Nanoscopic Zero-Valent Iron Supported on MgO for Lead Removal from Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of MgO_nZVI
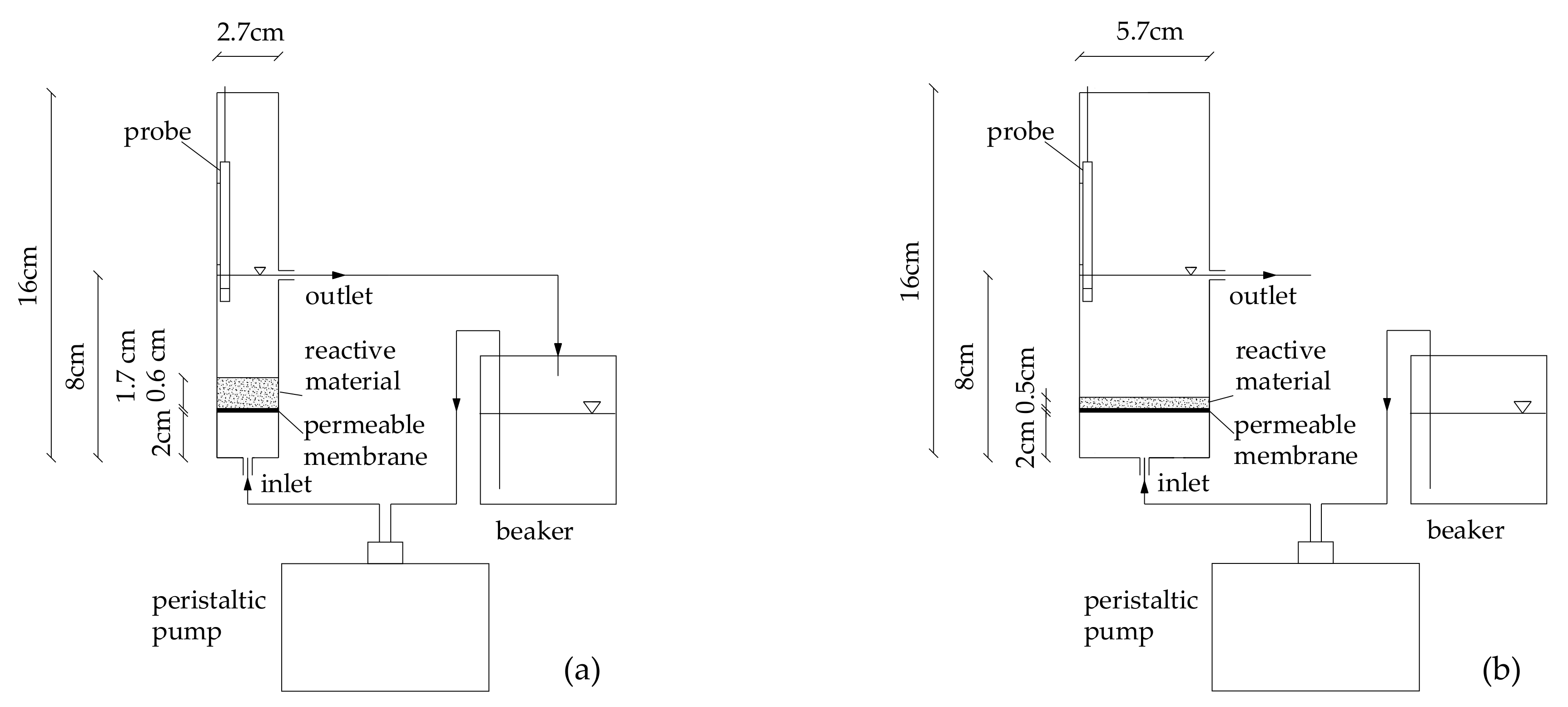
2.3. Column Tests
2.4. Analytical Methods and Presentation of Results
3. Results and Discussion
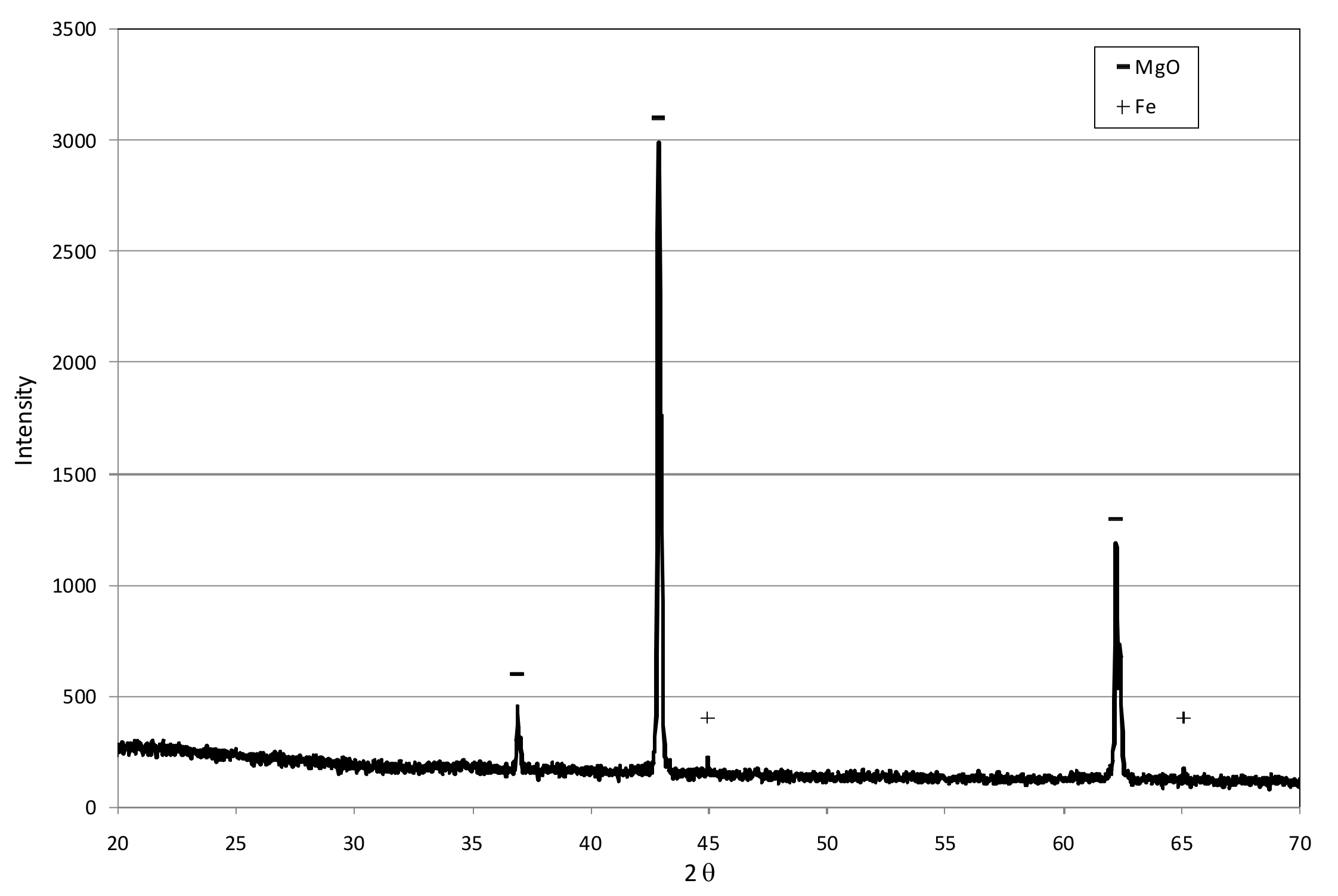
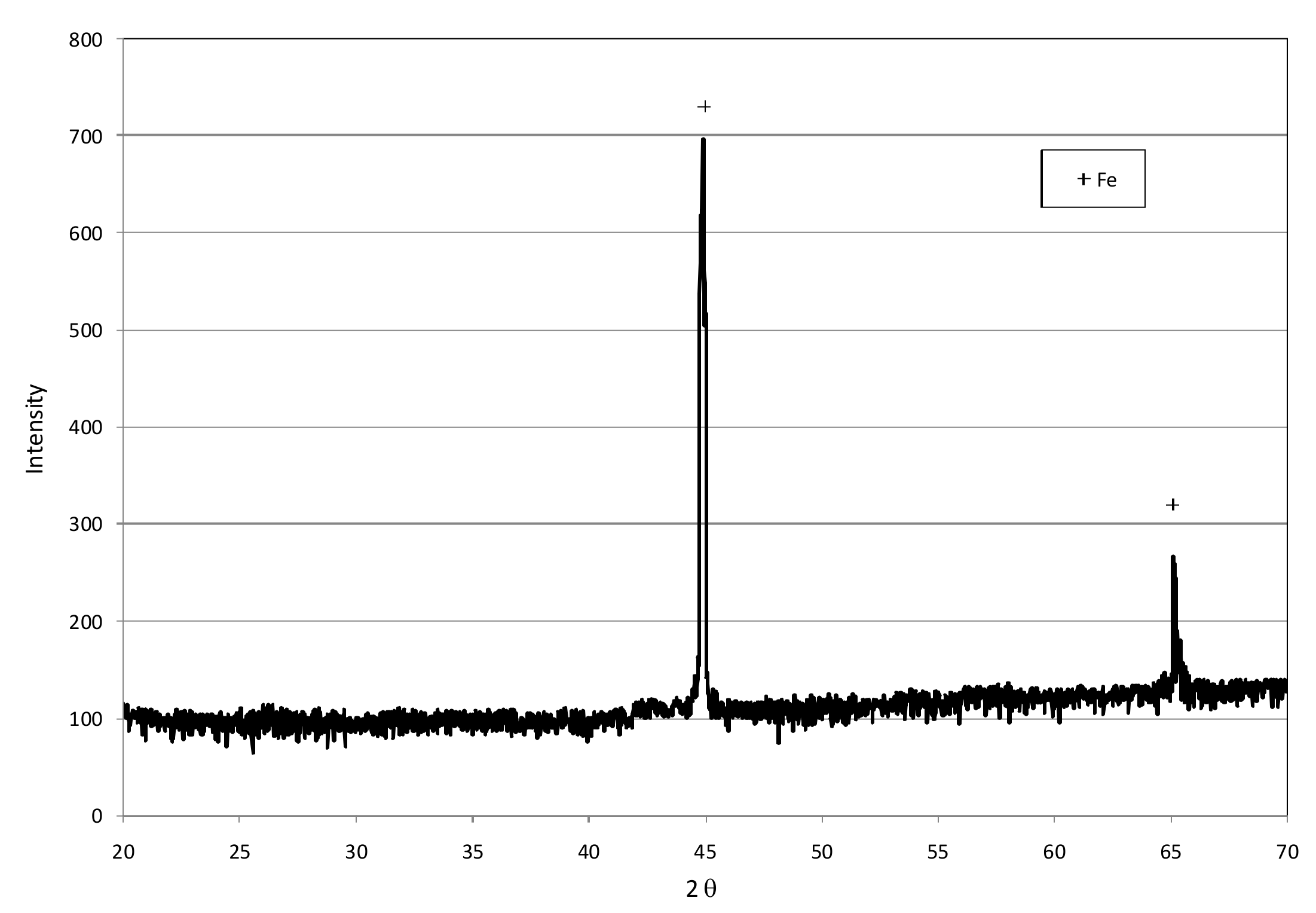
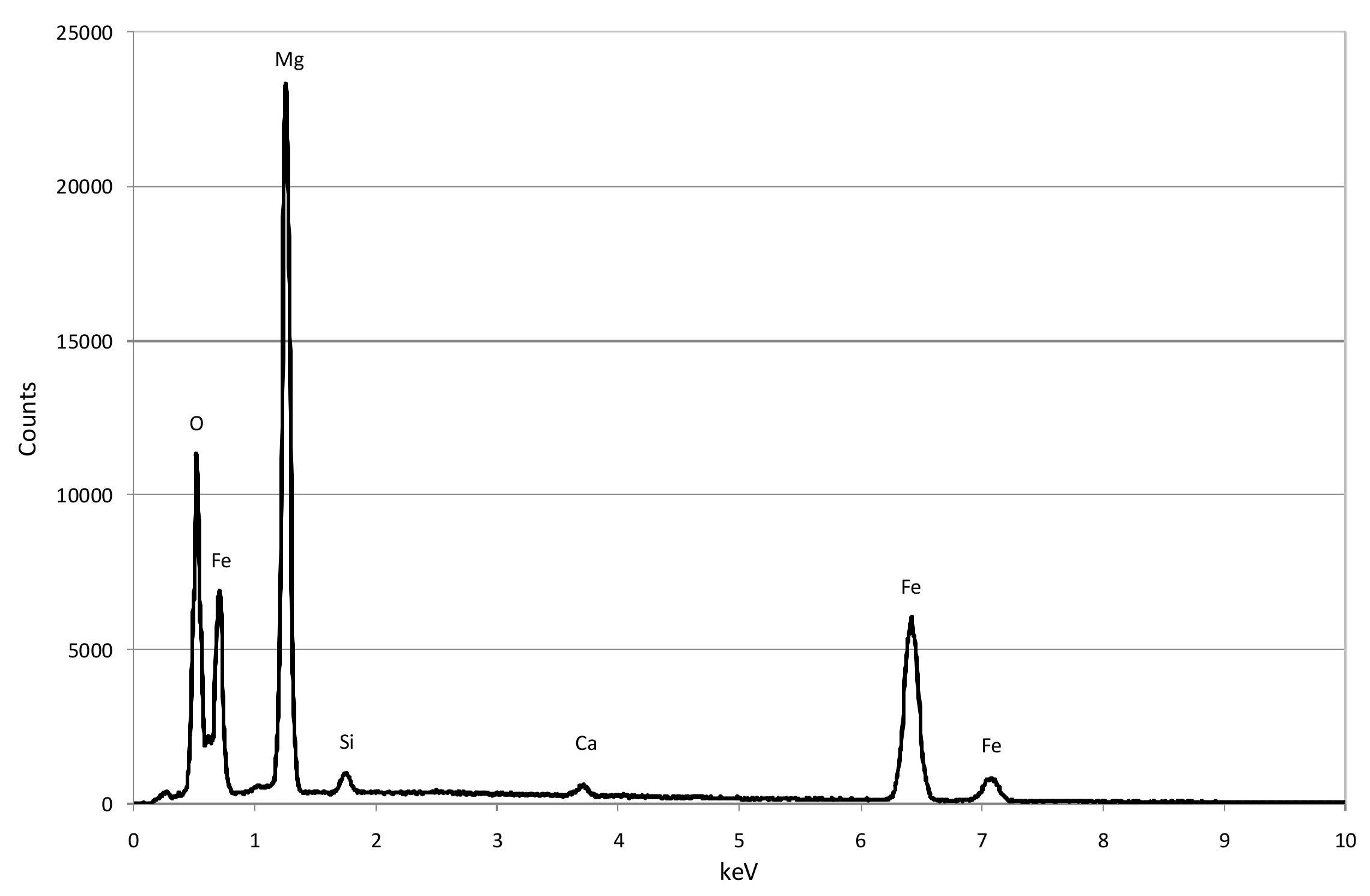
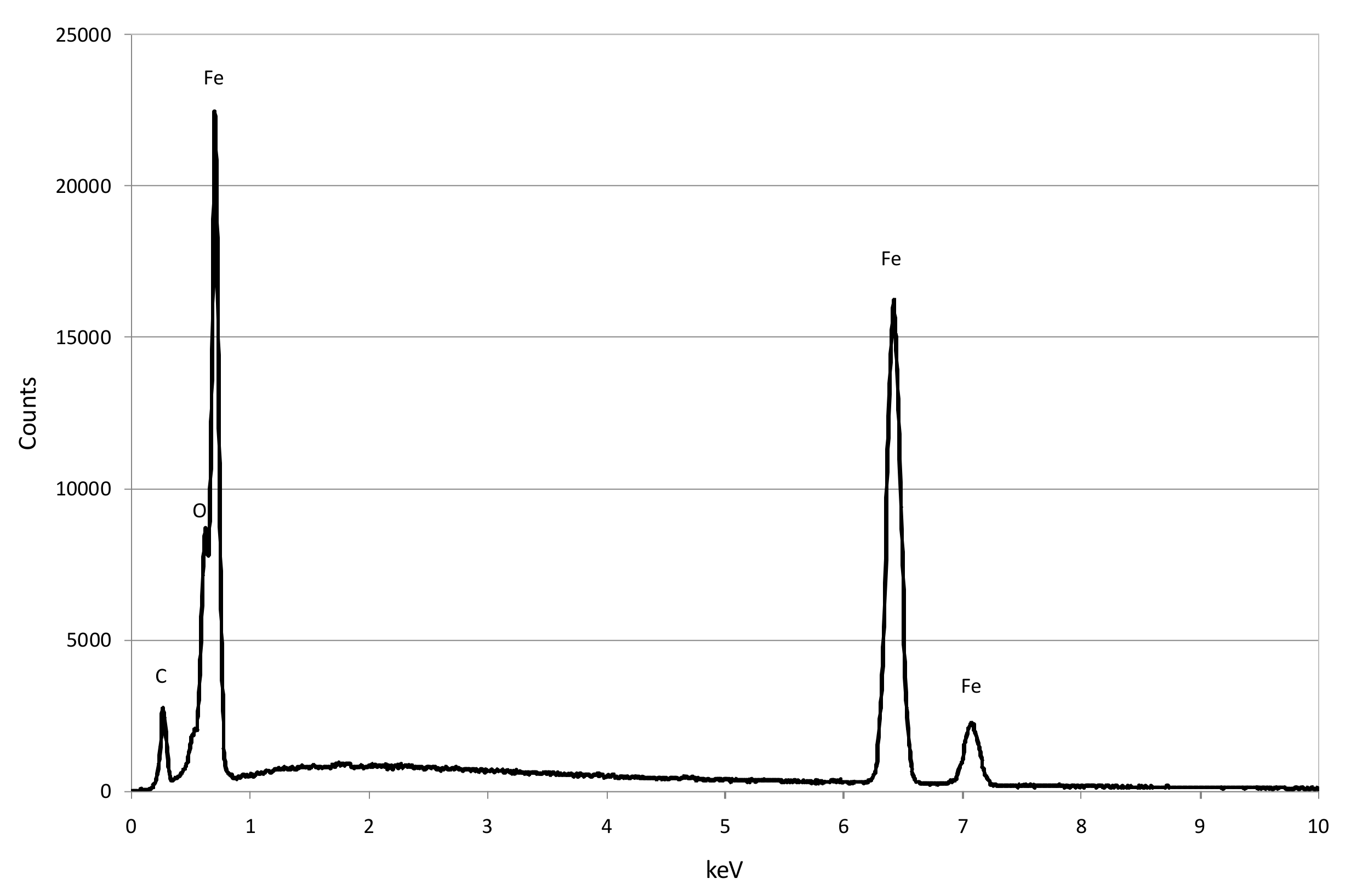
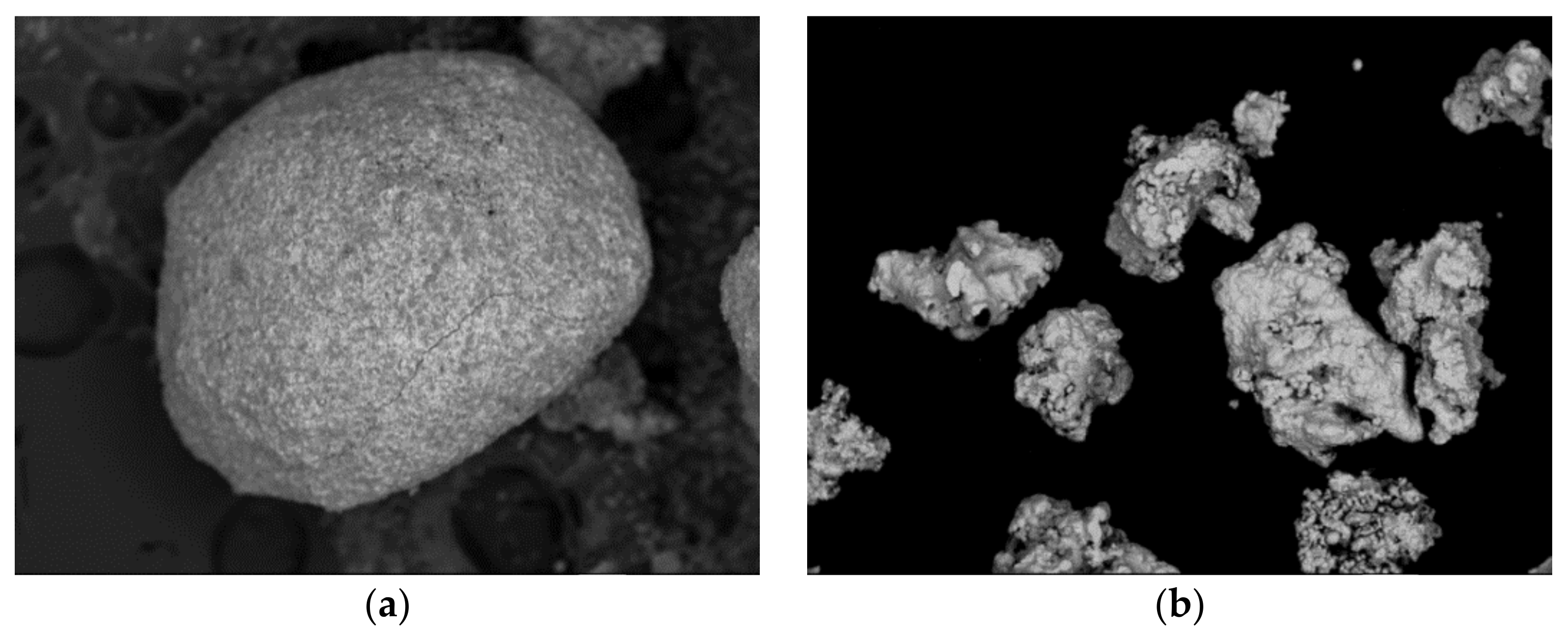
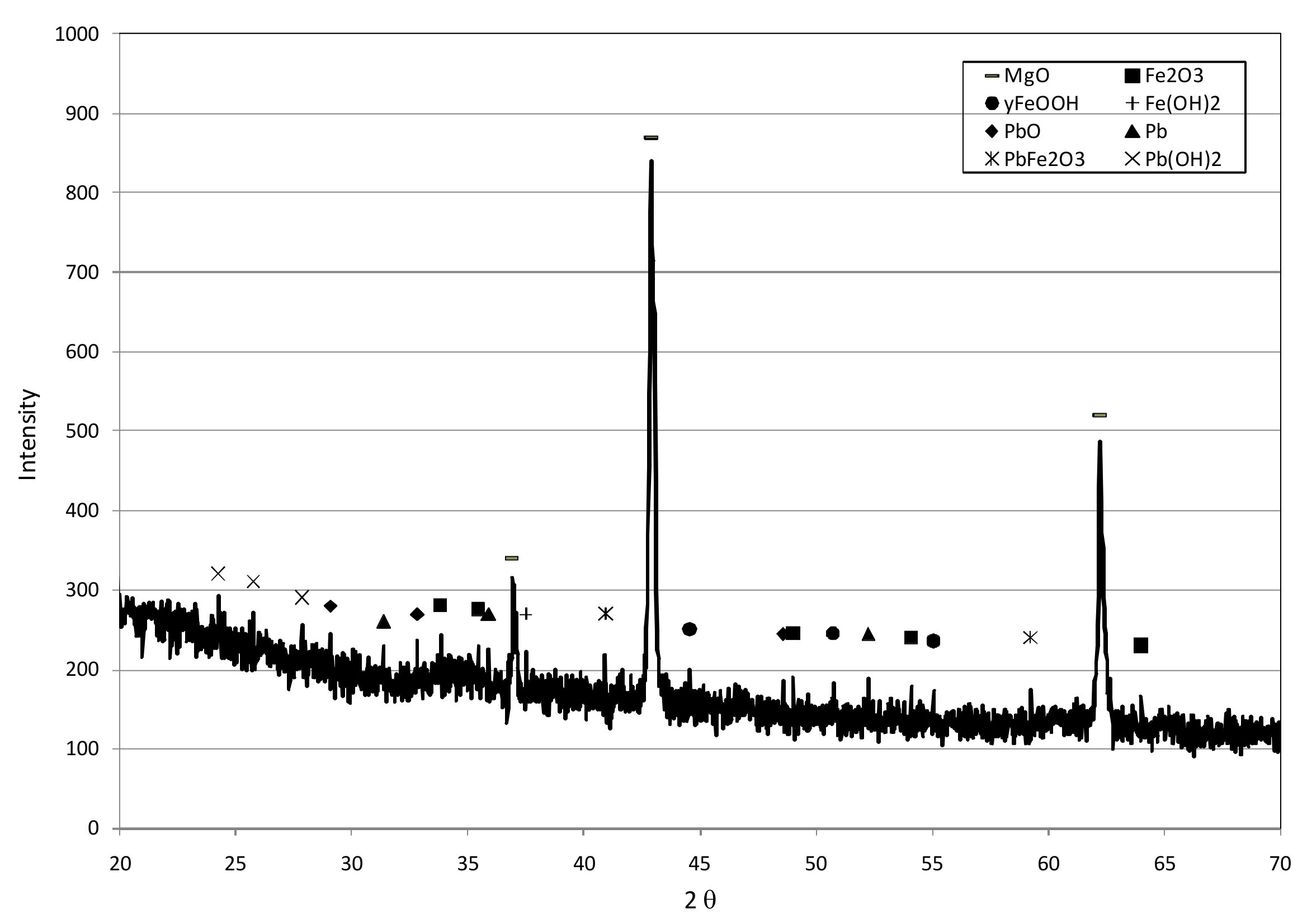
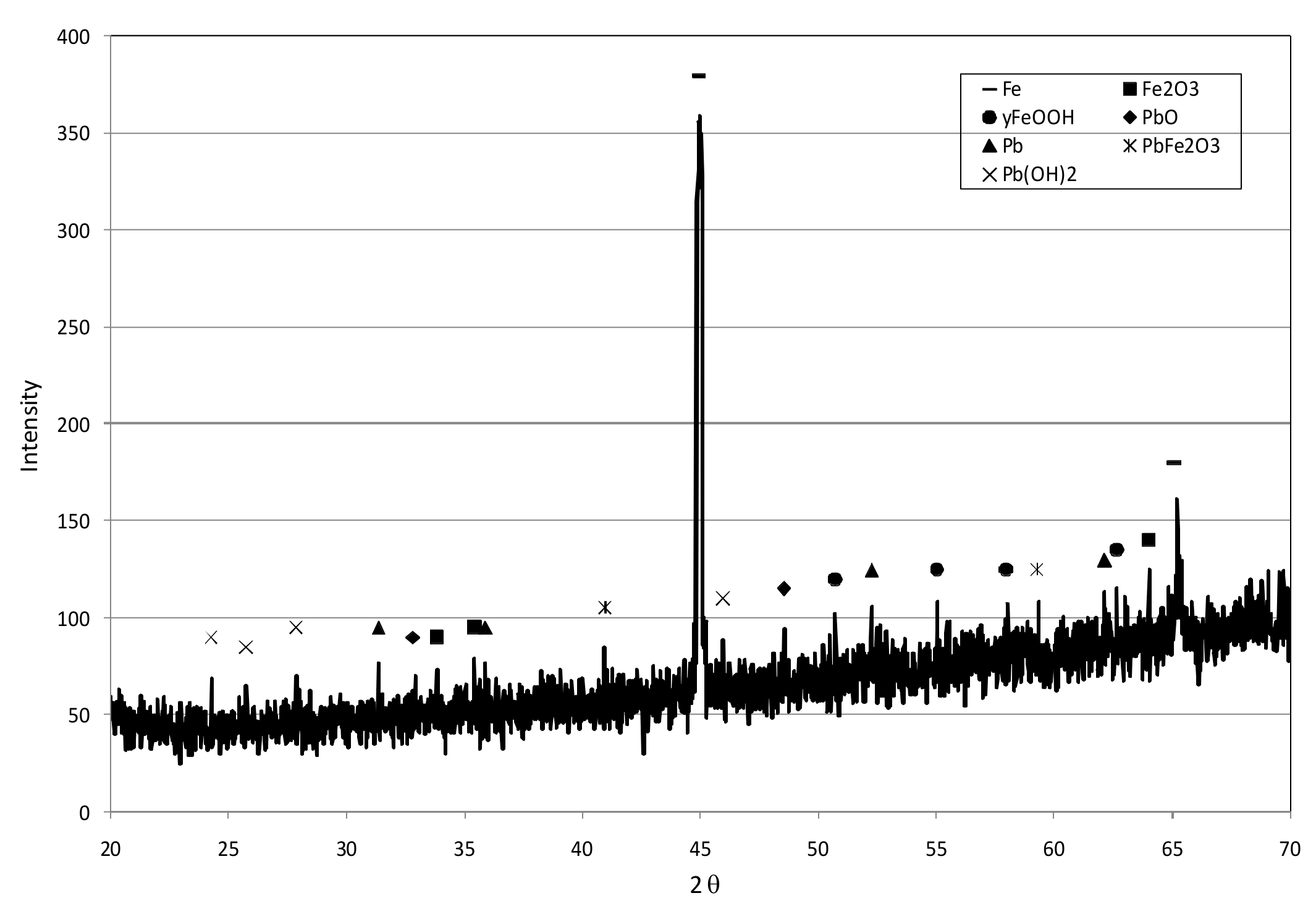
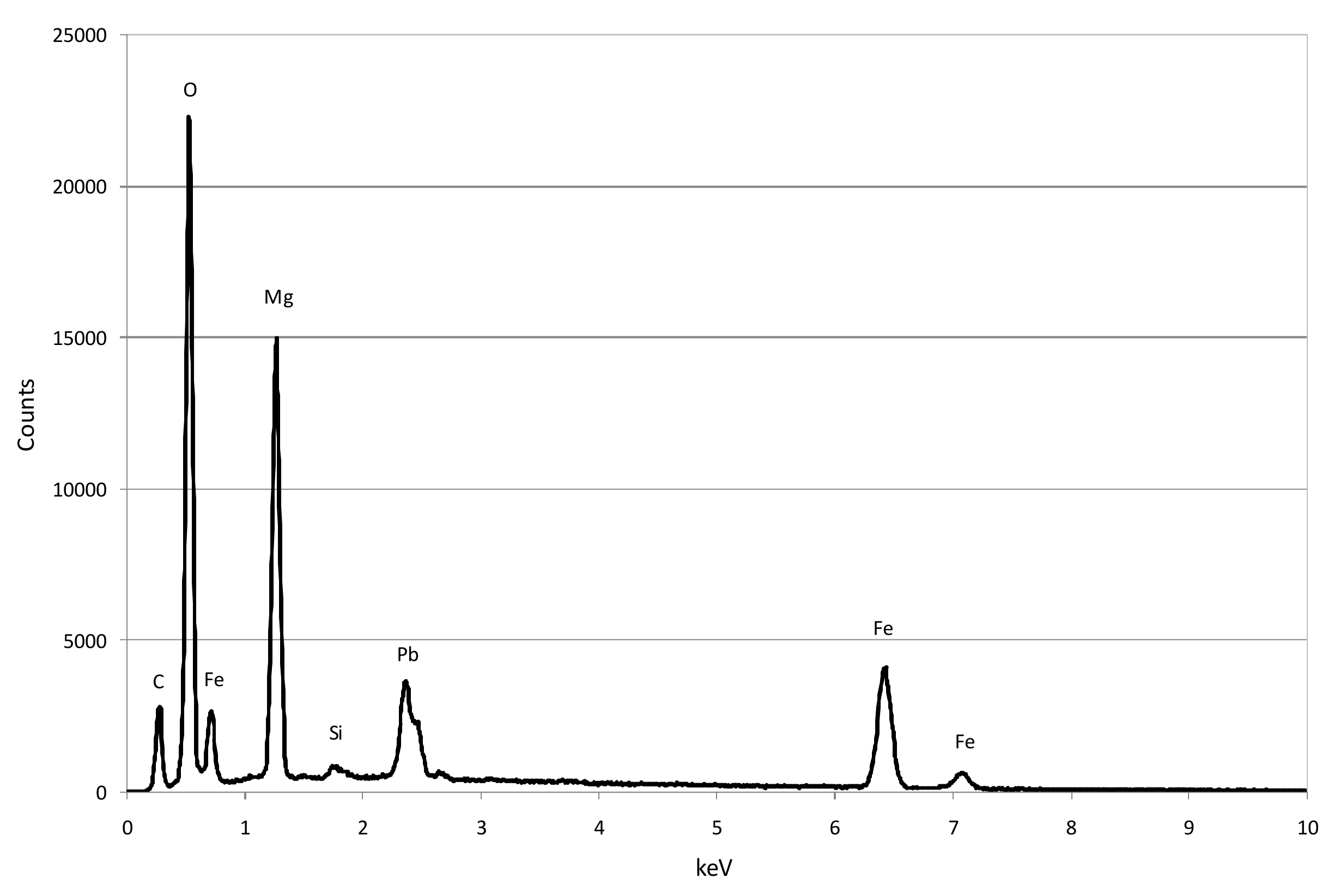
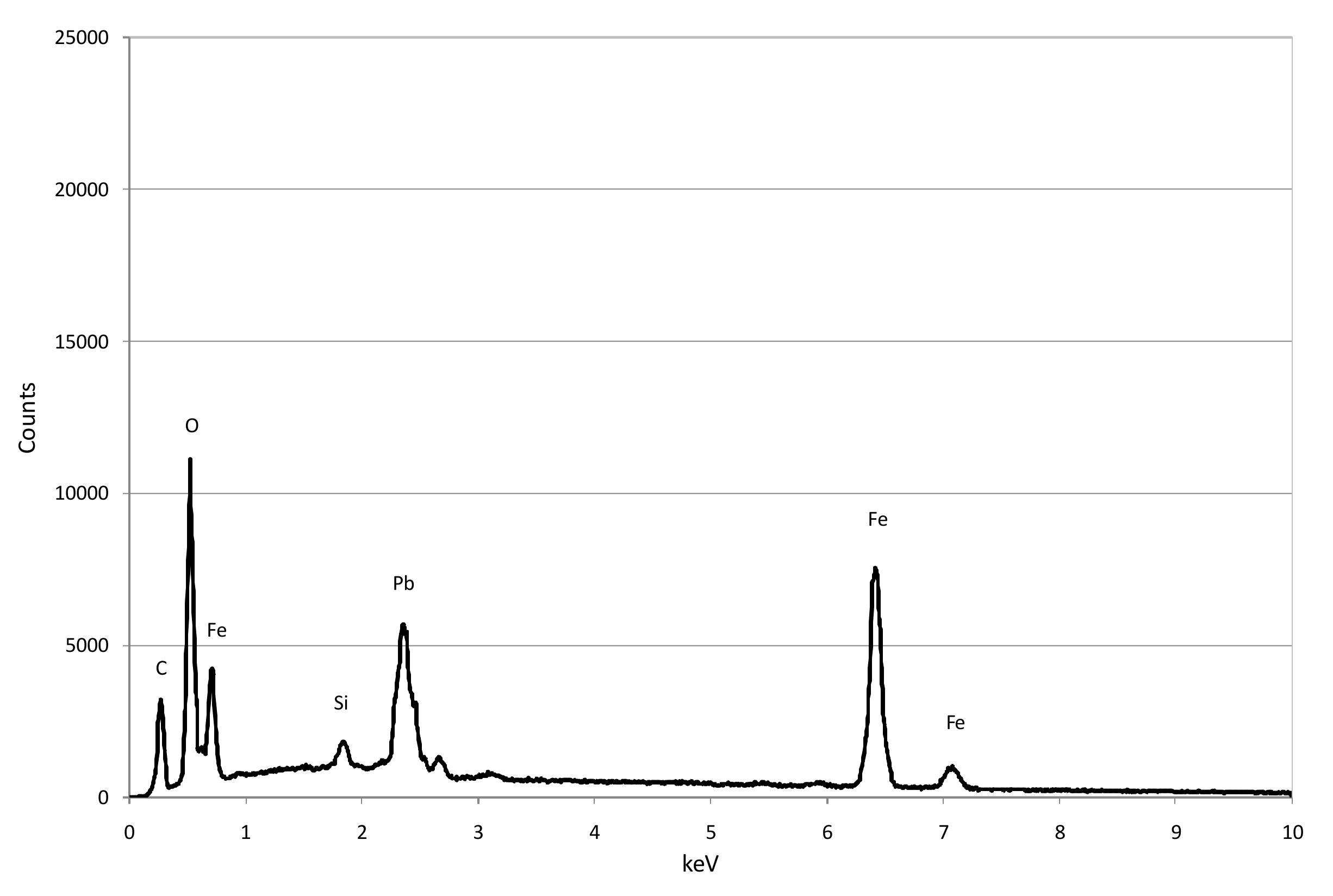
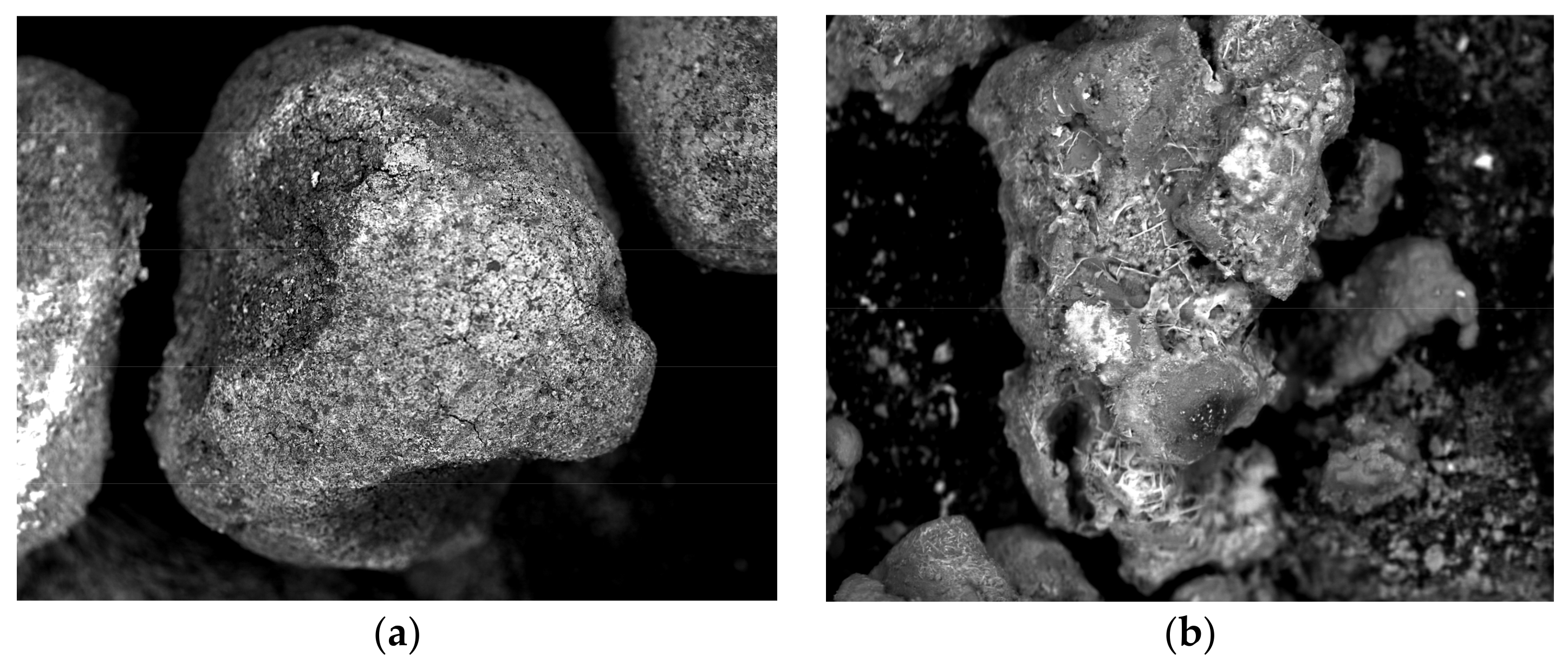
3.1. Reactive Materials
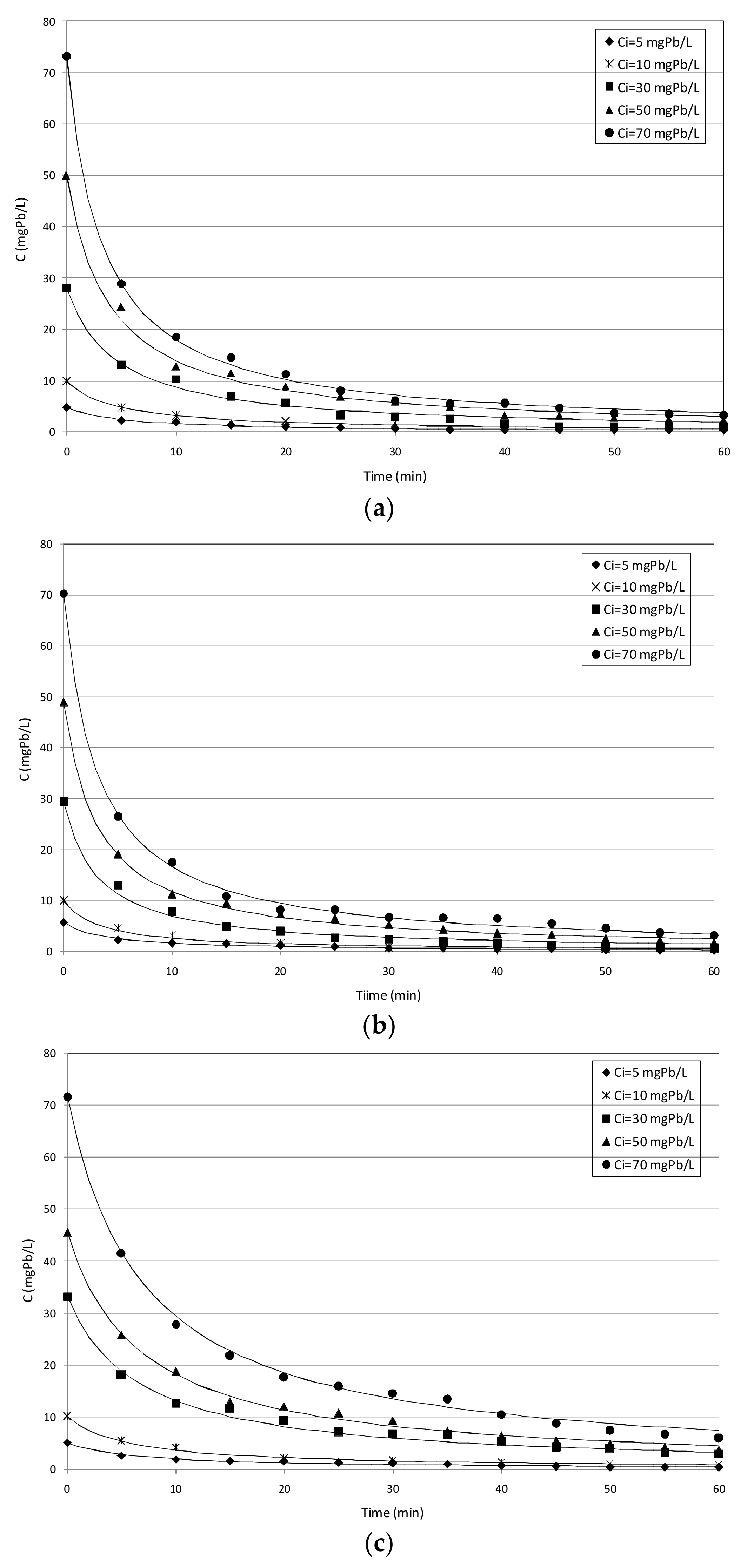
3.2. Batch Column Tests
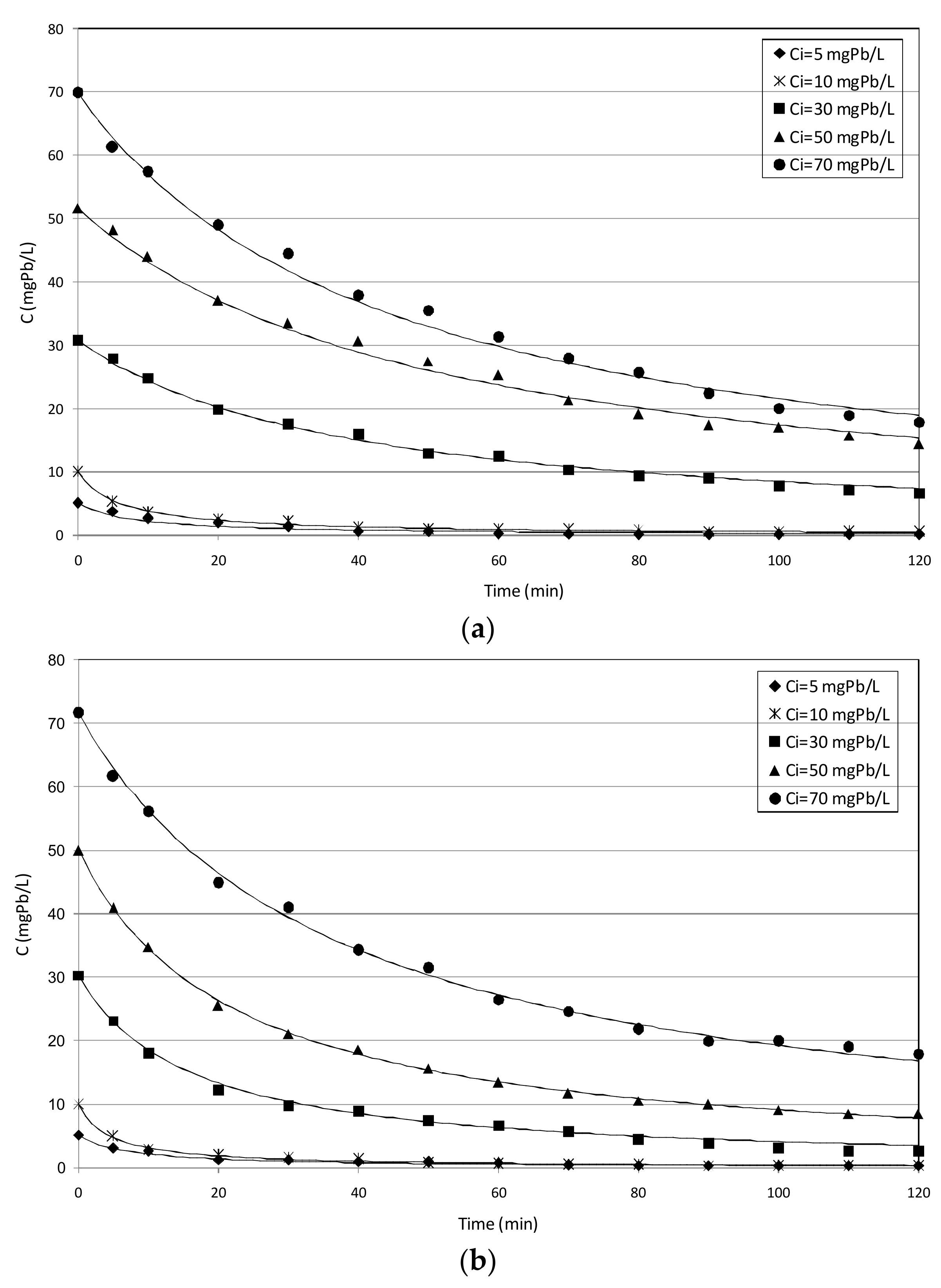
3.2.1. Tests with MgO_nZVI
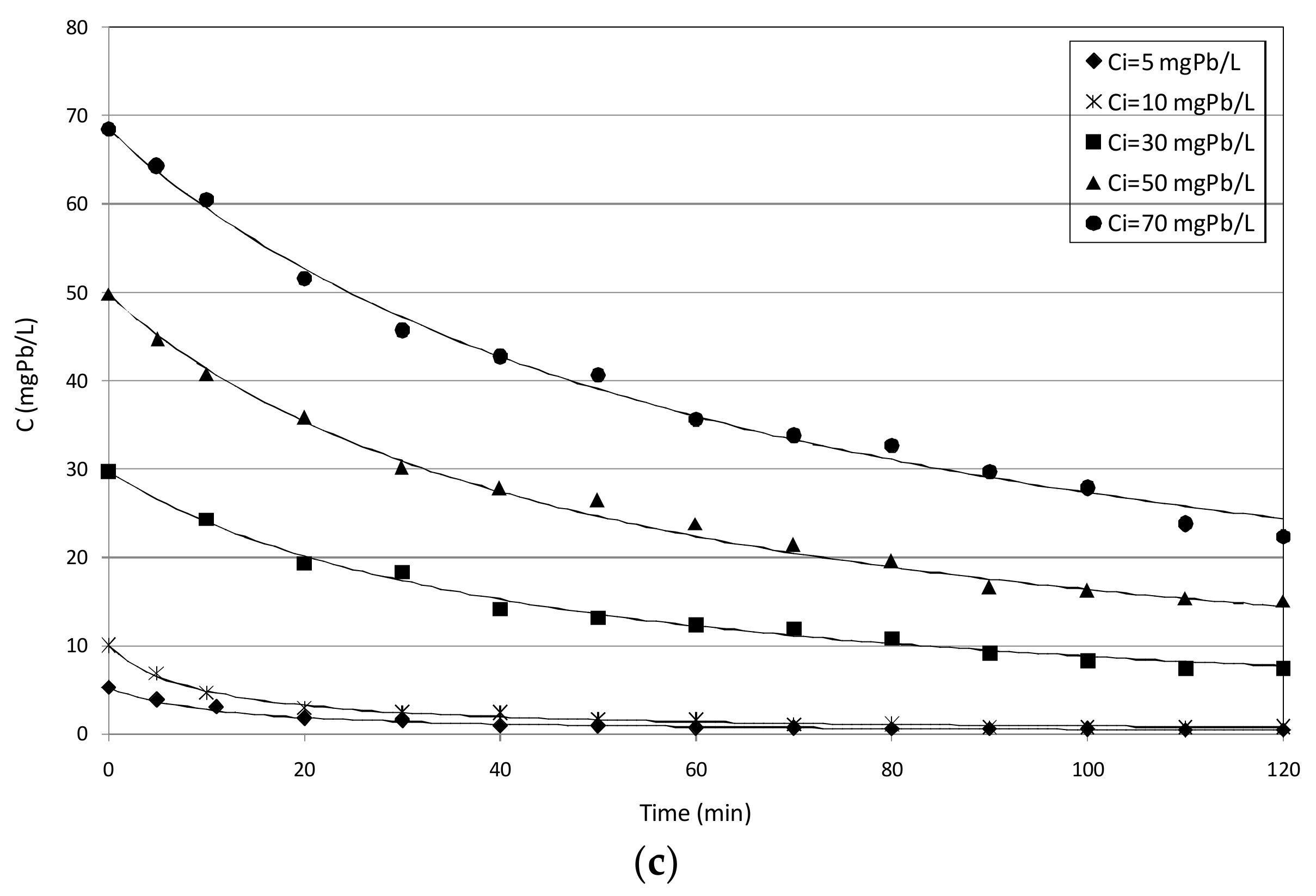
3.2.2. Tests with mZVI
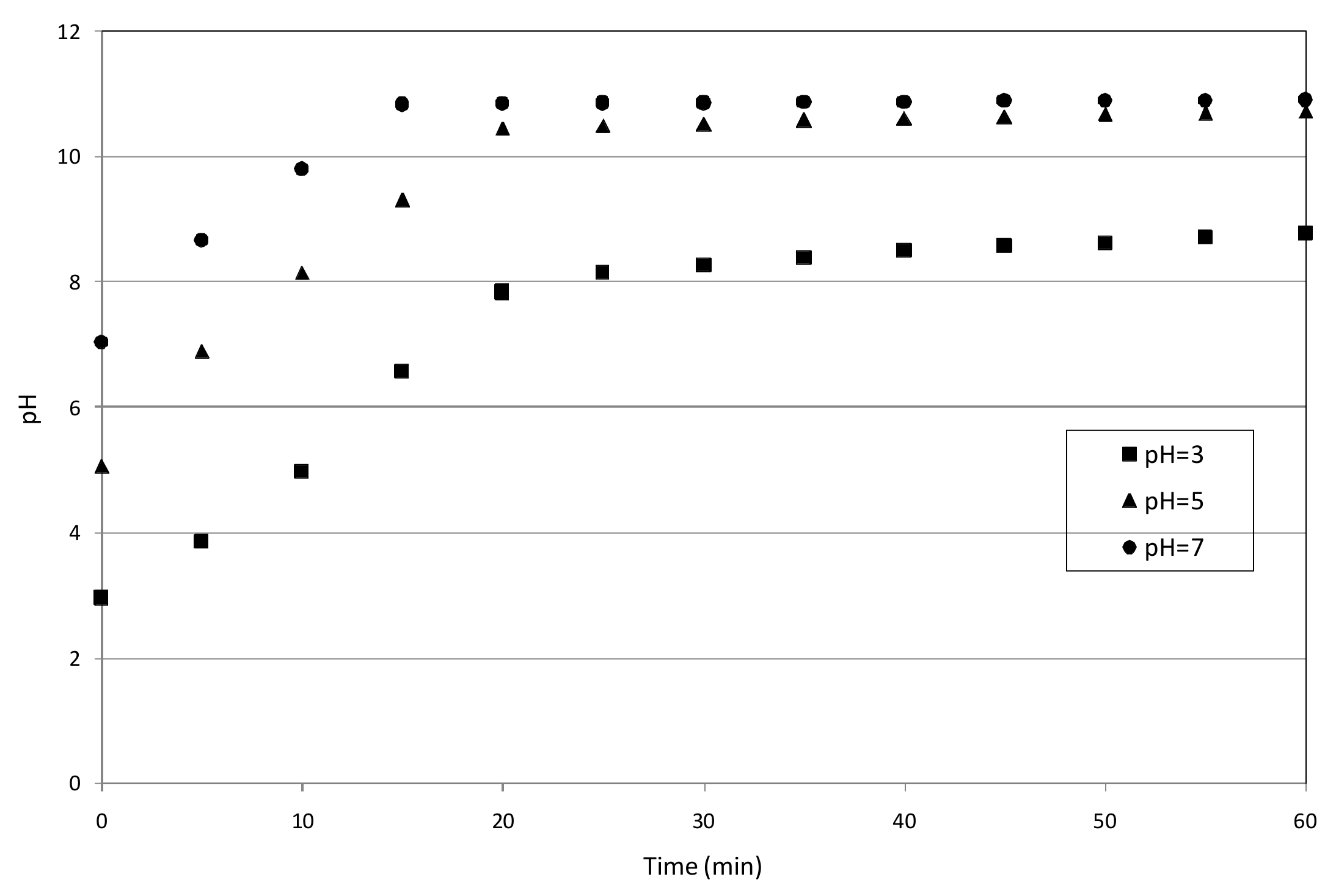
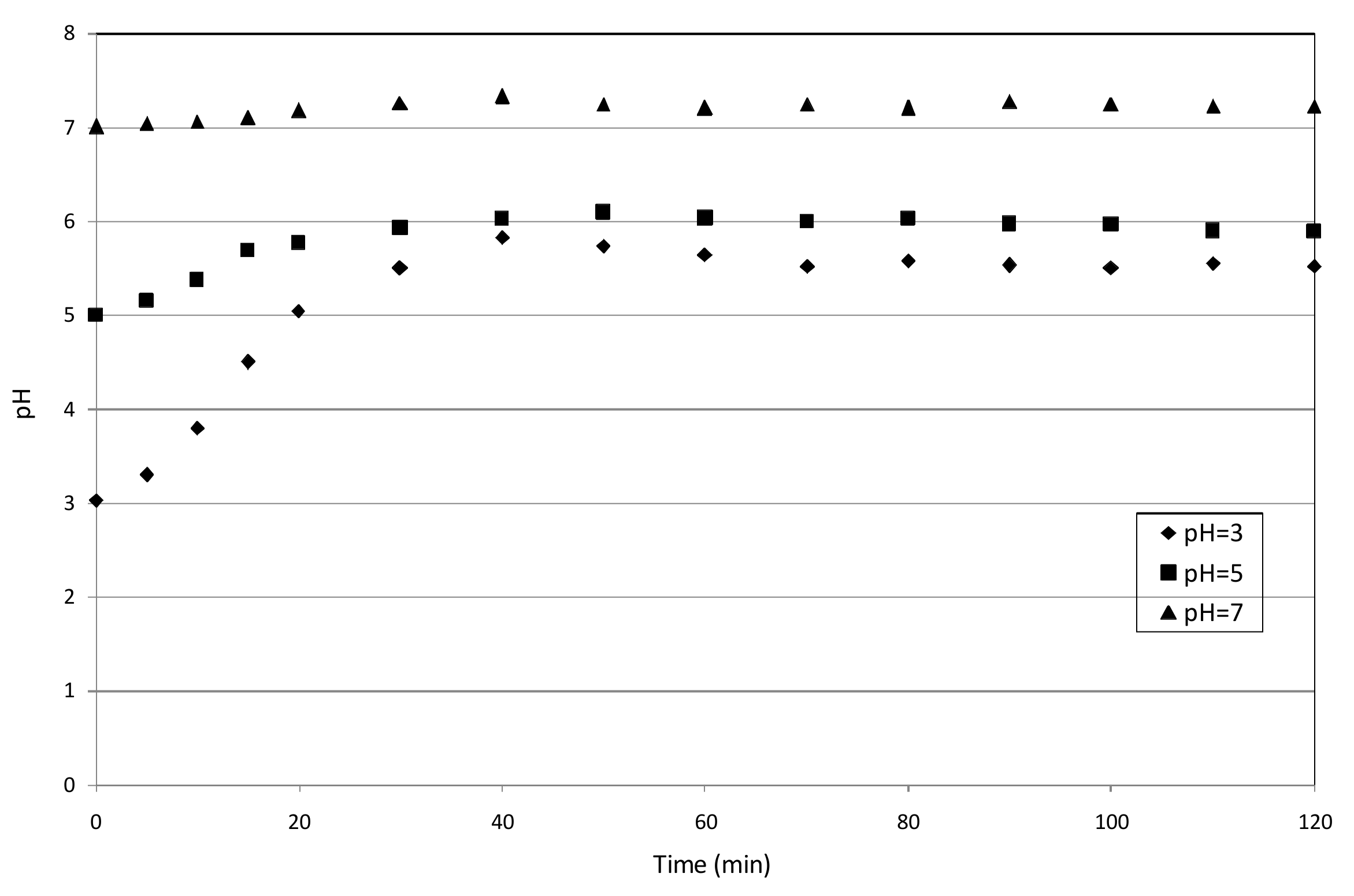
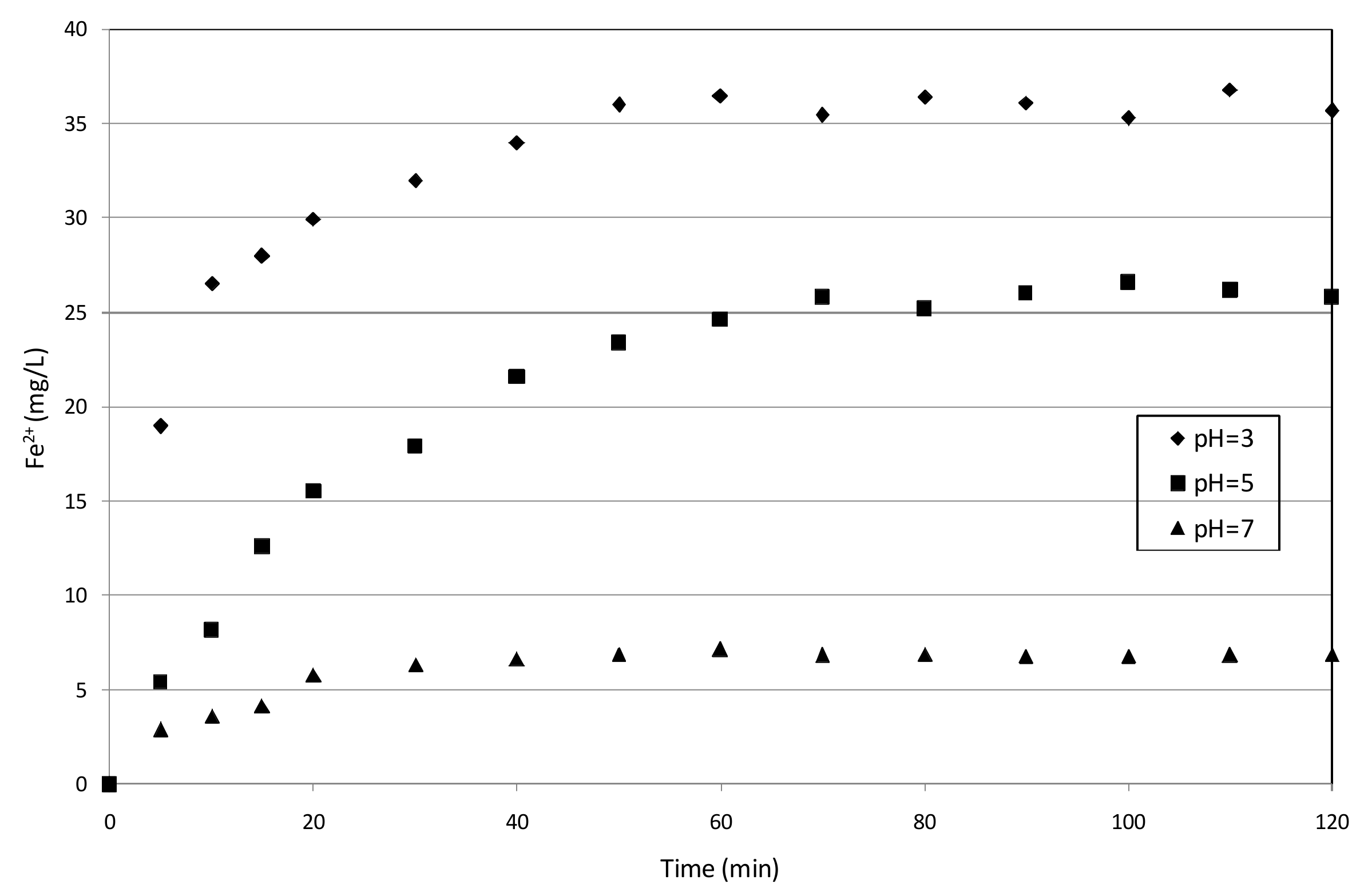
3.3. Reaction Mechanisms
3.4. Kinetic Analysis
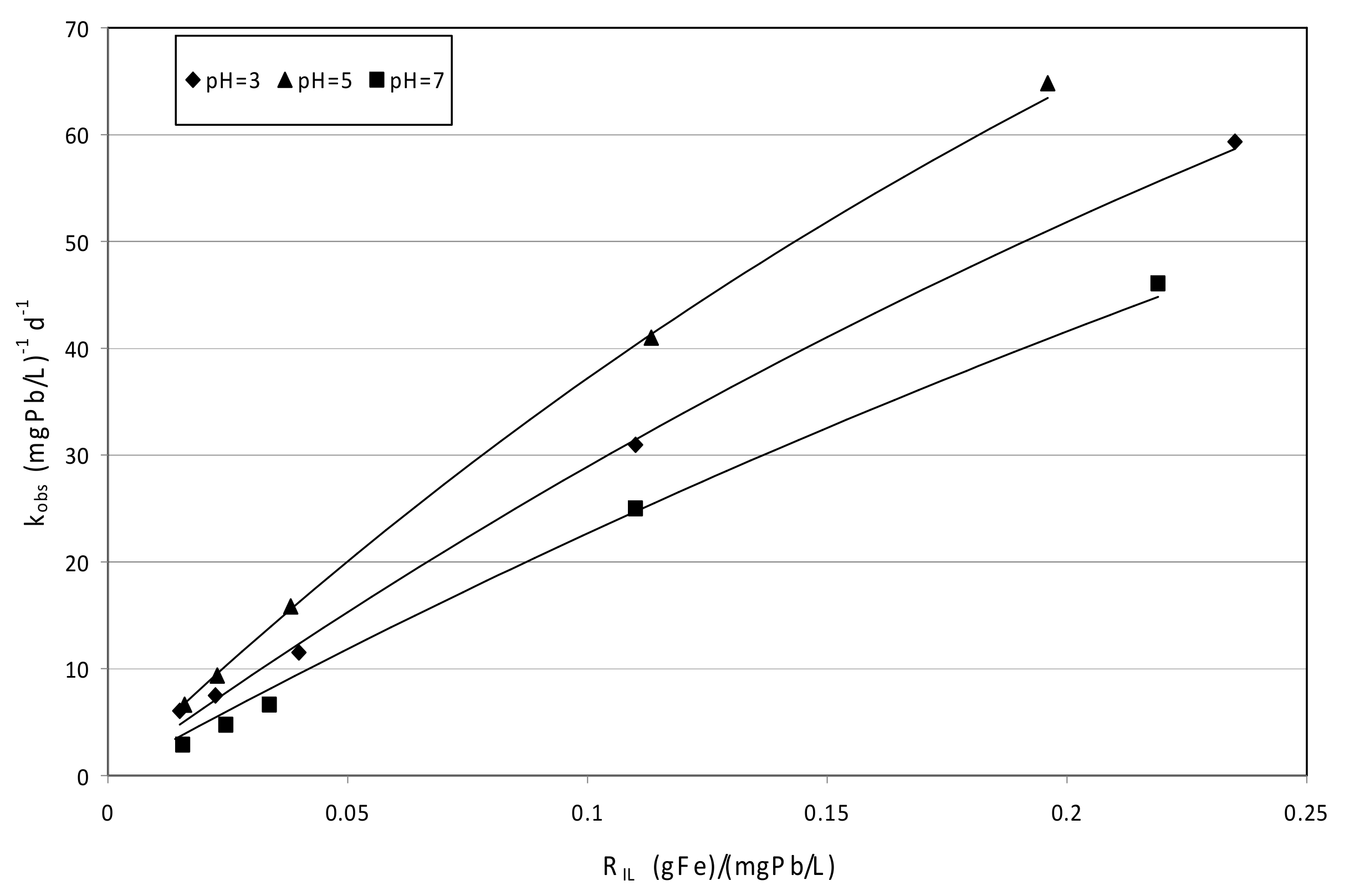
3.4.1. Tests Conducted with MgO_nZVI
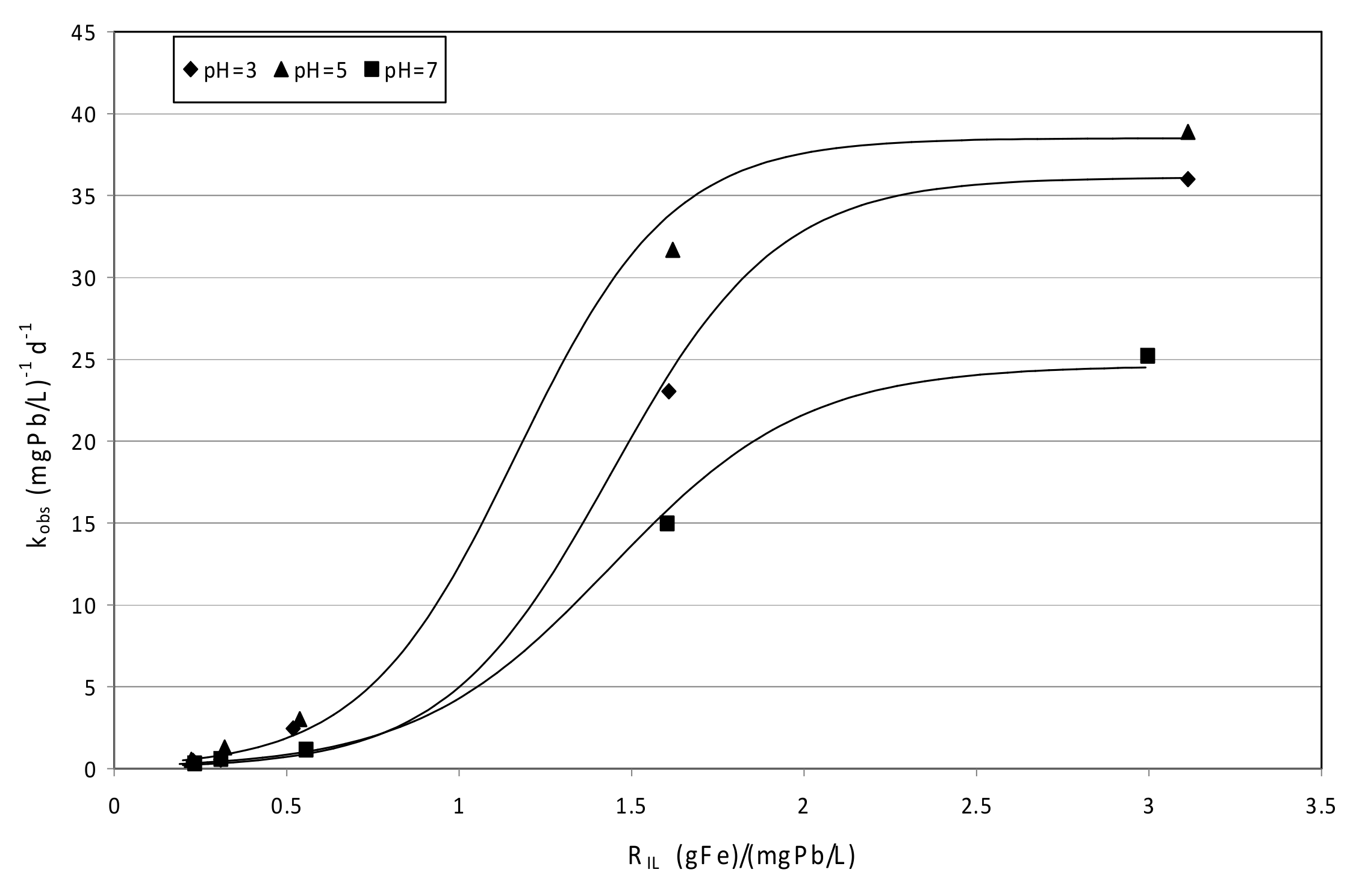
3.4.2. Tests Conducted with mZVI
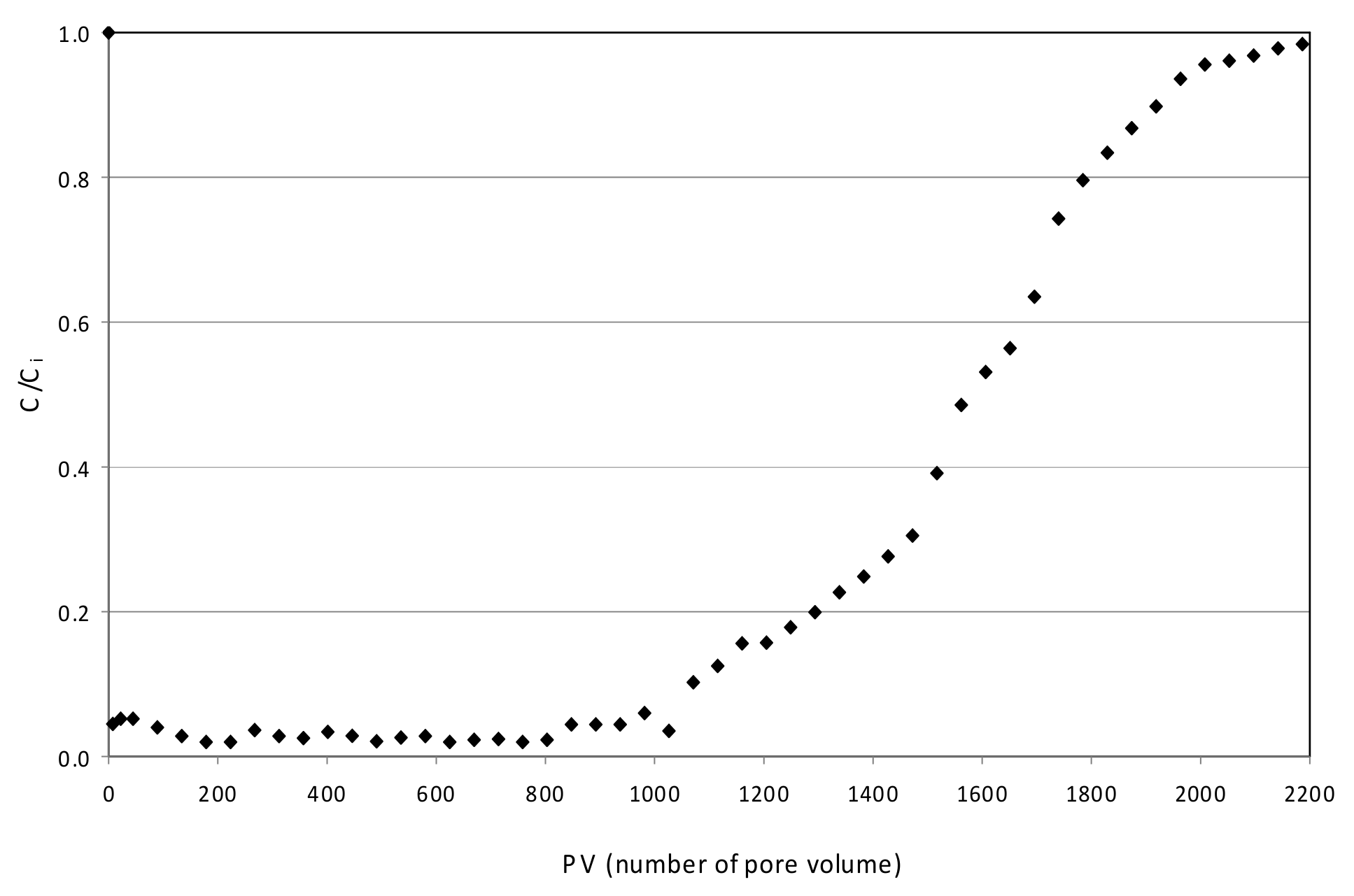
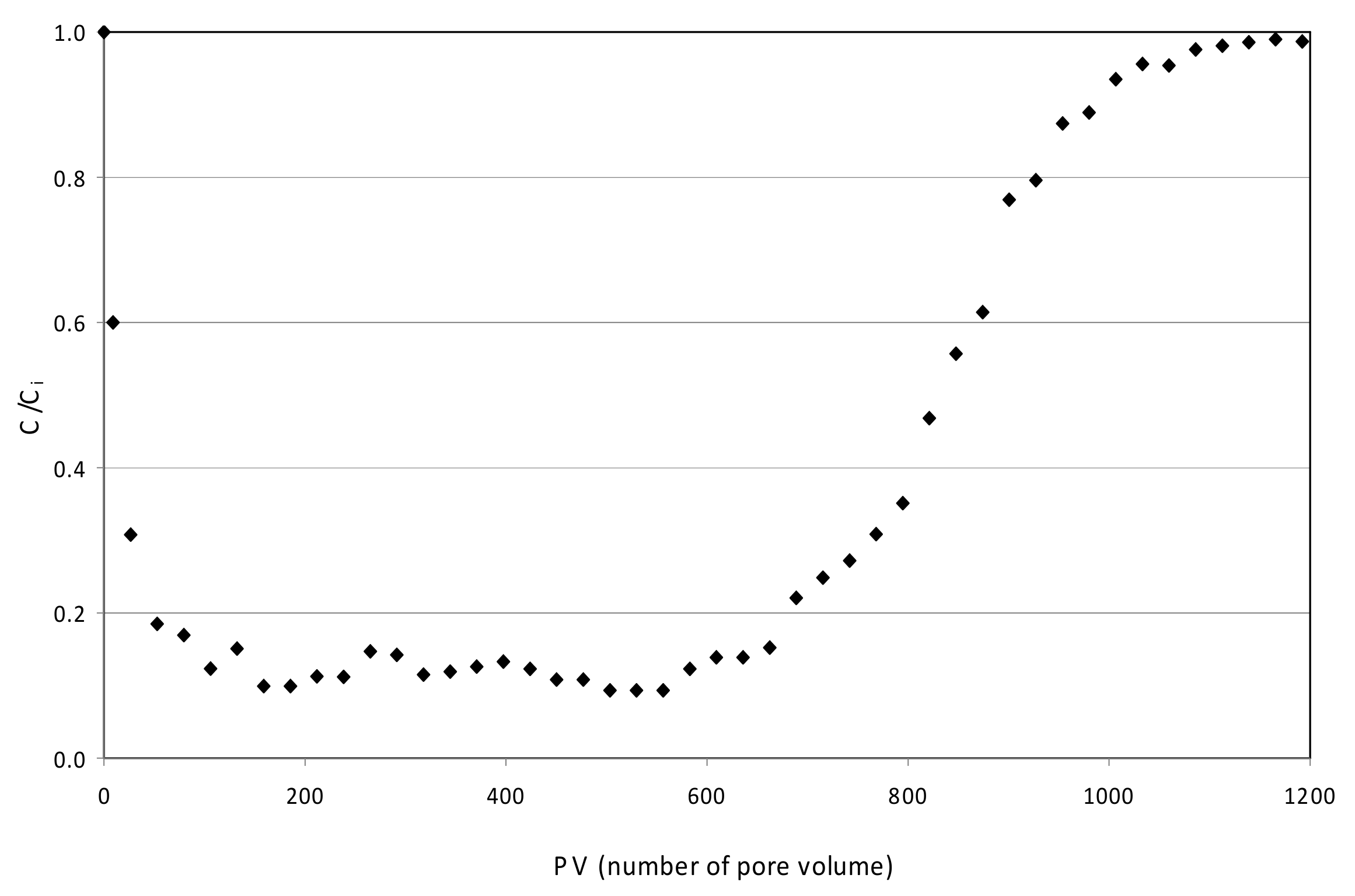
