Benthic Diatoms of the Ying River (Huaihe River Basin, China) and Their Application in Water Trophic Status Assessment
Abstract
1. Introduction
2. Materials and Methods
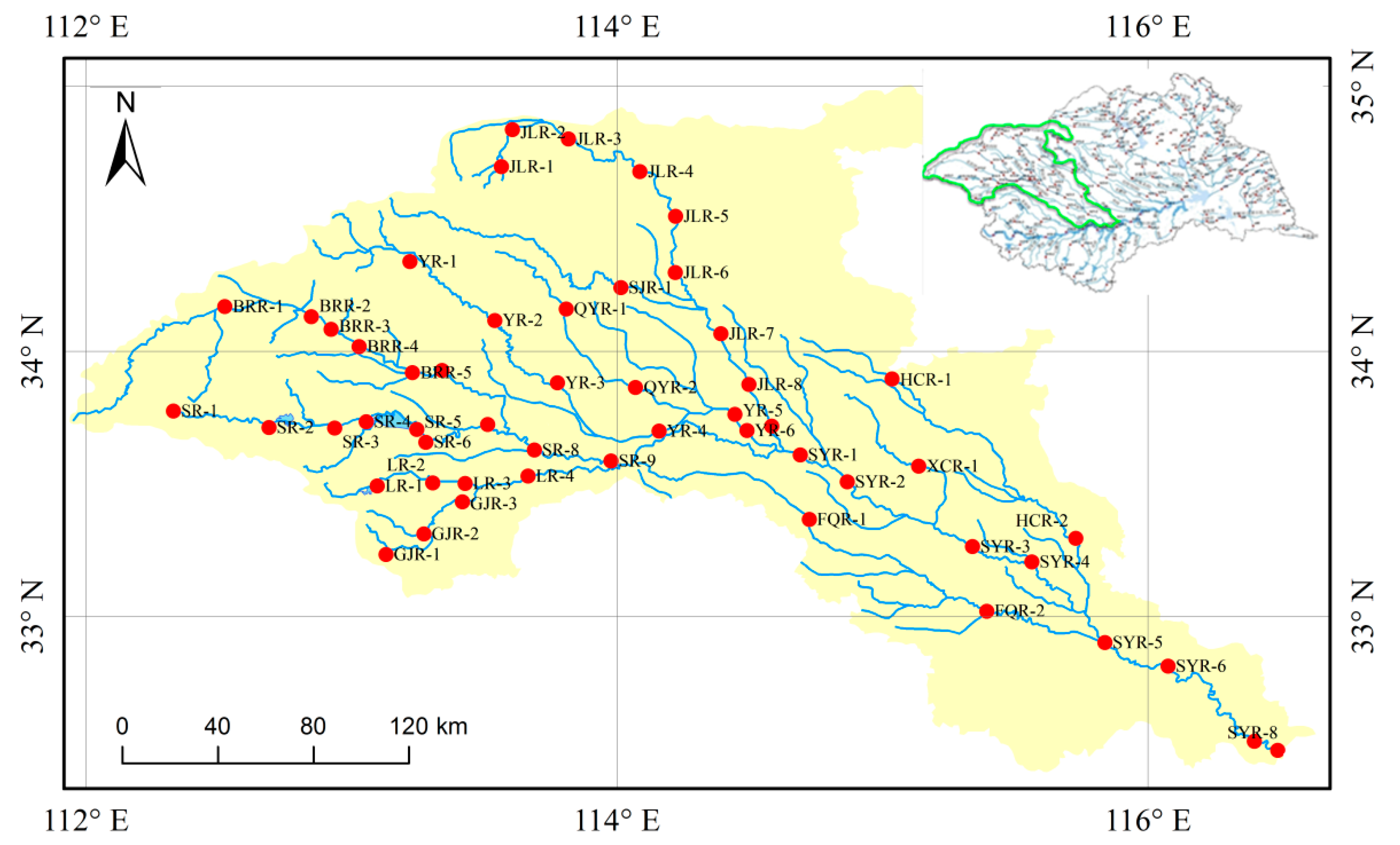
2.1. Study Region and Sampling Sites
2.2. Sampling and Measurement Procedures
2.3. Data Analysis
3. Results and Discussion
3.1. Benthic Diatom Assemblages
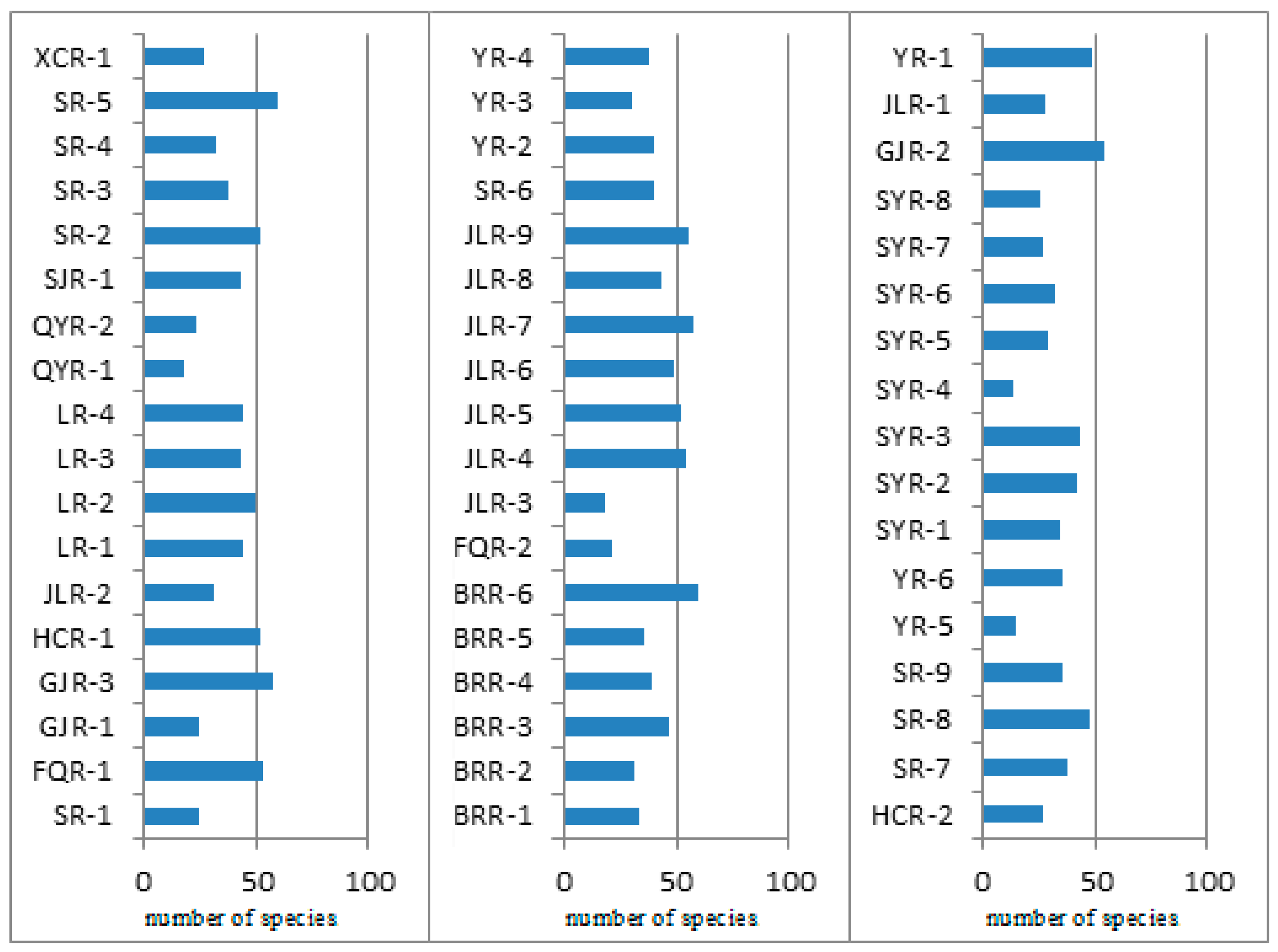
3.2. Dominant Species of Benthic Diatoms and Their Distribution
3.3. Distribution of Benthic Diatoms in Graded Rivers of the Ying River
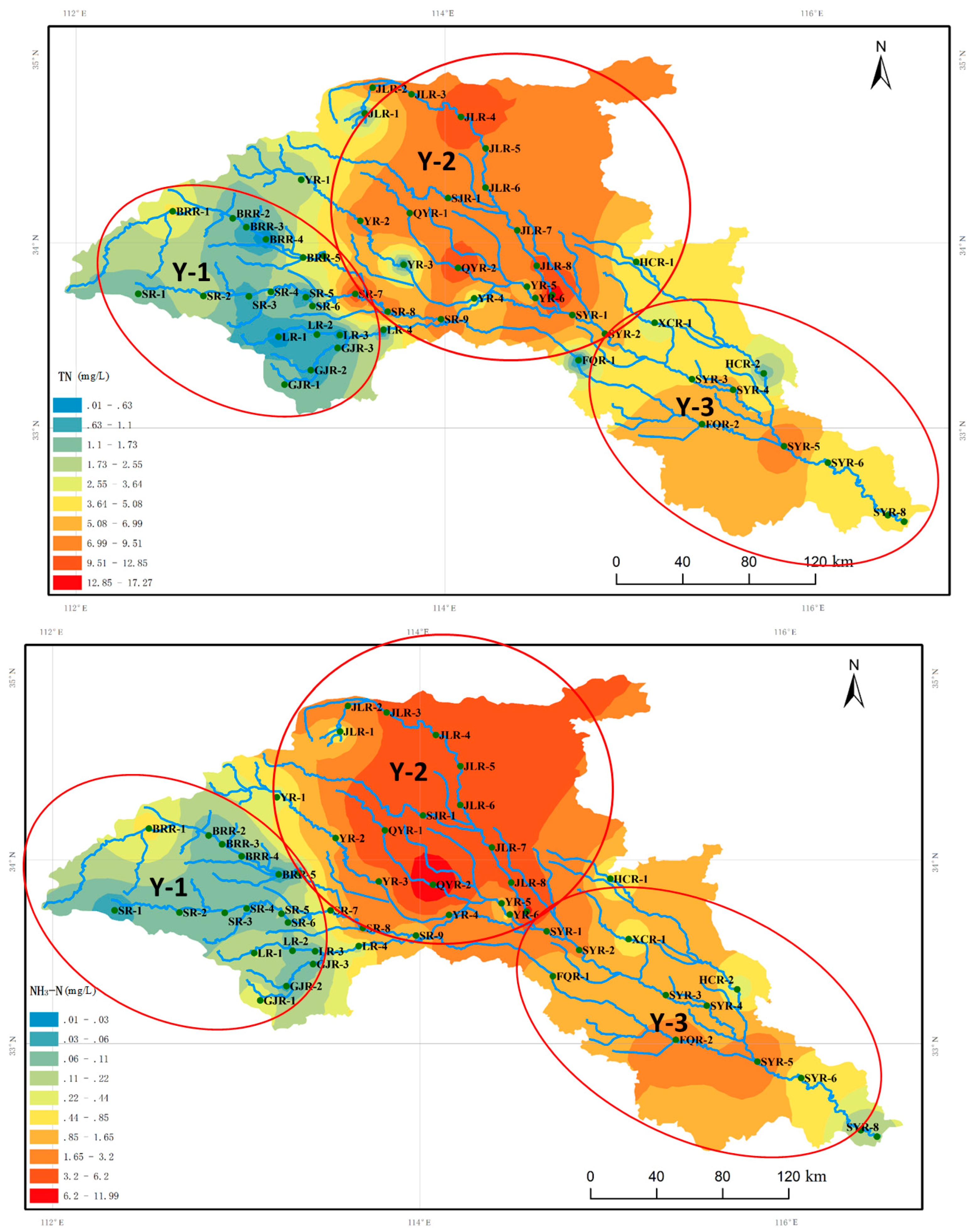
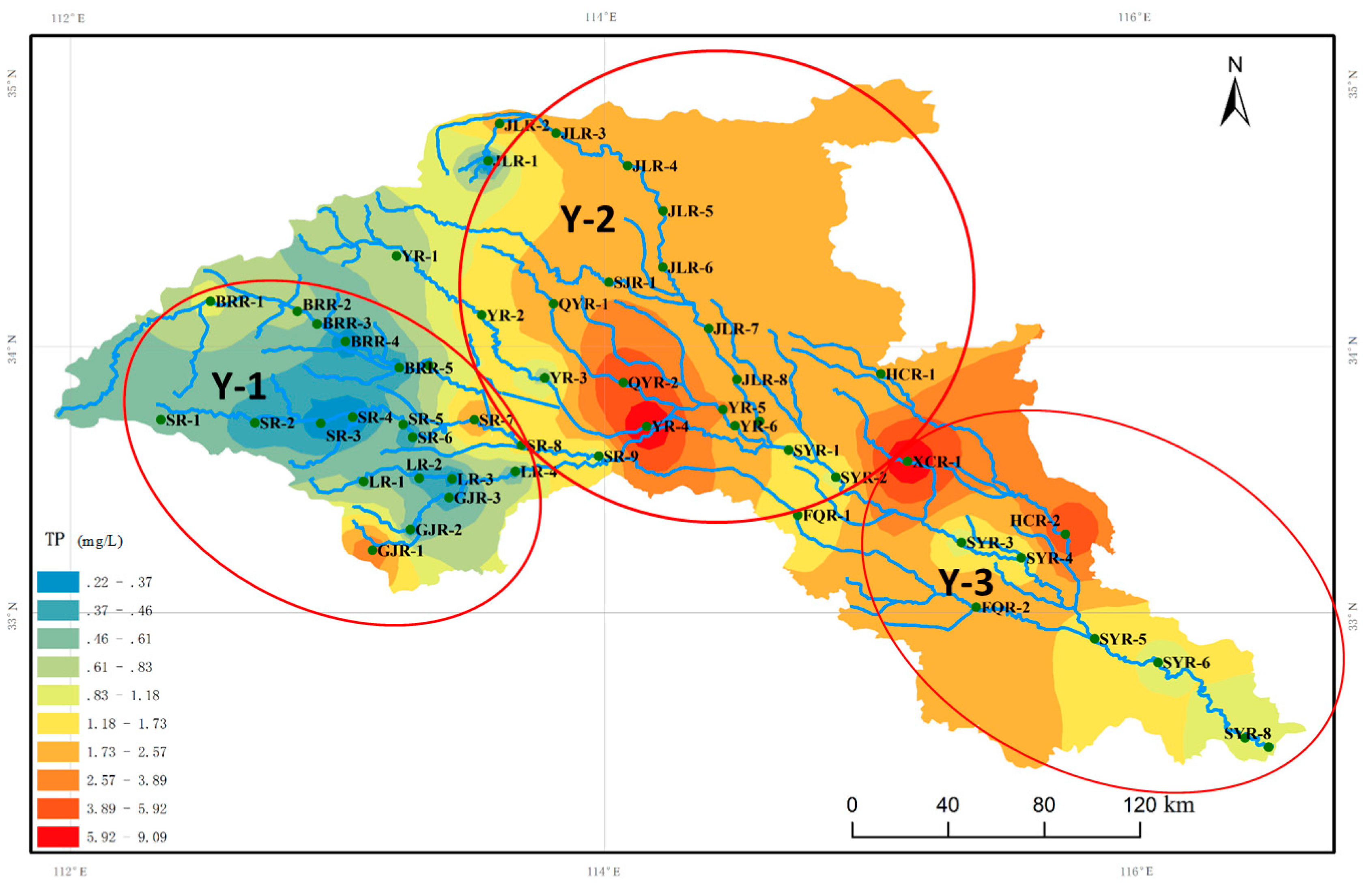
3.4. Distribution of Dominant Benthic Diatoms of the Ying River by Nutrients
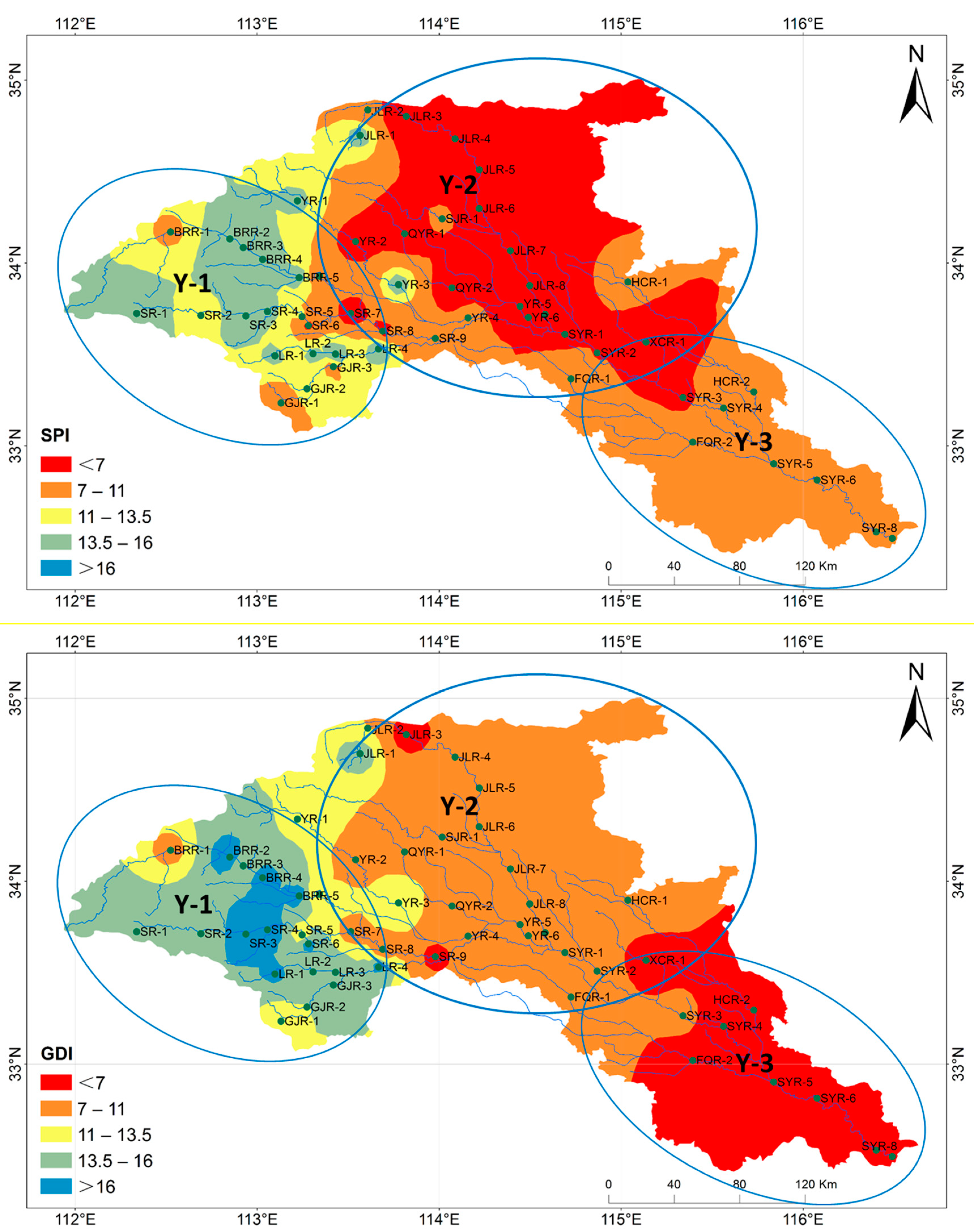
3.5. Distribution of Dominant Benthic Diatoms of the Ying River by Diatom Indices
3.6. Correlation between Benthic Diatoms and Environmental Parameters in the Ying River
3.6.1. Physicochemical Parameters of the Ying River
3.6.2. Pearson’s Correlation Analysis
3.6.3. Canonical Correspondence Analysis (CCA) of Benthic Diatoms with Environmental Factors at Various Orders of Rivers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mccormick, P.V.; Cairns, J. Algae as indicators of environmental change. J. Appl. Phycol. 1994, 6, 509–526. [Google Scholar] [CrossRef]
- Winter, J.G.; Duthie, H.C. Epilithic diatoms as indicators of stream total N and total P concentration. J. N. Am. Benthol. Soc. 2000, 19, 32–49. [Google Scholar] [CrossRef]
- Potapova, M.; Charles, D.F. Diatom metrics for monitoring eutrophication in rivers of the United States. Ecol. Indic. 2007, 7, 48–70. [Google Scholar] [CrossRef]
- Reid, M.A.; Tibby, J.C.; Penny, D.; Gell, P.A. The use of diatoms to assess past and present water quality. Aust. J. Ecol. 2010, 20, 57–64. [Google Scholar] [CrossRef]
- Vasiljević, B.; Krizmanić, J.; Ilić, M.; Marković, V.; Tomović, J.; Zorić, K.; Paunović, M. Water quality assessment based on diatom indices–small hilly streams case study. Water Res. Manag. 2014, 4, 31–35. [Google Scholar]
