Abstract
More economical and effective technology is being developedin the wastewater treatment process to deal with the products of phenylhydrazine hydrochloride (PHH). Fixed ammonium in the effluent is converted to free ammonia by utilizing the neutralization reaction, and the sulfate is removed in the form of gypsum. Meanwhile, the toxic PHH is recycled according to the extraction and re-extraction technology. The raffinate phase is reused through boiling off ammonia vapor. The recovery rates of PHH reach 93.3% in the laboratory and 92.9% at the pilot scale, respectively. Compared with our previous work, the cost of the new technology is ~1/10 of the original, and the profit increases ~3.5-fold. Consequently, it has great potential to be applied to industrial production.
1. Introduction
Pollution problems involving industrial wastewater have aroused worldwide concern in recent years [1,2,3,4,5]. Various treatment technologies have been investigated, including physicochemical methods [6,7,8], incineration [9,10], and advanced oxidation processes [11,12,13,14]. The technology is available depending on the composition of wastewater [15,16,17]. Consequently, a promising treatment technology needs to be specific to a particular type of wastewater [10].
Phenylhydrazine hydrochloride (PHH) has been widely applied to synthesize multiple fine chemicals [18,19,20,21]. Nowadays, there are 212 PHH suppliers in the global range, and around 70% of the enterprises are located in China [22]. The main raw materials in PHH production are aniline and hydrochloric acid (HCl). In the processes of diazotization, reduction, acid precipitation analysis, and salt formation [23,24,25], a large amount of wastewater is generated, which includes multiple ammonium salts, dissolved PHH, and other organic pollutants. As a result, the wastewater is highly toxic. The arbitrary discharge of the wastewater can lead not only to environmental pollution but also to resource loss. Therefore, it is urgent for scientists and engineers to develop suitable, inexpensive, and rapid water treatment and recycling technology in the process of PHH production.
To date, there have been few reports about treatment technology for wastewater created during PHH production. The only reported technology was resin adsorption, combined with Fenton’s oxidation process [26]. In the treatment process, the flow rate and pH of wastewater had important effects on adsorption efficiency. Fenton’s reagent (H2O2 and Fe2+ catalyst) [27,28] was used to further purify the wastewater. However, traditional Fenton processes are costly and consume a lot of energy. Moreover, these results were based on the laboratory scale. Very recently, we presented an extraction and re-extraction technology to recycle PHH, and ammonium salts were reused as industrial raw materials [25]. In practical industrial applications, however, it was found that the economic benefit was limited.
A major restriction when changing or implementing a new process is the operating costs [29]. Therefore, economical and effective wastewater treatment and reclamation at a commercial level is still a challenging problem. In this contribution, we aim to develop a new treatment technology to further simplify prior technology and reduce the operating costs. The new technology is first explored in the laboratory, and then extended to the pilot scale.
2. Materials and Methods
All reagents used in this study are industrial grade. These chemicals are used for experiments without further purification. The wastewater is collected from an enterprise in Shandong province of China.
The density of the wastewater (1.15 g/mL) is determined by the hydrometer. The contents of ammonium bisulfite and sulfate ions are determined by iodimetry (HG/T2785-2012) and spectrophotometry (GB 535-1995), respectively. The total quantity of ammonium salts is analyzed by the distillation‒titration method [30]. The concentration of HCl is measured by acid‒base titration. High-performance liquid chromatography [31] is used to determine the content of PHH. The components and the corresponding content are shown in Table 1. It can be seen that ammonium salts in the wastewater account for 27.6%. Even a small amount of PHH (1.15%) is toxic and can probably cause cancer [22]. In addition, due to the existence of PHH and organic by-products, the wastewater is characterized by a pink color. Meanwhile, because of the addition of excessive HCl in the process of acid precipitation analysis, the wastewater is strongly acidic (pH = 0.5).

Table 1.
Chemical components of the wastewater.
To realize the harmless treatment and the reclamation of resources, the wastewater is treated by oxidation, neutralization, extraction and re-extraction, and evaporation. The treatment process of PHH effluent is sketched out in Figure 1. It is shown that toxic PHH is recycled for sale. Sulfate is converted to gypsum. Recycled xylene and HCl can be reused as extraction and re-extraction agents, respectively. The raffinate phase is heated. Free ammonia is obtained, and ammonia liquor is formed after cooling and water absorption. Meanwhile, the remaining water is used to prepared lime milk by adding CaO. Therefore, there are no hazardous streams formed in the treatment process. The specific processes are as follows.
