Effect of Water Deficit on Morphoagronomic and Physiological Traits of Common Bean Genotypes with Contrasting Drought Tolerance
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Experimental Design
2.2. Induction and Monitoring of Water Deficit
2.3. Morphoagronomic Evaluations
2.4. Physiological Evaluations
2.5. Data Analyses
3. Results
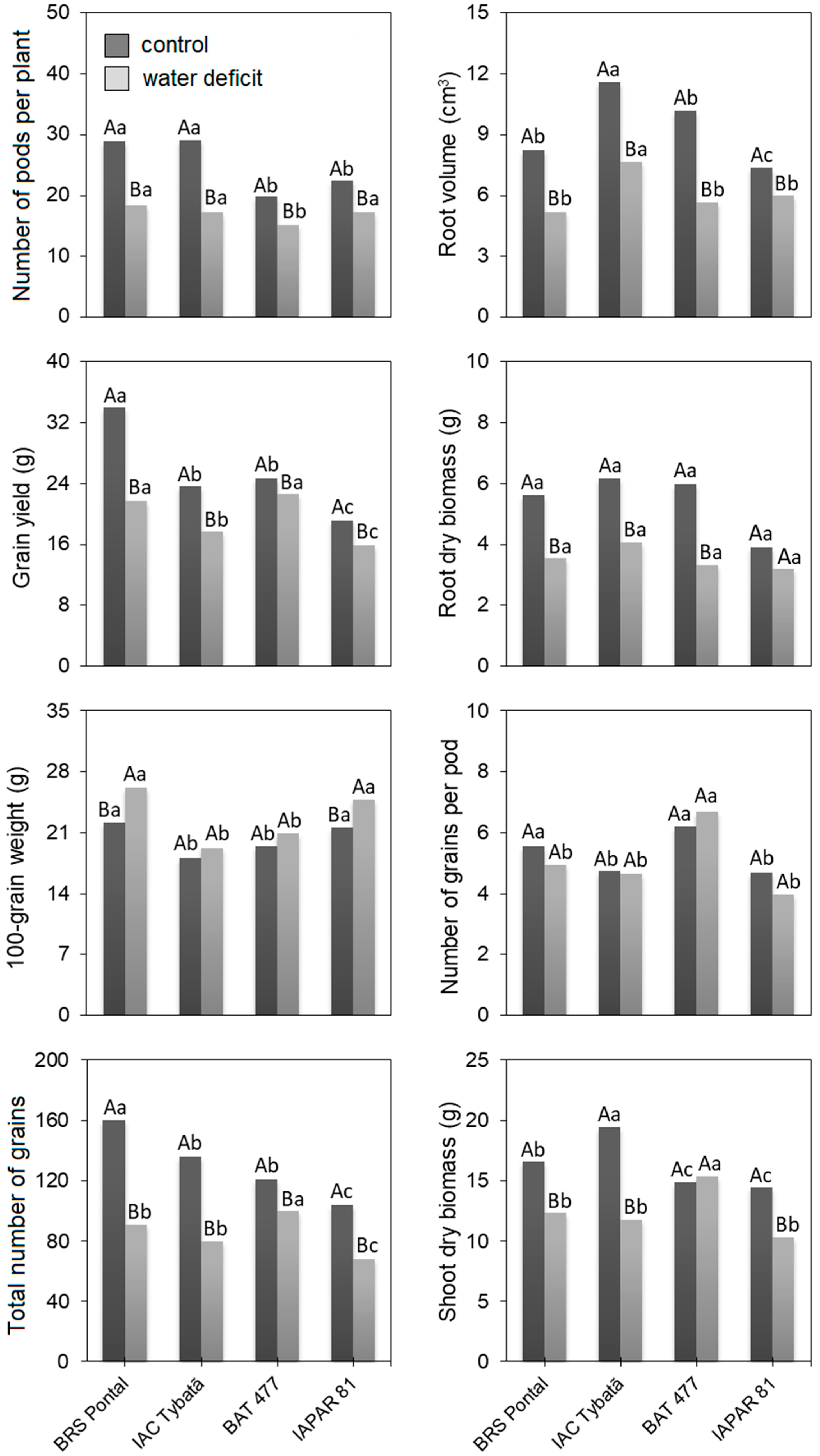
3.1. Morphoagronomic Traits
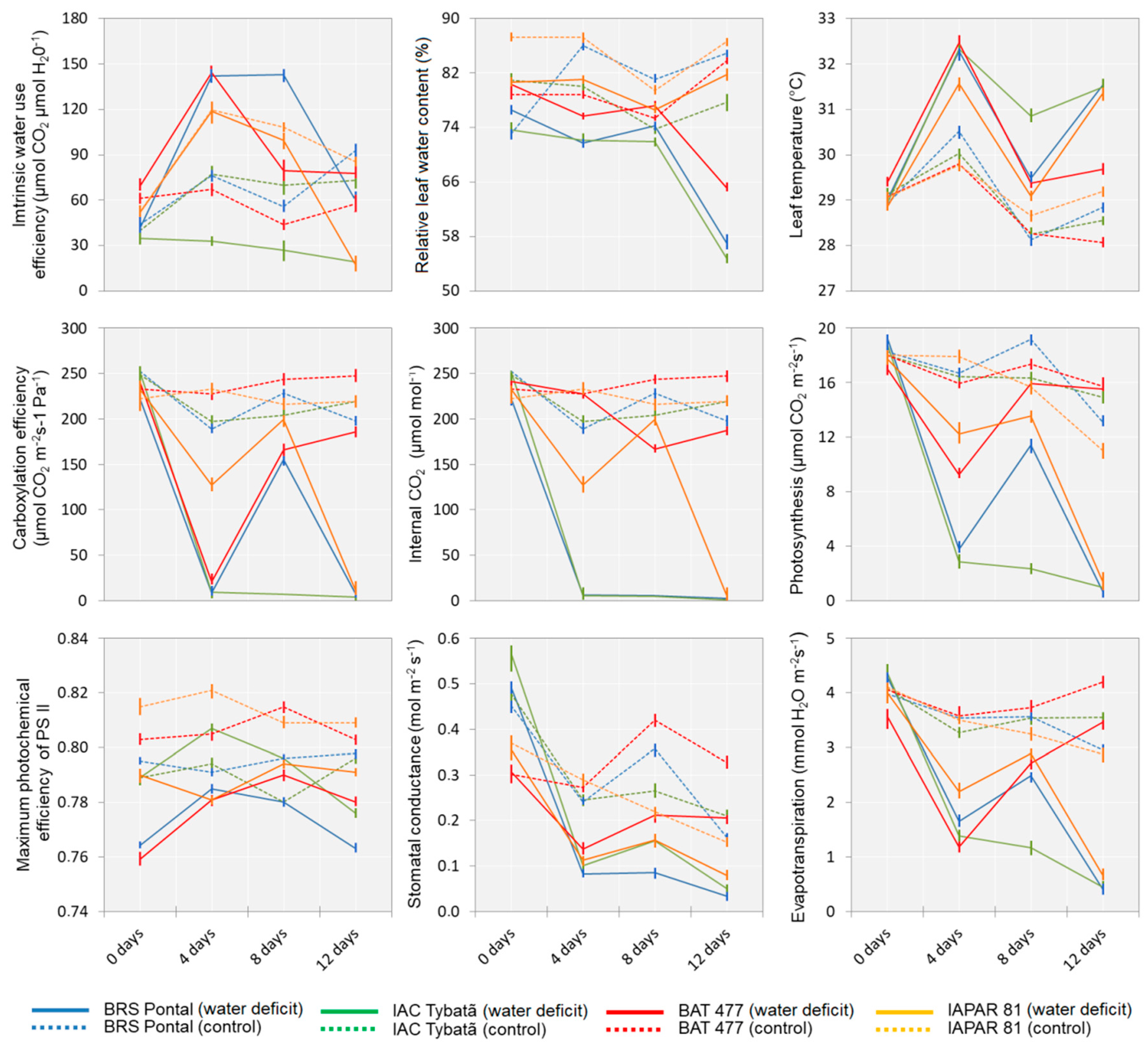
3.2. Physiological Traits
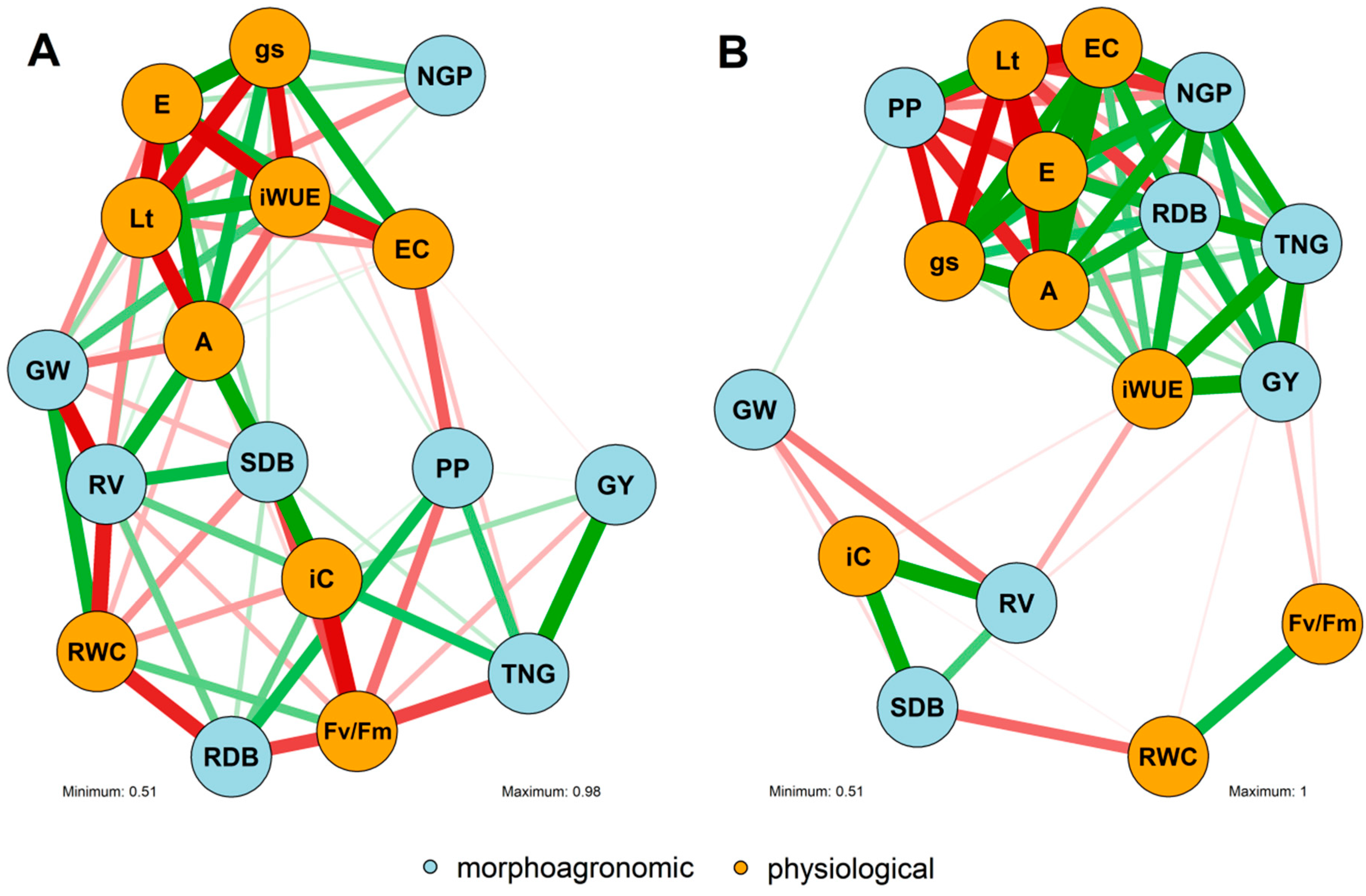
3.3. Correlation Network
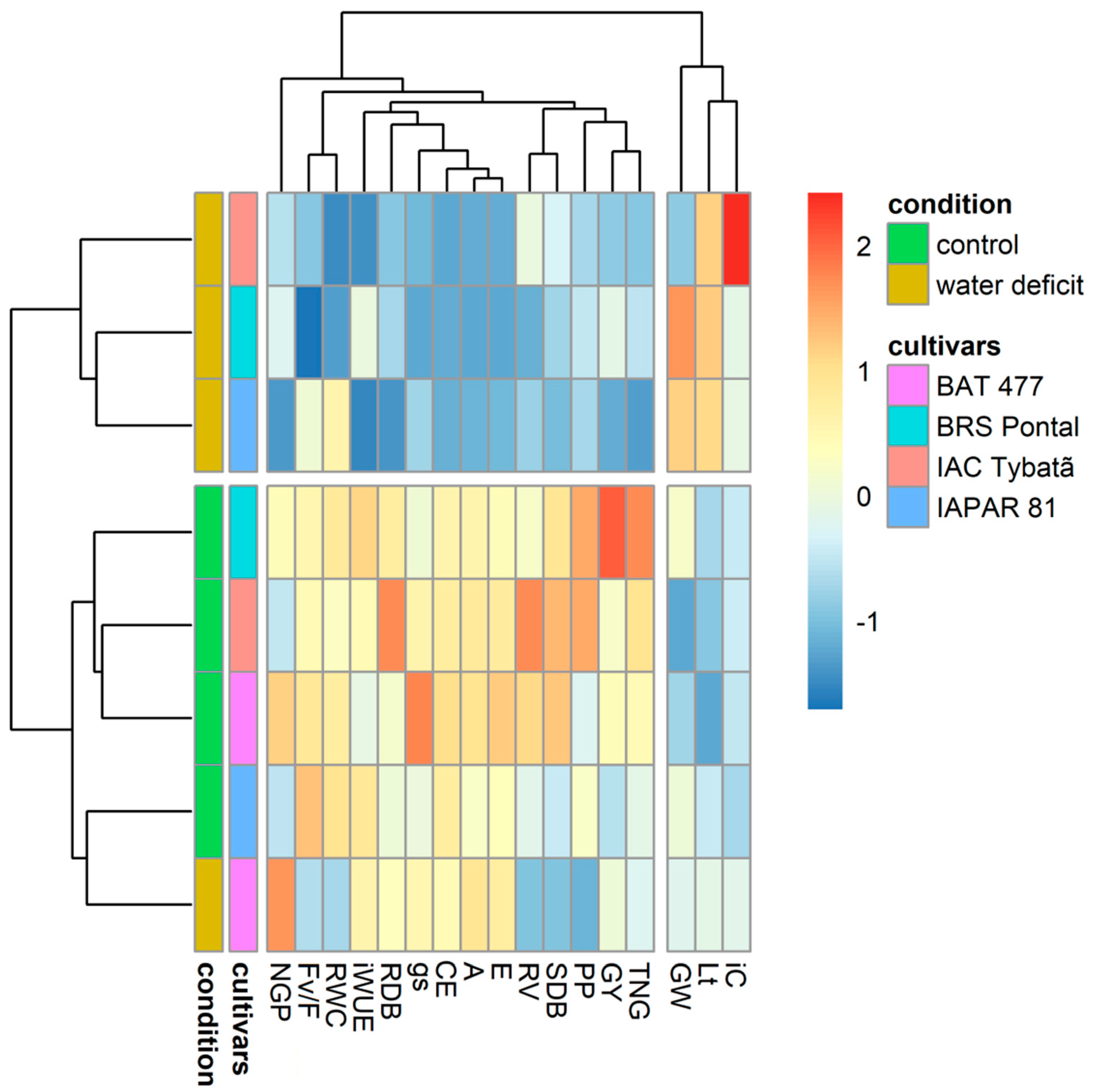
3.4. Heatmap Representation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Taylor, S. Crop niche modeling projects major shifts in common bean growing areas. Agric. For. Meteorol. 2016, 218, 102–113. [Google Scholar] [CrossRef]
- Hummel, M.; Hallahan, B.F.; Brychkova, G.; Ramirez-Villegas, J.; Guwela, V.; Chataika, B.; Curley, E.; McKeown, P.C.; Morrison, L.; Talsma, E.F.; et al. Reduction in nutritional quality and growing area suitability of common bean under climate change induced drought stress in Africa. Sci. Rep. 2018, 8, 16187. [Google Scholar] [CrossRef]
- Mukeshimana, G.; Butare, L.; Cregan, P.B.; Blair, M.W.; Kelly, J.D. Quantitative trait loci associated with drought tolerance in common bean. Crop. Sci. 2004, 54, 923–938. [Google Scholar] [CrossRef]
- Assefa, T.; Mahama, A.A.; Brown, A.V.; Cannon, E.K.S.; Rubyogo, J.C.; Rao, I.M.; Blair, M.W.; Cannon, S.B. A review of breeding objectives, genomic resources, and marker-assisted methods in common bean (Phaseolus vulgaris L.). Mol. Breed. 2019, 39, 1–23. [Google Scholar] [CrossRef]
- Asfaw, A.; Blair, M.W. Quantitative trait loci for rooting pattern traits of common beans grown under drought stress versus non stress conditions. Mol. Breed. 2012, 30, 681–695. [Google Scholar] [CrossRef]
