How Does Season Affect Passage Performance and Fatigue of Potamodromous Cyprinids? An Experimental Approach in a Vertical Slot Fishway
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Ethical Statement
References
- Branco, P.; Segurado, P.; Santos, J.M.; Pinheiro, P.J.; Ferreira, M.T. Does longitudinal connectivity loss affect the distribution of freshwater fish? Ecol. Eng. 2012, 48, 70–78. [Google Scholar] [CrossRef]
- Calles, O.; Greenberg, L. Connectivity is a two-way street—The need for a holistic approach to fish passage problems in regulated rivers. River Res. Appl. 2009, 25, 1268–1286. [Google Scholar] [CrossRef]
- Poff, N.L.; Hart, D.D. How dams vary and why it matters for the emerging science of dam removal. BioScience 2002, 52, 659–668. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Sabater, S. (Eds.) Global Change and River Ecosystems-Implications for Structure, Function and Ecosystem Services; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2011; Volume 215. [Google Scholar]
- Katopodis, C.; Williams, J.G. The development of fish passage research in a historical context. Ecol. Eng. 2012, 28, 407–417. [Google Scholar] [CrossRef]
- Puertas, J.; Cea, L.; Bermúdez, M.; Pena, L.; Rodríguez, Á.; Rabuñal, J.R.; Balairón, L.; Lara, Á.; Aramburu, E. Computer application for the analysis and design of vertical slot fishways in accordance with the requirements of the target species. Ecol. Eng. 2012, 48, 51–60. [Google Scholar] [CrossRef]
- DVWK FAO. Fish Passes: Design, Dimensions, and Monitoring; DVWK FAO: Rome, Italy, 2002. [Google Scholar]
- Santos, J.M.; Silva, A.; Katopodis, C.; Pinheiro, P.; Pinheiro, A.; Bochechas, J.; Ferreira, M.T. Ecohydraulics of pool-type fishways: Getting past the barriers. Ecol. Eng. 2012, 48, 38–50. [Google Scholar] [CrossRef]
- Sanz-Ronda, F.J.; Bravo-Córdoba, F.J.; Fuentes-Pérez, J.F.; Castro-Santos, T. Ascent ability of brown trout, Salmo trutta, and two Iberian cyprinids—Iberian barbel, Luciobarbus bocagei, and northern straight-mouth nase, Pseudochondrostoma duriense—in a vertical slot fishway. Knowl. Manag. Aquat. Ecosyst. 2016, 417, 10. [Google Scholar] [CrossRef]