- Chen, X.; Bu, Z.; Stevenson, M.A.; Cao, Y.; Zeng, L.; Qin, B. Variations in diatom communities at genus and species levels in peatlands (central China) linked to microhabitats and environmental factors. Sci. Total Environ. 2016, 568, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Liboriussen, L.; Jeppesen, E. Structure, biomass, production and depth distribution of periphyton on artificial substratum in shallow lakes with contrasting nutrient concentrations. Freshw. Biol. 2006, 51, 95–109. [Google Scholar] [CrossRef]
- Ponader, K.C.; Charles, D.F.; Belton, T.J. Diatom-based TP and TN inference models and indices for monitoring nutrient enrichment of New Jersey streams. Ecol. Indic. 2007, 7, 79–93. [Google Scholar] [CrossRef]
- Wagenhoff, A.; Clapcott, J.E.; Lau, K.E.M.; Lewis, G.D.; Young, R.G. Identifying congruence in stream assemblage thresholds in response to nutrient and sediment gradients for limit setting. Ecol. Appl. 2017, 27, 469. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, I.; Campeau, S.; Zugicdrakulic, N.; Winter, J.G.; Fortin, C. Using diatoms to monitor stream biological integrity in Eastern Canada: An overview of 10 years of index development and ongoing challenges. Sci. Total Environ. 2014, 475, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J.; Pan, Y.; Manoylov, K.M.; Parker, C.A.; Larsen, D.P.; Herlihy, A.T. Development of diatom indicators of ecological conditions for streams of the western Us. J. N. Am. Benthol. Soc. 2008, 27, 1000–1016. [Google Scholar] [CrossRef]
- Smucker, N.J.; Vis, M.L. Spatial factors contribute to benthic diatom structure in streams across spatial scales: Considerations for biomonitoring. Ecol. Indic. 2011, 11, 1191–1203. [Google Scholar] [CrossRef]
- Liu, J.; Soininen, J.; Han, B.P.; Declerck, S.A.J. Effects of connectivity, dispersal directionality and functional traits on the metacommunity structure of river benthic diatoms. J. Biogeogr. 2013, 40, 2238–2248. [Google Scholar] [CrossRef]
- Rimet, F.; Bouchez, A.; Tapolczai, K. Spatial heterogeneity of littoral benthic diatoms in a large lake: Monitoring implications. Hydrobiologia 2016, 771, 179–193. [Google Scholar] [CrossRef]
