Influence of an Extended Domestic Drinking Water System on the Drinking Water Quality
Abstract
1. Introduction
2. Materials and Methods
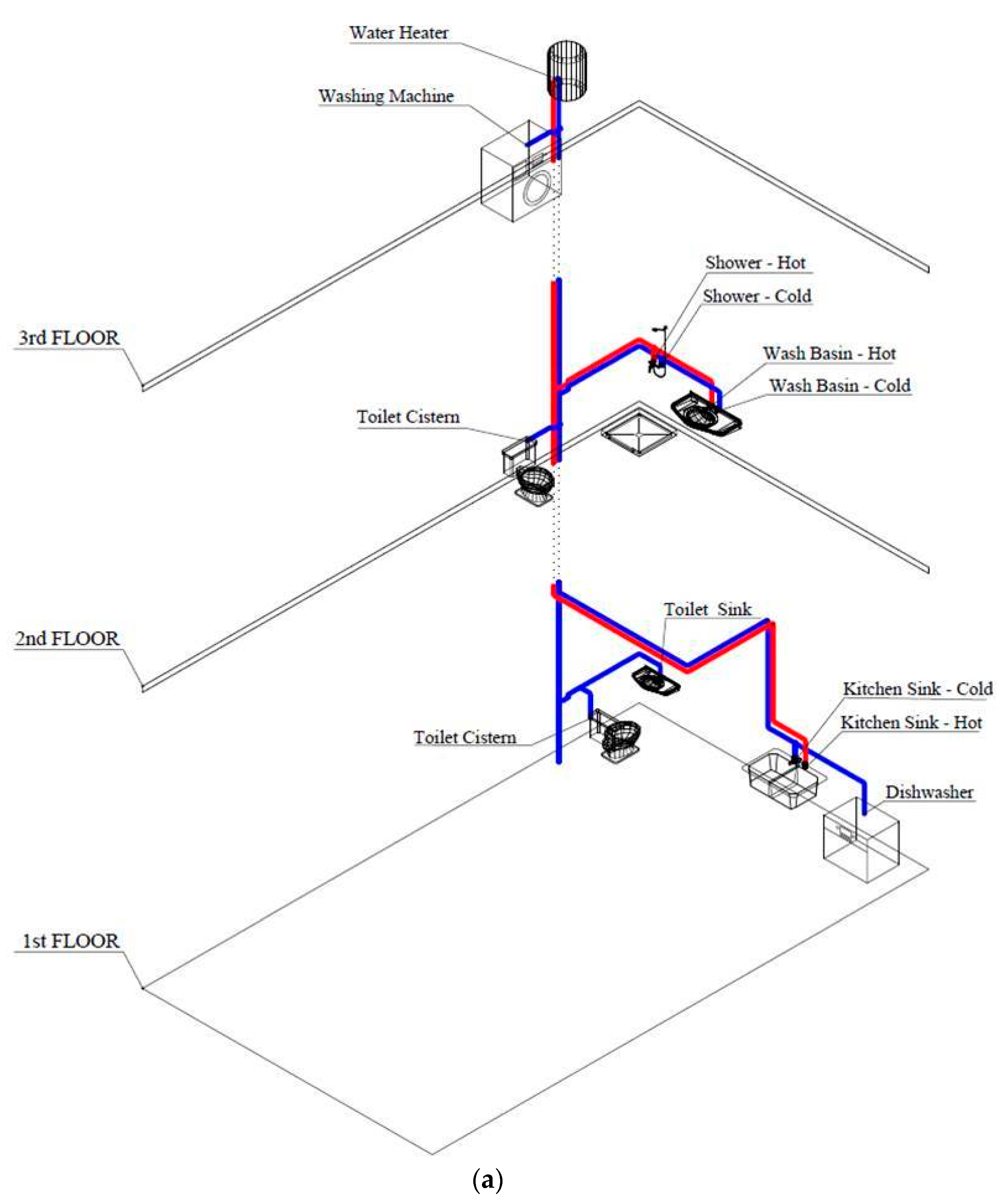
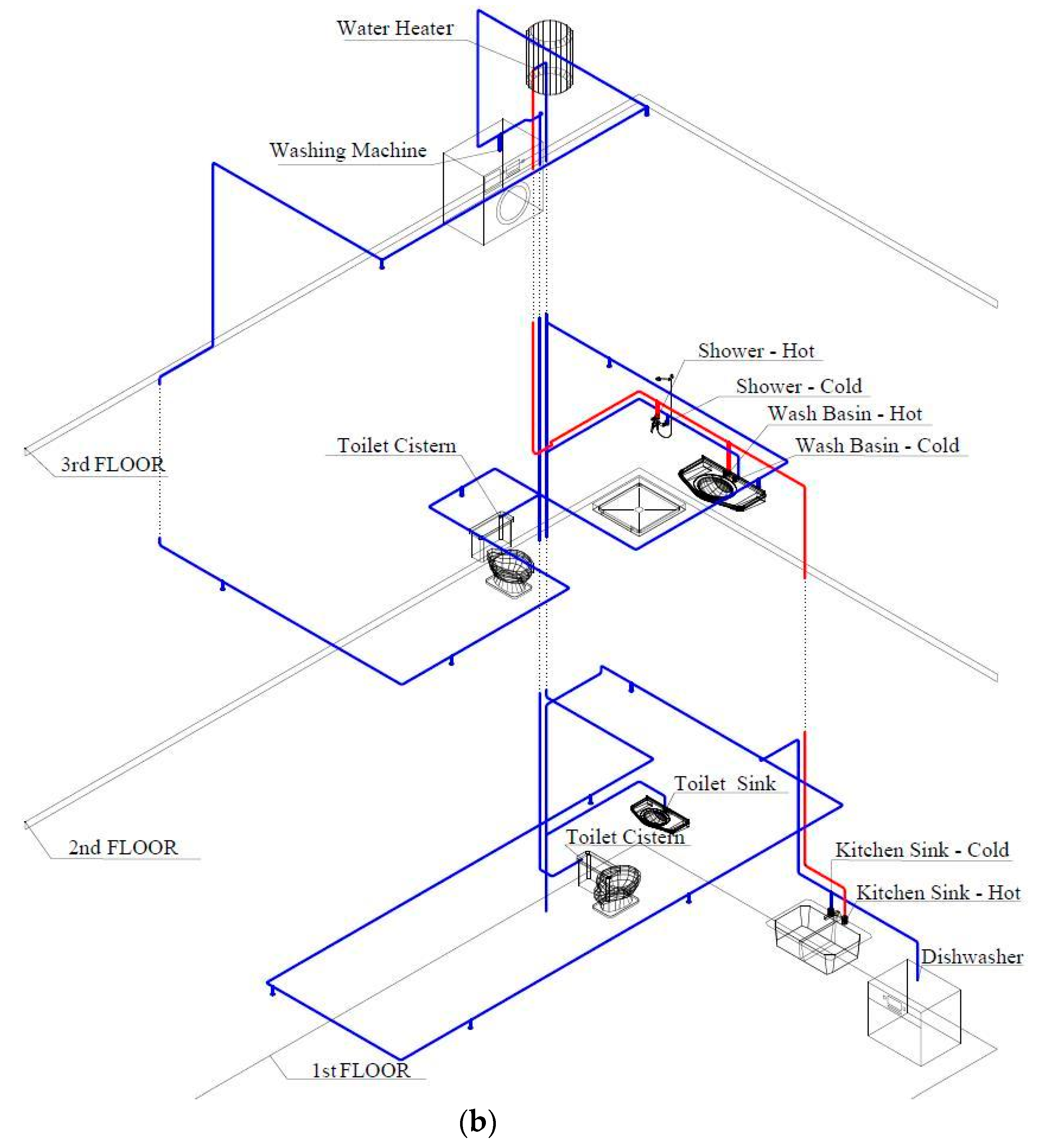
Description of the DDWS Experimental Rigs
3. Results
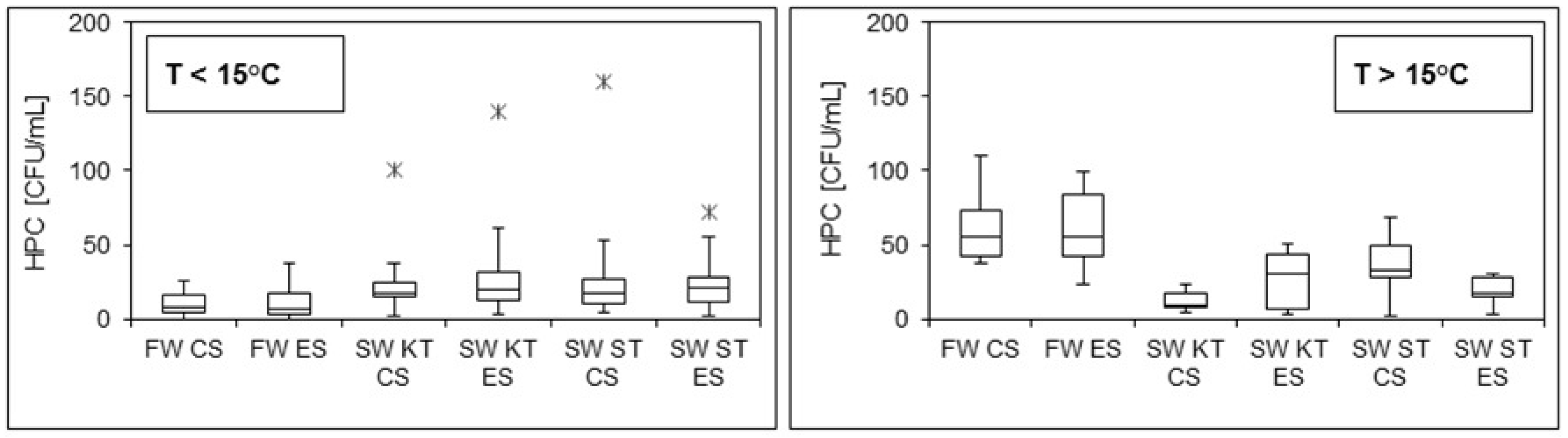
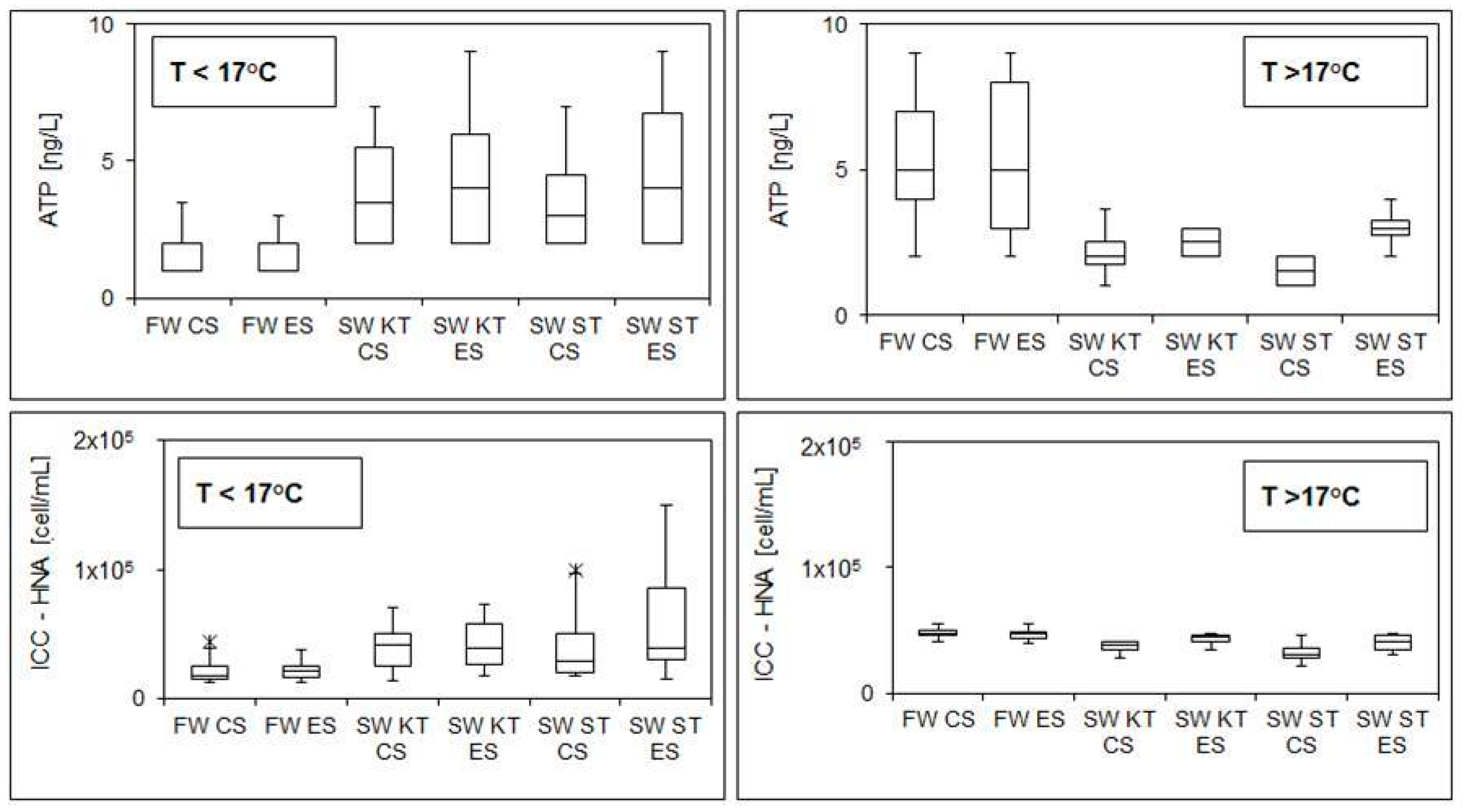
3.1. Overnight Stagnation Experiments
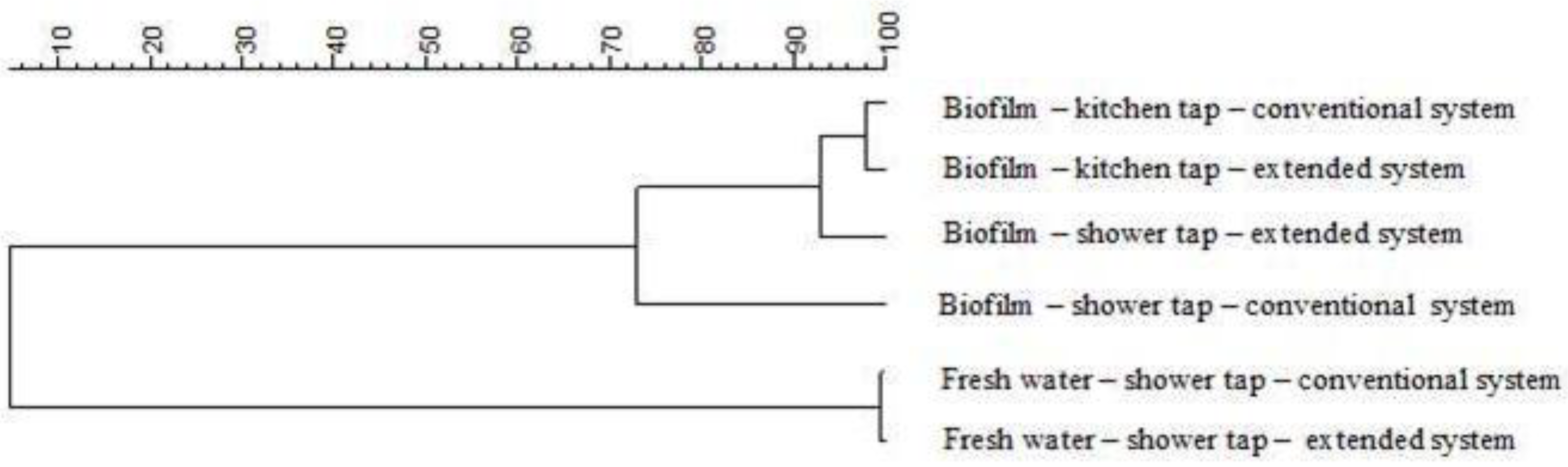
3.2. Biofilm Measurements
4. Discussion
4.1. Water Quality
4.2. Biofilm Measurements
5. Conclusions
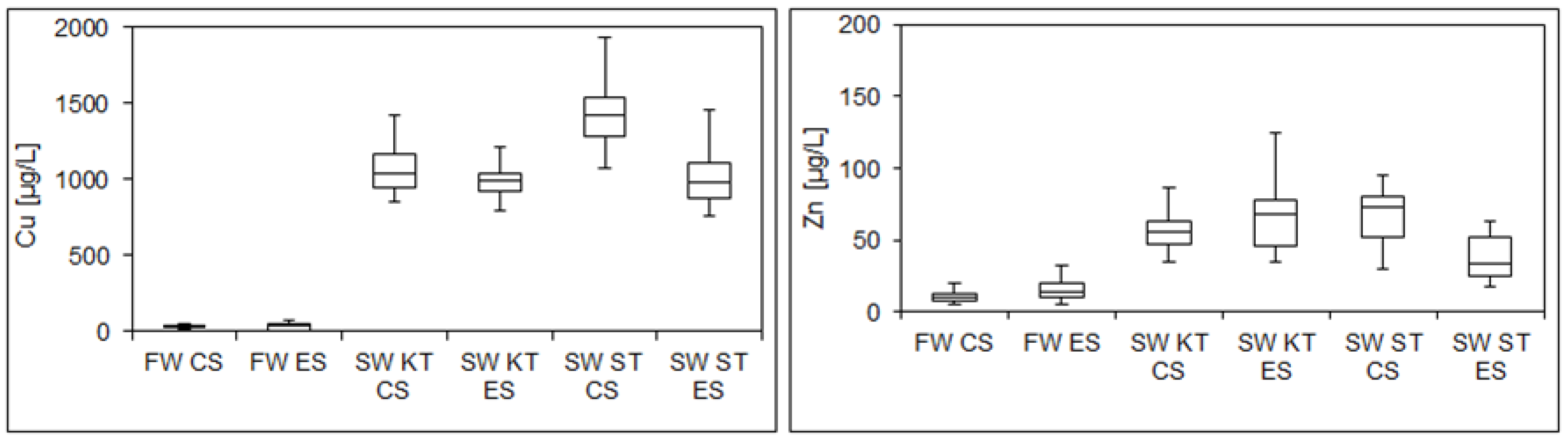
- Overnight stagnation of water in the conventional and extended DWDSs stimulated leaching of copper and zinc from the copper pipes and brass elements. However, the measured copper and zinc concentrations in stagnant water samples were below the WHO maximum contaminant levels.
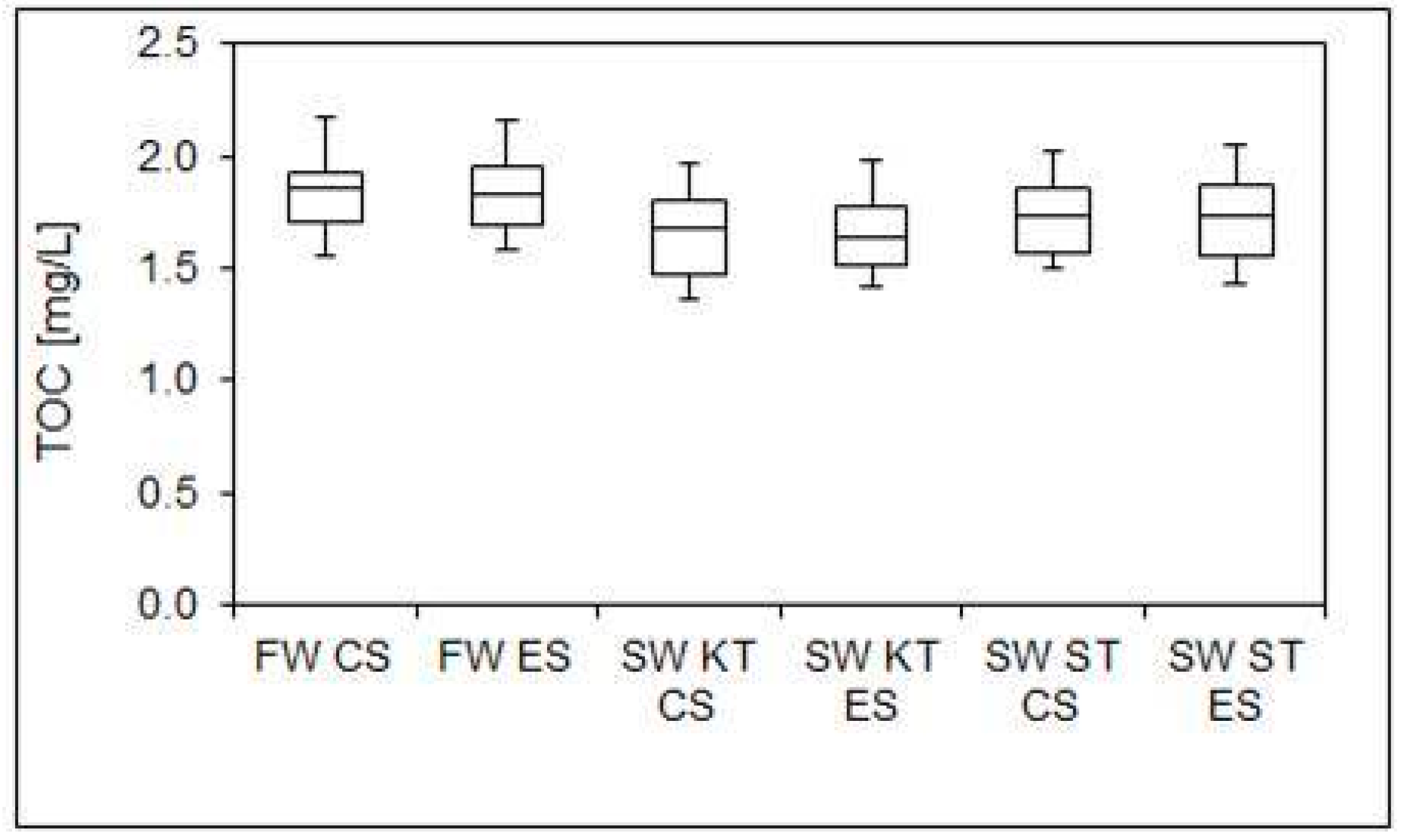
