Degradation of Methylene Blue via Dielectric Barrier Discharge Plasma Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
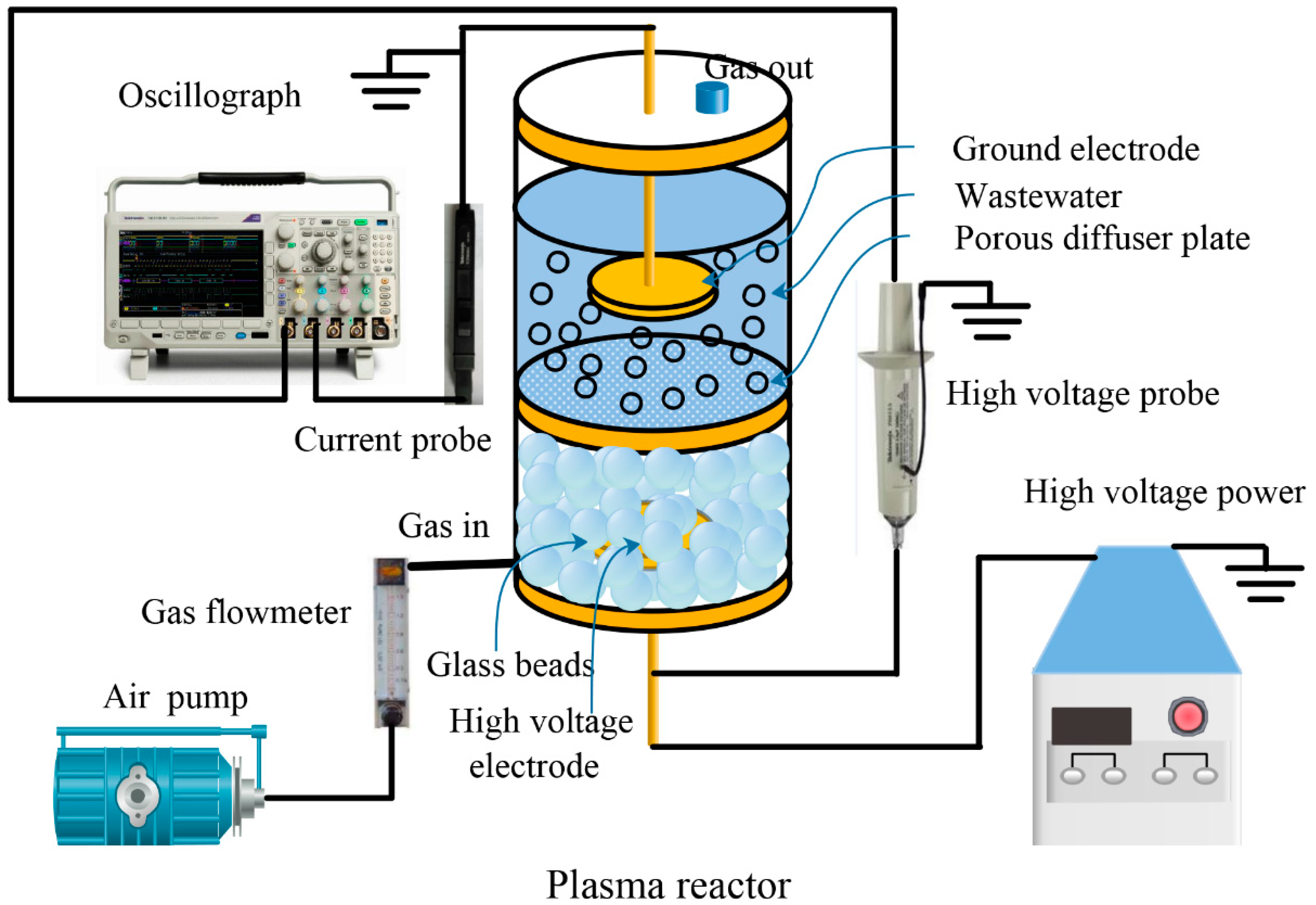
2.2. Experimental Setup
2.3. Analysis Methods
3. Results and Discussion
3.1. MB Degradation Using the DBD Plasma Reactor
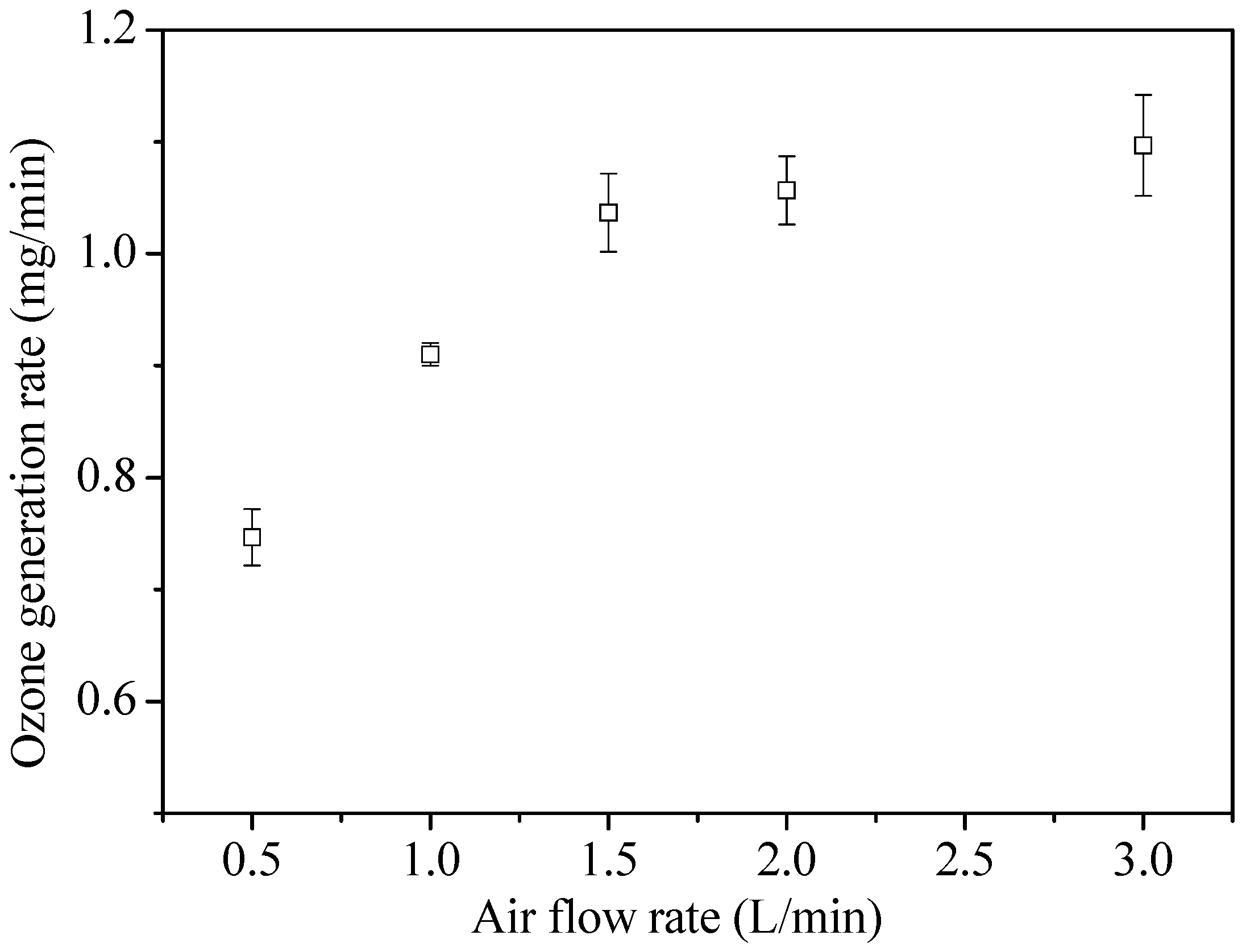
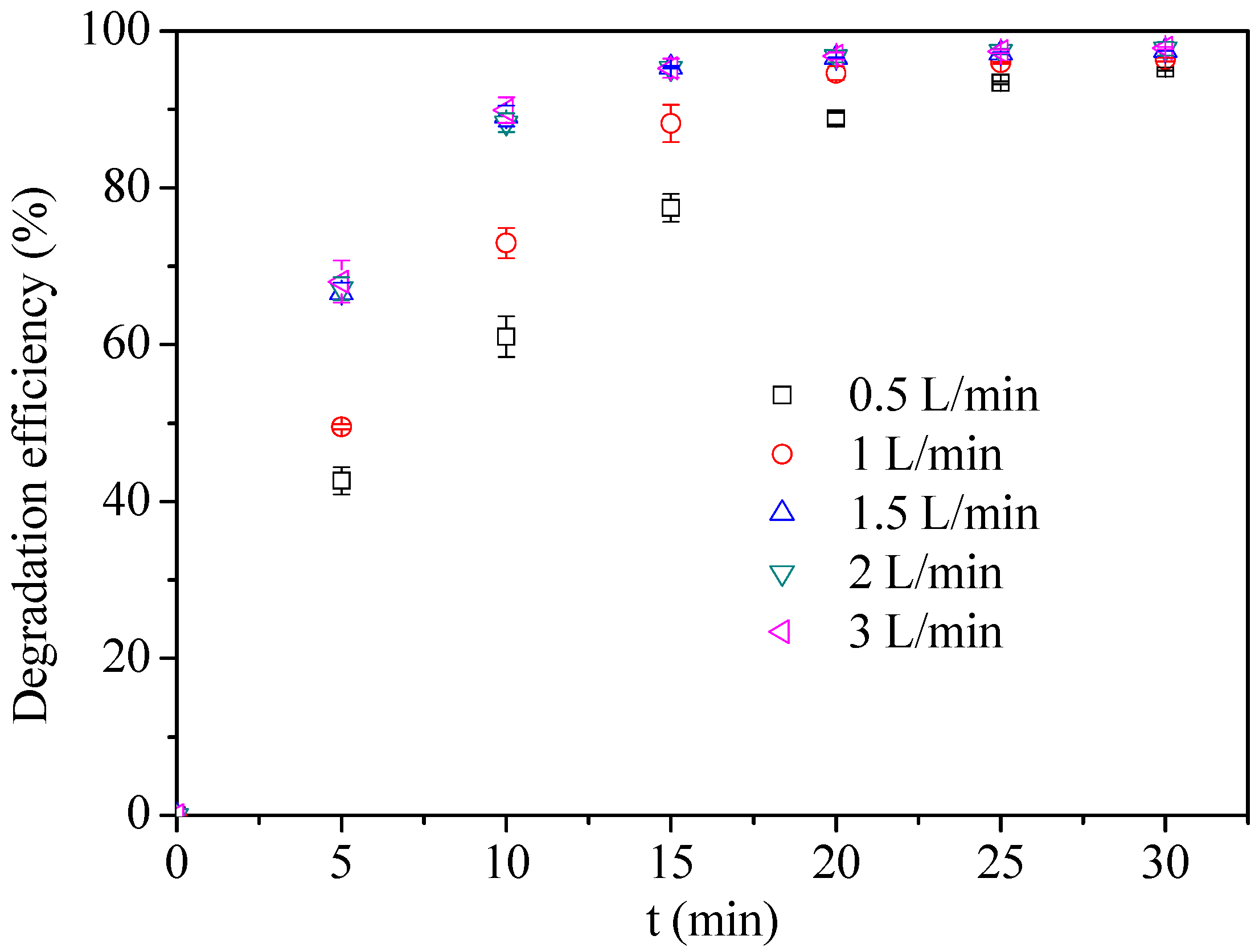
3.1.1. Effect of Air Flow Rate
3.1.2. Effect of Initial MB Concentration
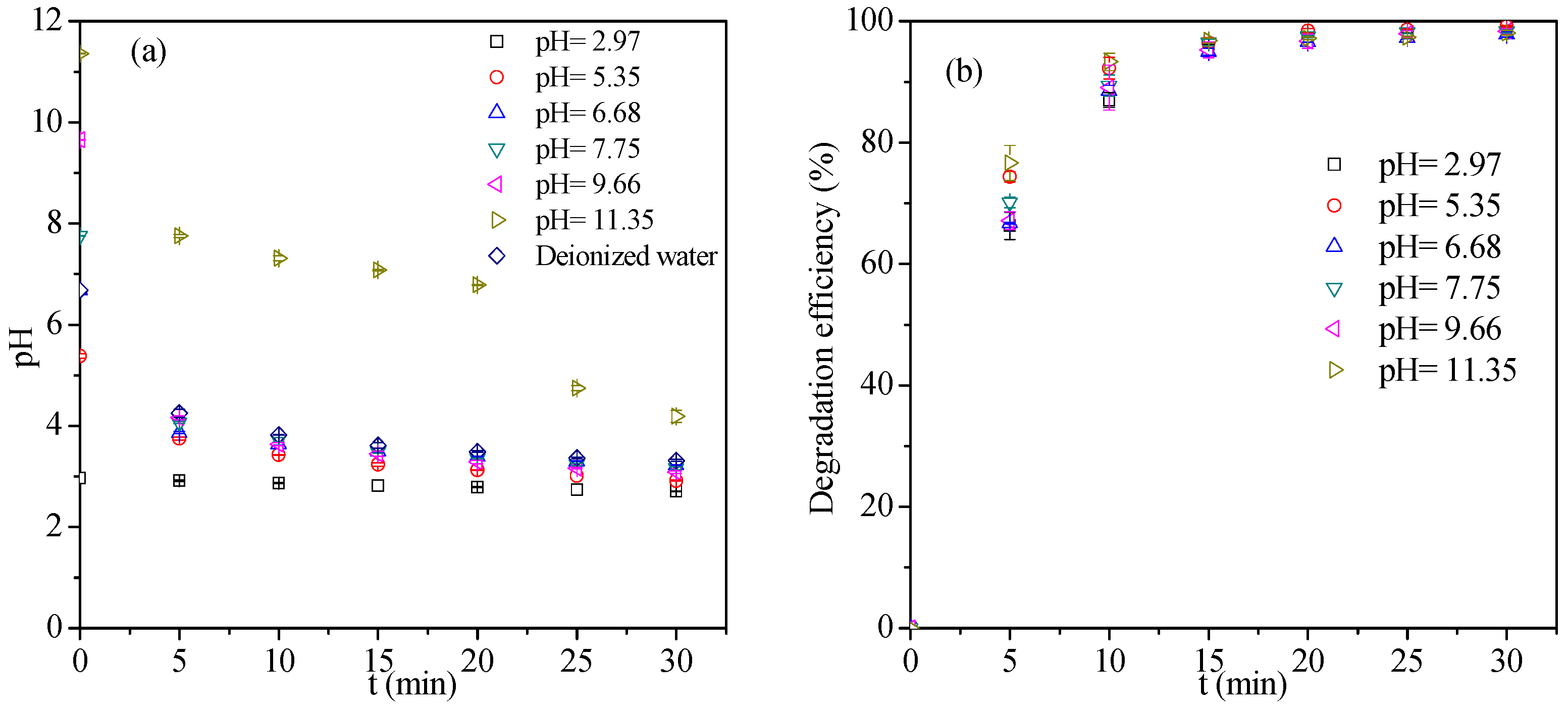
3.1.3. Effect of Initial Solution pH
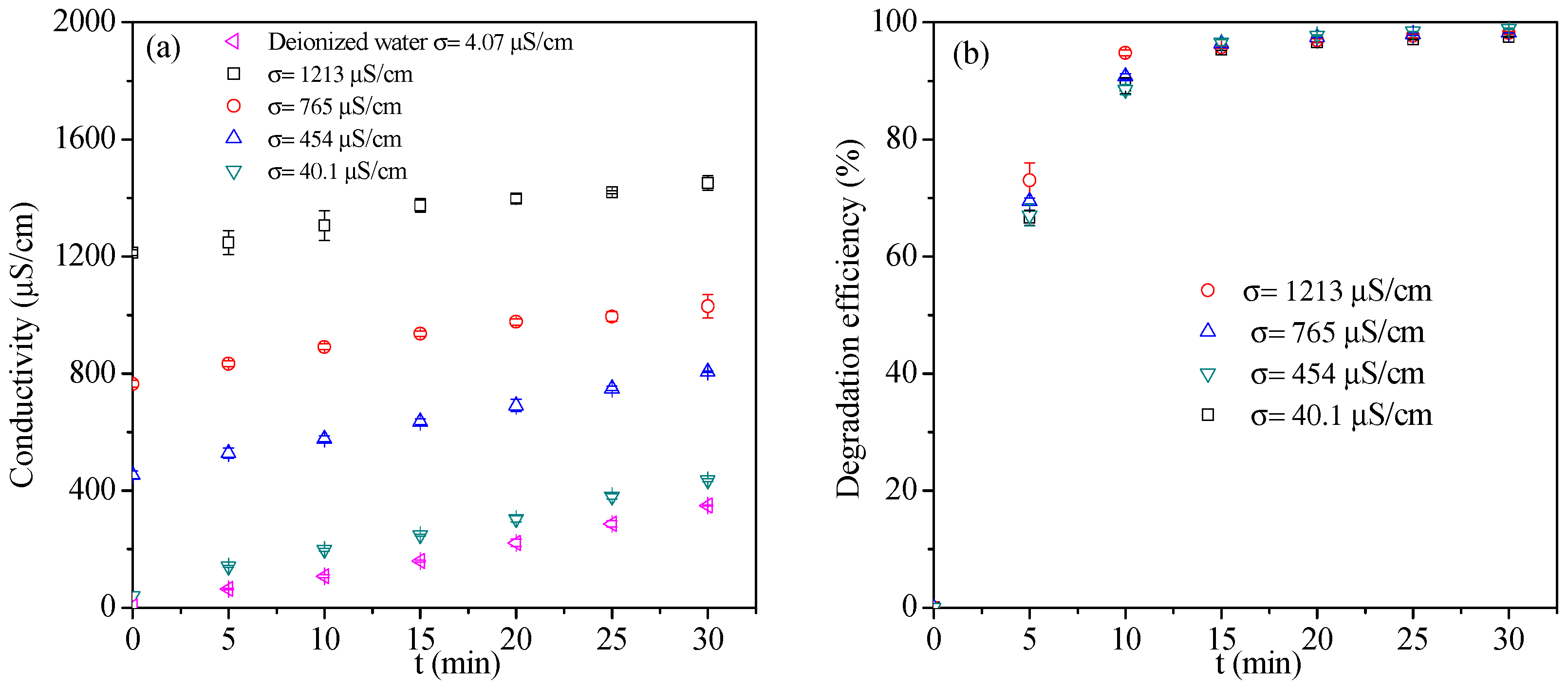
3.1.4. Effect of Initial Solution Conductivity
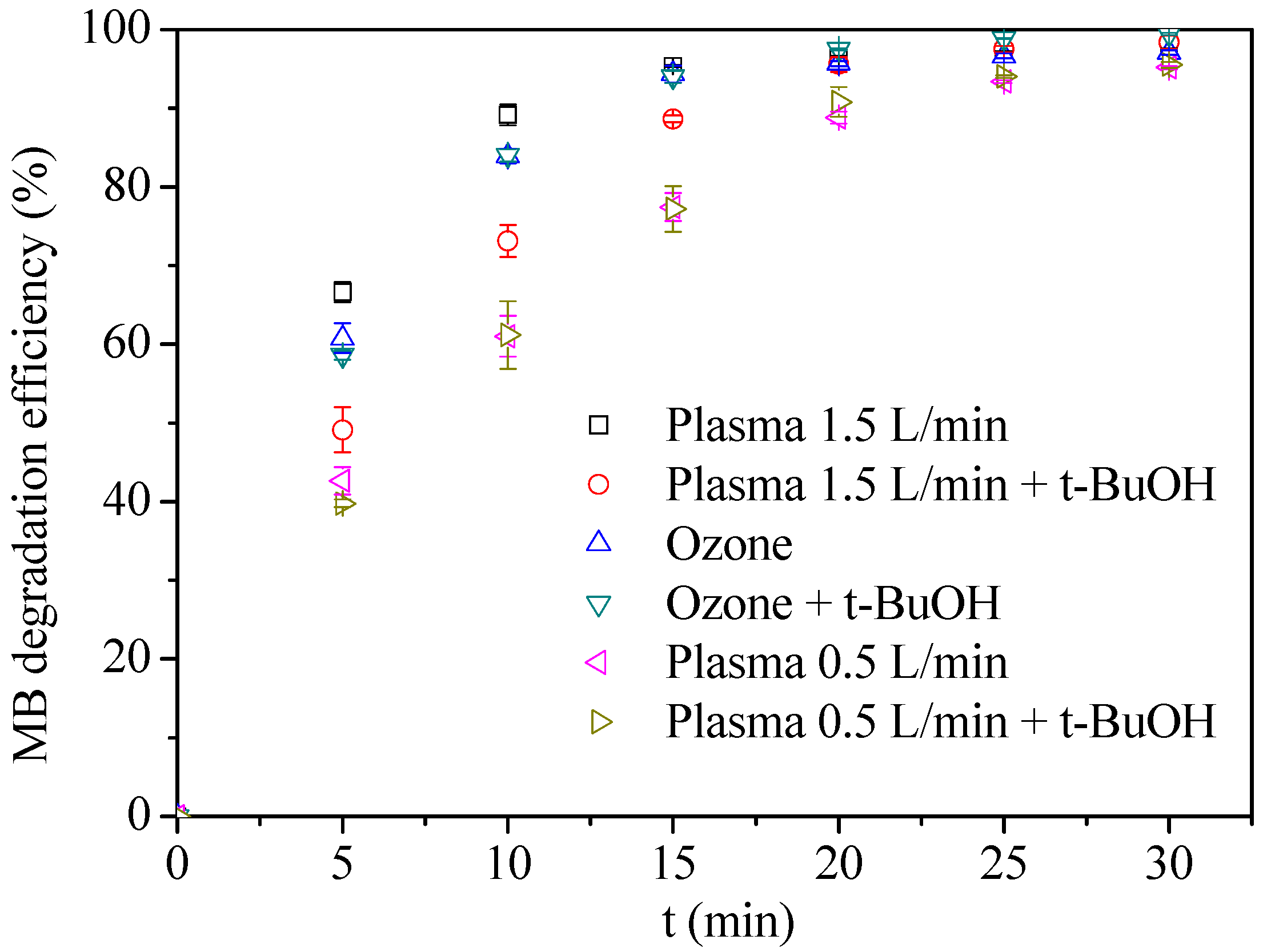
3.2. Comparison of Ozone and Plasma on MB Degradation
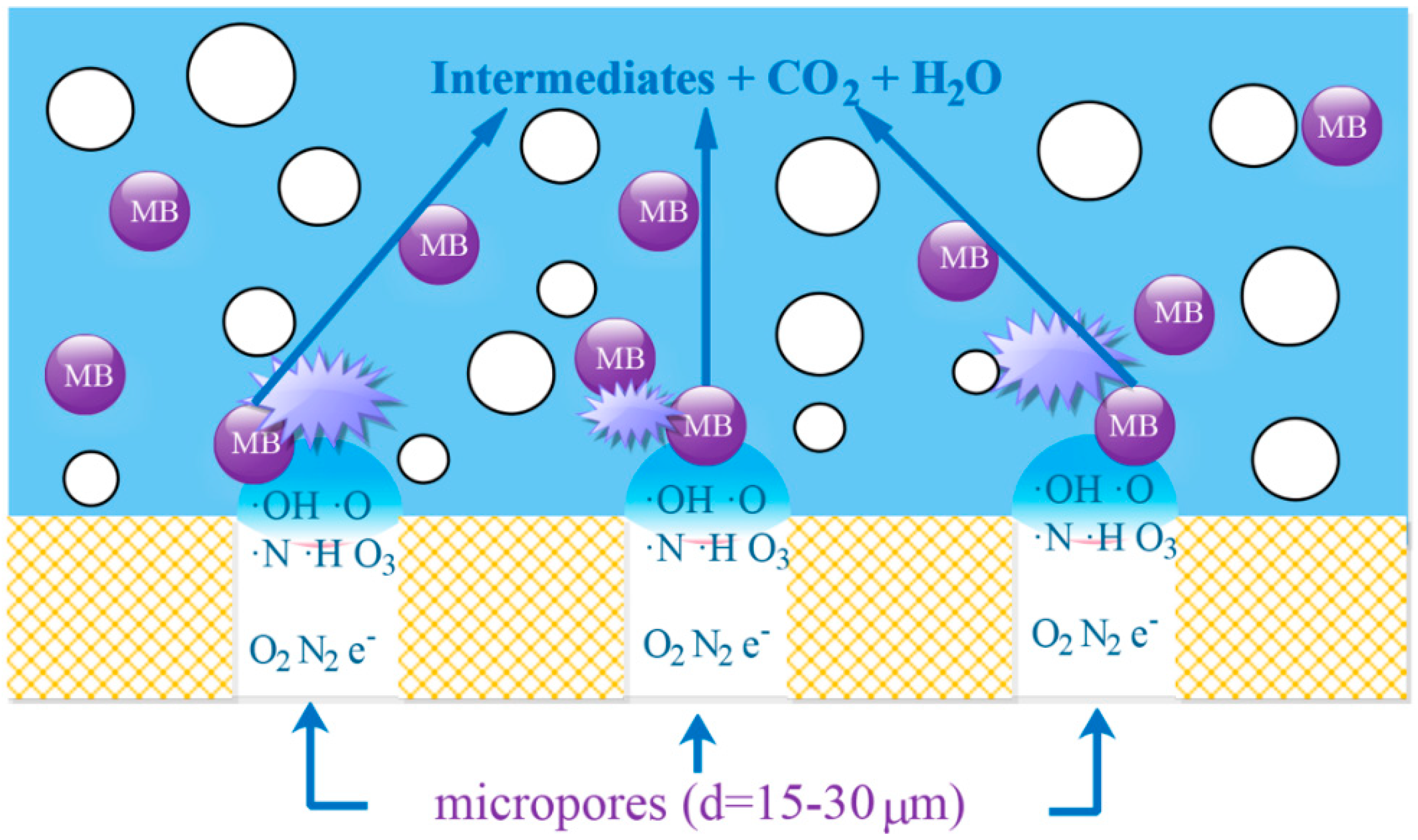
3.3. Possible Mechanism of Generation of Plasma Active Species
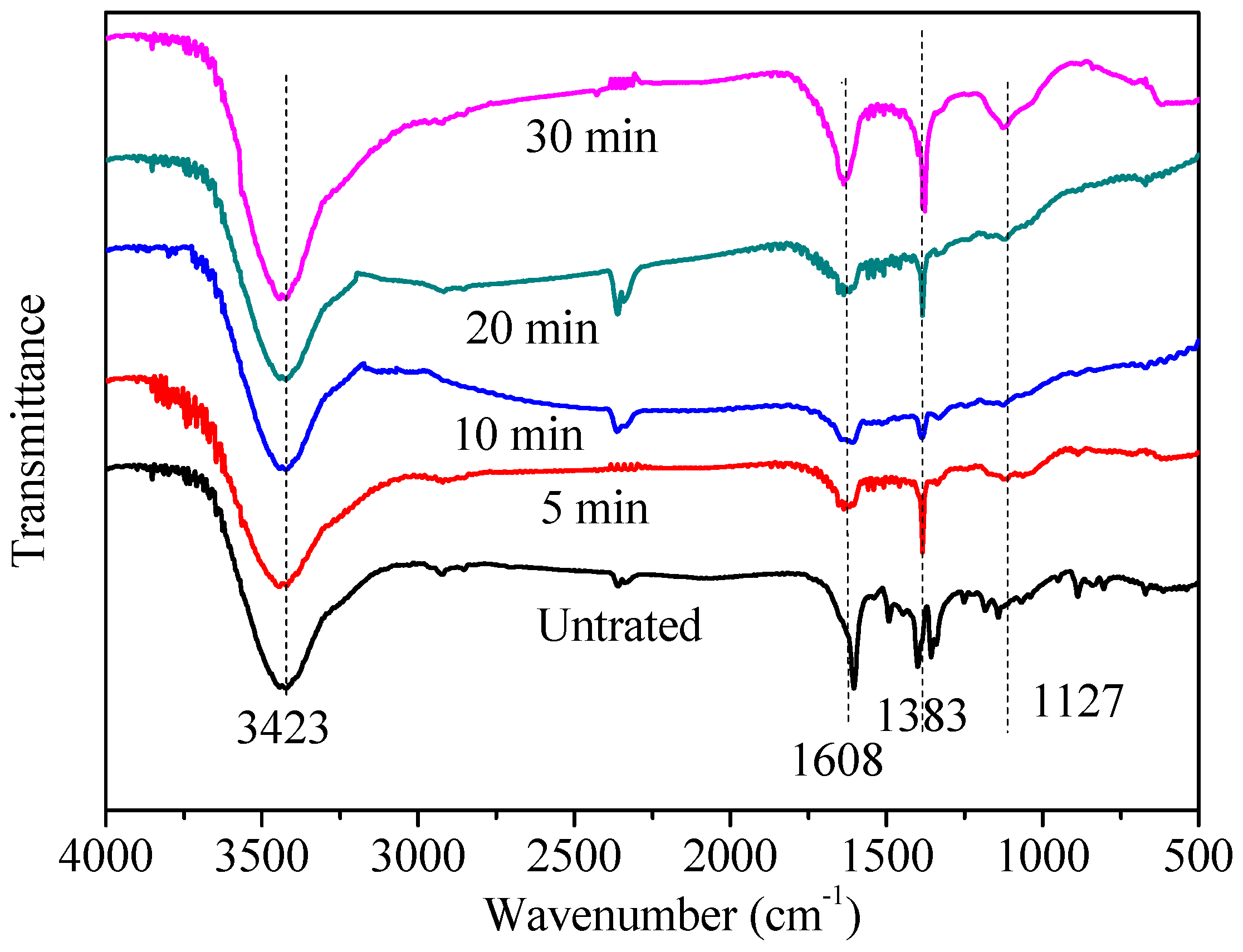
3.4. Mineralization of MB
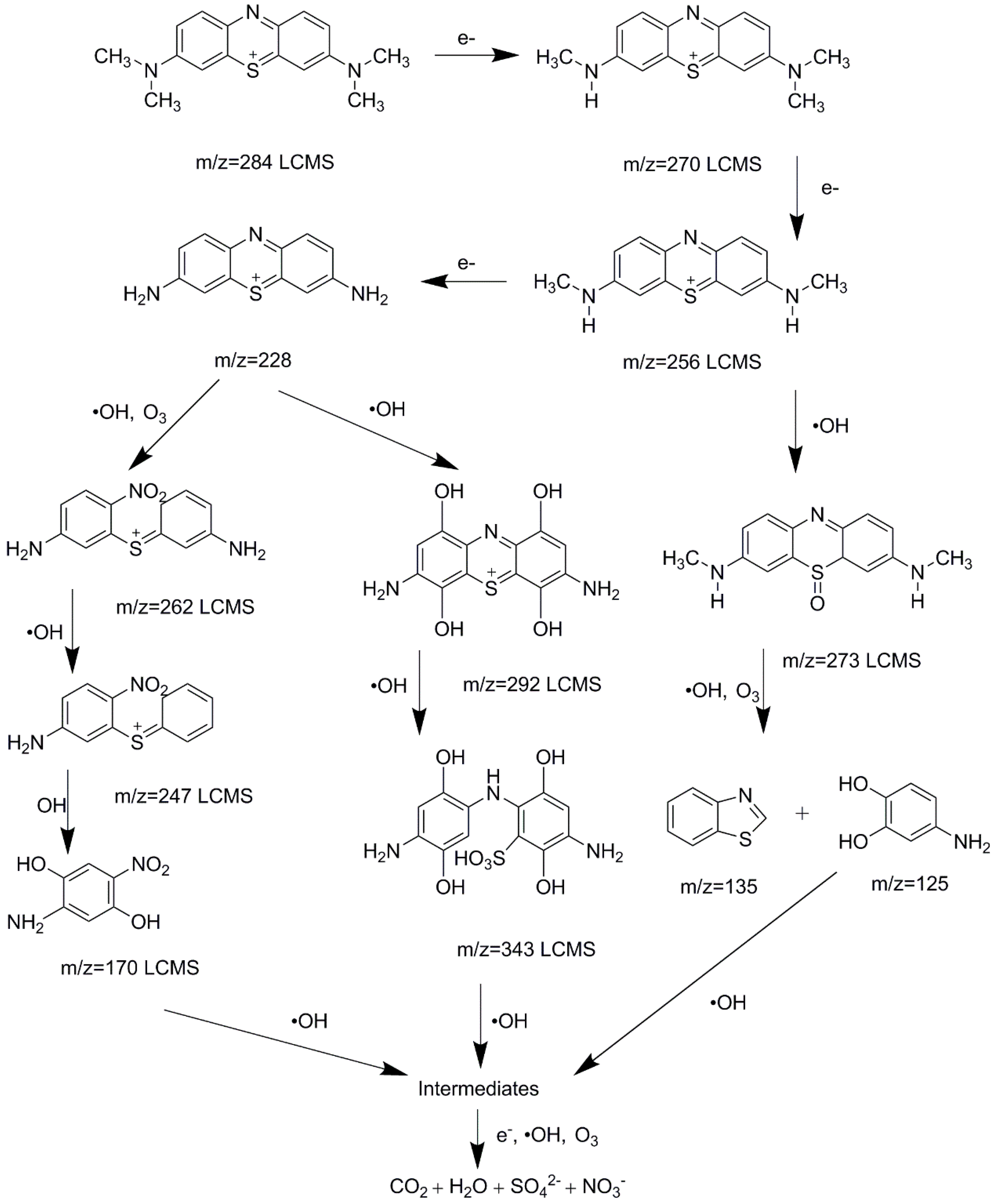
