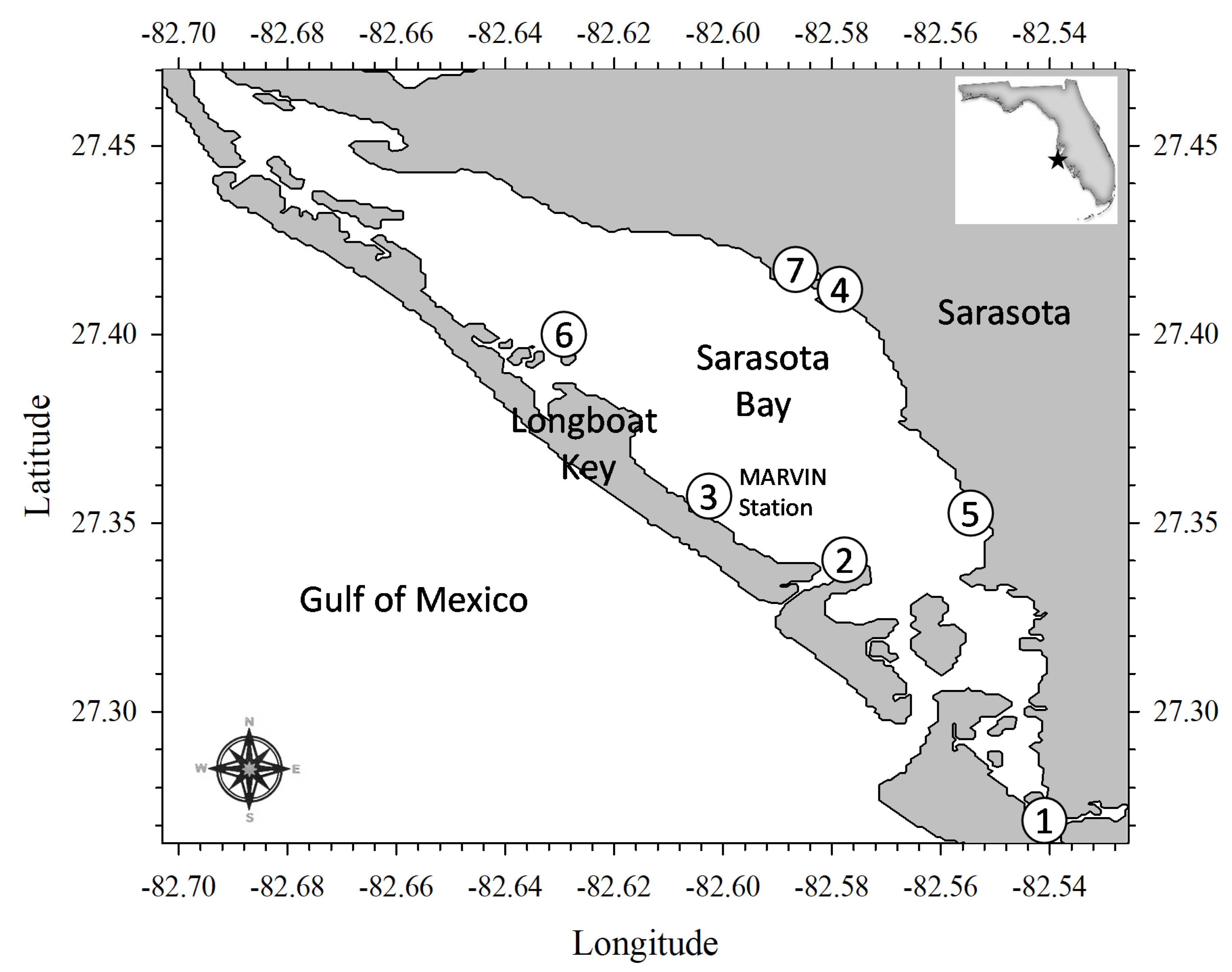
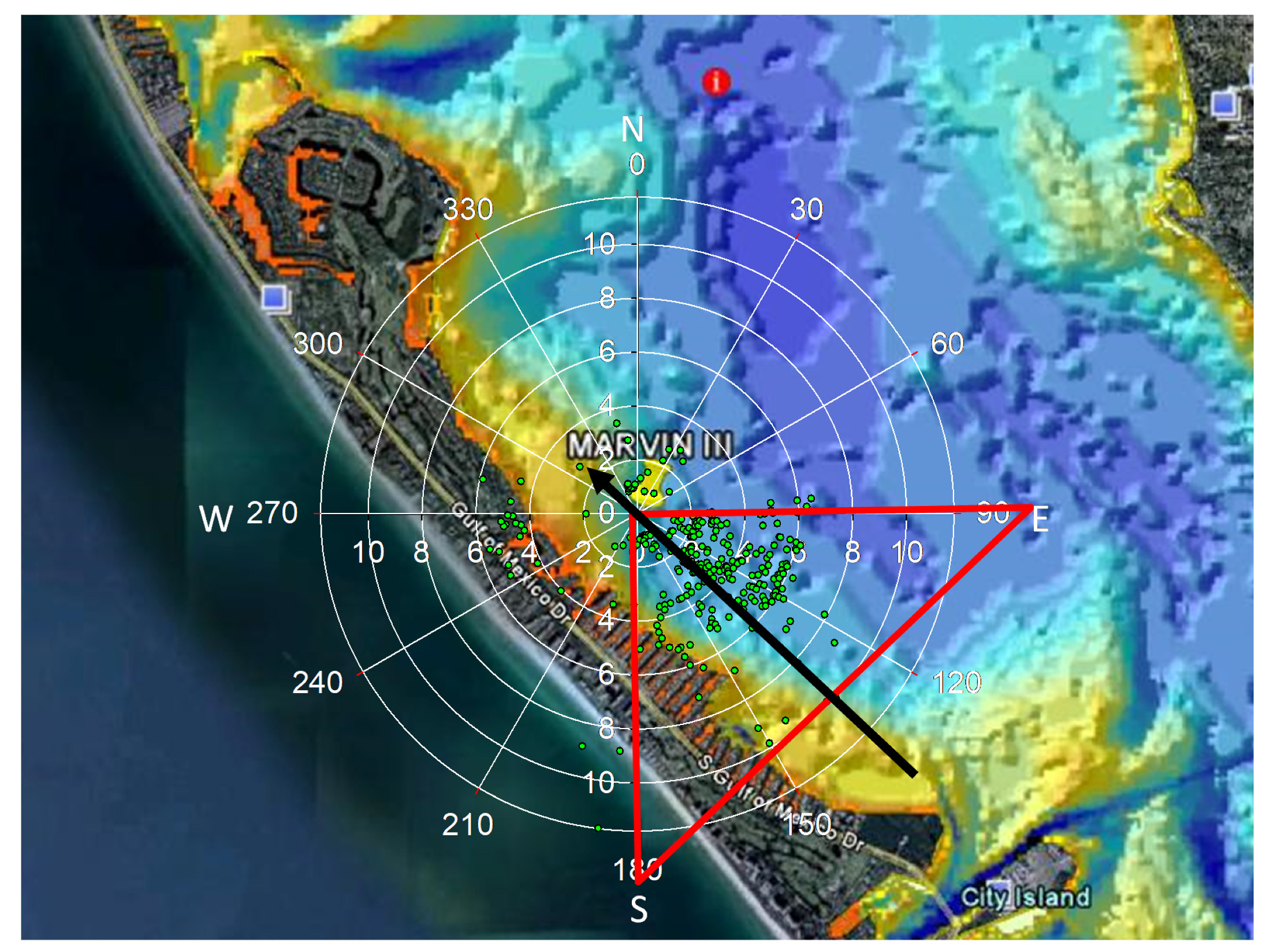
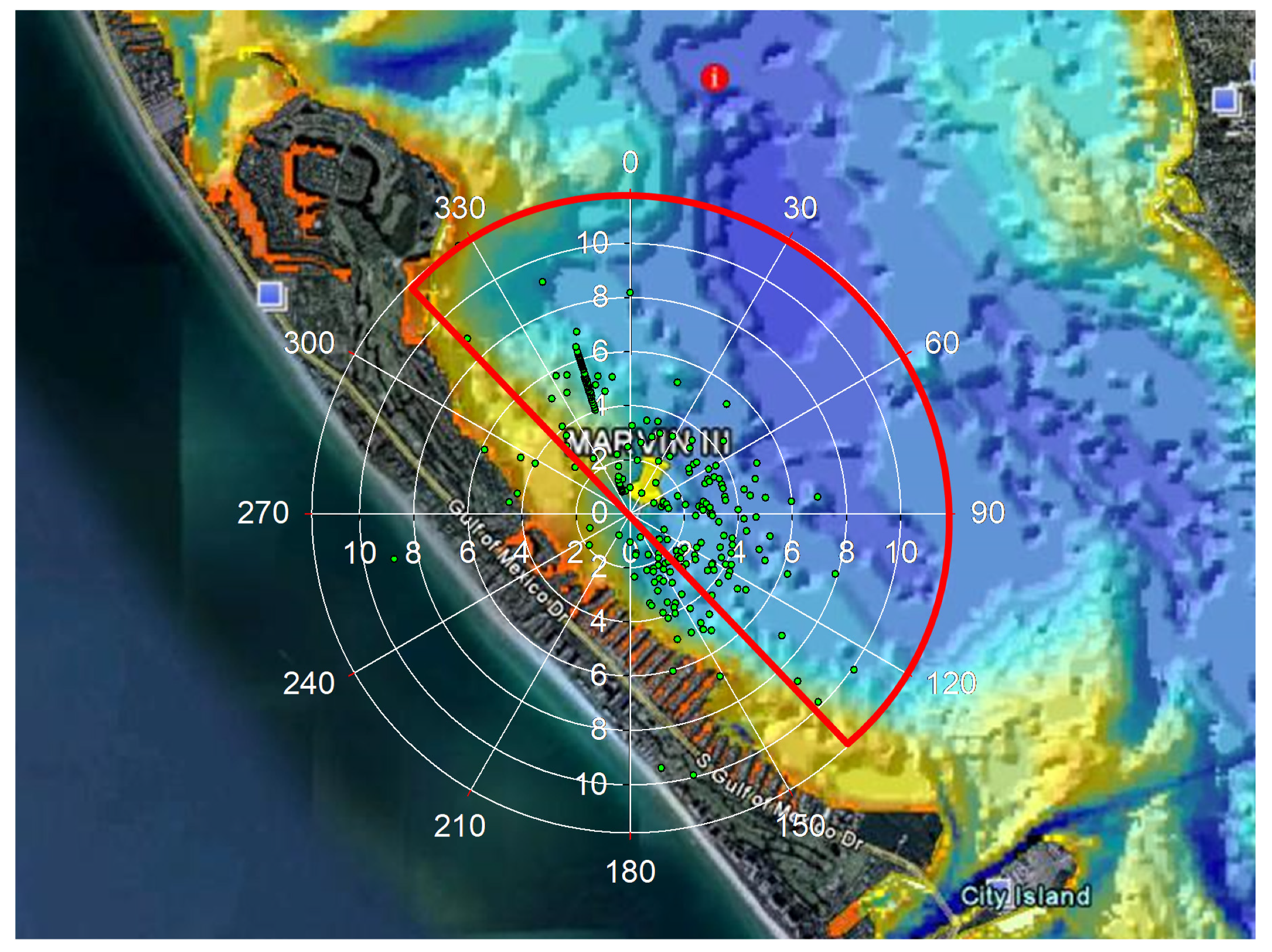
2.1. Sampling Stations
2.2. Autonomous Water Quality Platform
2.3. Laboratory Analyses
2.4. Urea Enrichment Experiments
2.5. Historical Data
2.6. Statistical Analysis of Wind Influence
3. Results
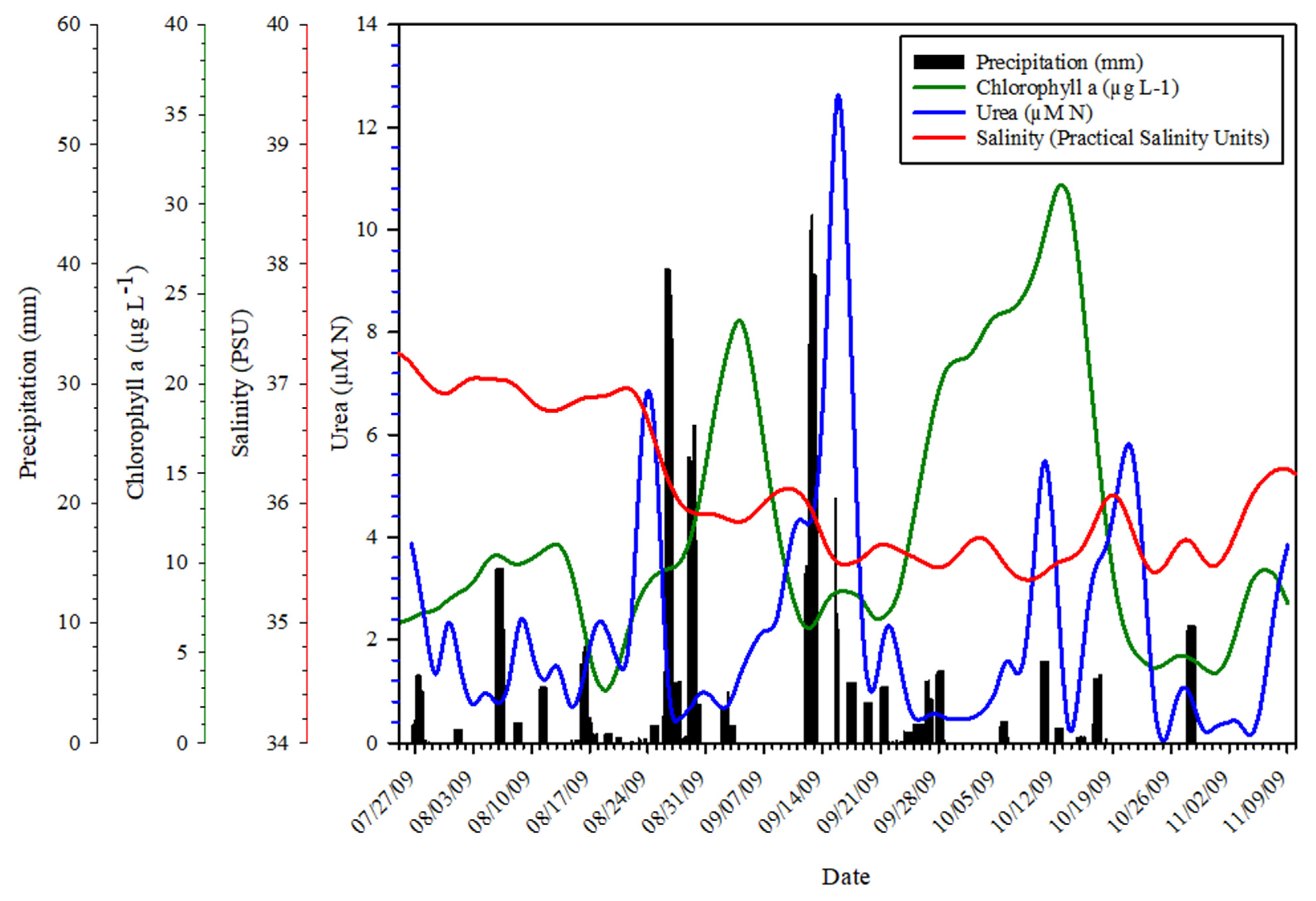
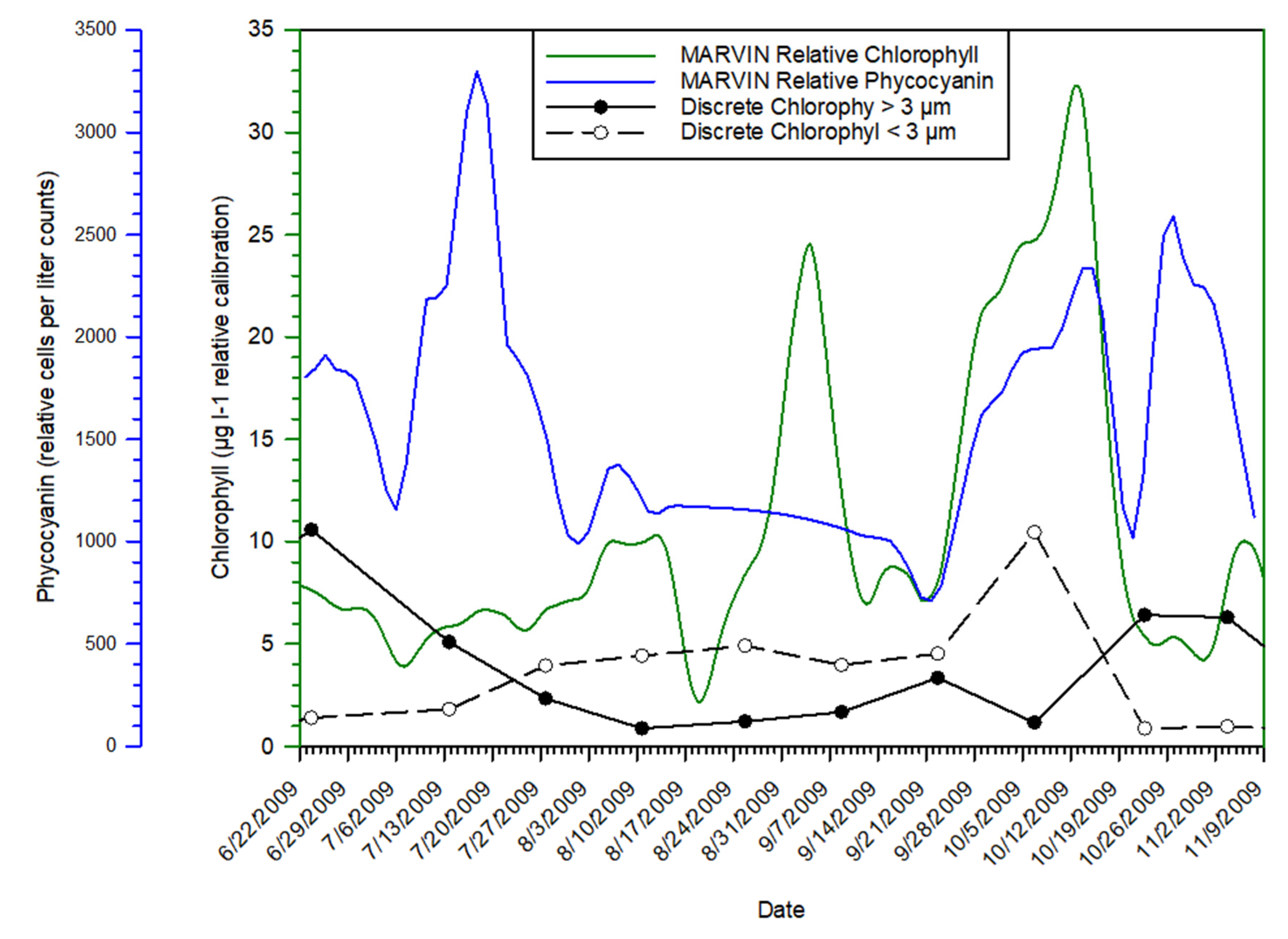
3.1. Time Series Trends
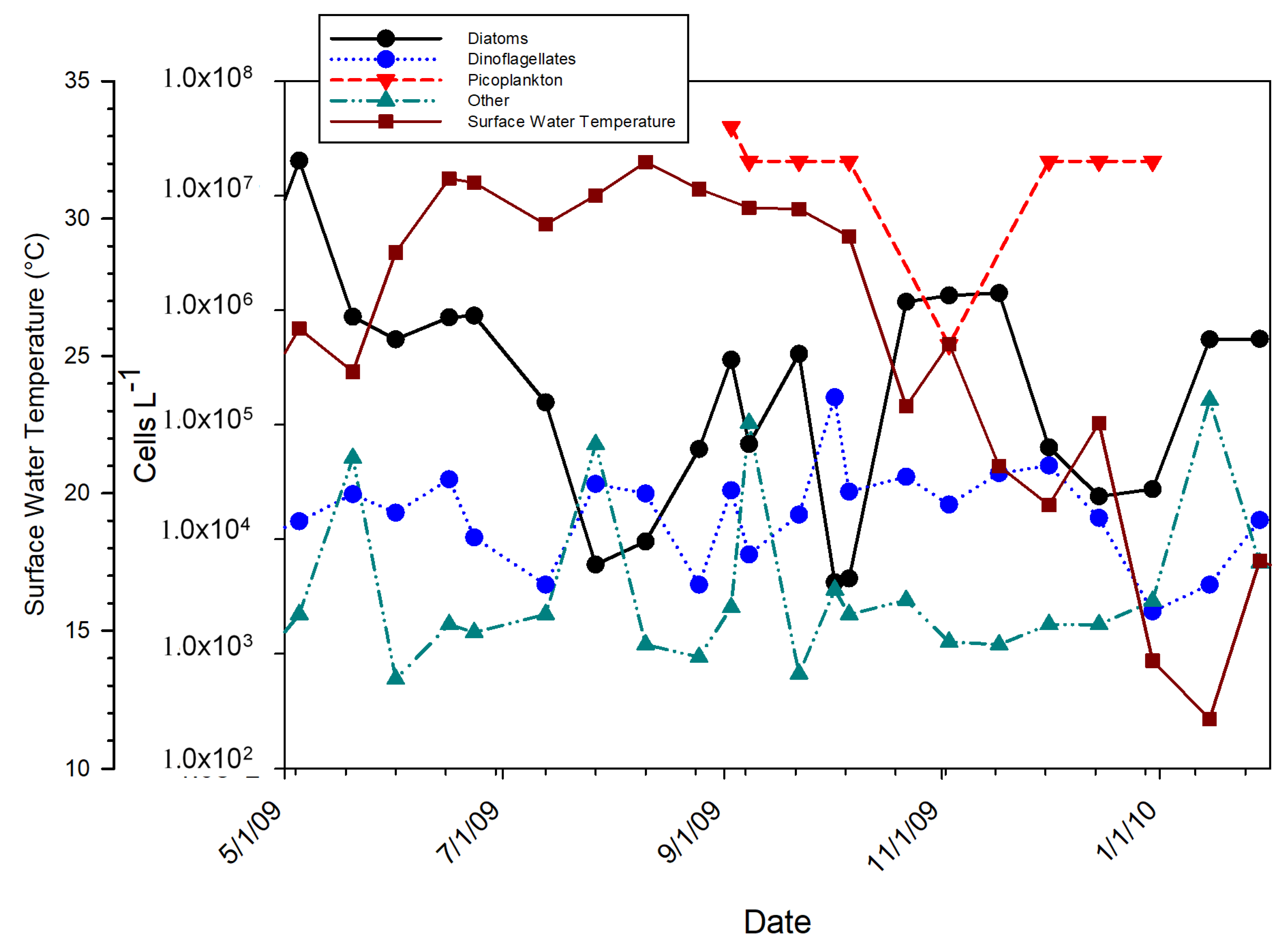
3.2. Phytoplankton Data
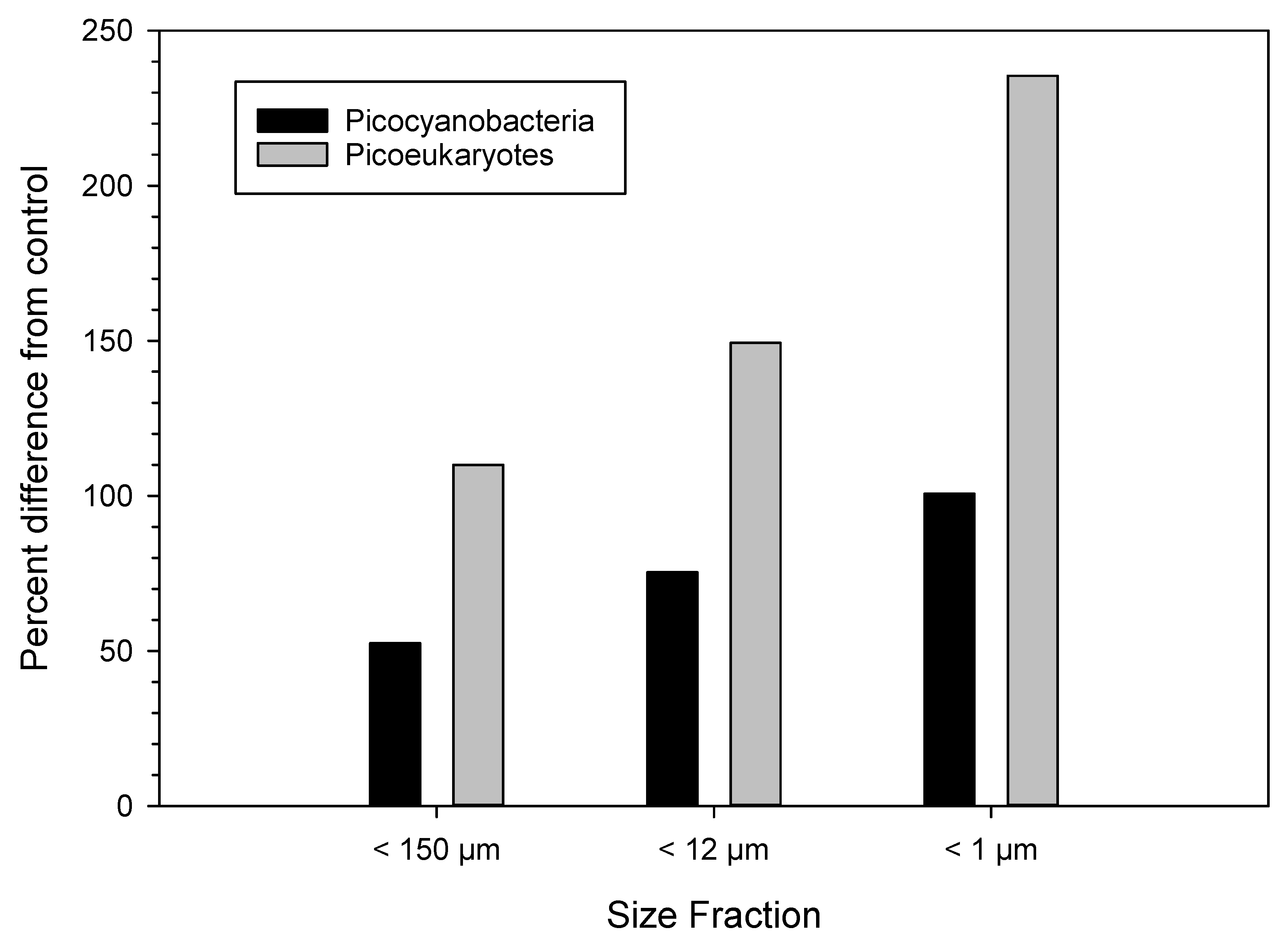
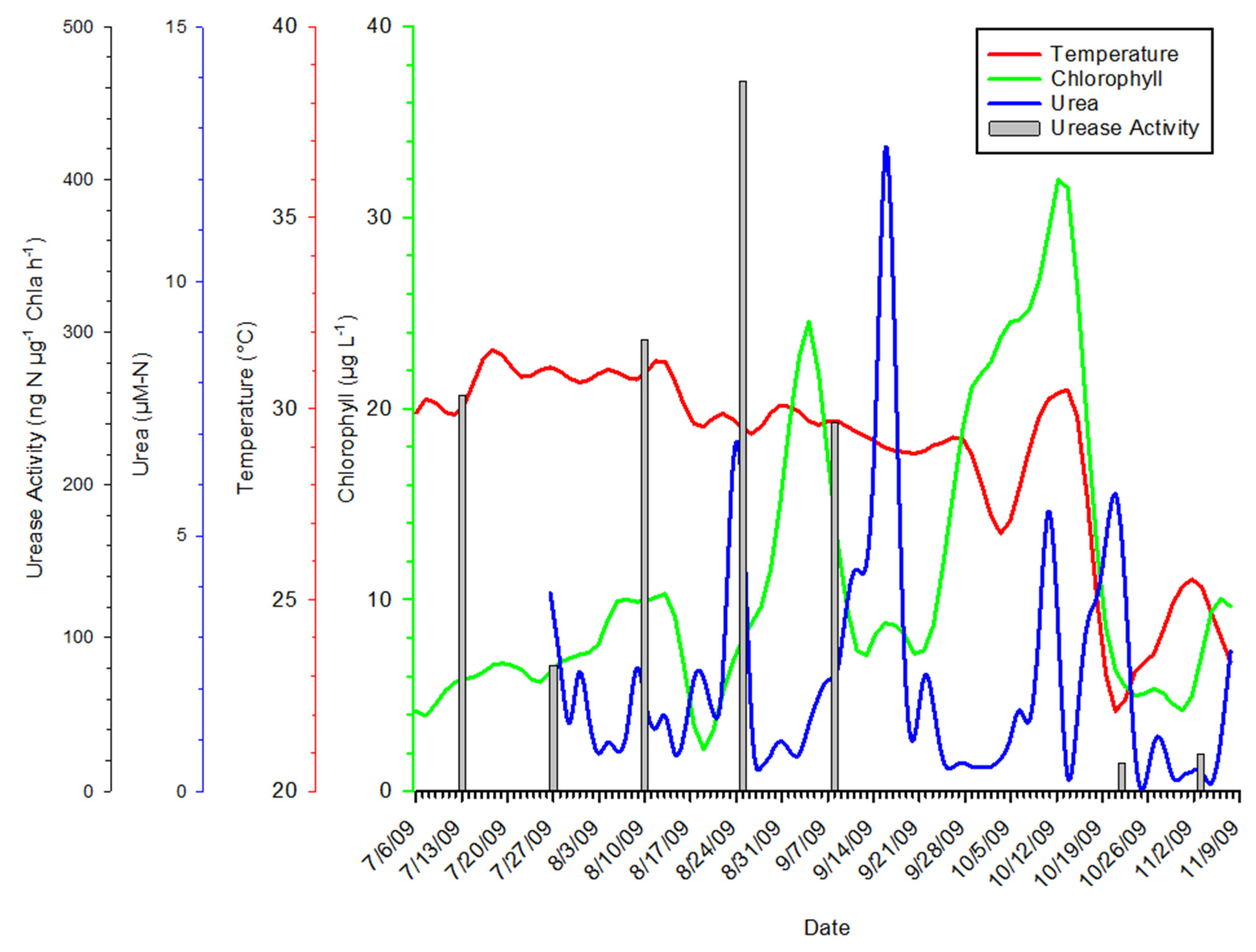
3.3. Urea Enrichment Experiments
3.4. Discrete Sample Data
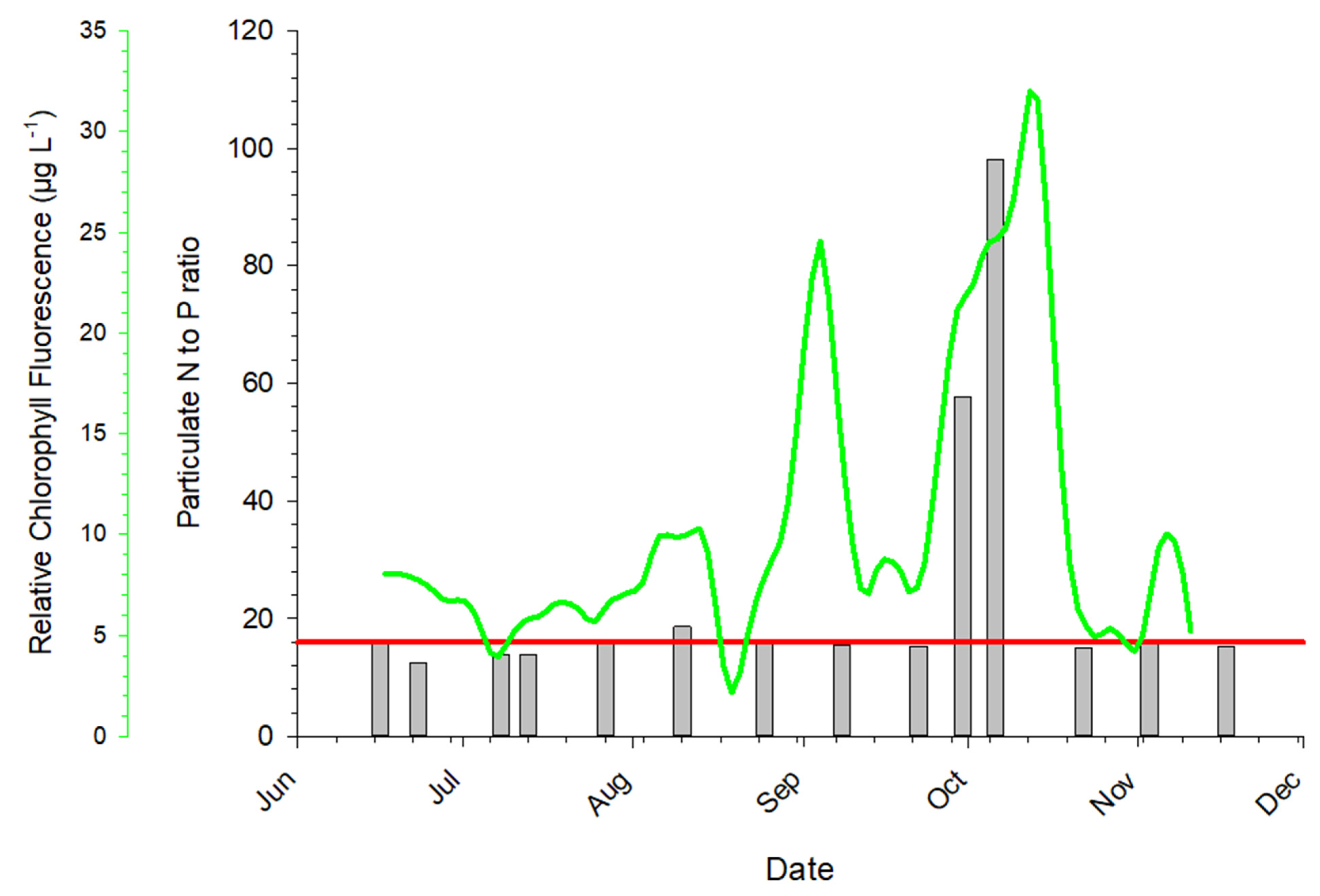
3.5. Abiotic Data Relationships to Urea Concentrations
4. Discussion
4.1. Species Composition Shifts
4.2. Nutrient Fluxes
4.3. Response to Urea Pulses
4.4. Possible Urea Sources
4.5. Mitigation
4.6. Potential Trophic Cascades
4.7. Autonomous Sampling
4.8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nixon, S.W. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 1995, 41, 199–219. [Google Scholar] [CrossRef]
- Bricker, S.B.; Clement, C.G.; Pirhalla, D.E.; Orlando, S.P.; Farrow, D.R.G. National Estuarine Eutrophication Assessment: Effects of Nutrient Enrichment in the Nation’s Estuaries; US National Oceanographic and Atmospheric Administration, National Ocean Service, Special Projects Office and the National Center for Coastal Ocean Science: Silver Spring, MD, USA, 1999; p. 71. [Google Scholar]
- Howarth, R.W. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 2008, 8, 14–20. [Google Scholar] [CrossRef]
- Bricker, S.B.; Longstaff, B.; Dennison, W.; Jones, A.; Boicourt, K.; Wicks, C.; Woerner, J. Effects of nutrient enrichment in the nation’s estuaries: A decade of change. Harmful Algae 2009, 8, 21–32. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Peierls, B.L.; Rossignol, K.L. Evolving paradigms and challenges in estuarine and coastal eutrophication dynamics in a culturally and climatically stressed world. Estuaries Coasts 2014, 37, 243–258. [Google Scholar] [CrossRef]
- Howarth, R.; Anderson, D.; Cloern, J.; Elfring, C.; Hopkinson, C.; Lapointe, B.; Malone, T.; Marcus, N.; McGlathery, K.; Sharpley, A.; et al. Nutrient pollution of coastal rivers, bays, and seas. Issues Ecol. 2000, 7, 1–15. [Google Scholar]
- Smil, V. Enriching the Earth. In Transformation of World Food Production; Haber, F., Bosch, C., Eds.; The MIT Press: Cambridge, UK, 2001; p. 358. [Google Scholar]
- Anderson, D.M.; Burkholder, J.M.; Cochlan, W.P.; Glibert, P.M.; Gobler, C.J.; Heil, C.A.; Kudela, R.; Parsons, M.L.; Rensel, J.E.; Townsend, D.W.; et al. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae 2008, 8, 39–53. [Google Scholar] [CrossRef]
- Gowen, R.J.; Tett, P.; Bresnan, E.; Davidson, K.; McKinney, A. Anthropogenic nutrient enrichment and blooms of harmful phytoplankton. Oceanogr. Mar. Biol. 2012, 50, 65–126. [Google Scholar]
- Cloern, J.E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 2001, 210, 223–253. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Glibert, P.M.; Seitzinger, S.; Heil, C.A.; Burkholder, J.M.; Parrow, M.W.; Codispoti, L.A.; Kelly, V. The role of eutrophication in the global proliferation of harmful algal blooms: New perspectives and approaches. Oceanography 2005, 18, 196–207. [Google Scholar] [CrossRef]
- Glibert, P.M.; Trice, T.M.; Michael, B.; Lane, L. Urea in the tributaries of the Chesapeake and coastal bays of Maryland. Water Air Soil Pollut. 2005, 160, 229–243. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Tomasko, D.A.; Touchette, B.W. Seagrasses and eutrophication. J. Exp. Mar. Biol. Ecol. 2007, 350, 46–72. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. UK 2015. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Harrison, J.; Heil, C.; Seitzinger, S. Escalating worldwide use of urea–a global change contributing to coastal eutrophication. Biogeochemistry 2006, 77, 441–463. [Google Scholar] [CrossRef]