- Tang, T.; Tang, T.; Tan, L.; Gu, Y.; Jiang, W.; Cai, Q. Identifying community thresholds for lotic benthic diatoms in response to human disturbance. Sci. Rep. 2017, 7, 4134. [Google Scholar] [CrossRef] [PubMed]
- Solak, C.N.; Àcs, É. Water quality monitoring in European and Turkish rivers using diatoms. Turk. J. Fish. Aquat. Sci. 2011, 11, 329–337. [Google Scholar] [CrossRef]
- Szczepocka, E.; Szulc, B.; Szulc, K.; Rakowska, B.; Żelazna-Wieczorek, J. Diatom indices in the biological assessment of the water quality based on the example of a small lowland river. Oceanol. Hydrobiol. Stud. 2014, 43, 265–273. [Google Scholar] [CrossRef]
- Jakovljević, O.; Krizmanić, J.; Cvijan, M. Water quality assessment of the DTD hydrosystem by diatom indices. Matica Srpska J. Nat. Sci. Novi. Sad. 2014, 127, 22–33. [Google Scholar]
- Kolkwitz, R.; Marsson, M. Ökologie der pflanzlichen Saprobien. Ber. Dt. Bot. Ges. 1908, 26A, 505–519. [Google Scholar]
- Sládeček, V. Diatoms as indicators of organic pollution. Acta Hydrochim. Hydrobiol. 1986, 14, 555–566. [Google Scholar] [CrossRef]
- Kelly, M.G.; Whitton, B.A. The trophic diatom index: A new index for monitoring eutrophication in rivers. J. Appl. Phycol. 1995, 7, 433–444. [Google Scholar] [CrossRef]
- Dell’Uomo, A. L’indice Diatomico Di Eutrofizzazione/Polluzione (EPI-D) Nel Monitoraggio Delle Acque Correnti. Linee guida; Agenzia per la protezione dell’ambiente e per i servizi tecnici: Roma, Italy, 2004. [Google Scholar]
- Schiefele, S.; Schreiner, C. Use of diatoms for monitoring nutrient enrichment, acidification and impact of salt in rivers in Germany and Austria. In Use of Algae for Monitoring Rivers; Studia Student G.m.b.H.: Innsbruck, Austria, 1991. [Google Scholar]
- Coste, M. Étude Des Méthodes Biologiques D'appréciation Quantitative De La Qualité Des Eaux; Bassin Rhône-Méditérannée-Corse, Cemagref: Lyon, France, 1982. [Google Scholar]
- Lenoir, A.; Coste, M. Development of a practical diatom index of overall water quality applicable to the French national water board network. In Use of Algae for Monitoring Rivers II; Studia Student G.m.b.H: Innsbruck, Austria, 1996. [Google Scholar]