- Significant differences in water quality were observed between the stagnant water samples from the shower taps in the conventional and extended DWDSs. Insignificant differences between all fresh water samples were found for all examined parameters, indicating that tap flushing can restore the quality of the drinking water quality in DDWSs.
- Alteration in microbial parameters was evident after overnight stagnation in both systems, while the overall trend appeared to be a function of fresh water temperature (i.e., increased cell concentrations and viability were observed at low temperatures of fresh water, while at high temperatures of fresh water, we detected a reduction in the cell concentrations and viability in stagnant water samples).
- Characterization of microbiota in water samples and biofilms showed that Proteobacteria dominated among phyla sequences. The high level of similarity at the genus level between fresh water and biofilm communities for both systems, >98% and >70–94% respectively, indicates that the extension of the DWDS affects neither microbial water quality, nor the biofilm compositions.
- Extension of the copper DWDS for residential fire sprinklers accommodation had no effect on the drinking water quality parameters of fresh water during the 14 months of the experimental study.
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- VEWIN. Water Supply Statistics 2007; VEWIN: Rijswijk, The Netherlands, 2008. [Google Scholar]
- Bartram, J. Heterotrophic Plate Counts and Drinking-Water Safety: The Significance of Hpcs for Water Quality and Human Health; World Health Organization: London, UK, 2003. [Google Scholar]
- Vreeburg, J.H.G. Discolouration in Drinking Water Systems: A particular Approach (Chapters 4 and 5). Ph.D. Thesis, Technical University of Delft, Delft, The Netherlands, 2007. [Google Scholar]
- Kerneïs, A.; Nakache, F.; Deguin, A.; Feinberg, M. The effects of water residence time on the biological quality in a distribution network. Water Res. 1995, 29, 1719–1727. [Google Scholar] [CrossRef]
- Uhl, W.; Schaule, G. Establishment of HPC(R2A) for regrowth control in non-chlorinated distribution systems. Int. J. Food Microbiol. 