- Brodersen, J.; Nilsson, P.A.; Hansson, L.A.; Skov, C.; Brönmark, C. Condition-dependent individual decision-making determines cyprinid partial migration. Ecology 2008, 89, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, C.M.; Almeida, P.R.; Neves, T.; Mateus, C.S.; Costa, J.L.; Quintella, B.R. Effects of flow regulation on the movement patterns and habitat use of a potamodromous cyprinid species. Ecohydrology 2016, 9, 326–340. [Google Scholar] [CrossRef]
- Domenici, P. (Ed.) Fish Locomotion: An Eco-Ethological Perspective; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Lennox, R.J.; Chapman, J.M.; Souliere, C.M.; Tudorache, C.; Wikelski, M.; Metcalfe, J.D.; Cooke, S.J. Conservation physiology of animal migration. Conserv. Physiol. 2016, 4, cov072. [Google Scholar] [CrossRef] [PubMed]
- Castro-Santos, T.; Sanz-Ronda, F.J.; Ruiz-Legazpi, J. Breaking the speed limit—comparative sprinting performance of brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 2013, 70, 280–293. [Google Scholar] [CrossRef]
- Bizzotto, P.M.; Godinho, A.L.; Vono, V.; Kynard, B.; Godinho, H.P. Influence of seasonal, diel, lunar, and other environmental factors on upstream fish passage in the Igarapava Fish Ladder, Brazil. Ecol. Freshw. Fish. 2009, 18, 461–472. [Google Scholar] [CrossRef]
- Cooke, S.J.; Hinch, S.G. Improving the reliability of fishway attraction and passage efficiency estimates to inform fishway engineering, science, and practice. Ecol. Eng. 2013, 58, 123–132. [Google Scholar] [CrossRef]
- Castro-Santos, T. Adaptive fishway design: A framework and rationale for effective evaluations. Bundesanstalt für Gewässerkunde Veranstaltungen 2012, 7, 76–89. [Google Scholar]
- Roscoe, D.W.; Hinch, S.G. Effectiveness monitoring of fish passage facilities: Historical trends, geographic patterns and future directions. Fish Fish 2010, 11, 12–33. [Google Scholar] [CrossRef]
- Lucas, M.C.; Baras, E. Migration of Freshwater Fishes; Blackwell Science Ltd.: London, UK, 2001. [Google Scholar]