- Rumeau, A.; Coste, M. Initiation à la systématique des diatoméesd’eau douce. Bull. Fr. Pêche Piscic. 1988, 309, 1–69. [Google Scholar] [CrossRef]
- Descy, J.P.; Coste, M. A test of methods for assessing water quality based on diatoms. Int. Ver. für theor. und angew. Limnol. Verh. 1991, 24, 2112–2116. [Google Scholar] [CrossRef]
- Pipp, E. A regional diatom-based trophic state indication system for running water sites in Upper Austria and its overregional applicability. Int. Ver. für theor. und angew. Limnol. Verh. 2002, 27, 3376–3380. [Google Scholar] [CrossRef]
- Kelly, M.G.; Cazaubon, A.; Coring, E.; Dell’Uomo, A.; Ector, L.; Goldsmith, B.; Guasch, H.; Hu¨rlimann, J.; Jarlman, A.; Kawecka, B.; et al. Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J. Appl. Phycol. 1998, 10, 215–224. [Google Scholar] [CrossRef]
- Hustedt, F. Bacillariophyta (Diatomeae). In Die Süsswasserflora Mitteleuropas, Heft 10. (2. Aufl.); Pascher, A., Ed.; Gustav Fischer: Jena, LA, USA, 1930. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil: Naviculaceae. In Süßwasserflora von Mitteleuropa, (Band 2/1); Spektrum Akademischer Verlag: Heidelberg, Germany, 1986. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süßwasserflora von Mitteleuropa, (Band 2/2); Spektrum Akademischer Verlag: Heidelberg, Germany, 1988. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Süßwasserflora von Mitteleuropa, (Band 2/3); Spektrum Akademischer Verlag: Heidelberg, Germany, 1991. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Achnanthes s. 1., Navicula s. str., Gomphonema. Gesamtliteraturverzeichnis. Teil 1-4. In Süßwasserflora von Mitteleuropa, (Band 2/4); Spektrum Akademischer Verlag: Heidelberg, Germany, 1991. [Google Scholar]
- McNaughton, S.J. Relationship among functional properties of California grassland. Nature 1967, 216, 168–169. [Google Scholar] [CrossRef]
- Zelinka, M.; Marvan, P. Zur Prazisierung der biologischen Klassifikation des Reinheit fliessender Gewässer. Archiv für Hydrobiologie 1961, 57, 389–407. [Google Scholar]
