First-Principles Study on the Migration of Heavy Metal Ions in Ice-Water Medium from Ulansuhai Lake
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experiment
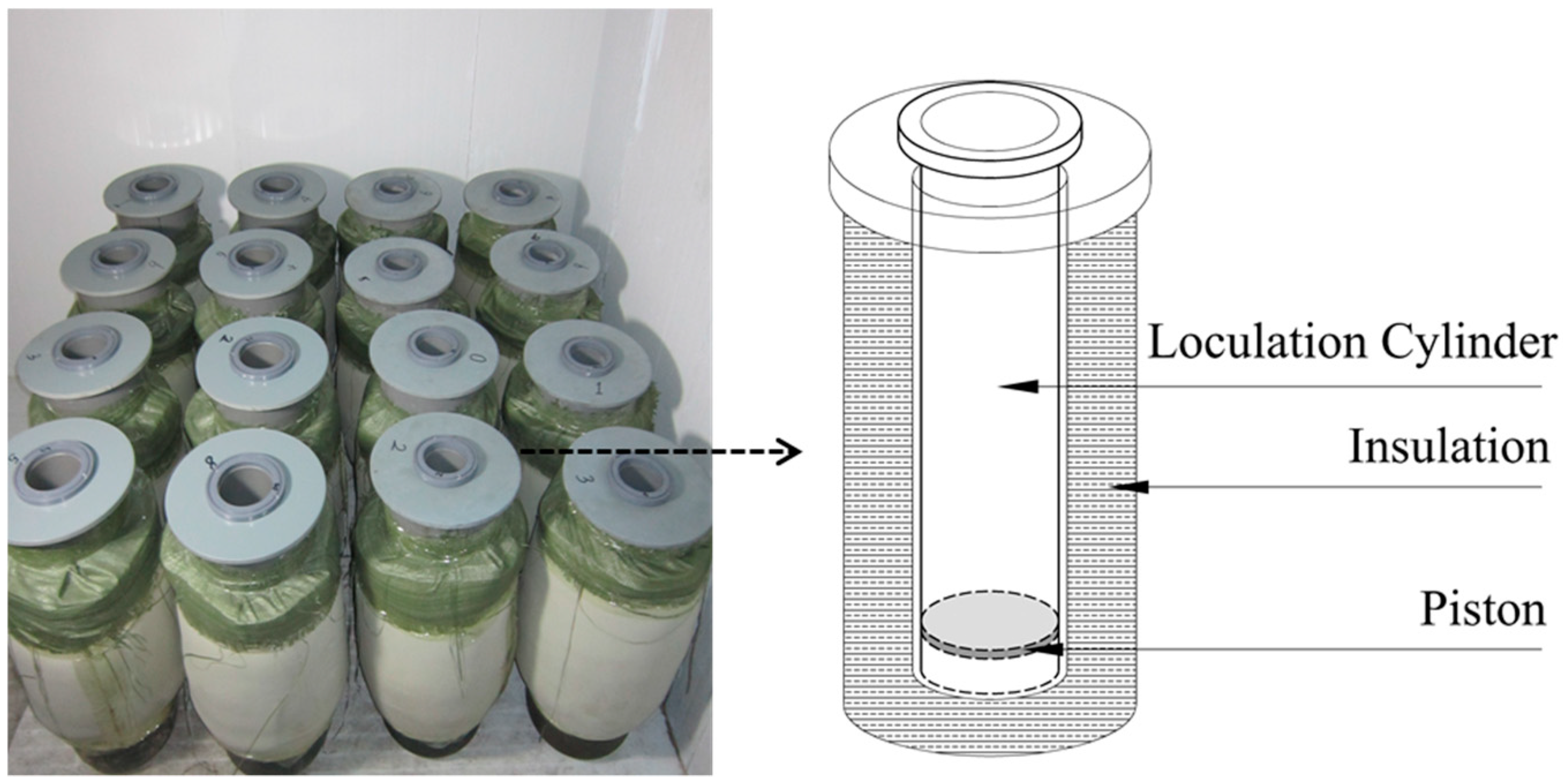
2.2.1. Simulation Experiment Device
2.2.2. Experimental Design and Methods
2.3. Calculation
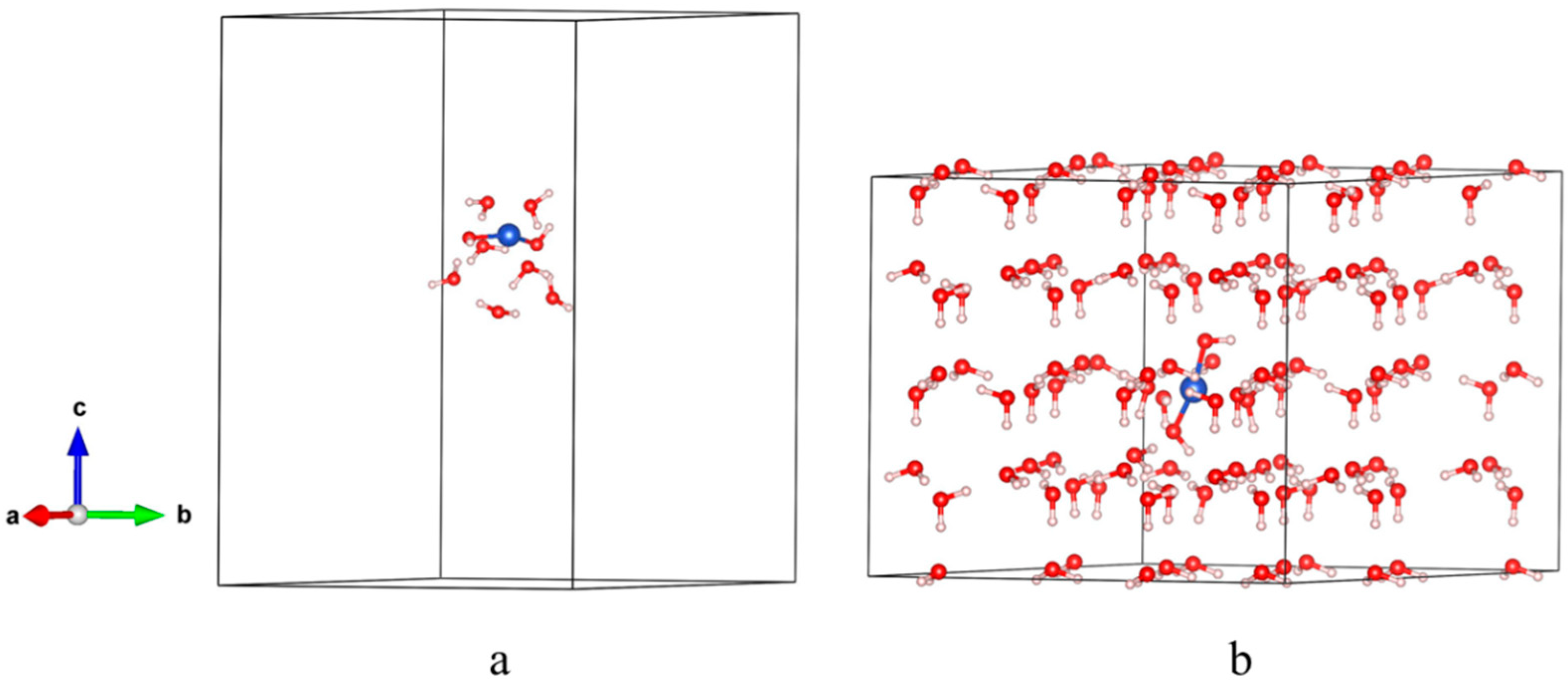
2.3.1. Microstructure Model
2.3.2. Binding Energy Calculation Method
3. Results
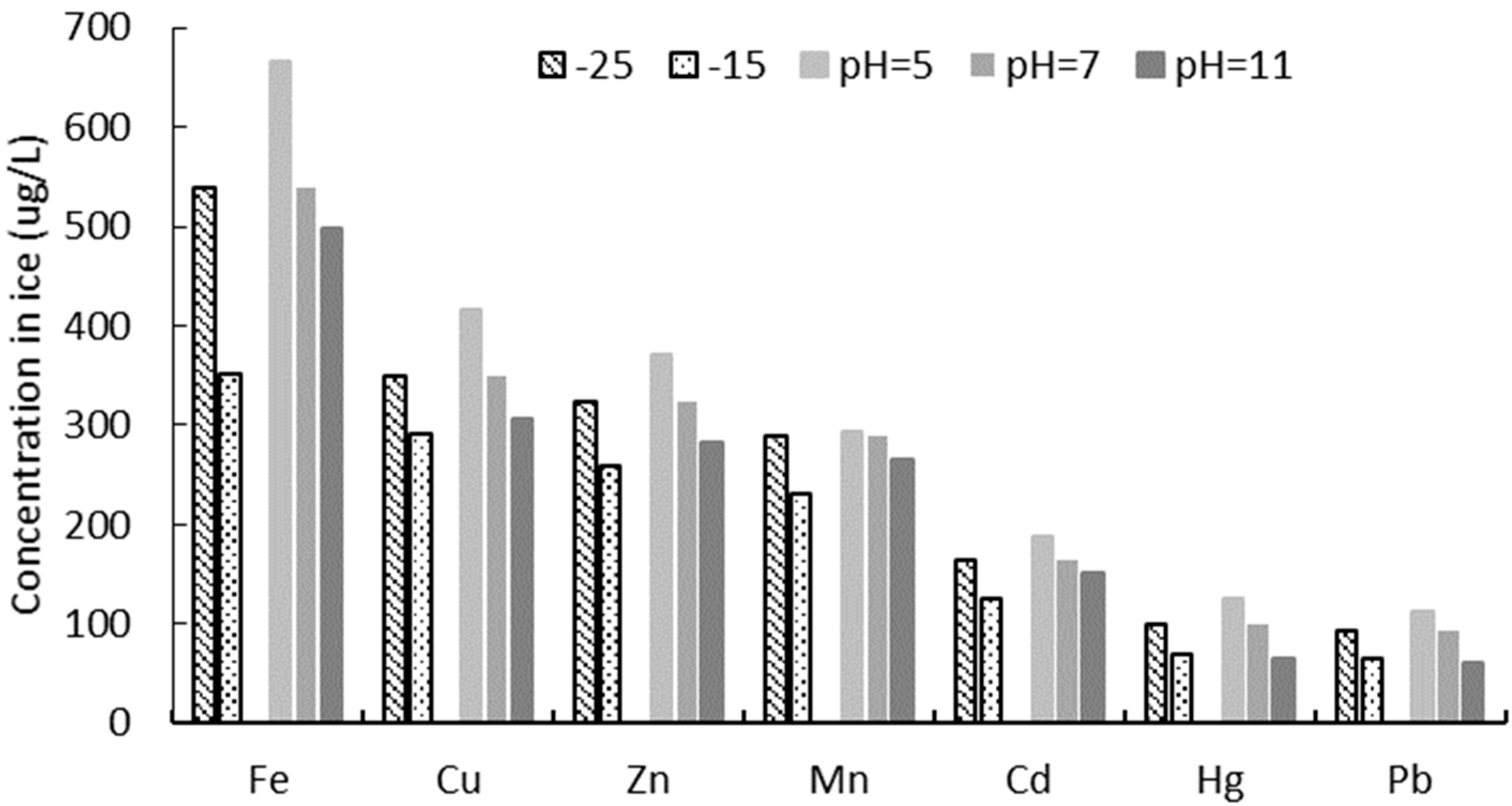
3.1. The Distribution and Transfer of HMIs in Ice and Water
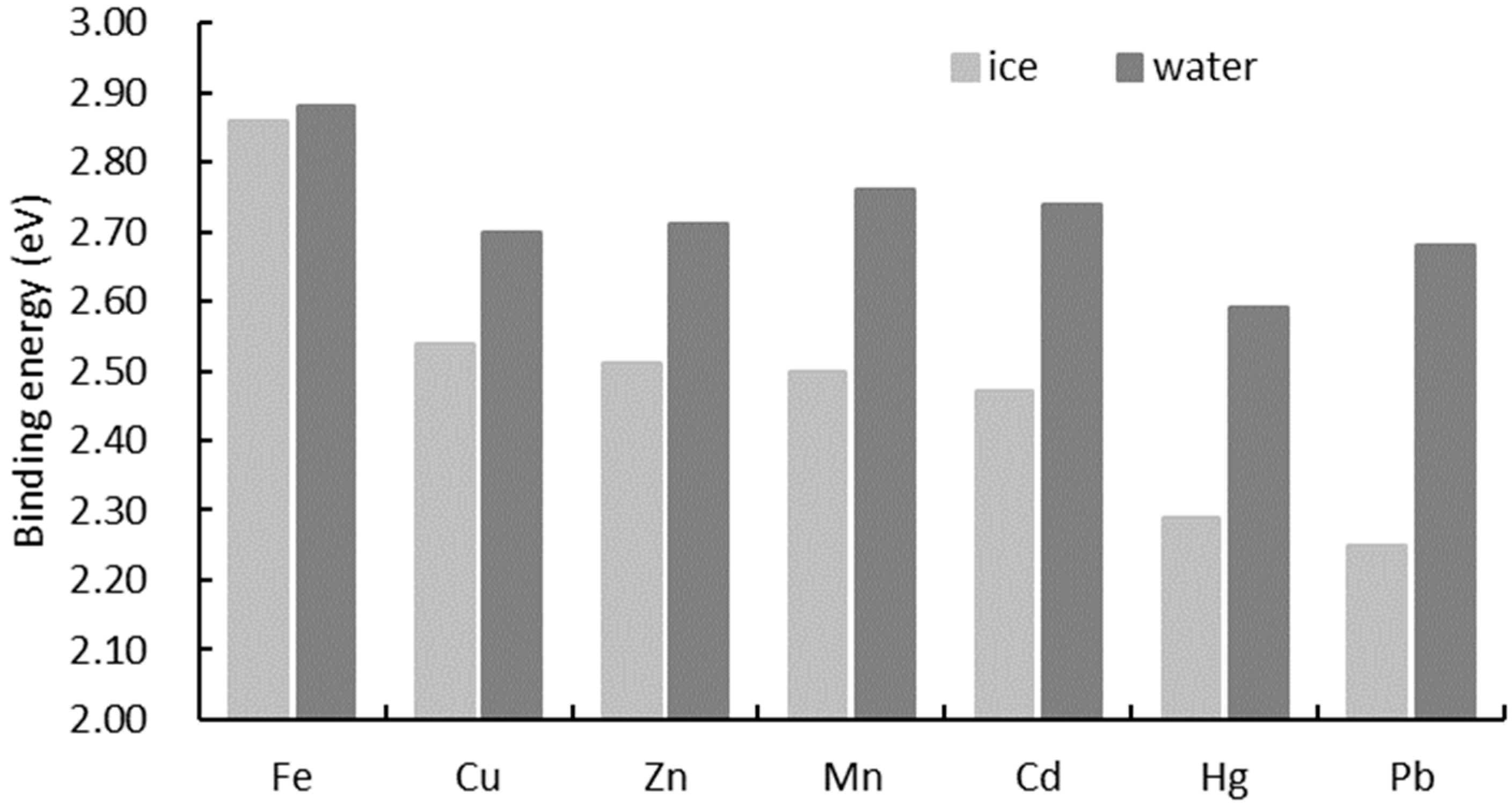
3.2. Energy of HMIs in Ice and Water
4. Discussion
4.1. Mechanism of HMIs Migration in Ice and Water
4.1.1. Stability of HMIs in Ice and Water
4.1.2. Binding Energy of HMIs in Ice and Water
4.2. Mechanism of Different HMIs in Ice
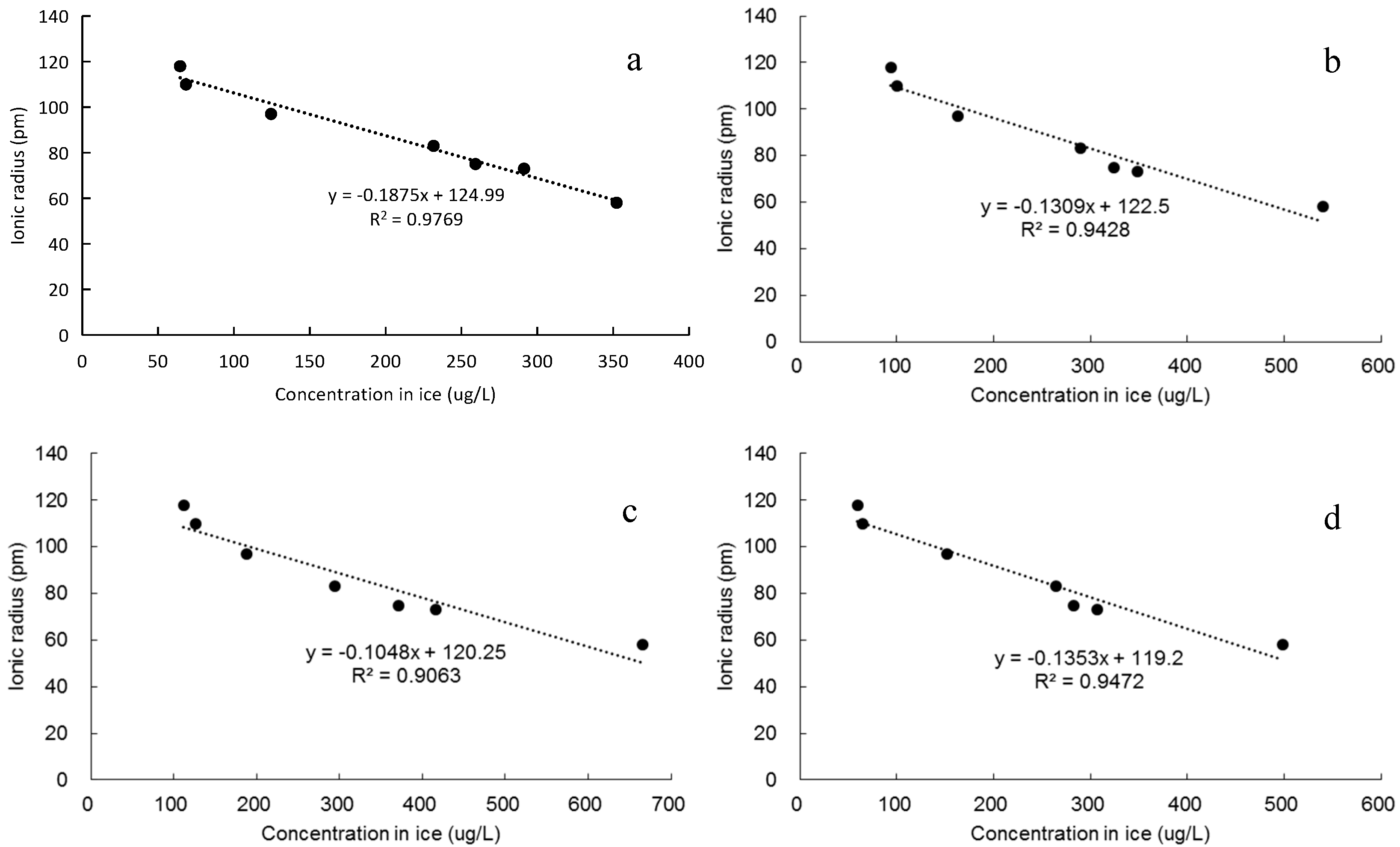
4.2.1. Effect of Ionic Radius on Concentration in Ice
4.2.2. Effect of Binding Energy on Concentration in Ice
5. Conclusions
6. Implications and Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, A.; Zeng, G.; Zhang, J.; Lu, L.; Chen, M.; Xu, P. Global landscape of total organic carbon, nitrogen and phosphorus in lake water. Sci. Rep. 2015, 5, 15043. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Fan, C.; Zhong, J.; Zhang, Y.; Wang, C.; Lei, Z. Evaluation of in situ simulated dredging to reduce internal nitrogen flux across the sediment-water interface in lake Taihu, China. Environ. Pollut. 