2004, 92, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Nescerecka, A.; Rubulis, J.; Vital, M.; Juhna, T.; Hammes, F. Biological instability in a chlorinated drinking water distribution network. PLoS ONE 2014, 9, e96354. [Google Scholar] [CrossRef] [PubMed]
- Prest, E.I.E.D. Biological Stability in drinking Water Distribution Systems: A Novel Approach for Systematic Microbial Water Quality Monitoring. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2015. [Google Scholar]
- Andrade, M.A.; Choi, C.Y.; Lansey, K.; Jung, D. Enhanced artificial neural networks estimating water quality constraints for the optimal water distribution systems design. J. Water Resour. Plan. Manag. 2016, 142, 04016024. [Google Scholar] [CrossRef]
- Quintiliani, C.; Alfonso, L.; Di Cristo, C.; Leopardi, A.; de Marinis, G. Exploring the use of operational interventions in water distribution systems to reduce the formation of TTHMs. Procedia Eng. 2017, 186, 475–482. [Google Scholar] [CrossRef]
- Shokoohi, M.; Tabesh, M.; Nazif, S.; Dini, M. Water quality based multi-objective optimal design of water distribution systems. Water Resour. Manag. 2017, 31, 93–108. [Google Scholar] [CrossRef]
- Prasad, T.D.; Walters, G.A. Minimizing residence times by rerouting flows to improve water quality in distribution networks. Eng. Optim. 2006, 38, 923–939. [Google Scholar] [CrossRef]
- NIFV. Fire Fatalities during the Period from 2001 to 2015 (In Dutch: Dodelijke Slachtoffers bij Branden Periode 2001 t/m 2015); Nederlands Instituut Fysieke Veiligheid (NIFV): Arnhem, The Netherlands, 2001–2015. [Google Scholar]
- Bukowski, R.W.; Waterman, T.E.; Christian, W.J. Detector Sensitivity and Siting Requirements for Dwellings: A Report of the NBS Indiana Dunes Tests; NFPA: Quincy, MA, USA, 1975. [Google Scholar]
- Bukowski, R.W.; Peacock, R.D.; Averill, J.D.; Cleary, T.G.; Bryner, N.P.; Walton, W.D.; Reneke, P.A.; Kuligowski, E.D. Performance of Home Smoke Alarms Analysis of the Response of Several Available Technologies in Residential Fire Settings; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2007.