3.5. Continuous Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chandraiah, M.R. Facile synthesis of zero valent iron magnetic biochar composites for Pb(II) removal from the aqueous medium. Alexandria Eng. J. 2016, 55, 619–625. [Google Scholar]
- Fu, R.; Yang, Y.; Xu, Z.; Zhang, X.; Guo, X.; Bi, D. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015, 138, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Soleymanzadeh, M.; Salvacion, J.W.L.; SalimiVahid, F. Nanoscale Zero-Valent Iron (NZVI) supported on sineguelas waste for Pb(II) removal from aqueous solution: Kinetics, thermodynamic and mechanism. J. Colloid Interface Sci. 2014, 426, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Cundy, A.B.; Hopkinson, L.; Whitby, R.L.D. Use of iron-based technologies in contaminated land and groundwater remediation: A review. Sci. Total Environ. 2008, 400, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Meng, Q. Removal of nitrate by zero-valent iron and pillared bentonite. J. Hazard. Mater. 2010, 174, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, R.T.; Su, C.; Ford, R.G.; Paul, C.J. Chromium-Removal Processes during Groundwater Remediation by a Zerovalent Iron Permeable Reactive Barrier. Environ. Sci. Technol. 2005, 39, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Alowitz, M.J.; Scherer, M.M. Kinetics of Nitrate, Nitrite, and Cr(VI) Reduction by Iron Metal. Environ. Sci. Technol. 2002, 36, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Elliott, D.