- Switzer, T. Urea loading from a spring storm—Knysna estuary, South Africa. Harmful Algae 2008, 8, 66–69. [Google Scholar] [CrossRef]
- Cozzi, S.; Mistaro, A.; Sparnocchia, S.; Colugnati, L.; Bajt, O.; Toniatti, L. Anthropogenic loads and biogeochemical role of urea in the Gulf of Trieste. Sci. Total Environ. 2014, 493, 271–281. [Google Scholar] [CrossRef]
- Antia, N.J.; Harrison, P.J.; Oliveira, L. The role of dissolved organic nitrogen in phytoplankton nutrition, cell biology and ecology. Phycologia 1991, 30, 1–89. [Google Scholar] [CrossRef]
- Berman, T.; Bronk, D.A. Dissolved organic nitrogen: A dynamic participant in aquatic ecosystems. Aquat. Microb. Ecol. 2003, 31, 279–305. [Google Scholar] [CrossRef]
- Heil, C.A.; Revilla, M.; Glibert, P.M.; Murasko, S. Nutrient quality drives differential phytoplankton community composition on the southwest Florida Shelf. Limnol. Oceanogr. 2007, 52, 1067–1078. [Google Scholar] [CrossRef]
- Killberg-Thoreson, L.; Mulholland, M.R.; Heil, C.A.; Sanderson, M.P.; O’Neil, J.M.; Bronk, D.A. Nitrogen uptake kinetics in field populations and cultured strains of Karenia brevis. Harmful Algae 2014, 38, 73–85. [Google Scholar] [CrossRef]
- Lomas, M.W.; Trice, T.M.; Glibert, P.M.; Bronk, D.A.; McCarthy, J.J. Temporal and spatial dynamics of urea uptake and regeneration rates, and concentrations in Chesapeake Bay. Estuaries 2002, 25, 469–482. [Google Scholar] [CrossRef]
- Beman, J.M.; Arrigo, K.R.; Matson, P.A. Agricultural runoff fuels large phytoplankton blooms in vulnerable areas of the ocean. Nature 2005, 434, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Obreza, T.A.; Alva, A.K.; Calvert, D.V. Citrus Fertilizer Management on Calcareous Soils; Report CIR1127; Cooperative Extension Service, University of Florida: Gainesville, FL, USA, 1993; p. 8. [Google Scholar]
- Paramasivam, S.; Alva, A.K. Leaching of nitrogen forms from controlled-release nitrogen fertilizers. Commun. Soil Sci. Plant Anal. 1997, 28, 1663–1674. [Google Scholar] [CrossRef]
- Glibert, P.M.; Heil, C.A.; Hollander, D.; Revilla, M.; Hoare, A.; Alexander, J.; Murasko, S. Evidence for dissolved organic nitrogen and phosphorus uptake during a cyanobacterial bloom in Florida Bay. Mar. Ecol. Prog. Ser. 2004, 280, 73–83. [Google Scholar] [CrossRef]
- Crandall, J.B.; Teece, M.A. Urea is a dynamic pool of bioavailable nitrogen in coral reefs. Coral Reefs 2012, 31, 207–214. [Google Scholar] [CrossRef]
- McCarthy, J.J. The uptake of urea by natural populations of marine phytoplankton. Limnol. Oceanogr. 1972, 17, 738–748. [Google Scholar] [CrossRef]
- McCarthy, J.J. The uptake of urea by marine phytoplankton. J. Phycol. 1972, 8, 216–222. [Google Scholar] [CrossRef]
- Twomey, L.J.; Piehler, M.F.; Paerl, H.W. Phytoplankton uptake of ammonium, nitrate and urea in the Neuse River Estuary, NC. Hydrobiologia 2005, 533, 123–134. [Google Scholar] [CrossRef]
- Collos, Y.; Jauzein, C.; Ratmaya, W.; Souchu, P.; Abadie, E.A.; Vaquer, A. Comparing diatom and Alexandrium catenella/tamarense blooms in Thau lagoon: Importance of dissolved organic nitrogen in seasonally N-limited systems. Harmful Algae 2014, 37, 84–91. [Google Scholar] [CrossRef]
- Solomon, C.M.; Collier, J.L.; Berg, G.M.; Glibert, P.M. Role of urea in microbial metabolism in aquatic systems: A biochemical and molecular review. Aquat. Microb. Ecol. 2010, 59, 67–88. [Google Scholar] [CrossRef]