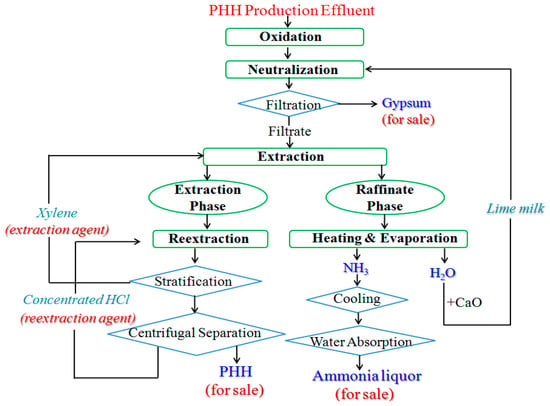
Figure 1.
Schematic diagram of treatment process of PHH effluent.
2.1. Sulfite Oxidation
The air is introduced into the wastewater, and the solution is heated to 60–75 °C in order to promote the reaction efficiency. Accordingly, sulfite in the effluent is oxidized to stable sulfate, which means that ammonium bisulfite is transformed into ammonium hydrogen sulfate. The corresponding reaction equation can be expressed by
2.2. Lime Milk Neutralization
In order to reclaim ammonium salts (NH4HSO4 and NH4Cl), the neutralization reactions are performed. Lime milk solution, in which the mass fraction of CaO is 15%, is added into the oxidation reaction solution. The addition of lime milk solution continues until the pH value of the solution reaches 9.0–9.5. During this process, fixed ammonium is decomposed into free ammonia. At the same time, PHH is transformed into phenylhydrazine (PHZ), and gypsum (i.e., CaSO4·2H2O precipitation) is formed under the alkaline conditions. Subsequently, the reaction is continued with stirring (50 rpm) for 30 min.
The neutralization reaction equations can be written as follows:
Because the solubility of CaSO4·2H2O decreases with the increase of the temperature, the reaction solution should be immediately filtrated while hot. The filtrate is delivered to the next process.
2.3. Xylene Extraction and HCl Re-Extraction
In order to recycle PHZ in the filtrate, a suitable extractant must be adopted. Due to the insolubility in water and approximately equal molecule weight (xylene 106.17, PHZ 108.14), xylene is selected as the extraction agent in this study.
In the extraction process, the flow rates of the filtrate and xylene should be strictly controlled, and the extraction temperature is controlled in the range of 30–40 °C. The extraction operation is performed continuously. Finally, PHZ in the filtrate is extracted.
In the re-extraction process, PHZ in the extract phase is separated out in the form of PHH. As the re-extraction agent, concentrated HCl is added under the stirring condition (40–50 rpm). The addition of HCl is in excess of 40% so that PHZ and HCl can react with each other, and then PHH is crystallized out. After the addition of HCl, the reaction is continued for 10 min with stirring (40–50 rpm). At the end of the re-extraction operation, the reaction solution is stratified. Xylene in the upper layer is recycled as the extraction agent, and HCl in the lower layer is reused as the re-extraction agent. At the same time, PHH crystals are obtained by filtration.
The re-extraction reaction equation is expressed as
2.4. Ammonia Evaporation
The raffinate phase is heated to evaporate ammonia. In this stage, the steam pressure is controlled between 0.16 MPa and 0.18 MPa, and the evaporating temperature is limited to the range of 110–120 °C. The process is carried on until the free ammonia is totally evaporated. The free ammonia is cooled and absorbed by water to prepare ammonia liquor. Subsequently, part of the ammonia liquor can be sent back to the reduction process to adjust the pH value of the solution, and the rest can be reused as raw materials to produce NH4HSO3. The water after ammonia evaporation can be applied to prepare the lime milk.
3. Results and Discussion
3.1. Lab-Scale Test
To evaluate the effects of the parameters in the extraction process on the recovery rate of PHH, the pH value, extraction temperature, and extractant dosage are investigated. In each lab-scale test, 100 mL effluent was selected.