-W.; Zhang, W.-X. Zero-Valent Iron Nanoparticles for Abatement of Environmental Pollutants: Materials and Engineering Aspects. Crit. Rev. Solid State Mater. Sci. 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Liou, Y.H.; Lo, S.-L.; Lin, C.-J.; Kuan, W.H.; Weng, S.C. Chemical reduction of an unbuffered nitrate solution using catalyzed and uncatalyzed nanoscale iron particles. J. Hazard. Mater. 2005, B127, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A. Use of nanoscale zero-valent iron (NZVI) particles for chemical denitrification under different operating conditions. Metals 2015, 5, 1507–1519. [Google Scholar] [CrossRef]
- Luo, S.; Qin, P.; Shao, J.; Peng, L.; Zeng, Q.; Gu, J.-D. Synthesis of reactive nanoscale zero valent iron using rectorite supports and its application for Orange II removal. Chem. Eng. J. 2013, 223, 1–7. [Google Scholar] [CrossRef]
- Kim, H.; Hong, H.-J.; Jung, J.; Kim, S.-H.; Yang, J.-W. Degradation of trichloroethylene (TCE) by nanoscale zero-valent iron (nZVI) immobilized in alginate bead. J. Hazard. Mater. 2010, 176, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Kamala-Kannan, S.; Lee, K.-J.; Park, Y.-J.; Shea, P.J.; Lee, W.-H.; Kim, H.-M.; Oha, B.-T. Removal of Pb(II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chem. Eng. J. 2013, 217, 54–60. [Google Scholar] [CrossRef]