- Hoyos-Villegas, V.; Song, Q.; Kelly, J.D. Genome-wide association analysis for drought tolerance and associated traits in common bean. Plant Gen. 2016, 10, 1–17. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop. Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Cortés, A.J.; Blair, M.W. Genotyping by sequencing and genome–environment associations in wild common bean predict widespread divergent adaptation to drought. Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.L.; Waskom, R.M.; Niu, Y.; Siddique, K.H. Regulated stress irrigation for crop production under drought stress. A review. Agron. Sust. Devel. 2016, 36, 1–21. [Google Scholar]
- Arruda, I.M.; Moda-Cirino, V.; Koltun, A.; dos Santos, O.J.A.P.; Moreira, R.S.; Moreira, A.F.P.; Gonçalves, L.S.A. Physiological, biochemical and morphoagronomic characterization of drought-tolerant and drought-sensitive bean genotypes under water stress. Physiol. Mol. Biol. Plants 2018, 24, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Polania, J.; Poschenrieder, C.; Rao, I.; Beebe, S. Estimation of phenotypic variability in symbiotic nitrogen fixation ability of common bean under drought stress using 15N natural abundance in grain. Eur. J. Agron. 2016, 79, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Manjeru, P.; Madanzi, T.; Makeredza, B.; Nciizah, A.; Sithole, M. Effect of Water Stress at Different Growth Stage on Components and Grain Yield of Common Bean (Phaseolus vulgaris L.). Afr. Crop. Sci. Conf. Proc. 2007, 8, 299–303. [Google Scholar]
- Ambachew, D.; Mekbib, F.; Asfaw, A.; Beebe, S.E.; Blair, M.W. Trait associations in common bean genotypes grown under drought stress and field infestation by BSM bean fly. Crop. J. 2015, 3, 305–316. [Google Scholar] [CrossRef]
- Fleta-Soriano, E.; Munné-Bosch, S. Stress memory and the inevitable effects of drought: A physiological perspective. Front. Plant Sci. 2016, 7, 143. [Google Scholar] [CrossRef]