2016, 214, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.Y.; Shi, X.H.; Chao, L.I. The migration of total dissolved solids during natural freezing process in Ulansuhai Lake. J. Arid. Land 2012, 4, 85–94. [Google Scholar] [CrossRef]
- Bengtsson, L. Mixing in ice-covered lakes. Hydrobiologia 1996, 322, 91–97. [Google Scholar] [CrossRef]
- Fedotov, V.I.; Cherepanov, N.V.; Tyshko, K.P. Some features of the growth, structure and metamorphism of East Antarctic landfast sea ice. In Antarctic Sea Ice: Physical Processes, Interactions and Variability; Jeffries, M.O., Ed.; American Geophysical Union: Washington, DC, USA, 2013; pp. 343–354. [Google Scholar]
- Baker, R.A. Trace organic contaminant concentration by freezing-IV: Ionic effects. Water Res. 1970, 4, 559–573. [Google Scholar] [CrossRef]
- Huige, N.J.J.; Thijssen, H.A.C. Production of large crystals by continuous ripening in a stirrer tank. J. Cryst. Growths 1972, 13–14, 483–487. [Google Scholar] [CrossRef]
- Muller, M.; Sekoulov, I. Waste water reuse by freeze concentration with falling film reactor. Water Sci. Technol. 1992, 26, 1475–1482. [Google Scholar] [CrossRef]
- Weeks, W. Sea ice: The potential of remote sensing. Oceanus 1981, 24, 39–48. [Google Scholar]