- Ponder, S.M.; Darag, J.G.; Mallouk, T.E. Remediation of Cr(VI) and Pb(II) Aqueous Solutions Using Supported, Nanoscale Zero-valent Iron. Environ. Sci. Technol. 2000, 34, 2564–2569. [Google Scholar] [CrossRef]
- Shu, H.-Y.; Chang, M.-C.; Chen, C.-C.; Chen, P.-E. Using resin supported nano zero-valent iron particles for decoloration of Acid Blue 113 azo dye solution. J. Hazard. Mater. 2010, 184, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, T.; Jin, Z. Stabilization of Fe0 nanoparticles with silica fume for enhanced transport and remediation of hexavalent chromium in water and soil. J. Environ. Sci. 2011, 23, 1211–1218. [Google Scholar] [CrossRef]
- Fan, M.; Yuan, P.; Zhu, J.; Chen, T.; Yuan, A.; He, H.; Chen, K.; Liu, D. Core–shell structured iron nanoparticles well dispersed on montmorillonite. J. Magn. Magn. Mater. 2009, 321, 3515–3519. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Li, J.; Hu, L.; Zheng, X. Enhanced removal of nitrate by a novel composite: Nanoscale zero valent iron supported on pillared clay. Chem. Eng. J. 2011, 171, 526–531. [Google Scholar] [CrossRef]
- Geng, B.; Jin, Z.; Li, T.; Qi, X. Kinetics of hexavalent chromium removal from water by chitosan-Fe0 nanoparticles. Chemosphere 2009, 75, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.X.; Gan, B.J.; Zhang, L. Electrochemical deposition of iron nanoneedles on titanium oxide nanotubes. Mater. Lett. 2011, 65, 2992–2994. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, H.; Peng, F.; Wang, H. Facile synthesis of porous hollow iron oxide nanoparticles supported on carbon nanotubes. Mater. Lett. 2012, 67, 245–247. [Google Scholar] [CrossRef]