Under alkaline conditions, PHH can be converted into PHZ, and then PHZ is extracted by xylene. The pH value of the solution was adjusted within the range of 7.3–10.0. In addition, the temperature and extractant dosage were tuned within the range of 20–55 °C and 20–500 mL, respectively, whereas the other conditions were kept the same. The experimental data are displayed in Table 2. It should be noted that the mass of recycled PHH and the recovery rate η increases with the increase of the pH value. However, they first increase and then decrease as the temperature rises. The maximum appears at 35 °C. In the meantime, the recovery rate of PHH increases monotonically, and the effect of the dosage of xylene is the most significant. In consideration of the experimental results as well as economic factors, the optimum extraction conditions in the laboratory are pH = 9.3, T = 35°C, and volume of xylene 5 times that of the effluent. Under these operating conditions, the recovery rate is up to 93.3%.

Table 2.
Experimental data in the extraction process at lab scale.
3.2. Pilot-Scale Test
The pilot-scale tests were carried out in the same enterprise in Shandong province. Firstly, the production process of PHH was performed in a reaction kettle. The masses of raw materials and PHH are shown in Table 3. According to the data, we can see that 370 kg PHH can be obtained in each kettle. The corresponding theoretical output is 414 kg, and the yield is 89.4%. Ultimately, the purity of PHH reaches 91.6%.

Table 3.
Mass of raw materials and product.
Subsequently, 3500 kg effluent were formed, in which 42 kg PHH was dissolved. Before the pilot-scale tests for effluent treatment, the extraction process is simulated by using process simulation software (CHEMCAD 5.6.0, Chemstations Inc., Houston, TX, USA). The number of theoretical plates (NT), extraction temperature, pH value, and solvent ratio are considered to optimize operating conditions. The simulation results are displayed in Figure 2.
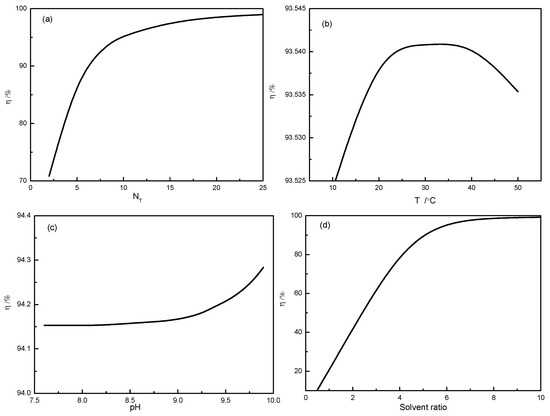
Figure 2.
Effects of (a) number of theoretical plates (NT), (b) extraction temperature (T), (c) pH value, and (d) solvent ratio on the extraction efficiency in the simulation process.
Figure 2a shows that the extraction efficiency increases with the increase of NT. When NT is larger than 15, the extraction efficiency remains constant. The increase in NT can increase the equipment investment. For this reason, NT is set to 8 in subsequent simulations. The extraction efficiency is still high (95%) at NT = 8. Figure 2b shows that the extraction efficiency initially increases and then decreases with the increase of temperature. The extraction efficiency reaches a maximum and stays almost unchanged in the range of 25 °C to 35 °C. The pH value of the solution in Figure 2c affects the extraction efficiencyslightly. When pH > 9.0, the extraction efficiency shows little improvement. Figure 2d shows that the solvent ratio has significant impacts on the extraction process as solvent ratio < 6.0. The increase in the solvent ratio hardly influences the extraction efficiency.
In view of the above discussion and in consideration of the laboratory results as well as economic factors, the adopted operation conditions for extraction process are T = 35°C, pH = 9.0, and solvent ratio 6.0 in pilot-scale tests.
In the following work, effluent treatment processes are performed at the pilot scale. A simplified scheme is drawn in Figure 3. The effluent is pumped into the reaction kettle and oxidized by the air. The neutralization reactions are carried out with lime milk solution from the mixing tank. The reaction solution is filtrated immediately while hot, and gypsum is obtained. The filtrate is then pumped into the top of the extraction column. Meanwhile, the extractant xylene is fed from the bottom of the column. In the extraction process, the extraction phase from the top of the extraction column is sent to the reaction kettle to re-extract PHH. After filtration, PHH is recycled. The raffinate phase from the bottom of the extraction column is sent to the evaporator to heat and evaporate ammonia. The free ammonia is pumped into the cooling tower and then sent to the absorption tower to prepare ammonia liquor.