- Wang, S. Study on Distribution Law and Laboratory Simulation Experiment of Heavy Metals during the Icebound Season in Wulansuhai Lake; Inner Mongolia Agriculture University: Inner Mongolia, China, 2012. (In Chinese) [Google Scholar]
- Lv, H.Z. Studies on Pollutant Distribution in Ice-Water System of Ulansuhai Lake; Inner Mongolia Agriculture University: Inner Mongolia, China, 2015. (In Chinese) [Google Scholar]
- Liu, Y.; Li, C.Y.; Anderson, B.; Zhang, S.; Shi, X.H.; Zhao, S.N. A modified QWASI model for fate and transport modeling of mercury between the water-ice-sediment in Lake Ulansuhai. Chemosphere 2017, 176, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, Y.; Li, Y.G.; Meng, X.F.; Song, Q.L. First-principles studies of the effects of microalloy elements on Fe/Al interface. J. At. Mol. Phys. 2007, 24, 853–857. (In Chinese) [Google Scholar]
- Hu, X.L.; Zhang, Y.; Lu, G.H.; Wang, T.M.; Xiao, P.H.; Yin, P.G.; Xu, H.B. Effect of impurity on structure and mechanical properties of NiAl intermetallics: A first-principles study. Intermetallics 2009, 17, 358–364. [Google Scholar] [CrossRef]
- Xu, Q.C.; Ven, A.V. First-principles investigation of migration barriers and point defect complexes in B2-NiAl. Intermetallics 2009, 17, 319–329. [Google Scholar] [CrossRef]
- Wei, H.; Liang, J.J.; Sun, B.Z.; Zheng, Q.; Sun, X.F.; Peng, P.; Yao, X.; Dargusch, M.S. Site preference of Re in NiAl and valence band structure of NiAl containing Re: First-principles study and photoelectron spectrum. Appl. Phys. Lett. 2009, 94, 1731–1756. [Google Scholar]