3.5. Degradation Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, P.Y.; Zhang, Y.J.; Chen, H.; Liu, L.C. Development of an eco-efficient CaMoO4/electroconductive geopolymer composite for recycling silicomanganese slag and degradation of dye wastewater. J. Clean. Prod. 2019, 208, 1476–1487. [Google Scholar] [CrossRef]
- Li, W.; Mu, B.; Yang, Y. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Tichonovas, M.; Krugly, E.; Racys, V.; Hippler, R.; Kauneliene, V.; Stasiulaitiene, I.; Martuzevicius, D. Degradation of various textile dyes as wastewater pollutants under dielectric barrier discharge plasma treatment. Chem. Eng. J. 2013, 229, 9–19. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2018, in press. [Google Scholar] [CrossRef]
- Bruggeman, P.; Leys, C. Non-thermal plasmas in and in contact with liquids. J. Phys. D Appl. Phys. 2009, 42, 53001. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Sun, J.; Liu, J.; Shen, X.; Zhan, J.; Zhang, A.; Ognier, S.; Cavadias, S.; Li, P. Degradation of aniline in aqueous solution using non-thermal plasma generated in microbubbles. Chem. Eng. J. 2018, 345, 679–687. [Google Scholar] [CrossRef]
- Ceriani, E.; Marotta, E.; Shapoval, V.; Favaro, G.; Paradisi, C. Complete mineralization of organic pollutants in water by treatment with air non-thermal plasma. Chem. Eng. J. 2018, 337, 567–575. [Google Scholar] [CrossRef]
- Huang, F.; Chen, L.; Wang, H.; Yan, Z. Analysis of the degradation mechanism of methylene blue by atmospheric pressure dielectric barrier discharge plasma. Chem. Eng. J. 2010, 162, 250–256. [Google Scholar] [CrossRef]
- Foster, J.E.; Mujovic, S.; Groele, J.; Blankson, I.M. Towards high throughput plasma based water purifiers: Design considerations and the pathway towards practical application. J. Phys. D Appl. Phys. 2018, 51, 293001. [Google Scholar] [CrossRef]
- Li, J.; Sato, M.; Ohshima, T. Degradation of phenol in water using a gas-liquid phase pulsed discharge plasma reactor. Thin Solid Films 2007, 515, 4283–4288. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.; Qiu, S.; Wu, M.; Zhang, Q.; Yan, Z.; Xue, Q. Review on electrical discharge plasma technology for wastewater remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar] [CrossRef]