- Siciliano, A. Removal of Cr(VI) from water using a new reactive material: Magnesium Oxide supported nanoscale zero-valent iron. Materials 2016, 9, 666. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.; Molloy, K.C.; Stone, F.S. Reduction of MgO-supported iron oxide: Formation and characterization of Fe/MgO catalysts. Solid State Ion. 1997, 101–103, 697–705. [Google Scholar] [CrossRef]
- Jung, K.-D.; Joo, O.-S.; Kim, C.-S. Study on the structure of Fe/MgO catalysts for H2S wet oxidation. Catal. Lett. 2002, 84, 53–57. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Kim, D.-G.; Shin, H.-S. Mechanism study of nitrate reduction by nano zero valent iron. J. Hazard. Mater. 2011, 185, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association and Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]
- Liu, T.; Wang, Z.-L.; Yan, X.; Zhang, B. Removal of mercury (II) and chromium (VI) from wastewater using a new and effective composite: Pumice-supported nanoscale zero-valent iron. Chem. Eng. J. 2014, 245, 34–40. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.; Lu, X.-Q.; Chen, Z.-L. Removal of Pb(II) from water using synthesized kaolin supported nanoscale zero-valent iron. Chem. Eng. J. 2010, 163, 243–248. [Google Scholar] [CrossRef]