- Hatry, C.; Thiem, J.D.; Binder, T.R.; Hatin, D.; Dumont, P.; Stamplecoskie, K.M.; Molina, J.M.; Smokorowski, K.E.; Cooke, S.J. Comparative Physiology and Relative Swimming Performance of Three Redhorse (Moxostoma spp.) Species: Associations with Fishway Passage Success. Physiol. Biochem. Zool. 2013, 87, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Pon, L.B.; Hinch, S.G.; Cooke, S.J.; Patterson, D.A.; Farrell, A.P. A comparison of the physiological condition, and fishway passage time and success of migrant adult sockeye salmon at Seton River Dam, British Columbia, under three operational water discharge rates. N. Am. J. Fish. Manag. 2009, 29, 1195–1205. [Google Scholar] [CrossRef]
- Weber, J.M.; Choi, K.; Gonzalez, A.; Omlin, T. Metabolic fuel kinetics in fish: Swimming, hypoxia and muscle membranes. J. Exp. Biol. 2016, 219, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Pon, L.B.; Hinch, S.G.; Suski, C.D.; Patterson, D.A.; Cooke, S.J. The effectiveness of tissue biopsy as a means of assessing the physiological consequences of fishway passage. River Res. Appl. 2012, 28, 1266–1274. [Google Scholar] [CrossRef]
- Kieffer, J.D. Limits to exhaustive exercise in fish. Compar. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 126, 161–179. [Google Scholar] [CrossRef]
- Farrell, A.P. Comparisons of swimming performance in rainbow trout using constant acceleration and critical swimming speed tests. J. Fish Biol. 2008, 72, 693–710. [Google Scholar] [CrossRef]
- Cooke, S.J.; Hinch, S.G.; Donaldson, M.R.; Clark, T.D.; Eliason, E.J.; Crossin, G.T.; Raby, G.D.; Jeffries, K.M.; Lapointe, M.; Miller, K.; et al. Conservation physiology in practice: How physiological knowledge has improved our ability to sustainably manage Pacific salmon during up-river migration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Ferreira, M.T.; Godinho, F.N.; Bochechas, J. Efficacy of a nature-like bypass channel in a Portuguese lowland river. J. Appl. Ichthyol. 2005, 21, 381–388. [Google Scholar] [CrossRef]
- Romão, F.; Quaresma, A.L.; Branco, P.; Santos, J.M.; Amaral, S.; Ferreira, M.T.; Katopodis, C.; Pinheiro, A.N. Passage performance of two cyprinids with different ecological traits in a fishway with distinct vertical slot configurations. Ecol. Eng. 2017, 108, 180–188. [Google Scholar]
- Romão, F.; Branco, P.; Quaresma, A.L.; Amaral, S.; Pinheiro, A.N. Effectiveness of a multi-slot vertical slot fishway versus a standard vertical slot fishway for potamodromous cyprinids. Hydrobiologia 2018. [Google Scholar] [CrossRef]