- Solomon, C.M.; Glibert, P.M. Urease activity in five phytoplankton species. Aquat. Microb. Ecol. 2008, 52, 149–157. [Google Scholar] [CrossRef]
- Davidson, K.; Gowen, R.J.; Harrison, P.J.; Fleming, L.; Hoagland, P.; Moschonas, G. Anthropogenic nutrients and harmful algae in coastal waters. J. Environ. Manag. 2014, 146, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Donald, D.B.; Bogard, M.J.; Finlay, K.; Leavitt, P.R. Comparative effects of urea, ammonium, and nitrate on phytoplankton abundance, community composition, and toxicity in hypereutrophic freshwaters. Limnol. Oceanogr. 2011, 56, 2161–2175. [Google Scholar] [CrossRef]
- Glibert, P.M.; Terlizzi, D.E. Cooccurrence of elevated urea levels and dinoflagellate blooms in temperate estuarine aquaculture ponds. Appl. Environ. Microbiol. 1999, 65, 5594–5596. [Google Scholar] [CrossRef]
- Glibert, P.M.; Magnien, R.; Lomas, M.W.; Alexander, J.; Fan, C.; Haramoto, E.; Trice, T.M.; Kana, T.M. Harmful algal blooms in the Chesapeake and Coastal Bays of Maryland, USA: Comparison of 1997, 1998, and 1999 events. Estuaries 2001, 24, 875–883. [Google Scholar] [CrossRef]
- Fan, C.; Glibert, P.M.; Burkholder, J.M. Characterization of the nitrogen uptake kinetics of Prorocentrum minimum in natural blooms and laboratory cultures. Harmful Algae 2003, 2, 283–299. [Google Scholar] [CrossRef]
- Fan, C.; Glibert, P.M.; Alexander, J.M.; Lomas, M.W. Characterization of urease activity in three marine phytoplankton species, Aureococcus anophagefferens, Prorocentrum minimum, and Thalassiosira weissflogii. Mar. Biol. 2003, 142, 949–958. [Google Scholar] [CrossRef]
- Kudela, R.M.; Lane, J.Q.; Cochlan, W.P. The potential role of anthropogenically derived nitrogen in the growth of harmful algae in California, USA. Harmful Algae 2008, 8, 103–110. [Google Scholar] [CrossRef]
- Brody, S.D.; Carrasco, V.; Highfield, W.E. Measuring the adoption of local sprawl reduction planning policies in Florida. J. Plan. Educ. Res. 2006, 25, 294–310. [Google Scholar] [CrossRef]
- Kahrl, A.W. The Sunbelt’s sandy foundation: Coastal development and the making of the modern South. South. Cult. 2014, 20, 24–42. [Google Scholar] [CrossRef]
- Bronk, D.A.; Sanderson, M.P.; Mulholland, M.R.; Heil, C.A.; O’Neil, J.M. Organic and inorganic nitrogen uptake kinetics in field populations dominated by Karenia brevis. In Harmful Algae 2002; Steidinger, K.A., Landsberg, J.H., Tomas, C.R., Vargo, G.A., Eds.; Florida Fish and Wildlife Conservation Commission, IOC-UNESCO: St Petersburg, FL, USA, 2004; pp. 80–82. [Google Scholar]
- Lindall, W.N., Jr.; Fable, W.A., Jr.; Collins, L.A. Additional studies of the fishes, macroinvertebrates, and hydrological conditions of upland canals in Tampa Bay, Florida. Fish. Bull 1975, 73, 81–85. [Google Scholar]
- Ma, S.; Whereat, E.; Luther III, G.W. Shift of algal community structure in dead end lagoons of the Delaware Inland Bays during seasonal anoxia. Aquat. Microb. Ecol. 2006, 44, 279–290. [Google Scholar] [CrossRef][Green Version]
- Lewitus, A.J.; Brock, L.B.; Burke, M.K.; DeMattio, K.A.; Wilde, S.B. Lagoonal stormwater detention ponds as promoters of harmful algal blooms and eutrophication along the South Carolina coast. Harmful Algae 2008, 8, 60–65. [Google Scholar] [CrossRef]
- Phlips, E.J.; Badylak, S.; Christman, M.; Wolny, J.; Brame, J.; Garland, J.; Hall, L.; Hart, J.; Landsberg, J.; Lasi, M.; et al. Scales of temporal and spatial variability in the distribution of harmful algae species in the Indian River Lagoon, Florida, USA. Harmful Algae 2011, 10, 277–290. [Google Scholar] [CrossRef]
- LaPointe, B.E.; Herren, L.W.; Debortoli, D.D.; Vogel, M.A. Evidence of sewage-driven eutrophication and harmful algal blooms in Florida’s Indian River Lagoon. Harmful Algae 2015, 43, 82–102. [Google Scholar] [CrossRef]