- Pojananukij, N.; Wantala, K.; Neramittagapong, S.; Neramittagapong, A. Equilibrium, kinetics, and mechanism of lead adsorption using zero-valent iron coated on diatomite. Desal. Water Treat. 2016, 57, 18475–18489. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Y.; Zhang, Y.; Zhang, C.; Li, X.; Chen, Z.; Huang, J.; Li, X.; Flores, G.; Kamon, M. Column test-based optimization of the permeable reactive barrier (PRB) technique for remediating groundwater contaminated by landfill leachates. J. Contam. Hydrol. 2014, 168, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Li, J.; Bao, Q.; Li, L.; Guan, X. Role of dissolved oxygen in metal(loid) removal by zerovalent iron at different pH: Its dependence on the removal mechanisms. RSC Adv. 2016, 6, 50144. [Google Scholar] [CrossRef]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–108. [Google Scholar] [CrossRef]
- Jeen, S.-W.; Blowes, D.W.; Gillham, R.W. Performance evaluation of granular iron for removing hexavalent chromium under different geochemical conditions. J. Contam. Hydrol. 2008, 95, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Pratt, A.R.; Blowes, D.W.; Ptacek, C.J. Products of chromate reduction on proposed subsurface remediation material. Environ. Sci. Technol. 1997, 31, 2492–2498. [Google Scholar] [CrossRef]