- Branco, P.; Santos, J.M.; Katopodis, C.; Pinheiro, A.; Ferreira, M.T. Pool-type fishways: Two different morpho-ecological cyprinid species facing plunging and streaming flows. PLoS ONE 2013, 8, e65089. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, C.; Quintella, B.R.; Silva, A.T.; Mateus, C.S.; Romão, F.; Branco, P.; Ferreira, M.T.; Almeida, P.R. Use of electromyogram telemetry to assess the behavior of the Iberian barbel (Luciobarbus bocagei Steindachner, 1864) in a pool-type fishway. Ecol. Eng. 2013, 51, 191–202. [Google Scholar] [CrossRef]
- Rajaratnam, N.; Katopodis, C.; Solanki, S. New designs for vertical slot fishways. Can. J. Civ. Eng. 1992, 19, 402–414. [Google Scholar] [CrossRef]
- Rajaratnam, N.; Van der Vinne, G.; Katopodis, C. Hydraulics of vertical slot fishways. J. Hydraul. Eng. 1986, 112, 909–927. [Google Scholar] [CrossRef]
- Rodríguez, T.T.; Agudo, J.P.; Mosquera, L.P.; González, E.P. Evaluating vertical-slot fishway designs in terms of fish swimming capabilities. Ecol. Eng. 2006, 27, 37–48. [Google Scholar] [CrossRef]
- Benitez, J.P.; Matondo, B.N.; Dierckx, A.; Ovidio, M. An overview of potamodromous fish upstream movements in medium-sized rivers, by means of fish passes monitoring. Aquat. Ecol. 2015, 49, 481–497. [Google Scholar] [CrossRef]
- European Committee for Standardization 2003 CEN. Water Quality—Sampling of Fish with Electricity; Document EN 14011:2003 E; The European Commission—Legis: Brussels, Belgium, 2003; Volume 327, pp. 1–72. [Google Scholar]
- Doadrio, I. Ictiofauna Continental Española: Bases para su Seguimiento; Ministerio de Medio Ambiente y Medio Rural y Marino, Centro de Publicaciones: Madrid, Spain, 2011. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Berlin, Germany, 2007. [Google Scholar]
- Plaut, I. Critical swimming speed: Its ecological relevance. Compar. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 131, 41–50. [Google Scholar] [CrossRef]
- Mateus, C.S.; Quintella, B.R.; Almeida, P.R. The critical swimming speed of Iberian barbel Barbus bocagei in relation to size and sex. J. Fish Biol. 2008, 73, 1783–1789. [Google Scholar] [CrossRef]
- Day Length Photoperiod Data. Available online: http://www.sunrise-and-sunset.com/ (accessed on 25 September 2016).