- Kalyoncu, H.; ÇİCek, N.L.; Akkoz, C.; Yorulmaz, B. Comparative performance of diatom indices in aquatic pollution assessment. Afr. J. Agric. Res. 2009, 4, 1032–1040. [Google Scholar]
- Gomà, J.; Rimet, F.; Cambra, J.; Hoffmann, L.; Ector, L. Diatom communities and water quality assessment in mountain rivers of the upper segre basin (la cerdanya, oriental pyrenees). Hydrobiologia 2005, 551, 209–225. [Google Scholar] [CrossRef]
- Simkhada, B. Diatoms as Indicators of Environmental Change in Lakes and Ponds of the Lowlands, Middle Hills and High Himalaya of Nepal. Ph.D. Thesis, University of Bielefeld Faculty, Kathmandu, Népal, 2006. [Google Scholar]
- Qian, N.; Zhang, R.; Zhou, Z. Fluvial Process Theory; Science Press: Beijing, China, 1987. [Google Scholar]
- Strahlar, A.N. Quantitative analysis of watershed geomorphology. Eos Trans. 1957, 38, 913–920. [Google Scholar] [CrossRef]
- Stevenson, R.J. Effects of current and conditions stimulating autogenically changing microhabitats on benthic diatom immigration. Ecology 1983, 64, 1514–1524. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The Ecology of Running Waters; Livepool University Press: Liverpool, UK, 1970; Volume 555. [Google Scholar]
- Pan, Y.; Stevenson, R.J.; Hill, B.H.; Kaufmann, P.R.; Herlihy, A.T. Spatial Patterns and ecological determinants of benthic algal assemblages in Mid-Atlantic streams, USA. J. Phycol. 1999, 35, 460–468. [Google Scholar] [CrossRef]
- Douglas, B. The ecology of attached diatoms and other algae in a small stony stream. J. Ecol. 1958, 46, 295–322. [Google Scholar] [CrossRef]
- Hoagland, K.D.; Peterson, C.G. Effects of light and wave disturbance on vertical zonation of attached microalgage in a large reservoir. J. Phycol. 2010, 26, 450–457. [Google Scholar] [CrossRef]
- Boyd, C.E.; Mnnsiri, P. Phosphorous absorption capacity and availability of added phosphorous in soils from aquaculture areas in Tailand. J. World Aquacult. Soc. 1996, 27, 160–167. [Google Scholar] [CrossRef]
- Balod, M.; Purina, I.; Becllemin, C.; Maestrini, S.Y. Effects of nutrient enrichment on the growth rates and community structure of summer phytoplankton from the Gulf of Riga, Baltic Sea. J. Plankton Res. 1998, 20, 2251–2271. [Google Scholar] [CrossRef]