- Bullerjahn, G.S.; McKay, R.M.; Davis, T.W.; Baker, D.B.; Boyer, G.L.; D’Anglada, L.V.; Doucette, G.J.; Ho, J.C.; Irwin, E.G.; Kling, C.L.; et al. Global solutions to regional problems: Collecting global expertise to address the problem of harmful cyanobacterial blooms. A Lake Erie Case Study. Harmful Algae 2016, 54, 223–238. [Google Scholar] [CrossRef]
- Reidmiller, D.R.; Avery, C.W.; Easterling, D.R.; Kunkel, K.E.; Lewis, K.L.M.; Maycock, T.K.; Stewart, B.C. Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment; U.S. Global Change Research Program: Washington, DC, USA, 2018; p. 1526. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Pniewski, F.; Latała, A. Allelopathic interactions between Synechococcus sp. and Nodularia spumigena under different light conditions. Allelopath. J. 2016, 37, 241–252. [Google Scholar]
- Śliwińska-Wilczewska, S.; Maculewicz, J.; Barreiro, A.; Latała, A. Allelopathic and bloom-forming picocyanobacteria in a changing world. Toxins 2018, 10, 48. [Google Scholar] [CrossRef]
- Ellison, R.M.; Bendis, B. Keeping constant watch on harmful algal blooms: Environmental use a pontoon-mounted autonomous monitoring system in Florida waters. Sea Technol. 2007, 48, 10–14. [Google Scholar]
- Millie, D.F.; Weckman, G.R.; Young, W.A.; Ivey, J.E.; Fries, D.P.; Ardjmand, E.; Fahnenstiel, G.L. Coastal ‘Big Data’ and nature-inspired computation: Prediction potentials, uncertainties, and knowledge derivation of neural networks for an algal metric. Estuar. Coast. Shelf Sci. 2013, 125, 57–67. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Boyd, T.R.C. Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinization. Clin. Chim. Acta 1980, 107, 3–9. [Google Scholar] [CrossRef]
- Glibert, P.M.; Kelly, V.; Alexander, J.; Codipoti, L.A.; Boicourt, W.C.; Trice, T.M.; Michael, B. In situ nutrient monitoring: A tool for capturing nutrient variability and the antecedent conditions that support algal blooms. Harmful Algae 2008, 8, 175–181. [Google Scholar] [CrossRef]
- Heil, C.A.; Steidinger, K.A. Monitoring, management and mitigation of Karenia blooms in the eastern Gulf of Mexico. Harmful Algae 2009, 8, 611–617. [Google Scholar] [CrossRef]
- Corcoran, A.A.; Wolny, J.; Leone, E.; Ivey, J.; Murasko, S. Drivers of phytoplankton dynamics in Old Tampa Bay, FL (USA), a subestuary lagging in ecosystem recovery. Estuar. Coast. Shelf Sci. 2017, 185, 130–140. [Google Scholar] [CrossRef]
- Wehr, J.D.; Sheath, R.G. (Eds.) Freshwater Algae of North America; Academic Press: New York, NY, USA, 2003; p. 917. [Google Scholar] [CrossRef]
- Tomas, C.R. (Ed.) Identifying Marine Phytoplankton; Academic Press: San Diego, CA, USA, 1997; p. 858. [Google Scholar]
- Steidinger, K.A.; Wolny, J.L.; Haywood, A.J. Identification of Kareniaceae (Dinophyceae) in the Gulf of Mexico. Nova Hedwigia 2008, 133, 269–284. [Google Scholar]
- Marshall, H.G. Autotrophic picoplankton distribution and abundance in Chesapeake Bay, U.S.A. Mar. Nat. 1995, 4, 33–42. [Google Scholar]
- Affronti, L.F.; Marshall, H.G. Diel abundance and productivity patterns of autotrophic picoplankton in the lower Chesapeake Bay. J. Plankton Res. 1993, 15, 1–8. [Google Scholar] [CrossRef]
- Holm-Hansen, O. Determination of microbial biomass in ocean profiles. Limnol. Oceanogr. 1969, 14, 740–747. [Google Scholar] [CrossRef]
- Solozano, L.; Sharp, J.H. Determination of total dissolved phosphorus and particulate phosphorus in natural waters. Limnol. Oceanogr. 1980, 25, 756–760. [Google Scholar]
- Bronk, D.A.; Lomas, M.W.; Glibert, P.M.; Schukert, K.J.; Sanderson, M.P. Total dissolved nitrogen analysis: Comparisons between the persulfate, UV and high temperature oxidation methods. Mar. Chem. 2000, 69, 163–178. [Google Scholar] [CrossRef]