- Chen, H.L.; Lee, H.M.; Chen, S.H.; Chang, M.B. Review of packed-bed plasma reactor for ozone generation and air pollution control. Ind. Eng. Chem. Res. 2008, 47, 2122–2130. [Google Scholar] [CrossRef]
- Wang, W.; Kim, H.H.; Van Laer, K.; Bogaerts, A. Streamer propagation in a packed bed plasma reactor for plasma catalysis applications. Chem. Eng. J. 2018, 334, 2467–2479. [Google Scholar] [CrossRef]
- Hensel, K. Microdischarges in ceramic foams and honeycombs. Eur. Phys. J. D 2009, 54, 141–148. [Google Scholar] [CrossRef]
- Hong, Y.C.; Jeon, H.W.; Lee, B.J.; Uhm, H.S. Generation of plasma using capillary discharge in water. IEEE Trans. Plasma Sci. 2010, 38, 3464–3466. [Google Scholar] [CrossRef]
- Hensel, K.; Machala, Z.; Tardiveau, P. Capillary microplasmas for ozone generation. Eur. Phys. J. Appl. Phys. 2009, 47, 22813. [Google Scholar] [CrossRef]
- Eden, J.G.; Kim, M.H.; Cho, J.H.; Park, S.J. Modular Microplasma Microchannel Reactor Devices, Miniature Reactor Modules and Ozone Generation Devices. U.S. Patent 9,390,894 B2, 12 July 2016. [Google Scholar]
