Influence of Carbon Source on the Efficiency of Nitrogen Removal and Denitrifying Bacteria in Biofilm from Bioelectrochemical SBBRs
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Wastewater Fed to Reactors
2.2. Experimental Stand and Organization
2.3. Analytical Procedures
2.4. Molecular Analyses of Biofilm
3. Results and Discussion
3.1. The Denitrifying Bacteria Community
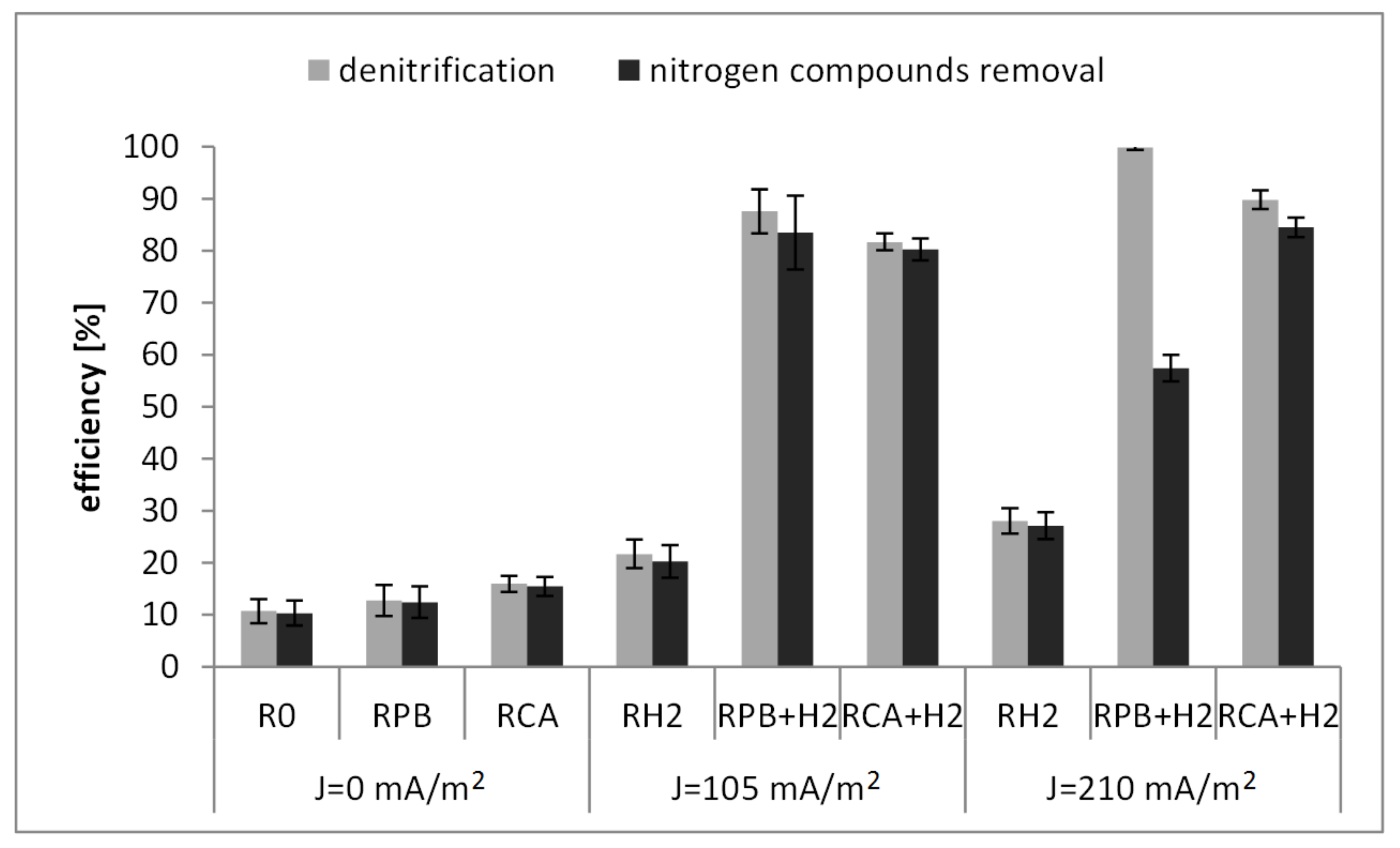
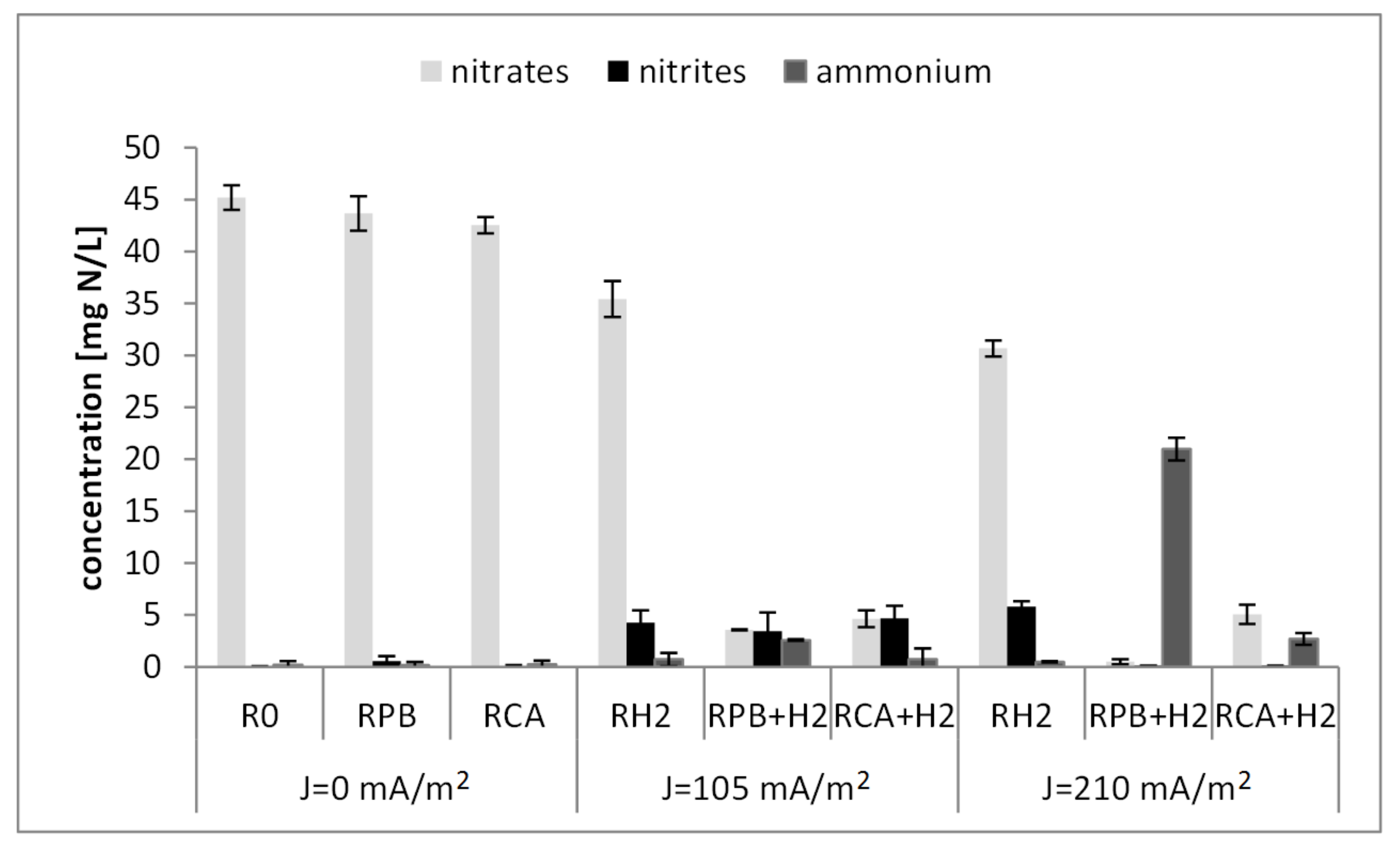