- Pitcher, T.J.; Parrish, J.K. Functions of shoaling behaviour in teleosts. In Behaviour of Teleost Fishes; Pitcher, T.J., Ed.; Chapman and Hall: London, UK, 1993; pp. 363–439. [Google Scholar]
- Boyd, G.L.; Parsons, G.R. Swimming Performance and Behavior of Golden Shiner, Notemigonus crysoleucas, While Schooling. Copeia 1998, 1998, 467–471. [Google Scholar] [CrossRef]
- Johansen, J.L.; Vaknin, R.; Steffensen, J.F.; Domenici, P. Kinematics and energetic benefits of schooling in the labriform fish, striped surfperch Embiotoca lateralis. Mar. Ecol. Progress Ser. 2010, 420, 221–229. [Google Scholar] [CrossRef]
- Ficke, A.D.; Myrick, C.A.; Jud, N. The Swimming and Jumping Ability of Three Small Great Plains Fishes: Implications for Fishway Design. Trans. Am. Fish. Soc. 2011, 140, 1521–1531. [Google Scholar] [CrossRef]
- Wagner, R.L.; Makrakis, S.; Castro-Santos, T.; Makrakis, M.C.; Dias, J.H.P.; Belmont, R.F. Passage performance of long-distance upstream migrants at a large dam on the Paraná River and the compounding effects of entry and ascent. Neotrop. Ichthyol. 2012, 10, 785–795. [Google Scholar] [CrossRef]
- Schmutz, S.; Giefing, C.; Wiesner, C. The efficiency of a nature-like bypass channel for pike-perch (Stizostedion lucioperca) in the Marchfeldkanalsystem. Hydrobiologia 1998, 371, 355. [Google Scholar] [CrossRef]
- Haro, A.; Castro-Santos, T.; Noreika, J.; Odeh, M. Swimming performance of upstream migrant fishes in open-channel flow: A new approach to predicting passage through velocity barriers. Can. J. Fish. Aquat. Sci. 2004, 61, 1590–1601. [Google Scholar] [CrossRef]
- Roscoe, D.W.; Hinch, S.G.; Cooke, S.J.; Patterson, D.A. Fishway passage and post-passage mortality of up-river migrating sockeye salmon in the Seton River, British Columbia. River Res. Appl. 2011, 27, 693–705. [Google Scholar] [CrossRef]
- Stoot, L.J.; Cairns, N.A.; Cull, F.; Taylor, J.J.; Jeffrey, J.D.; Morin, F.; Mandelman, J.W.; Clark, T.D.; Cooke, S.J. Use of portable blood physiology point-of-care devices for basic and applied research on vertebrates: A review. Conserv. Physiol. 2014, 2, cou011. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Lennox, R.J.; Katopodis, C.; Cooke, S.J. Is there evidence for flow variability as an organism-level stressor in fluvial fish? J. Ecohydraul. 2017, 2, 68–83. [Google Scholar] [CrossRef]