- Kim, K.-S.; Yang, C.-S.; Mok, Y.S. Degradation of veterinary antibiotics by dielectric barrier discharge plasma. Chem. Eng. J. 2013, 219, 19–27. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Sundar Rajan, P. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Fahmy, A.; El-Zomrawy, A.; Saeed, A.M.; Sayed, A.Z.; Ezz El-Arab, M.A.; Shehata, H.A. Modeling and optimizing Acid Orange 142 degradation in aqueous solution by non-thermal plasma. Chemosphere 2018, 210, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Manoj Kumar Reddy, P.; Rama Raju, B.; Karuppiah, J.; Linga Reddy, E.; Subrahmanyam, C. Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem. Eng. J. 2013, 217, 41–47. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, Z.; Sun, Y.; Luan, J.; Wang, Z.; Wang, L.; Feng, J. Degradation of diuron in aqueous solution by dielectric barrier discharge. J. Hazard. Mater. 2008, 154, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, L.R.; Van Veldhuizen, E.M.; Pemen, A.J.M.; Rutgers, W.R. Corona above water reactor for systematic study of aqueous phenol degradation. Plasma Chem. Plasma Process. 2006, 26, 3–17. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Aguinaco, A.; Rey, A.; García-Araya, J.F. Kinetic studies on black light photocatalytic ozonation of diclofenac and sulfamethoxazole in water. Ind. Eng. Chem. Res. 2012, 51, 4533–4544. [Google Scholar] [CrossRef]
- Wang, B.; Dong, B.; Xu, M.; Chi, C.; Wang, C. Degradation of methylene blue using double-chamber dielectric barrier discharge reactor under different carrier gases. Chem. Eng. Sci. 2017, 168, 90–100. [Google Scholar] [CrossRef]
- Malik, M.A.; Rehman, U.; Ghaffar, A.; Ahmed, K. Synergistic effect of pulsed corona discharges and ozonation on decolourization of methylene blue in water. Plasma Sour. Sci. Technol. 2002, 11, 236. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J.; Qu, X.; Chen, H. Design of a novel non-equilibrium plasma-based water treatment reactor. Chemosphere 2008, 70, 1518–1524. [Google Scholar] [CrossRef]