3.2. Nitrogen Compound Removal
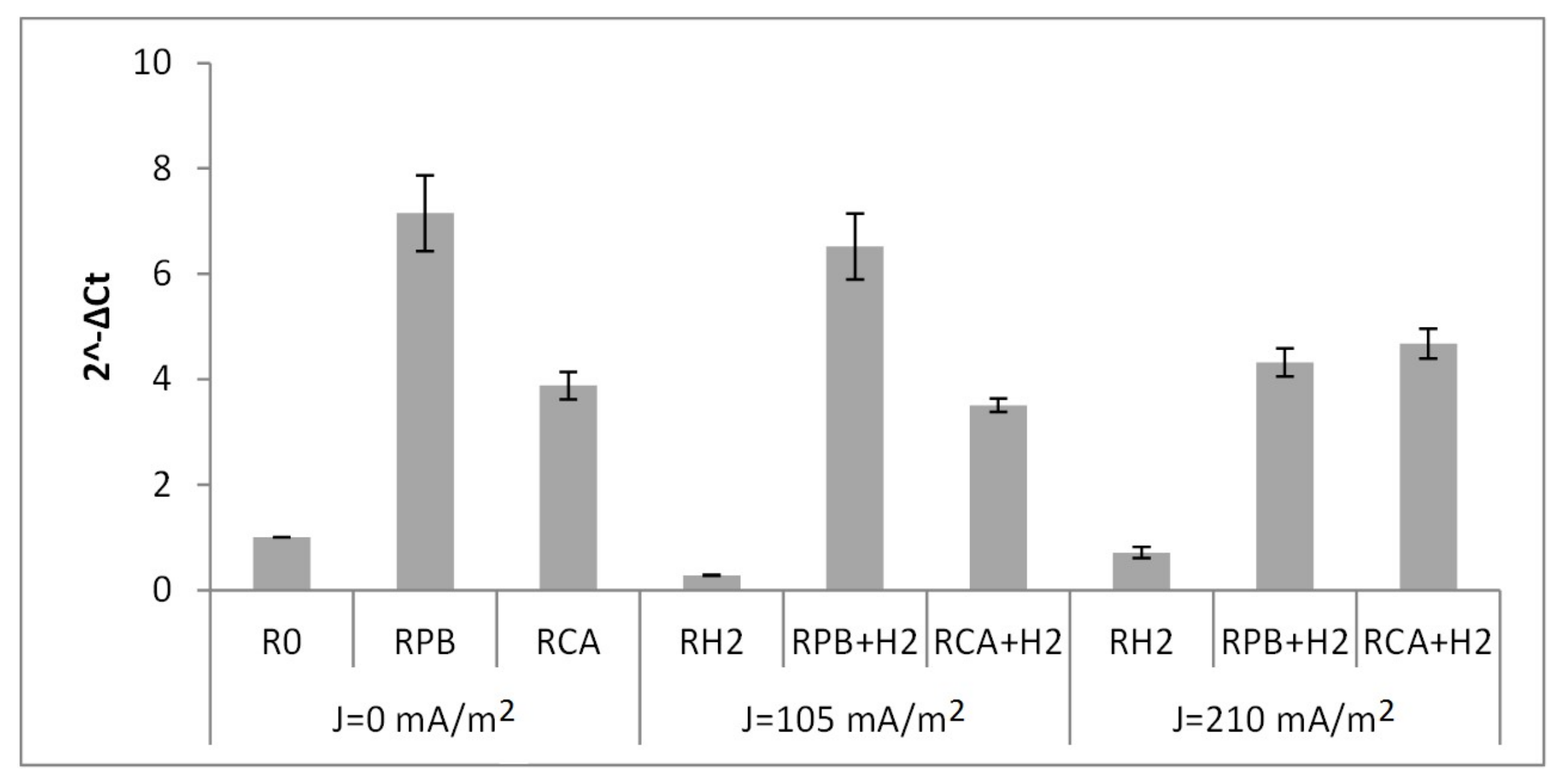
3.3. The Number of Denitrifying Bacteria
3.4. Total Organic and Total Inorganic Carbon Concentrations
4. Conclusions
- The efficiency of nitrogen compound removal in the bioelectrochemical SBBR depended on the density of the electric current and the external carbon source. The highest efficiency of 84.53(±1.88)% was obtained in the reactor with the organic carbon in the form of citric acid and an electric current density of 210 mA/m2. The lowest efficiency (57.42(±2.51)%) was recorded in the reactor with inorganic carbon in the form of potassium bicarbonate and an electric current density of 210 mA/m2.
- Citric acid, as a simple organic carbon source, was directly used by heterotrophs in heterotrophic denitrification. The produced carbon dioxide could additionally be a source of inorganic carbon for autotrophs.