- Kogita, T.; Kohyama, M.; Kido, Y. Structure and dynamics of NiAl(110) studied by high-resolution ion scattering combined with density functional calculations. Phys. Rev. B 2009, 80, 308–310. [Google Scholar] [CrossRef]
- Tu, T.S.; Fang, H.P. The microstructure of liquid water. J. Phys. Chem. 2010, 39, 79–84. (In Chinese) [Google Scholar]
- Zhou, P. A Theoretical Calculated Investigation on Hydrogen Bonding Clusters of Water-Ammonia Molecules; Shandong Normal University: Shandong, China, 2008. (In Chinese) [Google Scholar]
- Michalarias, I.; Beta, I.; Ford, R.; Ruffle, S.; Li, J.C. Inelastic neutron scattering studies of water in DNA. Appl. Phys. A 2002, 74, s1242–s1244. [Google Scholar] [CrossRef]
- Isakov, S.V.; Moessner, R.; Sondhi, S.L.; Tennant, D.A. Analytical theory for proton correlations in common water ice Ih. Phys. Rev. B 2015, 91, 245152. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.Y.; Zhang, C.F.; Shi, X.H.; Bourque, C.P.A.; Zhao, S.N. Evaluation of the applicability of the SWAT model in an arid piedmont plain oasis. Water Sci. Technol. 2016, 73, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.N.; Shi, X.H.; Li, C.Y.; Zhang, S.; Sun, B.; Wu, Y.; Zhao, S.X. Diffusion flux of phosphorus nutrients at the sediment-water interface of the Ulansuhai Lake in northern China. Water Sci. Technol. 