- Keck, F.; Bouchez, A.; Franc, A.; Rimet, F. Linking phylogenetic similarity and pollution sensitivity to develop ecological assessment methods: A test with river diatoms. J. Appl. Ecol. 2016, 53, 856–864. [Google Scholar] [CrossRef]
- Braak, C.J.F.T. Canonical correspondence analysis: A new eigenvector method for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Braak, C.J.F.T. Unimodal Models to Related Species to Environment; Agricultural Math Group: Wageningen, The Netherlands, 1987. [Google Scholar]



| Dominant Species | Dominance Y |
|---|---|
| Nitzschia inconspicua | 0.05 |
| Achnanthidium minutissimum | 0.05 |
| Navicula aitchelbee | 0.05 |
| Nitzschia palea | 0.05 |
| Cyclotella meneghiniana | 0.03 |
| Navicula submuralis | 0.02 |
| Mayamaea atomus | 0.02 |
| Region | Dominant Species | Dominance Y |
|---|---|---|
| Y-1 | Achnanthidium minutissimum | 0.15 |
| Achnanthidium sp. 2 | 0.03 | |
| Nitzschia palea | 0.03 | |
| Achnanthidium cf. eutrophilum | 0.03 | |
| Navicula submuralis | 0.02 | |
| Nitzschia amphibian | 0.02 | |
| Encyonopsis microcephala | 0.02 | |
| Y-2 | Navicula aitchelbee | 0.11 |
| Cyclotella meneghiniana | 0.09 | |
| Nitzschia palea | 0.09 | |
| Gomphonema parvulum | 0.05 | |
| Mayamaea atomus | 0.03 | |
| Achnanthidium minutissimum | 0.03 | |
| Nitzschia paleacea | 0.02 | |
| Y-3 | Nitzschia inconspicua | 0.31 |
| Navicula aitchelbee | 0.09 | |
| Nitzschia palea | 0.04 | |
| Navicula submuralis | 0.02 | |
| Navicula subminuscula | 0.02 |
| Sampling | Diatom Indices | Sampling | Diatom Indices | Sampling | Diatom Indices | |||
|---|---|---|---|---|---|---|---|---|
| Sites | SPI | GDI | Sites | SPI | GDI | Sites | SPI | GDI |
| LR-1 | 14.38 | 16.74 | SR-9 | 7.85 | 4.94 | JLR-6 | 2.95 | 7.25 |
| LR-2 | 14.97 | 16.07 | GJR-1 | 6.26 | 10.07 | JLR-7 | 5.28 | 8.76 |
| LR-3 | 17.39 | 16.61 | GJR-2 | 13.21 | 13.43 | JLR-8 | 6.73 | 10.32 |
| LR-4 | 17.29 | 17.49 | GJR-3 | 8.71 | 13.7 | JLR-9 | 5.11 | 9.01 |
| BRR-1 | 9.95 | 9.45 | YR-1 | 14.2 | 12.34 | SYR-1 | 5.81 | 9.27 |