- The main factor determining the composition of the denitrifying bacteria was the electric current flow. In the reactors with an electric current flow, the determining factor of the denitrifiers’ community was the external carbon source. In all biofilms from the experimental reactors, heterotrophic facultative bacteria of the genus Alicycliphilus were identified.
- Regardless of the electric current density, the external carbon sources in the form of citric acid and potassium bicarbonate resulted in a 4-fold and 4–7-fold increase in the abundance of full-denitrifiers in the biofilm, respectively.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hassard, F.; Biddle, J.; Cartmell, E.; Jefferson, B.; Tyrrel, S.; Stephenson, T. Rotating biological contactors for wastewater treatment—A review. Process Saf. Environ. Prot. 2015, 94, 285–306. [Google Scholar] [CrossRef]
- Ignatowicz, K.; Puchlik, M. Rotary biological contactor as alternative for small amount of wastewater treatment. Rocz. Ochr. Sr. 2011, 13, 1385–1404. [Google Scholar]
- Lu, C.; Yeh, A.C.; Lin, M.R. Treatment of high–strength organic wastewaters using an anaerobic rotating biological contactors. Environ. Int. 1995, 21, 313–323. [Google Scholar] [CrossRef]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Rotating biological contactors: A review of main factors affecting performance. Rev. Environ. Sci. Bio/Tecnnol. 2008, 7, 155–172. [Google Scholar] [CrossRef]
- Teixeira, P.; Oliveira, R. Denitrification in a closed rotating biological contactor: Effect of disk submergence. Process Biochem. 2001, 37, 345–349. [Google Scholar] [CrossRef]
- Karanasios, K.A.; Vasiliadou, I.A.; Pavlou, S.; Vayenas, D.V. Hydrogenotrophic denitrification of potable water: A review. J. Hazard. Mater. 2010, 180, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Kłodowska, I.; Rodziewicz, J.; Janczukowicz, W. Removal of nitrogen compounds in the process of autotrophic denitrification in a Sequencing Batch Biofilm Reactor (SBBR). Pol. J. Nat. Sci. 2014, 29, 359–369. [Google Scholar]
- Kłodowska, I.; Rodziewicz, J.; Janczukowicz, W. Effect of technological and environmental parameters on electrolytically-aided denitrification using the anaerobic rotating multi-disc reactor. Ecol. Eng. 2015, 85, 223–225. [Google Scholar] [CrossRef]
- Kłodowska, I.; Rodziewicz, J.; Janczukowicz, W.; Cydzik-Kwiatkowska, A.; Parszuto, K. Effect of citric acid on the efficiency of the removal of nitrogen and phosphorus compounds during simultaneous heterotrophic-autotrophic denitrification (HAD) and electrocoagulation. Ecol. Eng. 2016, 95, 30–35. [Google Scholar] [CrossRef]
- Kłodowska, I.; Rodziewicz, J.; Janczukowicz, W.; Gotkowska-Płachta, A.; Cydzik-Kwiatkowska, A. Hydrogenotrophic denitrification process efficiency and the number of denitrifying bacteria (MPN) in the sequencing batch biofilm reactor (SBBR) with platinum and carbon anodes. J. Environ. Sci. Health Part A 2016, 51, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feng, C.; Wang, Q.; Yang, Y.; Zhang, Z.; Sugiura, N. Nitrate removal from groundwater by cooperating heterotrophic with autotrophic denitrification in a biofilm-electrode reactor. J. Hazard. Mater. 2011, 192, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- ISO 7890-3:1988 Determination of Nitrate—Part 3: Spectrometric Method Using Sulfosalicylic Acid; International Organization for Standardization: Geneva, Switzerland, 1988.
- ISO 6777:1984 Determination of Nitrite—Molecular Absorption Spectrometric Method; International Organization for Standardization: Geneva, Switzerland, 1984.
- PN-C-04576-04:1994 Determination of Ammonium by Spectrophotometric Method; International Organization for Standardization: Geneva, Switzerland, 1994.