- Manning, B.A.; Kiser, J.R.; Kwon, H.; Kanel, S.R. Spectroscopic investigation of Cr(III)- and Cr(VI)-treated nanoscale zerovalent Iron. Environ. Sci. Technol. 2007, 41, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; De Rosa, S. Experimental formulation of a kinetic model describing the nitrification process in biological aerated filters filled with plastic elements. Environ. Technol. 2015, 36, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-N.; Lin, Y.-M.; Zhang, X.; Chen, Z.-L. Synthesis, characterization and kinetics of bentonite supported nZVI for the removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2011, 171, 612–617. [Google Scholar] [CrossRef]
- Siciliano, A.; De Rosa, S. An experimental model of COD abatement in MBBR based on biofilm growth dynamic and on substrates’ removal kinetics. Environ. Technol. 2016, 37, 2058–2071. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; Stillitano, M.A.; De Rosa, S. Biogas production from wet olive mill wastes pretreated with hydrogen peroxide in alkaline conditions. Renew. Energy 2016, 85, 903–916. [Google Scholar] [CrossRef]
- Cheng, F.; Muftikian, R.; Fernando, Q.; Korte, N. Reduction of nitrate to ammonia by zero-valent iron. Chemosphere 1997, 35, 2689–2695. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Kwiatkowska-Malina, J.; Malina, G.; Kasela, T. Geochemical modelling for predicting the long-term performance of zeolite-PRB to treat lead contaminated groundwater. J. Contam. Hydrol. 2015, 177–178, 76–84. [Google Scholar] [CrossRef] [PubMed]