2017, 75, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.Y.; Sun, J.C.; Jiang, C.Y. Current situation and advances in hydrogeochemical researches. Acta Geosci. Sin. 2002, 23, 477–482. (In Chinese) [Google Scholar]
- Zhao, S.N. Enviromental Geochemistry of Heavy Metal and Modelling Analysis of Their Speciation for Ulansuhai Lake in Inner Mongolia; Inner Mongolia Agriculture University: Inner Mongolia, China, 2013. (In Chinese) [Google Scholar]
- Cui, F.L.; Li, C.Y.; Shi, X.H.; Shi, Y.Q.; Fu, X.J. Seasonal changing characteristics of the major ions in Ulansuhai Lake. J. Arid. Land Resour. Environ. 2013, 8, 137–142. [Google Scholar]
- Environment Quality Standards for Surface Water; GB3838-2002; State Environmental Protection Administration of the People’s Republic of China: Beijing, China, 2002. (In Chinese)
- Li, W.P.; Xu, J.; Yu, L.H.; Han, P.J.; Zhao, Z.; Jing, J. Distribution characteristics of nutrients and phytoplankton in Ulansuhai Lake during the icebound season. Ecol. Environ. Sci. 2014, 23, 1007–1013. (In Chinese) [Google Scholar]
- Yang, F.; Li, C.Y.; Leppäranta, M.; Shi, X.H.; Zhao, S.N.; Zhang, C.F. Notable increases in nutrient concentrations in a shallow lake during seasonal ice growth. Water Sci. Technol. 2016, 74, 2773–2783. [Google Scholar]