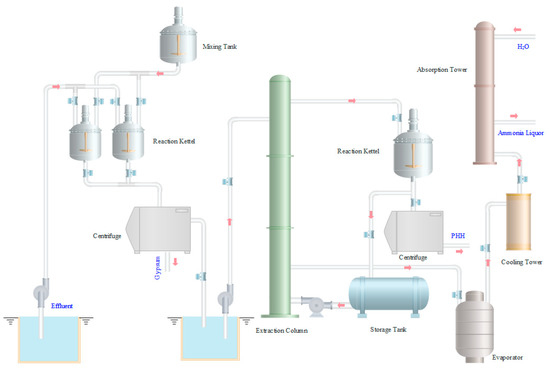
Figure 3.
Effluent treatment devices used in pilot tests.
Finally, the results of pilot tests are shown in Table 4. It should be noted that ammonia and gypsum are obtained in the treatment process. The recovery rate of PHH reaches 92.9%. The purity of PHH is 88.6%, which conforms with industrial requirements. After the effluent is treated, the solution becomes clear and colorless. It only contains a small amount of NaCl (2.25%) and CaCl2 (6.06%), which can be reused as raw materials to prepare lime milk solution.

Table 4.
Results of the treatment of PHH production waste.
To evaluate the economic benefits of the new technology, the operating costs, mainly including raw material costs and energy consumption costs, are estimated. The associated costs of depreciation of equipment, employee salary, management costs, and selling expenses are not considered. All prices are based on the current Chinese market. In the pilot-scale test, 3500 kg wastewater are treated. The cost of lime milk and xylene is ~724 CNY (108 USD). In addition, the cost of energy consumption is composed of the water rate, electricity charge, and steam expense, and corresponds to ~493 CNY (74 USD). Accordingly, the total operating cost is ~1217 CNY (182 USD). Meanwhile, the income of recycled PHH, liquid ammonia, and gypsum is ~3717 CNY (556 USD). Thus, the profit of the pilot scale is ~714 CNY (106 USD) per ton of wastewater. Compared with our previous work [25], the cost of this new technology has been reduced to ~1/10 of the original, and the profit increases by ~3.5-fold. The technology in this work can significantly improve the process of wastewater treatment for PHH production.
4. Conclusions
In the PHH production process, a large amount of liquid waste is formed, which can cause severe environmental pollution. The treatment of the effluent and recycling of useful resources are urgent. By using a neutralization reaction, sulfate is transformed into calcium sulfate dihydrate, which can be easily filtered out from a solution. According to the extraction and re-extraction technology, the residual PHH is recovered. The raffinate phase is treated with evaporation, cooling, and water absorption; ammonia liquor is obtained, which can be reused as a raw material for PHH production. Residual water after ammonia distillation can be recycled to prepare a lime milk solution. The treatment process fulfills the requirements of non-toxic treatment and low cost.
Author Contributions
Conceptualization, X.L.; formal analysis, X.L. and D.Z.; writing—original draft preparation, X.L.; writing—review and editing, D.Z.; Validation, C.C.
Funding
This research was funded by the National Natural Science Foundation of China (No.21506114) and the Scientific Research Foundation of Qufu Normal University (No.BSQD20130116).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Larsson, D.G.J.; De Pedro, C.; Paxeus, N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical industry wastewater: Review of the technologies for water treatment and reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, L.Y.; He, Z.; Song, Y. Treatment of metallurgical industry wastewater for organic contaminant removal in China: Status, challenges, and perspectives. Environ. Sci. Water Res. Technol. 2015, 3, 1015–1031. [Google Scholar] [CrossRef]
- Hung, Y.-T.; Aziz, H.A.; Ramli, S.F.; Yeh, R.Y.-L.; Liu, L.-H.; Huhnke, C.R. Chemical waste and allied products. Water Environ. Res. 2016, 88, 1374–1394. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Maken, S.; Jang, J.H.; Park, K.; Park, J.W. Development of physicochemical nitrogen removal process for high strength industrial wastewater. Water Res. 2006, 40, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Aloui, F.; Kchaou, S.; Sayadi, S. Physicochemical treatments of anionic surfactants wastewater: Effect on aerobic biodegradability. J. Hazard. Mater. 2009, 164, 353–359. [Google Scholar] [CrossRef]
- Shi, S.; Wang, C.; Fang, S.; Jia, M.; Li, X. Removal performance and water quality analysis of paper machine white water in a full-scale wastewater treatment plant. Environ. Technol. 2017, 38, 1443–1451. [Google Scholar] [CrossRef]