- Ye, H.; Roorkiwal, M.; Valliyodan, B.; Zhou, L.; Chen, P.; Varshney, R.K.; Nguyen, H.T. Genetic diversity of root system architecture in response to drought stress in grain legumes. J. Exp. Bot. 2018, 69, 3267–3277. [Google Scholar] [CrossRef]
- Arruda, I.M.; Moda-Cirino, V.; Koltun, A.; Zeffa, D.M.; Nagashima, G.T.; Gonçalves, L.S.A. Combining Ability for Agromorphological and Physiological Traits in Different Gene Pools of Common Bean Subjected to Water Stress. Agronomy 2019, 9, 371. [Google Scholar] [CrossRef]
- Polania, J.; Poschenrieder, C.; Rao, I.M.; Beebe, S.E. Root traits and their potential links to plant ideotypes to improve drought resistance in common bean. Theor. Exp. Plant Physiol. 2017, 29, 143–154. [Google Scholar] [CrossRef]
- Polania, J.; Rao, I.M.; Cajiao, C.; Grajales, M.; Rivera, M.; Velasquez, F.; Beebe, S.E. Shoot and root traits contribute to drought resistance in recombinant inbred lines of MD 23–24 × SEA 5 of common bean. Front. Plant Sci. 2017, 8, 296. [Google Scholar] [CrossRef]
- Rosales, M.A.; Ocampo, E.; Rodríguez-Valentín, R.; Olvera-Carrillo, Y.; Acosta-Gallegos, J.; Covarrubias, A.A. Physiological analysis of common bean (Phaseolus vulgaris L.) cultivars uncovers characteristics related to terminal drought resistance. Plant Physiol. Biochem. 2012, 56, 24–34. [Google Scholar] [CrossRef]
- Heinemann, A.B.; Ramirez-Villegas, J.; Souza, T.L.P.; Didonet, A.D.; di Stefano, J.G.; Boote, K.J.; Jarvis, A. Drought impact on rain fed common bean production areas in Brazil. Agric. For. Meteorol. 2016, 225, 57–74. [Google Scholar] [CrossRef]
- Darkwa, K.; Ambachew, D.; Mohammed, H.; Asfaw, A.; Blair, M.W. Evaluation of common bean (Phaseolus vulgaris L.) genotypes for drought stress adaptation in Ethiopia. Crop. J. 2016, 4, 367–376. [Google Scholar] [CrossRef]
- Blum, A. Plant Breeding for Stress Environments; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–223. [Google Scholar]