- The State Environmental Protection Administration the Water and Wastewater Monitoring Analysis Method Editorial Board. Water and Wastewater Monitoring Analysis Method, 4th ed.; China Environmental Science Press: Beijing, China, 2002; pp. 38–47. (In Chinese) [Google Scholar]
- Sergey, K.; Mikhail, N. Lake ladoga ice phenology: Mean condition and extremes during the last 65 years. Hydrol. Process. 2011, 25, 2859–2867. [Google Scholar]
- Wang, W.H.; Zhao, L.; Yan, B. Effects of ions on structure of liquid water. Chemistry 2010, 73, 491–498. (In Chinese) [Google Scholar]
- Frank, H.S.; Wen, W.Y. Ion-solvent interaction. Structural aspects of ion-solvent interaction in aqueous solutions: A suggested picture of water structure. Discuss. Faraday Soc. 1957, 24, 133–140. [Google Scholar] [CrossRef]
- Swift, T.J.; Sayre, W.G. Determination of hydration numbers of cations in aqueous solution by means of proton NMR. J. Chem. Phys. 1966, 44, 3567–3574. [Google Scholar] [CrossRef]
- Goto, A.; Hondoh, T.; Mae, S. The electron density distribution in ice Ih determined by single-crystal X-ray diffractometry. J. Chem. Phys. 1990, 93, 1412–1417. [Google Scholar] [CrossRef]
- Petrenko, V.F.; Whitworth, R.W. Physics of Ice; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Atoms-in-Molecules Molecular-Dynamics Study of Ion-Water Bonding of K+ Solvation in Water; North University of China: Taiyuan, China, 2010. (In Chinese) [Google Scholar]