- Paasche, E. Silicon content of five marine plankton diatom species measured with a rapid filter method. Limnol. Ocean. 1980, 25, 474–480. [Google Scholar] [CrossRef]
- Koroleff, F. Determination of Urea. In Methods of Seawater Analysis; Grasshoff, K., Ehrhardt, M., Kremling, K., Eds.; Weinheim Verlag Chemie: Deerfield Beach, FL, USA, 1983; pp. 158–162. [Google Scholar]
- Solomon, C.M.; Alexander, J.A.; Glibert, P.M. Measuring urease activity in aquatic environmental samples. Limnol. Oceanogr. Methods 2007, 5, 280–288. [Google Scholar] [CrossRef]
- Cleveland, W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 1979, 74, 829–836. [Google Scholar] [CrossRef]
- Davey, M.; Tarran, G.A.; Mills, M.M.; Ridame, C.; Geider, R.J.; LaRoche, J. Nutrient limitation of picophytoplankton photosynthesis and growth in the tropical North Atlantic. Limnol. Oceanogr. 2008, 53, 1722–1733. [Google Scholar] [CrossRef]
- Moore, L.R.; Ostrowski, M.; Scanlan, D.J.; Feren, K.; Sweetsir, T. Ecotypic variation in phosphorus-acquisition mechanisms within marine picocyanobacteria. Aquat. Microb. Ecol. 2005, 39, 257–269. [Google Scholar] [CrossRef]
- Lin, S.; Litaker, R.W.; Sunda, W.G. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J. Phycol. 2016, 52, 10–36. [Google Scholar] [CrossRef]
- Berg, G.M.; Glibert, P.M.; Jørgensen, N.O.G.; Balode, M.; Purina, I. Variability in inorganic and organic nitrogen uptake associated with riverine nutrient input in the Gulf of Riga, Baltic Sea. Estuaries 2001, 24, 204–214. [Google Scholar] [CrossRef]
- Glibert, P.M.; Berg, G.M. Nitrogen form, fate and phytoplankton composition. In Enclosed Experimental Ecosystems and Scale: Tools for Understanding and Managing Coastal Ecosystems; Petersen, J.E., Kennedy, V.S., Dennison, W.C., Kemp, W.M., Eds.; Springer: New York, NY, USA, 2009; pp. 183–189. [Google Scholar]
- Caraccia, K.S. A Preliminary Assessment of the Effects of Treated Sewer Discharge on the Phytoplankton Communities of Whitaker Bayou and Adjoining Sarasota Bay; technical report; Mote Marine Laboratory: Sarasota, FL, USA, 1981; p. 17. [Google Scholar]
- Millie, D.F.; Schofield, O.; Kirkpatrick, G.J.; Johnsen, G.; Tester, P.A.; Vinyard, B.T. Phytoplankton pigments and absorption spectra as potential biomarkers for harmful algal blooms: A case study of the Florida red-tide dinoflagellate, Gymnodinium breve. Limnol. Ocean. 1997, 42, 1240–1251. [Google Scholar] [CrossRef]
- Hicks, D.B.; Cavinder, T.R.; Raschke, R.L.; Murphy, P.M. Finger-Fill Canal Studies Florida and North Carolina; USEPA 904/9-76-017; National Technical Information Service: Springfield, VA, USA, 1975; p. 446. [Google Scholar]
- Zieliński, P.; Górniak, A.; Piekarski, M.K. The effect of hydrological drought on chemical quality of water and dissolved organic carbon concentrations in lowland rivers. Pol. J. Ecol. 2009, 57, 217–227. [Google Scholar]
- Vargo, G.A.; Heil, C.A.; Fanning, K.A.; Dixon, L.K.; Neely, M.B.; Lester, K.; Ault, D.; Murasko, S.; Havens, J.; Walsh, J.; et al. Nutrient availability in support of Karenia brevis blooms on the central West Florida Shelf: What keeps Karenia blooming? Cont. Shelf Res. 2008, 28, 73–98. [Google Scholar] [CrossRef]
- Southwest Florida Water Management District. Sarasota Bay Surface Water Improvement and Management (SWIM) Plan; Report VISKH 7-17; Southwest Florida Water Management District: Brooksville, FL, USA, 2002; p. 5. [Google Scholar]
- Sarasota Bay Conditions Report for 2010. Available online: https://www.sarasota.wateratlas.usf.edu/bay-conditions/report/1/sarasota-bay/2010/#waterchemistry (accessed on 1 June 2020).