- Moda-Cirino, V.; Oliari, L.; Lollato, M.A.; Fonseca Júnior, N.S. IAPAR 81—Common bean. Crop Breed. Appl. Biotechnol. 2001, 1, 203–204. [Google Scholar] [CrossRef]
- Aguiar, R.S.; Moda-Cirino, V.; Faria, R.T.; Vida, L.H.I. Avaliação de linhagens promissoras de feijoeiro (Phaseolus vulgaris L.) tolerantes ao déficit hídrico. Semin. Cienc. Agrar. 2008, 29, 1–14. [Google Scholar] [CrossRef]
- Androciolli, L.G. Fenotipagem Para Tolerância a Seca Em Cultivares De Feijão. Master’s Thesis, Agronomic Institute of Paraná, Londrina, Brazil, 2007. [Google Scholar]
- Weatherley, P.E. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. N. Phytol. 1950, 49, 81–87. [Google Scholar] [CrossRef]
- Bartlett, M.S. Properties of sufficiency and statistical tests. Proc. R. Soc. Lond. 1937, 1670, 268–282. [Google Scholar]
- Shapiro, S.S.; Wilks, M.B. An analysis of variance test for normality. Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Box, G.E.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Scott, A.J.; Knott, M. A cluster analysis method for grouping means in the analysis of variance. Biometrics 1974, 30, 507–512. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: An R package for ANOVA and experimental designs. Appl. Math. 2014, 5, 2952. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S.; Adler, D.; Bates, D.; Baud-Bovy, G.; Ellison, S.; Heiberger, R. Package ‘Car’. Available online: https://cran.microsoft.com/snapshot/2017-06-17/web/packages/car/car.pdf (accessed on 10 January 2020).
- Epskamp, S.; Cramer, A.O.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. qgraph: Network visualizations of relationships in psychometric data. J. Stat. Softw. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Galili, T.; O’Callaghan, A.; Sidi, J.; Sievert, C. heatmaply: An R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 2017, 34, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Mazengo, K.D.; Tryphone, G.M.; Tarimo, A.J.P. Identification of drought selection indices of common bean (Phaseolus vulgaris L.) genotypes in the Southern Highlands of Tanzania. Afr. J. Agric. Res. 2019, 14, 161–167. [Google Scholar]