| BRR-2 | 16.33 | 18.79 | YR-2 | 3.38 | 7.55 | SYR-2 | 6.82 | 9.28 |
| BRR-3 | 14.94 | 12.75 | YR-3 | 16.01 | 16.1 | SYR-3 | 6.49 | 8.77 |
| BRR-4 | 15.51 | 17.53 | YR-4 | 8.11 | 9.24 | SYR-4 | 9.33 | 2.6 |
| BRR-5 | 15.4 | 17.91 | YR-5 | 5.17 | 10.38 | SYR-5 | 9.39 | 4.26 |
| BRR-6 | 7.42 | 9.16 | YR-6 | 7.27 | 8.48 | SYR-6 | 8.92 | 6.79 |
| SR-1 | 16.25 | 16.01 | QYR-1 | 3.79 | 9.27 | SYR-7 | 7.05 | 6.6 |
| SR-2 | 12.06 | 13.75 | QYR-2 | 3.76 | 8.7 | SYR-8 | 9.22 | 4.49 |
| SR-3 | 15.67 | 17.84 | SJR-1 | 8.23 | 11.13 | FQR-1 | 13.16 | 10.72 |
| SR-4 | 14.98 | 19.13 | JLR-1 | 15.48 | 16.14 | FQR-2 | 8.43 | 5.39 |
| SR-5 | 11.39 | 10.17 | JLR-2 | 7.75 | 10.92 | XCR-1 | 2.38 | 4.27 |
| SR-6 | 5.35 | 17.38 | JLR-3 | 1.35 | 4.37 | HCR-1 | 9.29 | 10.47 |
| SR-7 | 3.03 | 8.44 | JLR-4 | 4.33 | 7.64 | HCR-2 | 7.32 | 3.19 |
| SR-8 | 4.89 | 7.5 | JLR-5 | 3.46 | 6.36 | |||
| Parameters | Mean | Range |
|---|---|---|
| Velocity (m/s) | 0.13 | 0–1.022 |
| WT (°C) | 23.48 | 17.28–31.02 |
| Cond (mS/cm) | 0.91 | 0.17–1.93 |
| Sal (‰) | 0.45 | 0.08–0.96 |
| pH | 8.34 | 4.41–9.41 |
| Turbidity (NTU) | 31.03 | 1.18–93.84 (111.50, 115.70) |
| TSS (mg/L) | 0.12 | 0–0.46 |
| SD (cm) | 51.65 | 9–214 |
| COD (mg/L) | 33.89 | 0–57.20 (320.10, 341.00, 348.70) |
| TN (mg/L) | 4.81 | 0.01–17.27 |
| TP (mg/L) | 1.67 | 0.22–9.09 |
| NH3-N (mg/L) | 1.58 | 0.01–5.50 (7.46, 8.55, 10.74, 11.99) |
| TN/TP | 3.70 | 0.3–12.96 |
| Dominant Species and Diatom Indices | Velocity | Cond | Sal | pH | Turbidity | WT | |||||
| Nitzschia inconspicua | −0.277 * | 0.265 | 0.288 * | 0.034 | 0.146 | 0.077 | |||||
| Achnanthidium minutissimum | −0.242 | −0.535 ** | −0.557 ** | 0.301 * | −0.241 | 0.345 * | |||||
| Navicula aitchelbee | 0.421 ** | 0.474 ** | 0.495 ** | −0.068 | 0.430 ** | −0.302 * | |||||
| Nitzschia palea | 0.310 * | 0.402 ** | 0.459 ** | −0.263 | 0.340 * | −0.355 ** | |||||
| Cyclotella meneghiniana | 0.322 * | 0.360 ** | 0.408 ** | −0.192 | 0.027 | −0.366 ** | |||||
| Navicula submuralis | 0.079 | 0.065 | 0.072 | 0.068 | −0.097 | 0.123 | |||||
| Mayamaea atomus | −0.083 | 0.250 | 0.063 | 0.027 | −0.070 | 0.095 | |||||