- Smith, S.V. Phosphorus versus nitrogen limitation in the marine environment. Limnol. Oceanogr. 1984, 29, 1149–1160. [Google Scholar] [CrossRef]
- Sheng, Y.P.; Peene, S.; Yassuda, E. Circulation and transport in Sarasota Bay, Florida: The effect of tidal inlets on estuarine circulation and flushing quality. In Mixing in Estuaries and Coastal Seas Coastal; Pattiaratchi, C., Ed.; American Geophysical Union: Washington, DC, USA, 1996; pp. 184–210. [Google Scholar]
- Cannizzaro, J.; Corcoran, A.; Wolny, J.; Hu, C. Light absorption properties of algal blooms in Old Tampa Bay: Implications for management. In BASIS 6, Tampa Bay Area Scientific Information Symposium; Burke, M., Ed.; Tampa Bay Regional Planning Council: Pinellas Park, FL, USA, 2016; pp. 84–95. [Google Scholar]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker. R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. USA 2017, 114, 4975–4980. [Google Scholar] [CrossRef] [PubMed]
- Trainer, V.L.; Moore, S.K.; Hallegraeff, G.; Kudela, R.M.; Clement, A.; Mardones, J.I.; Cochlan, W.P. Pelagic harmful algal blooms and climate change: Lessons from nature’s experiments with extremes. Harmful Algae 2020, 91, 101591. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Hunt, J.H.; Herrnkind, W.F.; Childress, M.J.; Bertelsen, R.; Sharp, W.; Matthews, T.; Field, J.M.; Marshall, H.G. Cascading disturbances in Florida Bay, USA: Cyanobacteria blooms, sponge mortality, and implications for juvenile spiny lobsters Panulirus argus. Mar. Ecol. Prog. Ser. 1995, 129, 119–125. [Google Scholar] [CrossRef]
- Glibert, P.M.; Heil, C.A.; Rudnick, D.T.; Madden, C.J.; Boyer, J.N.; Kelly, S. Florida Bay: Water quality status and trends, historic and emerging algal bloom problems. Contrib. Mar. Sci. 2009, 38, 5–17. [Google Scholar]
- Hall, M.O.; Durako, M.J.; Fourqurean, J.W.; Zieman, J.C. Decadal changes in seagrass distribution and abundance in Florida Bay. Estuaries 1999, 22, 445–459. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Robblee, M.B. Florida Bay: A history of recent ecological changes. Estuaries 1999, 22, 345–357. [Google Scholar] [CrossRef]
- Bronk, D.A.; See, J.H.; Bradley, P.; Killberg, L. DON as a source of bioavailable nitrogen for phytoplankton. Biogeoscience 2007, 4, 283–296. [Google Scholar] [CrossRef]
- Bronk, D.A.; Glibert, P.M.; Malone, T.C.; Banahan, S.; Sahlsten, E. Inorganic and organic nitrogen cycling in Chesapeake Bay: Autotrophic versus heterotrophic processes and relationships to carbon flux. Aquat. Microb. Ecol. 1998, 15, 177–189. [Google Scholar] [CrossRef]
- Hochmuth, G.; Nell, T.; Sartain, J.; Unruh, J.B.; Martinez, C.; Trenholm, L.; Cisar, J. Urban Water Quality and Fertilizer Ordinances: Avoiding Unintended Consequences: A Review of the Scientific Literature; Report SL 283; Cooperative Extension Service, University of Florida: Gainesville, FL, USA, 2011; p. 25. [Google Scholar]
- Hochmuth, G.; Nell, T.; Unruh, J.B.; Trenholm, L.; Sartain, J. Potential unintended consequences associated with urban fertilizer bans in Florida—A scientific review. Horttechnology 2012, 22, 600–616. [Google Scholar] [CrossRef]
- Dillon, K.S.; Chanton, J.P. Nutrient transformations between rainfall and stormwater runoff in an urbanized coastal environment: Sarasota Bay, Florida. Limnol. Oceanogr. 2005, 50, 62–69. [Google Scholar] [CrossRef]
- Dillon, K.S.; Chanton, J.P. Nitrogen stable isotopes of macrophytes assess stormwater nitrogen inputs to an urbanized estuary. Estuaries Coasts 2008, 31, 360–370. [Google Scholar] [CrossRef]
- PBS&J. EMC Monitoring in Support of Pollutant Load Modeling, Prepared for Sarasota County; Atkins Company: Tampa, FL, USA, 2010; p. 31. [Google Scholar]