- Kim, V.K.; Ihm, S. Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: a review. J. Hazard. Mater. 2011, 186, 16–34. [Google Scholar] [CrossRef]
- Kumari, M.; Saroha, A.K. Performance of various catalysts on treatment of refractory pollutants in industrial wastewater by catalytic wet air oxidation: A review. J. Environ. Manag. 2018, 228, 169–188. [Google Scholar]
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, L.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar]
- Balcioglu, I.A.; Alaton, I.A.; Otker, M.; Bahar, R.; Bakar, N.; Ikiz, M. Application of advanced oxidation processes to different industrial wastewaters. J. Environ. Sci. Health. Part Atoxic/Hazard. Subst. Environ. Eng. 2003, 38, 1587–1596. [Google Scholar] [CrossRef]
- Ghatak, H.R. Advanced xxidation processes for the treatment of biorecalcitrant organics in wastewater. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1167–1219. [Google Scholar] [CrossRef]
- Krysa, J.; Mantzavinos, D.; Pichat, P.; Poulios, I. Advanced oxidation processes for water/wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 34799–34800. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Rojas, M.R.; Leung, C.; Bonk, F.; Zhu, Y.; Edwards, L.; Arnold, R.G.; Sáez, A.E.; Klečka, G. Assessment of the effectiveness of secondary wastewater treatment technologies to remove trace chemicals of emerging concern. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1281–1314. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Angelidaki, I.; Zhang, Y. Bio-electro-Fenton processes for wastewater treatment: Advances and prospects. Chem. Eng. J. 2018, 354, 492–506. [Google Scholar] [CrossRef]
- Zhong, C.; Ye, W.; Cui, Y. Improvement on technology for synthesizing triazophos. Mod. Chem. Ind. 1997, 17, 19–20. [Google Scholar]
- Banerjee, M.; Ray, A.K. The role of thyroid hormone on phenylhydrazine hydrochloride mediated inhibitory effects on blood acetylcholinesterase: An in vivo and in vitro study. J. Biochem. Mol. Toxicol. 2002, 16, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Ramyashree, D.; Raghavendra, K.R.; Kumar, A.D.; Vagish, C.B.; Kumar, K.A. Synthesis, characterization and antimicrobial activities of chalcones and their post transformation to pyrazole derivatives. Asian J. Chem. 2017, 29, 1538–1542. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, F.; Li, Y.; Xu, T.; Xu, H.; Jia, J.; Ye, Q.; Gao, J. The aggregation-induced emission enhancement properties of BF2 complex isatin-phenylhydrazone: Synthesis and fluorescence characteristics. Dyes Pigments 2015, 113, 502–509. [Google Scholar] [CrossRef]
- ChemicalBook, CAS Database List, Phenylhydrazine Hydrochloride. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB0101763.htm (accessed on 28 February 2019).
- Heinz, H. Process for the Production of Phenylhydrazine Hydrochloride. U.S. Patent 3203989, 31 August 1965. [Google Scholar]
- Zhang, R.; Wu, Y.-Z.; Zheng, D.-M. The synthesis of phenylhydrazine hydrochloride. J. Nanchang Univ. (Nat. Sci.) 2002, 26, 394–396. [Google Scholar]
- Liu, X.; Zhou, D.; Zhang, H. Research on the treatment and comprehensive utilization of phenylhydrazine hydrochloride effluent. Water 2018, 10, 438. [Google Scholar]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Gilarranz, M.A.; Rodriaguez, J.J. Chemical pathway and kinetics of phenol oxidation by Fenton’s reagent. Environ. Sci. Technol. 2005, 39, 9295–9302. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.-C.; Wei, D.; Bao, X.-Z. Research on treatment of phenylhydrazine hydrochloride wastewater. Technol. Dev. Chem. Ind. 2013, 42, 39–44. [Google Scholar]
- Amaral-Silva, N.; Martins, R.C.; Nunes, P.; Castro-Silvac, S.; Quinta-Ferreira, R.M. From a lab test to industrial application: scale-up of Fenton process for real olive mill wastewater treatment. J. Chem. Technol. Biotechnol. 2017, 92, 1336–1344. [Google Scholar] [CrossRef]
- Mu, J.; Li, H. Determination of distillation-titration method for the ammonia nitrogen in waste water. J. Henan Univ. (Nat. Sci.) 2006, 36, 54–57. [Google Scholar]
- Zhao, X.; Shao, Y.; Lu, Z.; Yao, J.; Cao, H.; Fan, J. Determination of phenylhydrazine hydrochloride in industrial wastewater by HPLC. Ind. Water Treat. 2012, 32, 80–82. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).