- Cooke, S.J.; Crossin, G.T.; Patterson, D.A.; English, K.K.; Hinch, S.G.; Young, J.L.; Alexander, R.; Healey, M.C.; Van Der Kraak, G.; Farrell, A.P. Coupling non-invasive physiological assessments with telemetry to understand inter-individual variation in behaviour and survivorship of sockeye salmon: Development and validation of a technique. J. Fish Biol. 2005, 67, 1342–1358. [Google Scholar] [CrossRef]
- White, A.J.; Schreer, J.F.; Cooke, S.J. Behavioral and physiological responses of the congeneric largemouth (Micropterus salmoides) and smallmouth bass (M. dolomieu) to various exercise and air exposure durations. Fish. Res. 2008, 89, 9–16. [Google Scholar] [CrossRef]
- Taylor, M.K.; Cook, K.V.; Hasler, C.T.; Schmidt, D.C.; Cooke, S.J. Behaviour and physiology of mountain whitefish (Prosopium williamsoni) relative to short-term changes in river flow. Ecol. Freshw. Fish. 2012, 21, 609–616. [Google Scholar] [CrossRef]
- Brown, J.A.; Watson, J.; Bourhill, A.; Wall, T. Evaluation and use of the Lactate Pro, a portable lactate meter, in monitoring the physiological well-being of farmed Atlantic cod (Gadus morhua). Aquaculture 2008, 285, 135–140. [Google Scholar] [CrossRef]
- Anderson, M.J.; Robinson, J. Permutation tests for linear models. Aust. N. Z. J. Stat. 2001, 43, 75–88. [Google Scholar] [CrossRef]
- Anderson, M.; Gorley, R.N.; Clarke, R.K. Permanova+ for Primer: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Kemp, P.S. Bridging the gap between fish behaviour, performance and hydrodynamics: An ecohydraulics approach to fish passage research. River Res. Appl. 2012, 28, 403–406. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, A.; Granado-Lorencio, C. Spawning period and migration of three species of cyprinids in a stream with Mediterranean regimen (SW Spain). J. Fish Biol. 1992, 41, 545–556. [Google Scholar] [CrossRef]
- Hammer, C. Fatigue and exercise tests with fish. Compar. Biochem. Physiol. Part A Physiol. 1995, 112, 1–20. [Google Scholar] [CrossRef]
- Peake, S.J. Swimming Performance and Behaviour of Fish Species Endemic to Newfoundland and Labrador: A Literature Review for The Purpose of Establishing Design and Water Velocity Criteria for Fishways and Culverts; Fisheries and Oceans Canada: St. John’s, NL, Canada, 2008; Volume 2843, v+52p. [Google Scholar]