- Bansode, A.S.; More, S.E.; Siddiqui, E.A.; Satpute, S.; Ahmad, A.; Bhoraskar, S.V.; Mathe, V.L. Effective degradation of organic water pollutants by atmospheric non-thermal plasma torch and analysis of degradation process. Chemosphere 2017, 167, 396–405. [Google Scholar] [CrossRef]
- De Brito Benetoli, L.O.; Cadorin, B.M.; Baldissarelli, V.Z.; Geremias, R.; de Souza, I.G.; Debacher, N.A. Pyrite-enhanced methylene blue degradation in non-thermal plasma water treatment reactor. J. Hazard. Mater. 2012, 237–238, 55–62. [Google Scholar] [CrossRef]


| Used Dye | Type of Plasma Reactor | Conditions | Energy Yield (g/kWh) | COD Removal (%) | TOC Removal (%) | Refs. |
|---|---|---|---|---|---|---|
| Methylene blue | DBD | V0: 0.1 L, C0: 100 ppm, t: 30 min, V: 8 kV, F: 9.7 kHz, Air: 1.5 L/min | 9.3 | 48.3 | 38.5 | This study |
| Methylene blue | DBD | V0: 0.05 L, C0: 100 ppm, t: 40 min, V: 6 kV, F: 50 Hz, O2: 0.06 L/min | 0.14 | 67.1 | – | [27] |
| Methylene blue | Pulsed corona discharge | V0: 0.02 L, C0: 13.25 ppm, t: 20 min, V: 40 kV, F: 60 Hz, O2: 0.01 L/min | 0.341 | – | – | [28] |