- Terán, H.; Singh, S.P. Comparison of sources and lines selected for drought resistance in common bean. Crop. Sci. 2002, 42, 64–70. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop. Sci. 2008, 48, 582–592. [Google Scholar] [CrossRef]
- Oliveira-Neto, S.; Zeffa, D.M.; Sartori, M.M.P.; Moda-Cirino, V. Cultivars selection of Carioca beans type to be harvested in arid farmlands. IRRIGA 2017, 22, 775–788. [Google Scholar] [CrossRef]
- Asfaw, A.; Ambachew, D.; Shah, T.; Blair, M.W. Trait associations in diversity panels of the two common bean (Phaseolus vulgaris L.) gene pools grown under well-watered and water-stress conditions. Front. Plant Sci. 2017, 8, 733. [Google Scholar] [CrossRef]
- Rao, I.; Beebe, S.; Polania, J.; Ricaurte, J.; Cajiao, C.; Garcia, R.; Rivera, M. Can tepary bean be a model for improvement of drought resistance in common bean? Afr. Crop. Sci. J. 2013, 21, 265–281. [Google Scholar]
- Barrios, A.N.; Hoogenboom, G.; Nesmith, D.S. Drought sress and the distribution of vegetative and reproductive traits of a bean cultivar. Sci. Agric. 2005, 62, 18–22. [Google Scholar] [CrossRef]
- Sponchiado, B.N.; White, J.W.; Castillo, J.A.; Jones, P.G. Root growth of four common bean cultivars in relation to drought tolerance in environments with contrasting soil types. Exp. Agric. 1989, 25, 249–257. [Google Scholar] [CrossRef]
- Moda-Cirino, V.; Gerage, A.C.; Riede, C.R.; Sera, G.H.; Takahashi, M.; Abbud, N.S.; Nazareno, N.R.X.; Araújo, P.M.; Auler, P.M.; Yamaoka, R.S.; et al. Plant breeding at Instituto Agronômico do Paraná –IAPAR. Crop. Breed. Appl. Biotechnol. 2012, 2, 25–30. [Google Scholar] [CrossRef]