- Jonsson, B.; Jonsson, N. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J. Fish Biol. 2009, 75, 2381–2447. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, J.; Currie, S.; Tufts, B. Effects of environmental temperature on the metabolic and acid-base responses of rainbow trout to exhaustive exercise. J. Exp. Biol. 1994, 194, 299–317. [Google Scholar]
- Baudoin, J.M.; Burgun, V.; Chanseau, M.; Larinier, M.; Ovidio, M.; Sremski, W.; Steinbach, P.; Voegtle, B. Assessing the Passage of Obstacles by Fish. Concepts, Design and Application; The National Agency for Water and Aquatic Environments: Vincennes, France, 2015. [Google Scholar]
- Katopodis, C. Developing a toolkit for fish passage, ecological flow management and fish habitat works. J. Hydraul. Res. 2005, 43, 451–467. [Google Scholar] [CrossRef]
- Katopodis, C.; Aadland, L.P. Effective dam removal and river channel restoration approaches. Int. J. River Basin Manag. 2006, 4, 153–168. [Google Scholar] [CrossRef]
- Leonard, J.B.K.; McCormick, S.D. The effect of migration distance and timing on metabolic enzyme activity in an anadromous clupeid, the American shad (Alosa sapidissima). Fish Physiol. Biochem. 1999, 20, 163–179. [Google Scholar] [CrossRef]
- Pottinger, T.G. A multivariate comparison of the stress response in three salmonid and three cyprinid species: Evidence for inter-family differences. J. Fish Biol. 2010, 76, 601–621. [Google Scholar] [CrossRef] [PubMed]
- Young, P.S.; Swanson, C.; Cech, J.J., Jr. Close encounters with a fish screen III: Behavior, performance, physiological stress responses, and recovery of adult delta smelt exposed to two-vector flows near a fish screen. Trans. Am. Fish. Soc. 2010, 139, 713–726. [Google Scholar] [CrossRef]
- Williams, J.G.; Armstrong, G.; Katopodis, C.; Larinier, M.; Travade, F. Thinking like a fish: A key ingredient for development of effective fish passage facilities at river obstructions. River Res. Appl. 2012, 28, 407–417. [Google Scholar] [CrossRef]
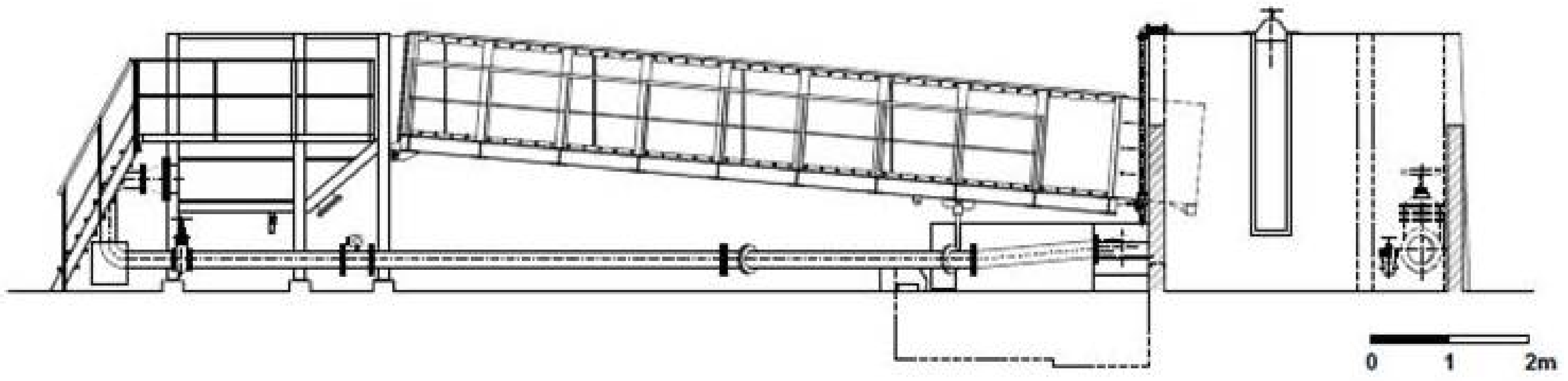
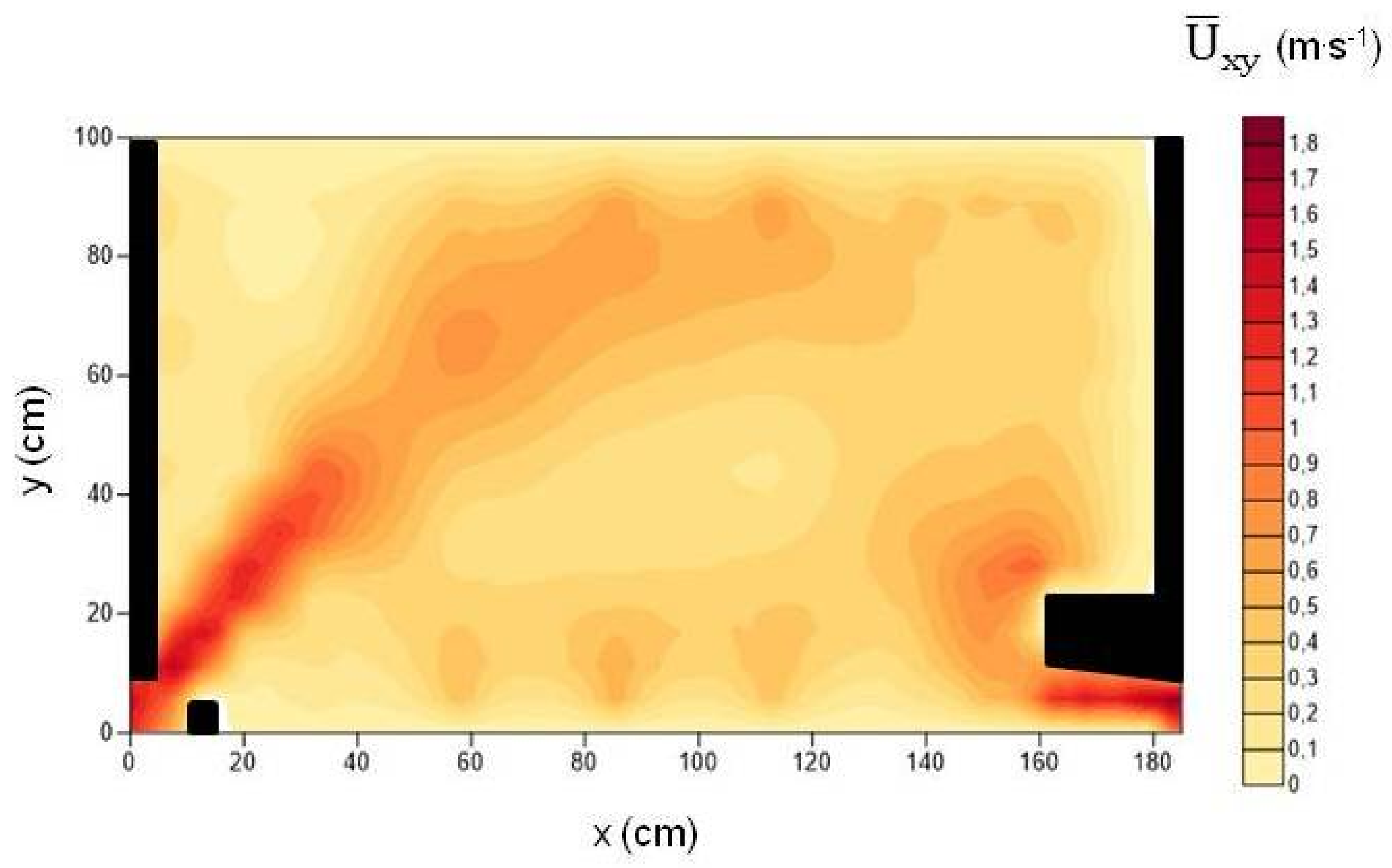
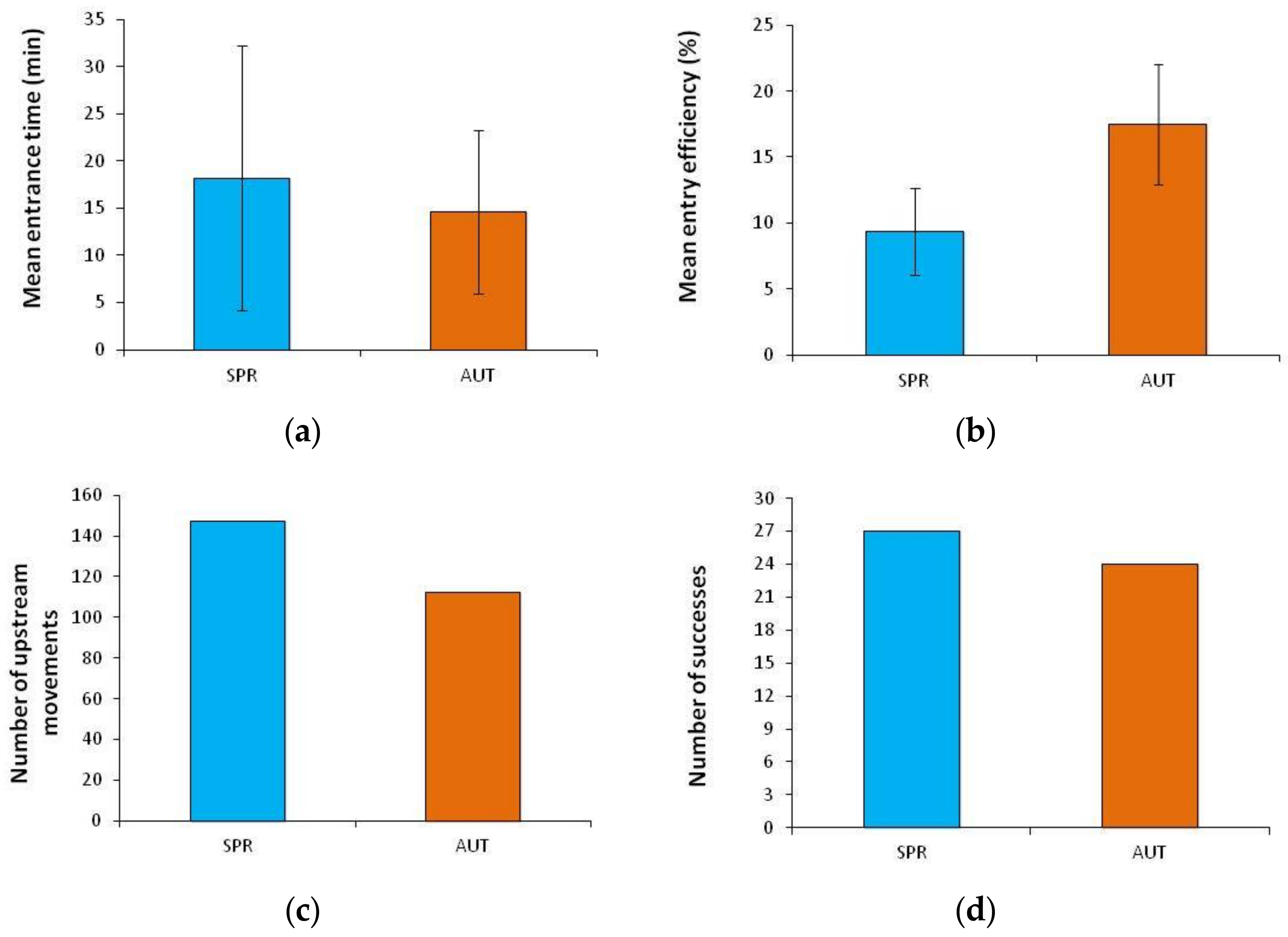