- Gadet, B. Private fire service performance. In Lessons to Learn for the Dutch Fire Service? Erasmus University: Rotterdam, The Netherlands, 2009. [Google Scholar]
- Hall, J.R.; Ahrens, M.; Evarts, B. Sprinkler impact on fire injury. In Sprinkler Impact on Fire Injury; NFPA: Quincy, MA, USA, 2012. [Google Scholar]
- Ford, J. Automatic Sprinklers: A 10 Year Study. A Detailed History of the Effects of the Automatic Sprinkler Code in Scottsdale, Arizona; Home Fire Sprinkler Coalition: Frankfort, IL, USA, 1997. [Google Scholar]
- Zlatanovic, L.; Vreeburg, J.; Van Der Hoek, J.P.; Poznakovs, I. An experimental study on the spray characteristics of residential sprinklers under low-flow and low-pressure conditions. Fire Saf. J. 2014, 68, 30–40. [Google Scholar] [CrossRef]
- Staatscourant. Drinking water decree 2011 (drinkwaterbesluit 2011). In Quality Standards; Official Journal of the Royal Kingdom of The Netherlands: The Hague, The Netherlands, 2011; Volume 293. [Google Scholar]
- Lautenschlager, K.; Boon, N.; Wang, Y.; Egli, T.; Hammes, F. Overnight stagnation of drinking water in household taps induces microbial growth and changes in community composition. Water Res. 2010, 44, 4868–4877. [Google Scholar] [CrossRef] [PubMed]
- Zlatanović, L.; van der Hoek, J.P.; Vreeburg, J.H.G. An experimental study on the influence of water stagnation and temperature change on water quality in a full-scale domestic drinking water system. Water Res. 2017, 123, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Whitaker, R.; LeChevallier, M.W.; Liu, W.-T. Drinking water microbiome assembly induced by water stagnation. ISME J. 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, M.; Miettinen, I.; Hirvonen, A.; Vartiainen, T.; Martikainen, P. Estimates of microbial quality and concentration of copper in distributed drinking water are highly dependent on sampling strategy. Int. J. Hyg. Environ. Health 2007, 210, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, S.; Liu, W. Investigation of organic matter migrating from polymeric pipes into drinking water under different flow manners. Environ. Sci. Process. Impacts 2014, 16, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Sarver, E.; Zhang, Y.; Edwards, M. Understanding dezincification failures in premise plumbing systems. In Proceedings of the Water Quality Technology Conference and Exposition 2009, Seattle, WA, USA, 15–18 November 2009; pp. 3592–3619. [Google Scholar]
- NEN. General Requirements for Water Supply Installations; NEN: Delft, The Netherlands, 2002; Volume 1006. [Google Scholar]
- Zlatanovic, L.; Moerman, A.; van der Hoek, J.P.; Vreeburg, J.; Blokker, M. Development and validation of a drinking water temperature model in domestic drinking water supply systems. Urban Water J. 2017, 14, 1031–1037. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Ludwig, J.A.; Reynolds, J.F. Statistical Ecology: A Primer in Methods and Computing; John Wiley & Sons: New York, NY, USA, 1988; Volume 1. [Google Scholar]
- Mulder, C.P.H.; Bazeley-White, E.; Dimitrakopoulos, P.G.; Hector, A.; Scherer-Lorenzen, M.; Schmid, B. Species evenness and productivity in experimental plant communities. Oikos 2004, 107, 50–63. [Google Scholar] [CrossRef]
- Good, I.J. The population frequencies of species and the estimation of population parameters. Biometrika 1953, 40, 237–264. [Google Scholar] [CrossRef]
- Lytle, D.A.; Schock, M.R. Impact of stagnation time on metal dissolution from plumbing materials in drinking water. J. Water Supply Res. Technol. AQUA 2000, 49, 243–257. [Google Scholar]
- Merkel, T.H.; Groß, H.J.; Werner, W.; Dahlke, T.; Reicherter, S.; Beuchle, G.; Eberle, S.H. Copper corrosion by-product release in long-term stagnation experiments. Water Res. 2002, 36, 1547–1555. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Miettinen, I.T.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. Estimates of microbial quality and concentration of copper in distributed drinking water are highly dependent on sampling strategy. Int. J. Hyg. Environ. Health 2007, 210, 725–732. [Google Scholar] [CrossRef] [PubMed]
- WHO. Copper in Drinking-Water: Background Document for Development of Who Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Lytle, D.A.; Schock, M.R. Stagnation Time, Composition, PH, and Orthophosphate Effects on Metal Leaching from Brass; National Risk Management Research Laboratory, Office of Research and Development, US Environmental Protection Agency: Washington, DC, USA, 1996.