- Kloos, K.; Mergel, A.; Rösch, C.; Bothe, H. Denitrification within the genus Azospirillum and other associative bacteria. Aust. J. Plant Physiol. 2001, 28, 991–998. [Google Scholar]
- Throbäck, I.N.; Enwall, K.; Jarvis, Ä.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Cydzik-Kwiatkowska, A.; Rusanowska, P.; Zielińska, M.; Bernat, K.; Wojnowska-Baryła, I. Structure of nitrogen-converting communities induced by hydraulic retention time and COD/N ratio in constantly aerated granular sludge reactors treating digester supernatant. Bioresour. Technol. 2014, 154, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 12 January 2018).
- Van Hannen, E.J.; Mooij, W.; Van Agterveld, M.P.; Gons, H.J.; Laanbroek, H.J. Detritus–dependent development of the microbial community in an experimental system: Qualitative analysis by denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 1999, 65, 2478–2484. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Oosterkamp, M.J.; Veuskens, T.; Talarico Saia, F.; Weelink, S.A.B.; Goodwin, L.A.; Daligault, H.E.; Bruce, D.C.; Detter, J.C.; Tapia, R.; Han, C.S.; et al. Genome analysis and physiological comparison of Alicycliphilus denitrificans strains BC and K601T. PLoS ONE 2013, 8, e66971. [Google Scholar] [CrossRef] [PubMed]
- Weelink, S.A.B.; Tan, N.C.G.; Broeke, H.; Kieboom, C.; Doesburg, W.; Langenhoff, A.A.M.; Gerritse, J.; Junca, H.; Stams, A.J.M. Isolation and characterization of Alicycliphilus denitrificans strain BC which grows on benzene with chlorate as the electron acceptor. Appl. Environ. Microbiol. 2008, 74, 6672–6681. [Google Scholar] [CrossRef] [PubMed]
- Mechichi, T.; Stackebrandt, E.; Fuchs, G. Alicycliphilus denitrificans gen. nov., sp. nov., a cyclohexanol-degrading nitrate-reducing β-proteobacterium. Int. J. Syst. Evol. Microbiol. 2003, 53, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Zhang, X.; Liu, B.; Mao, Y.; Liu, Y.; Zhao, L. Structural differentiation of bacterial communities in indole-degrading bioreactors under denitrifying and sulfate-reducing conditions. Res. Microbiol. 2010, 161, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Tsukuda, S.; Christianson, L.; Kolb, A.; Saito, K.; Summerfelt, S. Heterotrophic denitrification of aquaculture effluent using fluidized sand biofilters. Aquac. Eng. 2015, 64, 49–59. [Google Scholar] [CrossRef]
- Mao, Y.; Xia, Y.; Zhang, T. Characterization of Thauera-dominated hydrogen-oxidizing autotrophic denitrifying microbial communities by using high-throughput sequencing. Bioresour. Technol. 2013, 128, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, T.; Lee, T.K.; Shukla, S.K.; Tiedje, J.M.; Park, J. Increased nitrous oxide accumulation by bioelectrochemical denitrification under autotrophic conditions: Kinetics and expression of denitrification pathway genes. Water Res. 2013, 47, 7087–7097. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-J.; Wong, B.-T. Denitrifying sulfide removal by enriched microbial consortium: Kinetic diagram. Bioresour. Technol. 2014, 164, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Peng, J.; Feng, C.; Fang, F.; Chen, S.; Xu, Y.; Wang, X. Evaluation of simultaneous autotrophic and heterotrophic denitrification processes and bacterial community structure analysis. Appl. Microbiol. Biotechnol. 2015, 99, 6527–6536. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Sanseverino, J.; Chauhan, A.; Lucas, S.; Copeland, A.; Lapidus, A.; Del Rio, T.G.; Dalin, E.; Tice, H.; Bruce, D.; et al. Complete genome sequence of Thauera aminoaromatica strain MZ1T. Stand. Genom. Sci. 2012, 6, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, S.; Hasan, M.; Aroua, M.K. Effect of carbon dioxide and bicarbonate as inorganic carbon sources on growth and adaptation of autohydrogenotrophic denitrifying bacteria. J. Hazard. Mater. 2009, 162, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Buckwalter, S.P.; Repert, D.A.; Miller, D.N. Small-scale, hydrogen oxidizing denitrifying bioreactor for treatment of nitrate-contaminated drinking water. Water Res. 2005, 39, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Karanasios, K.A.; Michailidis, M.K.; Vasiliadou, I.A.; Pavlou, S.; Vayenas, D.V. Potable water hydrogenotrophic denitrification in packed-bed bioreactors coupled with a solar-electrolysis hydrogen production system. Desalin. Water Treat. 2011, 33, 86–96. [Google Scholar] [CrossRef]