| Season | Fish | Trials | Entrance Time (Minutes) | Entry Efficiency (%) | Number of Upstream Movements | Number of Successes | Lactate (mmol·L−1) | Total Length (cm) | Body Mass (g) |
|---|---|---|---|---|---|---|---|---|---|
| SPRING | Tested | A | 14 | 9.1 | 35 | 13 | 2.5 ± 1.4 | 20.1 ± 1.8 | 84.4 ± 21.8 |
| B | 4 | 8.6 | 37 | 4 | 2.4 ± 1.0 | 18.4 ± 2.8 | 70.2 ± 36.1 | ||
| C | 32 | 5.8 | 15 | 1 | 3.8 ± 2.0 | 17.8 ± 1.8 | 57.0 ± 22.1 | ||
| D | 7 | 14.8 | 36 | 5 | 3.6 ± 1.9 | 17.6 ± 1.5 | 52.6 ± 13.2 | ||
| E | 34 | 8.5 | 24 | 4 | 2.9 ± 1.1 | 16.8 ± 1.2 | 45.3 ± 8.0 | ||
| Control | - | - | - | - | - | 2.9 ± 0.8 | 19.8 ± 2.3 | 73.3 ± 30.0 | |
| EARLY-AUTUMN | Tested | A | 24 | 19.3 | 20 | 3 | 8.0 ± 1.5 | 17.4 ± 1.7 | 45.0 ± 17.0 |
| B | 4 | 10.2 | 26 | 7 | 5.0 ± 1.5 | 20.3 ± 2.7 | 71.9 ± 30.0 | ||
| C | 11 | 21.7 | 25 | 5 | 7.2 ± 2.3 | 20.3 ± 1.2 | 69.4 ± 13.1 | ||
| D | 11 | 20.0 | 23 | 4 | 8.3 ± 1.8 | 17.2 ± 1.5 | 43.4 ± 13.2 | ||
| E | 23 | 16.1 | 18 | 5 | 5.6 ± 0.4 | 21.4 ± 2.2 | 83.6 ± 33.8 | ||
| Control | - | - | - | - | - | 2.9 ± 1.2 | 17.5 ± 2.5 | 48.3 ± 21.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romão, F.; Santos, J.M.; Katopodis, C.; Pinheiro, A.N.; Branco, P. How Does Season Affect Passage Performance and Fatigue of Potamodromous Cyprinids? An Experimental Approach in a Vertical Slot Fishway. Water 2018, 10, 395. https://doi.org/10.3390/w10040395
Romão F, Santos JM, Katopodis C, Pinheiro AN, Branco P. How Does Season Affect Passage Performance and Fatigue of Potamodromous Cyprinids? An Experimental Approach in a Vertical Slot Fishway. Water. 2018; 10(4):395. https://doi.org/10.3390/w10040395
Chicago/Turabian StyleRomão, Filipe, José M. Santos, Christos Katopodis, António N. Pinheiro, and Paulo Branco. 2018. "How Does Season Affect Passage Performance and Fatigue of Potamodromous Cyprinids? An Experimental Approach in a Vertical Slot Fishway" Water 10, no. 4: 395. https://doi.org/10.3390/w10040395
APA StyleRomão, F., Santos, J. M., Katopodis, C., Pinheiro, A. N., & Branco, P. (2018). How Does Season Affect Passage Performance and Fatigue of Potamodromous Cyprinids? An Experimental Approach in a Vertical Slot Fishway. Water, 10(4), 395. https://doi.org/10.3390/w10040395