| SPI | −0.252 | −0.670 ** | −0.696 ** | 0.360 ** | −0.361 ** | 0.382 ** | |||||
| GDI | −0.115 | −0.685 ** | −0.722 ** | 0.175 | −0.356 ** | 0.329 * | |||||
| Dominant Species and Diatom Indices | TSS | SD | COD | TN | TP | NH3-N | TN/TP | ||||
| Nitzschia inconspicua | 0.138 | −0.089 | −0.091 | 0.056 | 0.015 | −0.120 | 0.181 | ||||
| Achnanthidium minutissimum | −0.242 | 0.135 | −0.003 | −0.594 ** | −0.413 ** | −0.339 * | −0.310 * | ||||
| Navicula aitchelbee | 0.431 ** | −0.195 | 0.158 | 0.525 ** | 0.213 | 0.294 * | 0.284 * | ||||
| Nitzschia palea | 0.341 * | −0.145 | 0.009 | 0.614 ** | 0.401 ** | 0.667 ** | 0.130 | ||||
| Cyclotella meneghiniana | 0.028 | −0.105 | 0.014 | 0.624 ** | 0.320 * | 0.762 ** | 0.150 | ||||
| Navicula submuralis | −0.084 | −0.006 | −0.027 | −0.041 | 0.239 | −0.117 | −0.161 | ||||
| Mayamaea atomus | −0.074 | 0.172 | −0.056 | 0.017 | 0.158 | −0.082 | −0.109 | ||||
| SPI | −0.363 ** | 0.180 | −0.132 | −0.719 ** | −0.492 ** | −0.491 ** | −0.272 * | ||||
| GDI | −0.355 ** | 0.214 | −0.071 | −0.535 ** | −0.422 ** | −0.289 * | −0.211 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, R.; Ren, H.; Yu, P.; You, Q.; Pang, W.; Wang, Q. Benthic Diatoms of the Ying River (Huaihe River Basin, China) and Their Application in Water Trophic Status Assessment. Water 2018, 10, 1013. https://doi.org/10.3390/w10081013
Shen R, Ren H, Yu P, You Q, Pang W, Wang Q. Benthic Diatoms of the Ying River (Huaihe River Basin, China) and Their Application in Water Trophic Status Assessment. Water. 2018; 10(8):1013. https://doi.org/10.3390/w10081013
Chicago/Turabian StyleShen, Rongrong, Hongye Ren, Pan Yu, Qingmin You, Wanting Pang, and Quanxi Wang. 2018. "Benthic Diatoms of the Ying River (Huaihe River Basin, China) and Their Application in Water Trophic Status Assessment" Water 10, no. 8: 1013. https://doi.org/10.3390/w10081013
APA StyleShen, R., Ren, H., Yu, P., You, Q., Pang, W., & Wang, Q. (2018). Benthic Diatoms of the Ying River (Huaihe River Basin, China) and Their Application in Water Trophic Status Assessment. Water, 10(8), 1013. https://doi.org/10.3390/w10081013