| Methyl orange | Non-equilibrium plasma | V0: 0.1 L, C0: 100 ppm, t: 15 min, V: 46 kV, F: 100 Hz, Air: 1.6 L/min | 3.6 | 45 | – | [29] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Xie, Q.; Lv, Y.; Wu, Z.; Liang, X.; Lu, M.; Nie, Y. Degradation of Methylene Blue via Dielectric Barrier Discharge Plasma Treatment. Water 2019, 11, 1818. https://doi.org/10.3390/w11091818
Wu L, Xie Q, Lv Y, Wu Z, Liang X, Lu M, Nie Y. Degradation of Methylene Blue via Dielectric Barrier Discharge Plasma Treatment. Water. 2019; 11(9):1818. https://doi.org/10.3390/w11091818
Chicago/Turabian StyleWu, Lihang, Qinglong Xie, Yongbo Lv, Zhenyu Wu, Xiaojiang Liang, Meizhen Lu, and Yong Nie. 2019. "Degradation of Methylene Blue via Dielectric Barrier Discharge Plasma Treatment" Water 11, no. 9: 1818. https://doi.org/10.3390/w11091818
APA StyleWu, L., Xie, Q., Lv, Y., Wu, Z., Liang, X., Lu, M., & Nie, Y. (2019). Degradation of Methylene Blue via Dielectric Barrier Discharge Plasma Treatment. Water, 11(9), 1818. https://doi.org/10.3390/w11091818