- Rosales, M.A.; Cuellar-Ortiz, S.M.; de la Paz Arrieta-Montiel, M.; Acosta-Gallegos, J.; Covarrubias, A.A. Physiological traits related to terminal drought resistance in common bean (Phaseolus vulgaris L.). J. Sci. Food Agric. 2013, 93, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Polania, J.; Rao, I.M.; Cajiao, C.; Rivera, M.; Raatz, B.; Beebe, S.E. Physiological traits associated with drought resistance in Andean and Mesoamerican genotypes of common bean (Phaseolus vulgaris L.). Euphytica 2016, 210, 17–29. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Chaszar, B.; Lewis, J.D.; Smith, R.A.; Huxman, T.E.; Tissue, D.T. CO2 and temperature effects on morphological and physiological traits affecting risk of drought-induced mortality. Tree Physiol. 2018, 38, 1138–1151. [Google Scholar] [CrossRef]
- Murtaza, G.; Rasool, F.; Habib, R.; Javed, T.; Sardar, K.; Ayub, M.M.; Rasool, A. A review of morphological, physiological and biochemical responses of plants under drought stress conditions. Imp. J. Interdiscip. Res. 2016, 2, 1600–1606. [Google Scholar]
- Blankenagel, S.; Yang, Z.; Avramova, V.; Schön, C.C.; Grill, E. Generating plants with improved water use efficiency. Agronomy 2018, 8, 194. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop. J. 2015, 3, 220–228. [Google Scholar] [CrossRef]
- Moshelion, M.; Halperin, O.; Wallach, R.; Oren, R.A.M.; Way, D.A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield. Plant Cell Environ. 2015, 38, 1785–1793. [Google Scholar] [CrossRef]
- Liu, B.; Liang, J.; Tang, G.; Wang, X.; Liu, F.; Zhao, D. Drought stress effects on growth, water use efficiency, gas exchange and chlorophyll fluorescence of Juglans rootstocks. Sci. Hortic. 2019, 250, 230–235. [Google Scholar] [CrossRef]