- Wang, W.H.; Zhao, L.; Yan, B.; Tan, X.; Qi, Y.; He, B. Effects of concentration and freeze-thaw on the first hydration shell structure of Zn2+ ions. J. Tianjin Univ. 2011, 17, 381–385. (In Chinese) [Google Scholar] [CrossRef]
- Marcus, Y. Effect of ions on the structure of water: Structure making and breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef] [PubMed]

| Standard Materials | Sample Number | C1 | C2 |
|---|---|---|---|
| Cu | GSB04-1725-2004 | 1.0 | 10.0 |
| Fe | GSB04-1726-2004 | 3.0 | 10.0 |
| Mn | GSB04-1736-2004 | 0.1 | 10.0 |
| Zn | GSB04-1761-2004 | 0.1 | 10.0 |
| Pb | GSB04-1742-2004 | 0.1 | 10.0 |
| Cd | GSB04-1721-2004 | 0.01 | 10.0 |
| Hg | GSB04-1729-2004 | 0.001 | 10.0 |
| HMIs | T = −25 °C | T = −15 °C | pH = 5 | pH = 11 | V-Type | 10,000 µg/L |
|---|---|---|---|---|---|---|
| Fe | 0.037 | 0.024 | 0.045 | 0.034 | 0.061 | 0.037 |
| Cu | 0.024 | 0.020 | 0.028 | 0.021 | 0.105 | 0.024 |
| Zn | 0.022 | 0.017 | 0.025 | 0.019 | 0.017 | 0.022 |
| Mn | 0.019 | 0.016 | 0.020 | 0.018 | 0.013 | 0.019 |
| Cd | 0.011 | 0.008 | 0.013 | 0.010 | 0.118 | 0.011 |
| Hg | 0.007 | 0.005 | 0.008 | 0.004 | 0.055 | 0.007 |
| Pb | 0.006 | 0.004 | 0.007 | 0.004 | 0.010 | 0.006 |
| Heavy Metals | E(M) | Calculation in Ice | Calculation in Water | ||
|---|---|---|---|---|---|
| E (M + H) | E (H) | E (M + H) | E (H) | ||