- Quevauviller, P.; Thompson, C. Analytical Methods for Drinking Water: Advances in Sampling and Analysis; John Wiley & Sons: New York, NY, USA, 2005; Volume 26. [Google Scholar]
- Sorlini, S.; Gialdini, F.; Collivignarelli, C. Metal leaching in drinking water domestic distribution system: An Italian case study. Int. J. Environ. Health Res. 2014, 24, 497–514. [Google Scholar] [CrossRef] [PubMed]
- Coker, A.K. Ludwig’s Applied Process Design for Chemical and Petrochemical Plants, 4th ed.; Elsevier: Burlington, MA, USA, 2010; Volume 2, pp. 1–961. [Google Scholar]
- WHO. Zinc in Drinking-Water: Background Document for Development of Who Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Edwards, M.; Sprague, N. Organic matter and copper corrosion by-product release: A mechanistic study. Corros. Sci. 2001, 43, 1–18. [Google Scholar] [CrossRef]
- Momba, M.N.B.; Kfir, R.; Venter, S.N.; Cloete, T.E. Overview of Biofilm Formation in Distribution Systems and Its Impact on the Deterioration of Water Quality; Water Research Commission: Gezina, South Africa, 2000. [Google Scholar]
- Tao, Q.; Liu, Z.; Dai, Y.; Zhan, X. Biosorption properties of extracellular polymeric substances towards Zn (II) and Cu (II). Desalin. Water Treat. 2012, 45, 40–47. [Google Scholar]
- Wang, Z.; Hessler, C.M.; Xue, Z.; Seo, Y. The role of extracellular polymeric substances on the sorption of natural organic matter. Water Res. 2012, 46, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Prest, E.I.; Hammes, F.; van Loosdrecht, M.; Vrouwenvelder, J.S. Biological stability of drinking water: Controlling factors, methods, and challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- LeChevallier, M.W.; Welch, N.J.; Smith, D.B. Full-scale studies of factors related to coliform regrowth in drinking water. Appl. Environ. Microbiol. 1996, 62, 2201–2211. [Google Scholar] [PubMed]
- Volk, C.; Dundore, E.; Schiermann, J.; LeChevallier, M.W. Practical evaluation of iron corrosion control in a drinking water distribution system. Water Res. 2000, 34, 1967–1974. [Google Scholar] [CrossRef]
- Liu, G.; Van Der Mark, E.J.; Verberk, J.Q.J.C.; Van Dijk, J.C. Flow cytometry total cell counts: A field study assessing microbiological water quality and growth in unchlorinated drinking water distribution systems. BioMed Res. Int. 2013, 2013, 595872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hammes, F.; Boon, N.; Chami, M.; Egli, T. Isolation and characterization of low nucleic acid (LNA)-content bacteria. ISME J. 2009, 3, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Prest, E.I.; Hammes, F.; Kötzsch, S.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Monitoring microbiological changes in drinking water systems using a fast and reproducible flow cytometric method. Water Res. 2013, 47, 7131–7142. [Google Scholar] [CrossRef] [PubMed]
- Lipphaus, P.; Hammes, F.; Kötzsch, S.; Green, J.; Gillespie, S.; Nocker, A. Microbiological tap water profile of a medium-sized building and effect of water stagnation. Environ. Technol. 2014, 35, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Chen, S.N.; Huang, T.L.; Shang, P.L.; Yang, X.; Ma, W.X. Indoor heating drives water bacterial growth and community metabolic profile changes in building tap pipes during the winter season. Int. J. Environ. Res. Public Health 2015, 12, 13649–13661. [Google Scholar] [CrossRef] [PubMed]
- Boe-Hansen, R.; Albrechtsen, H.J.; Arvin, E.; Jørgensen, C. Bulk water phase and biofilm growth in drinking water at low nutrient conditions. Water Res. 2002, 36, 4477–4486. [Google Scholar] [CrossRef]
- Morita, R.Y. Bacteria in Oligotrophic Environments: Starvation-Survival Lifestyle; Chapman & Hall: New York, NY, USA, 1997; Volume 1. [Google Scholar]
- Fang, H.; Chen, Y.; Huang, L.; He, G. Analysis of biofilm bacterial communities under different shear stresses using size-fractionated sediment. Sci. Rep. 2017, 7, 1299. [Google Scholar] [CrossRef] [PubMed]
- Kalmbach, S.; Manz, W.; Szewzyk, U. Isolation of new bacterial species from drinking water biofilms and proof of their in situ dominance with highly specific 16S rRNA probes. Appl. Environ. Microbiol. 1997, 63, 4164–4170. [Google Scholar] [PubMed]
- Liu, G. Microbiological Water Quality in Drinking Water Distribution Systems: Integral Study of Bulk Water, Suspended Solids, Loose Deposits, and Pipe Wall Biofilm (Chapter 5). Ph.D. Thesis, Technische Universiteit Delf, Delft, The Netherlands, 2013. [Google Scholar]