- Glass, C.; Silverstein, J. Denitrification kinetics of high nitrate concentration water: PH effect on inhibition and nitrite accumulation. Water Res. 1998, 32, 831–839. [Google Scholar] [CrossRef]
- Wilderer, P.; Jones, W.; Dau, U. Competition in denitrification systems affecting reduction rate and accumulation. Water Res. 1987, 21, 239–245. [Google Scholar] [CrossRef]
- Dhamole, P.B.; Nair, R.R.; D’Souza, S.F.; Lele, S.S. Denitrification of high strength nitrate waste. Bioresour. Technol. 2007, 98, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, S.; Hasan, M.; Aroua, M.K. Improvement of autohydrogenotrophic nitrite reduction rate through optimization of pH and sodium bicarbonate dose in batch experiments. J. Biosci. Bioeng. 2009, 107, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Huang, B.; Zou, Y.; Li, N.; Wang, M.; Yin, J.; Cong, Y.; Shen, D. The effect of carbon sources on nitrogen removal performance in bioelectrochemical systems. Bioresour. Technol. 2013, 128, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tong, S.; Chen, N.; Liu, Y.; Feng, C.; Hu, Q. Effect of electro-stimulation on the activity of heterotrophic denitrifying bacteria and denitrification performance. Bioresour. Technol. 2015, 196, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Feng, H.; Ding, Y.; Zheng, X.; Wang, M.; Li, N.; Shen, D.; Zhang, H. Microbial metabolism and activity in terms of nitrate removal in bioelectrochemical systems. Electrochim. Acta 2013, 113, 29–36. [Google Scholar] [CrossRef]
- Szewczyk, W.K. Biological Methods of Nitrogen Compounds Removal from Wastewater; Oficyna Wydawnicza Politechniki Warszawskiej: Warszawa, Poland, 2005; ISBN 8372075131. [Google Scholar]
- Park, J.-H.; Choi, O.; Lee, T.-H.; Kim, H.; Sang, B.-I. Pyrosequencing analysis of microbial communities in hollow fiber-membrane biofilm reactors system for treating high-strength nitrogen wastewater. Chemosphere 2016, 163, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, J. Removal of Nitrogen and Phosphorus Compounds from Wastewater Originating from Soil-Less Cultivation of Tomatoes in a Rotating Electrobiological Contactor; Dissertations and Monographs; Wydawnictwo Uniwersytetu Warmińsko-Mazurskiego: Olsztyn, Poland, 2017; ISBN 1509-3018. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłodowska, I.; Rodziewicz, J.; Janczukowicz, W.; Cydzik-Kwiatkowska, A.; Rusanowska, P. Influence of Carbon Source on the Efficiency of Nitrogen Removal and Denitrifying Bacteria in Biofilm from Bioelectrochemical SBBRs. Water 2018, 10, 393. https://doi.org/10.3390/w10040393
Kłodowska I, Rodziewicz J, Janczukowicz W, Cydzik-Kwiatkowska A, Rusanowska P. Influence of Carbon Source on the Efficiency of Nitrogen Removal and Denitrifying Bacteria in Biofilm from Bioelectrochemical SBBRs. Water. 2018; 10(4):393. https://doi.org/10.3390/w10040393
Chicago/Turabian StyleKłodowska, Izabella, Joanna Rodziewicz, Wojciech Janczukowicz, Agnieszka Cydzik-Kwiatkowska, and Paulina Rusanowska. 2018. "Influence of Carbon Source on the Efficiency of Nitrogen Removal and Denitrifying Bacteria in Biofilm from Bioelectrochemical SBBRs" Water 10, no. 4: 393. https://doi.org/10.3390/w10040393
APA StyleKłodowska, I., Rodziewicz, J., Janczukowicz, W., Cydzik-Kwiatkowska, A., & Rusanowska, P. (2018). Influence of Carbon Source on the Efficiency of Nitrogen Removal and Denitrifying Bacteria in Biofilm from Bioelectrochemical SBBRs. Water, 10(4), 393. https://doi.org/10.3390/w10040393