- Briñez, B.; Perseguini, J.M.K.C.; Rosa, J.S.; Bassi, D.; Gonçalves, J.G.R.; Almeida, C.; Valdisser, P.A.M.R. Mapping QTLs for drought tolerance in a SEA 5 × AND 277 common bean cross with SSRs and SNP markers. Genet. Mol. Biol. 2017, 40, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.M.; Ricaurte, J.; Tovar, E.; Cajiao, C.; Terán, H.; Grajales, M.; Raatz, B. QTL analyses for tolerance to abiotic stresses in a common bean (Phaseolus vulgaris L.) population. PLoS ONE 2018, 13, e0202342. [Google Scholar] [CrossRef] [PubMed]
| Cultivar | Origin | Market Group | Cycle (Days) | Growth Habit | Drought Reaction | Reference |
|---|---|---|---|---|---|---|
| BAT 477 | CIAT | Brown | 94 | III | Tolerant | [1] |
| IAPAR 81 | IAPAR | ‘Carioca’ | 92 | III | Tolerant | [24] |
| IAC Tybatã | IAC | ‘Carioca’ | 95 | II | Sensitive | [25] |
| BRS Pontal | EMBRAPA | ‘Carioca’ | 95 | II | Sensitive | [26] |
| Source of Variation | Mean Square of the Morphoagronomic Traits | |||||||
|---|---|---|---|---|---|---|---|---|
| SDB | RDB | RV | PP | TNG | NGP | GW | GY | |
| Genotype (G) | 24.2 ** | 5.2 ** | 23.1 ** | 104.0 ** | 3181.7 ** | 10.2 ** | 77.5 ** | 234.4 ** |
| Water deficit (W) | 54.8 ** | 12.2 ** | 41.6 ** | 94.1 ** | 2882.1 ** | 14.1 ** | 137.5 ** | 137.1 ** |
| G × W | 33.2 ** | 2.1 ns | 5.8 ns | 40.0 ** | 1372.9 ** | 0.9 ns | 5.5 ns | 63.3 ** |
| Error | 5.6 | 1.9 | 5.8 | 4.4 | 245.1 | 0.6 | 3.5 | 9.7 |
| Mean (control) | 25.3 | 22.7 | 24.96 | 130.3 | 5.2 | 9.3 | 5.4 | 16.3 |
| Mean (water deficit) | 19.3 | 20.3 | 16.9 | 84.5 | 5.0 | 6.0 | 3.5 | 12.4 |
| CV (%) | 16.5 | 31.5 | 31.4 | 10.1 | 14.6 | 14.5 | 8.7 | 13.9 |
| Source of Variation | Mean Square of Physiological Traits | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | E | gs | iC | iWUE | Fv/Fm | RWC | CE | Lt | |
| Genotype (G) | 149.9 ** | 3.03 * | 0.28 ns | 0.65 ** | 133.6 ** | 2.39 ** | 768.2 ** | 558.0 ** | 1.69 ns |
| Water deficit (W) | 1867.4 ** | 81.88 ** | 4.98 ** | 5.54 ** | 32.2 * | 16.18 ** | 2902.8 ** | 123.9 ** | 102.51 ** |
| Time (T) | 691.2 ** | 28.90 ** | 6.63 ** | 2.04 ** | 213.7 * | 0.69 ns | 285.6 ** | 272.7 ** | 45.82 ** |