- Wdowinski, S.; Bray, R.; Kirtman, B.P.; Wu, Z. Increasing flooding hazard in coastal communities due to rising sea level: Case study of Miami Beach, Florida. Ocean. Coast. Manag. 2016, 126, 1–8. [Google Scholar] [CrossRef]
- Lewitus, A.J.; Scmidt, L.B.; Mason, L.J.; Kempton, J.W.; Wilde, S.B.; Wolny, J.L.; Williams, B.J.; Hayes, K.C.; Hymel, S.N.; Keppler, C.J.; et al. Harmful algal blooms in South Carolina residential and golf course ponds. Popul. Environ. 2003, 24, 387–413. [Google Scholar] [CrossRef]
- Mwashote, B.M.; Murray, M.; Burnett, W.C.; Chanton, J.; Kruse, S.; Forde, A. Submarine groundwater discharge in the Sarasota Bay system: Its assessment and implications for the nearshore coastal environment. Cont. Shelf Res. 2013, 53, 63–76. [Google Scholar] [CrossRef]
- Konarzewska, Z.; Śliwińska-Wilczewska, S.; Barreiro Felpeto, A.; Vasconcelos, V.; Latała, A. Assessment of the allelochemical activity and biochemical profile of different phenotypes of picocyanobacteria from the genus Synechococcus. Mar. Drugs 2020, 18, 179. [Google Scholar] [CrossRef]
- Karjalainen, M.; Engström-Öst, J.; Korpinen, S.; Peltonen, H.; Pääkkönen, J.P.; Rönkkönen, S.; Suikkanen, S.; Viitasalo, M. Ecosystem consequences of cyanobacteria in the Northern Baltic Sea. AMBIO 2007, 36, 195–202. [Google Scholar] [CrossRef]
- Dickman, E.M.; Newell, J.M.; Gonzalez, M.J.; Vanni, M.J. Light, nutrients, and food-chain length constrain planktonic energy transfer efficiency across multiple trophic levels. Proc. Natl. Acad. Sci. USA 2008, 105, 18408–18412. [Google Scholar] [CrossRef] [PubMed]
- Wetz, M.S.; Paerl, H.W.; Taylor, J.C.; Leonard, J.A. Environmental controls upon picophytoplankton growth and biomass in a eutrophic estuary. Aquat. Microb. Ecol. 2011, 63, 133–143. [Google Scholar] [CrossRef]
- Glibert, P.M. Long-term changes in nutrient loading and stoichiometry and their relationships with changes in the food web and dominant pelagic fish species in the San Francisco Estuary, California. Rev. Fish. Sci. 2010, 18, 211–232. [Google Scholar] [CrossRef]
- McClenachan, L. Recreation and the “Right to Fish” movement: Anglers and ecological degradation in the Florida Keys. Environ. Hist. 2013, 18, 76–87. [Google Scholar] [CrossRef]
- Camp, E.; Garlock, T.; Anderson, J. Opportunities and Obstacles to Aquaculture in Florida; University of Florida: Gainesville, FL, USA, 2018; p. 6. [Google Scholar]
- Stauffer, B.A.; Bowers, H.A.; Buckley, E.; Davis, T.W.; Johengen, T.H.; Kudela, R.; McManus, M.A.; Purcell, H.; Smith, G.J.; Vander Woude, A.; et al. Considerations in harmful algal bloom research and monitoring: Perspectives from a consensus-building workshop and technology testing. Front. Mar. Sci. 2019, 6, 399. [Google Scholar] [CrossRef]
- Altman, J.C.; Paerl, H.W. Composition of inorganic and organic nutrient sources influences phytoplankton community structure in the New River Estuary, North Carolina. Aquat. Ecol. 2012, 46, 269–282. [Google Scholar] [CrossRef]
- Cira, E.K.; Paerl, H.W.; Wetz, M.S. Effects of nitrogen availability and form on phytoplankton growth in a eutrophied estuary (Neuse River Estuary, NC, USA). PLoS ONE 2016, 11, e0160663. [Google Scholar] [CrossRef]

| Instrument | Measurement | Final Output | Sample Location |
|---|---|---|---|
| YSI 6600 Data Sonde | Chlorophyll-a | µg L−1 | Surface, Bottom |
| Salinity | PSU | Surface, Bottom | |
| Temperature | °C | Surface, Bottom | |
| pH | pH | Surface, Bottom | |
| Phycocyanin | cells L−1 | Surface, Bottom | |
| Dissolved Oxygen | mg L−1 | Surface, Bottom | |
| Dissolved Oxygen | Percent saturation | Surface, Bottom | |
| Turbidity | Nephloid Turbidity Units | Surface, Bottom | |
| YSI 9600 | Nitrate | µg L−1 | Surface, Bottom |