| Fe | −28.09 | −1268.80 | −1237.85 | −118.12 | −87.15 |
| Cu | −16.10 | −1256.49 | −1237.85 | −105.95 | −87.15 |
| Zn | −15.41 | −1255.76 | −1237.85 | −105.27 | −87.15 |
| Mn | −18.98 | −1259.33 | −1237.85 | −108.89 | −87.15 |
| Cd | −14.10 | −1254.42 | −1237.85 | −103.99 | −87.15 |
| Hg | −13.50 | −1253.64 | −1237.85 | −103.24 | −87.15 |
| Pb | −17.63 | −1257.73 | −1237.85 | −107.46 | −87.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Li, C.; Liu, J.; Shi, X.; Zhao, S.; Wu, Y.; Tian, W. First-Principles Study on the Migration of Heavy Metal Ions in Ice-Water Medium from Ulansuhai Lake. Water 2018, 10, 1149. https://doi.org/10.3390/w10091149
Sun C, Li C, Liu J, Shi X, Zhao S, Wu Y, Tian W. First-Principles Study on the Migration of Heavy Metal Ions in Ice-Water Medium from Ulansuhai Lake. Water. 2018; 10(9):1149. https://doi.org/10.3390/w10091149
Chicago/Turabian StyleSun, Chi, Changyou Li, Jianjun Liu, Xiaohong Shi, Shengnan Zhao, Yong Wu, and Weidong Tian. 2018. "First-Principles Study on the Migration of Heavy Metal Ions in Ice-Water Medium from Ulansuhai Lake" Water 10, no. 9: 1149. https://doi.org/10.3390/w10091149
APA StyleSun, C., Li, C., Liu, J., Shi, X., Zhao, S., Wu, Y., & Tian, W. (2018). First-Principles Study on the Migration of Heavy Metal Ions in Ice-Water Medium from Ulansuhai Lake. Water, 10(9), 1149. https://doi.org/10.3390/w10091149