| pH | 3 | 5 | 7 | |
|---|---|---|---|---|
| MgO_nZVI | Α (gFe)−1(mgPb/L) | 2.33 | 3.14 | 1.81 |
| α (molFe)−1(mmolPb/L) | 0.629 | 0.848 | 0.489 | |
| kmax (mgPb/L)−1d−1 | 139.1 | 138 | 136.9 | |
| kmax (mmolPb/m3)−1d−1 | 28.8 | 28.6 | 28.4 | |
| mZVI | β (gFe)−1(mgPb/L) | 4.15 | 4.46 | 3.54 |
| β (molFe)−1(mmolPb/L) | 1.12 | 1.204 | 0.956 | |
| kmax (mgPb/L)−1d−1 | 36.1 | 38.5 | 24.6 | |
| kmax (mmolPb/m3)−1d−1 | 7.5 | 8 | 5.1 | |
| k0 (mgPb/L)−1d−1 | 0.091 | 0.21 | 0.15 | |
| kmax (mmolPb/m3)−1d−1 | 0.019 | 0.043 | 0.031 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siciliano, A.; Limonti, C. Nanoscopic Zero-Valent Iron Supported on MgO for Lead Removal from Waters. Water 2018, 10, 404. https://doi.org/10.3390/w10040404
Siciliano A, Limonti C. Nanoscopic Zero-Valent Iron Supported on MgO for Lead Removal from Waters. Water. 2018; 10(4):404. https://doi.org/10.3390/w10040404
Chicago/Turabian StyleSiciliano, Alessio, and Carlo Limonti. 2018. "Nanoscopic Zero-Valent Iron Supported on MgO for Lead Removal from Waters" Water 10, no. 4: 404. https://doi.org/10.3390/w10040404
APA StyleSiciliano, A., & Limonti, C. (2018). Nanoscopic Zero-Valent Iron Supported on MgO for Lead Removal from Waters. Water, 10(4), 404. https://doi.org/10.3390/w10040404