- Lehtola, M.J.; Laxander, M.; Miettinen, I.T.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. The effects of changing water flow velocity on the formation of biofilms and water quality in pilot distribution system consisting of copper or polyethylene pipes. Water Res. 2006, 40, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Boe-Hansen, R. Microbial Growth in Drinking Water Distribution Systems; Environment & Resources, DTU: Copenhagen, Denmark, 2001. [Google Scholar]
- Lehtola, M.J.; Miettinen, I.T.; Keinänen, M.M.; Kekki, T.K.; Laine, O.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. Microbiology, chemistry and biofilm development in a pilot drinking water distribution system with copper and plastic pipes. Water Res. 2004, 38, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Inkinen, J.; Kaunisto, T.; Pursiainen, A.; Miettinen, I.T.; Kusnetsov, J.; Riihinen, K.; Keinänen-Toivola, M.M. Drinking water quality and formation of biofilms in an office building during its first year of operation, a full scale study. Water Res. 2014, 49, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, M.M.; Korhonen, L.K.; Lehtola, M.J.; Miettinen, I.T.; Martikainen, P.J.; Vartiainen, T.; Suutari, M.H. The microbial community structure of drinking water biofilms can be affected by phosphorus availability. Appl. Environ. Microbiol. 2002, 68, 434–439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inkinen, J.; Jayaprakash, B.; Santo Domingo, J.W.; Keinänen-Toivola, M.M.; Ryu, H.; Pitkänen, T. Diversity of ribosomal 16S DNA-and rna-based bacterial community in an office building drinking water system. J. Appl. Microbiol. 2016, 120, 1723–1738. [Google Scholar] [CrossRef] [PubMed]
- Eichler, S.; Christen, R.; Höltje, C.; Westphal, P.; Bötel, J.; Brettar, I.; Mehling, A.; Höfle, M.G. Composition and dynamics of bacterial communities of a drinking water supply system as assessed by RNA- and DNA-based 16s rRNA gene fingerprinting. Appl. Environ. Microbiol. 2006, 72, 1858–1872. [Google Scholar] [CrossRef] [PubMed]
- Lührig, K.; Canbäck, B.; Paul, C.J.; Johansson, T.; Persson, K.M.; Rådström, P. Bacterial community analysis of drinking water biofilms in southern Sweden. Microbes Environ. 2015, 30, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Magic-Knezev, A.; Wullings, B.; Van der Kooij, D. Polaromonas and hydrogenophaga species are the predominant bacteria cultured from granular activated carbon filters in water treatment. J. Appl. Microbiol. 2009, 107, 1457–1467. [Google Scholar] [CrossRef] [PubMed]

| Biofilm Sample | HPC [CFU/cm2] | ATP [pg/cm2] | TCC [105 cell/cm2] | ICC [105 cell/cm2] |
|---|---|---|---|---|
| Kitchen tap—conventional system | 6.1 | 12 | 6.6 | 2.9 |
| Kitchen tap—extended system | 6.9 | 10 | 5.4 | 3.3 |
| Shower tap—conventional system | 3.7 | 11 | 10 | 5.7 |
| Shower tap—extended system | 0.3 | 8 | 2.1 | 1.5 |
| Sample | Number of Sequences | OTU (97%) | Chao Index | Shannon Index | Evenness | Coverage [%] |
|---|---|---|---|---|---|---|
| Fresh water—shower tap—CS | 185,546 | 3122 | 3509 | 6.17 | 0.76 | 89 |
| Fresh water—shower tap—ES | 178,081 | 3056 | 3480 | 6.15 | 0.76 | 88 |
| Biofilm—kitchen tap—CS | 115,046 | 1144 | 1959 | 2.67 | 0.38 | 58 |
| Biofilm—kitchen tap—ES | 132,662 | 1202 | 1627 | 2.95 | 0.41 | 60 |
| Biofilm—shower tap—CS | 79,962 | 1313 | 2206 | 2.37 | 0.33 | 67 |
| Biofilm—shower tap—ES | 79,383 | 736 | 1086 | 2.44 | 0.37 | 45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlatanović, L.; Knezev, A.; Van der Hoek, J.P.; Vreeburg, J.H.G. Influence of an Extended Domestic Drinking Water System on the Drinking Water Quality. Water 2018, 10, 582. https://doi.org/10.3390/w10050582
Zlatanović L, Knezev A, Van der Hoek JP, Vreeburg JHG. Influence of an Extended Domestic Drinking Water System on the Drinking Water Quality. Water. 2018; 10(5):582. https://doi.org/10.3390/w10050582
Chicago/Turabian StyleZlatanović, Ljiljana, Aleksandra Knezev, Jan Peter Van der Hoek, and Jan H. G. Vreeburg. 2018. "Influence of an Extended Domestic Drinking Water System on the Drinking Water Quality" Water 10, no. 5: 582. https://doi.org/10.3390/w10050582
APA StyleZlatanović, L., Knezev, A., Van der Hoek, J. P., & Vreeburg, J. H. G. (2018). Influence of an Extended Domestic Drinking Water System on the Drinking Water Quality. Water, 10(5), 582. https://doi.org/10.3390/w10050582