| G × W | 149.1 ** | 1.82 * | 0.07 ns | 0.55 ** | 151.8 ** | 2.38 * | 140.2 ns | 764.6 ** | 1.50 ns |
| W × T | 245.1 ** | 11.09 ** | 0.98 ** | 1.80 ** | 81.4 ** | 0.67 ns | 706.7 ** | 178.9 ** | 13.58 ** |
| G × T | 93.4 ** | 4.07 ** | 0.69 ** | 0.69 ** | 32.0 * | 0.33 ns | 107.2 ns | 126.6 ** | 2.10 ns |
| G × W × T | 40.3 ** | 1.93 * | 0.10 ns | 0.68 ** | 29.8 * | 0.47 ns | 117.7 ns | 91.1 ** | 0.92 ns |
| Error | 4.4 | 0.55 | 0.08 | 0.08 | 11.0 | 0.46 | 68.5 | 11.5 | 1.78 |
| Mean (control) | 16.4 | 3.6 | 0.29 | 220.1 | 70.2 | 0.80 | 80.9 | 223.7 | 29.0 |
| Mean (water deficit) | 10.1 | 2.3 | 0.19 | 109.9 | 73.3 | 0.78 | 73.1 | 115.8 | 30.4 |
| CV (%) | 15.7 | 25.1 | 36.3 | 27.3 | 32.1 | 5.8 | 10.7 | 4.8 | 5.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy Androcioli, L.; Mariani Zeffa, D.; Soares Alves, D.; Pires Tomaz, J.; Moda-Cirino, V. Effect of Water Deficit on Morphoagronomic and Physiological Traits of Common Bean Genotypes with Contrasting Drought Tolerance. Water 2020, 12, 217. https://doi.org/10.3390/w12010217
Godoy Androcioli L, Mariani Zeffa D, Soares Alves D, Pires Tomaz J, Moda-Cirino V. Effect of Water Deficit on Morphoagronomic and Physiological Traits of Common Bean Genotypes with Contrasting Drought Tolerance. Water. 2020; 12(1):217. https://doi.org/10.3390/w12010217
Chicago/Turabian StyleGodoy Androcioli, Leonardo, Douglas Mariani Zeffa, Daniel Soares Alves, Juarez Pires Tomaz, and Vânia Moda-Cirino. 2020. "Effect of Water Deficit on Morphoagronomic and Physiological Traits of Common Bean Genotypes with Contrasting Drought Tolerance" Water 12, no. 1: 217. https://doi.org/10.3390/w12010217
APA StyleGodoy Androcioli, L., Mariani Zeffa, D., Soares Alves, D., Pires Tomaz, J., & Moda-Cirino, V. (2020). Effect of Water Deficit on Morphoagronomic and Physiological Traits of Common Bean Genotypes with Contrasting Drought Tolerance. Water, 12(1), 217. https://doi.org/10.3390/w12010217