| Envirotech | Urea | µg L−1 | Bottom |
| Vaisala WXT-520 | Wind Speed | m s−1 | 4 m above surface |
| Temperature | °C | 4 m above surface | |
| Precipitation | inches h−1 | 4 m above surface | |
| Barometric Pressure | inches hg | 4 m above surface | |
| Relative Humidity | percent | 4 m above surface | |
| Licor LI-190 | Downwelling PAR | µeinsteins m−2 s−1 | 4 m above surface |
| Licor LI-192 | Downwelling PAR | µeinsteins m−2 s−1 | Surface, 1 m |
| Sontek ADCP | Current Flows | m s−1 | horizontal X and Y |
| Current Flows | m s−1 | vertical | |
| Water depth | m | Water column |
| Date Sampled | TN µM | TC µM | TP µM | Urea µM N/L | NO3 µM | NO2 µM | Si µM | NH4 µM | PO4- µM | TDN uM | DON µM | TDP µM | DOP µM | Biogenic Si µM | Urease N µg−1 Chl-a−1 h−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6/16/2009 | 15.01 | 121.58 | 0.94 | 1.19 | 0.44 | 0.02 | 6.30 | 0.29 | 0.05 | 24.81 | 24.06 | 0.54 | 0.50 | 17.80 | |
| 6/23/2009 | 12.54 | 99.23 | 1.00 | 0.44 | 0.64 | 0.01 | 4.24 | 0.48 | 0.04 | 23.51 | 22.37 | 0.41 | 0.37 | 22.51 | 58.71 |
| 7/8/2009 | 10.85 | 78.27 | 0.78 | 1.82 | 0.64 | 0.01 | 7.28 | 0.27 | 0.01 | 22.53 | 21.61 | 0.45 | 0.44 | 12.29 | 54.99 |
| 7/13/2009 | 10.06 | 69.33 | 0.72 | 0.81 | 0.65 | 0.01 | 10.34 | 0.29 | 0.01 | 22.03 | 21.07 | 1.06 | 1.06 | 13.04 | 259.01 |
| 7/27/2009 | 11.63 | 79.84 | 0.73 | 0.21 | 0.66 | 0.14 | 15.92 | 1.42 | 0.03 | 23.51 | 21.30 | 0.42 | 0.39 | 6.33 | 82.18 |
| 8/10/2009 | 11.74 | 86.59 | 0.63 | 0.33 | 0.65 | 0.02 | 20.91 | 0.34 | 0.06 | 23.03 | 22.02 | 0.45 | 0.39 | 3.88 | 295.08 |
| 8/25/2009 | 11.57 | 113.19 | 0.73 | 0.51 | 0.66 | 0.02 | 20.91 | 0.21 | 0.04 | 23.48 | 22.59 | 0.41 | 0.36 | 7.86 | 464.09 |
| 9/8/2009 | 11.95 | 93.78 | 0.77 | 1.11 | 0.64 | 0.03 | 29.79 | 0.47 | 0.05 | 26.97 | 25.83 | 0.65 | 0.60 | 3.33 | 241.23 |
| 9/22/2009 | 13.03 | 115.55 | 0.85 | 0.21 | 0.63 | 0.02 | 23.66 | 0.07 | 0.03 | 24.80 | 24.07 | 0.61 | 0.58 | 4.57 | 0.80 |
| 9/30/2009 | 18.45 | 149.75 | 0.32 | 1.15 | 30.70 | 0.09 | |||||||||
| 10/6/2009 | 18.64 | 171.31 | 0.19 | 0.52 | 0.64 | 0.02 | 36.07 | 0.07 | 0.04 | 24.74 | 24.02 | 0.09 | 0.05 | 2.09 | 0.00 |
| 10/22/2009 | 9.44 | 74.22 | 0.63 | 0.23 | 0.66 | 0.02 | 2.29 | 0.29 | 0.13 | 21.32 | 20.35 | 0.73 | 0.60 | 12.97 | 18.06 |
| 11/3/2009 | 11.51 | 99.70 | 0.71 | 0.92 | 0.66 | 0.14 | 6.66 | 0.45 | 0.05 | 30.74 | 29.49 | 0.63 | 0.58 | 13.16 | 24.17 |
| 11/17/2009 | 9.34 | 67.68 | 0.61 | 0.64 | 0.69 | 0.03 | 8.40 | 0.39 | 0.08 | 0.39 | 0.31 | 7.63 | 0.23 | ||
| Average | 12.55 | 101.43 | 0.69 | 0.72 | 0.63 | 0.04 | 14.83 | 0.39 | 0.05 | 24.78 | 23.23 | 0.50 | 0.48 | 9.80 | 124.88 |
| Standard Deviation | 2.92 | 30.59 | 0.22 | 0.47 | 0.06 | 0.05 | 10.63 | 0.34 | 0.03 | 3.00 | 2.52 | 0.25 | 0.23 | 6.11 | 152.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivey, J.E.; Wolny, J.L.; Heil, C.A.; Murasko, S.M.; Brame, J.A.; Parks, A.A. Urea Inputs Drive Picoplankton Blooms in Sarasota Bay, Florida, U.S.A. Water 2020, 12, 2755. https://doi.org/10.3390/w12102755
Ivey JE, Wolny JL, Heil CA, Murasko SM, Brame JA, Parks AA. Urea Inputs Drive Picoplankton Blooms in Sarasota Bay, Florida, U.S.A. Water. 2020; 12(10):2755. https://doi.org/10.3390/w12102755
Chicago/Turabian StyleIvey, James E., Jennifer L. Wolny, Cynthia A. Heil, Susan M. Murasko, Julie A. Brame, and Ashley A. Parks. 2020. "Urea Inputs Drive Picoplankton Blooms in Sarasota Bay, Florida, U.S.A." Water 12, no. 10: 2755. https://doi.org/10.3390/w12102755
APA StyleIvey, J. E., Wolny, J. L., Heil, C. A., Murasko, S. M., Brame, J. A., & Parks, A. A. (2020). Urea Inputs Drive Picoplankton Blooms in Sarasota Bay, Florida, U.S.A. Water, 12(10), 2755. https://doi.org/10.3390/w12102755





