Arsenic Removal Using Horizontal Subsurface Flow Constructed Wetlands: A Sustainable Alternative for Arsenic-Rich Acidic Waters
Abstract
:1. Introduction
2. Materials and Methods
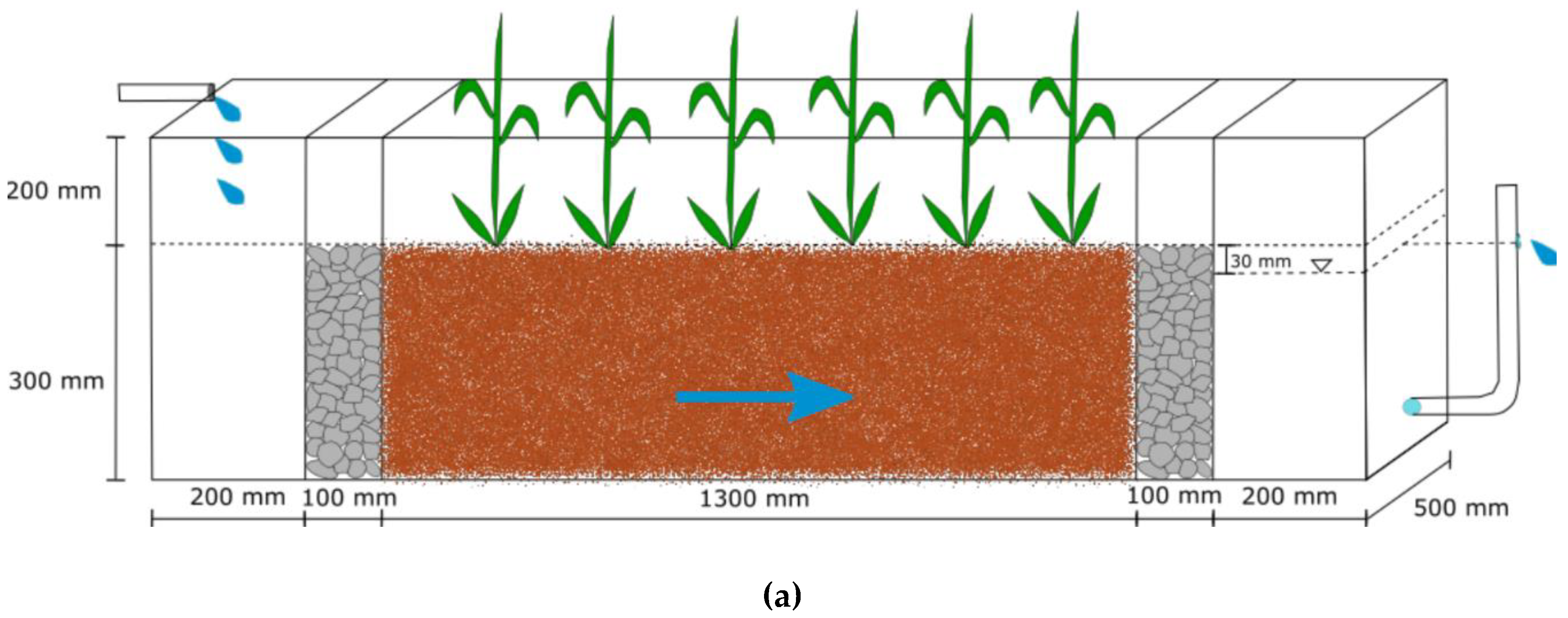
2.1. The Wetland System
2.2. Synthetic Water
2.3. Operation, Sampling, and Analysis
2.4. Water Losses
2.5. Geochemical Modeling
3. Results and Discussion
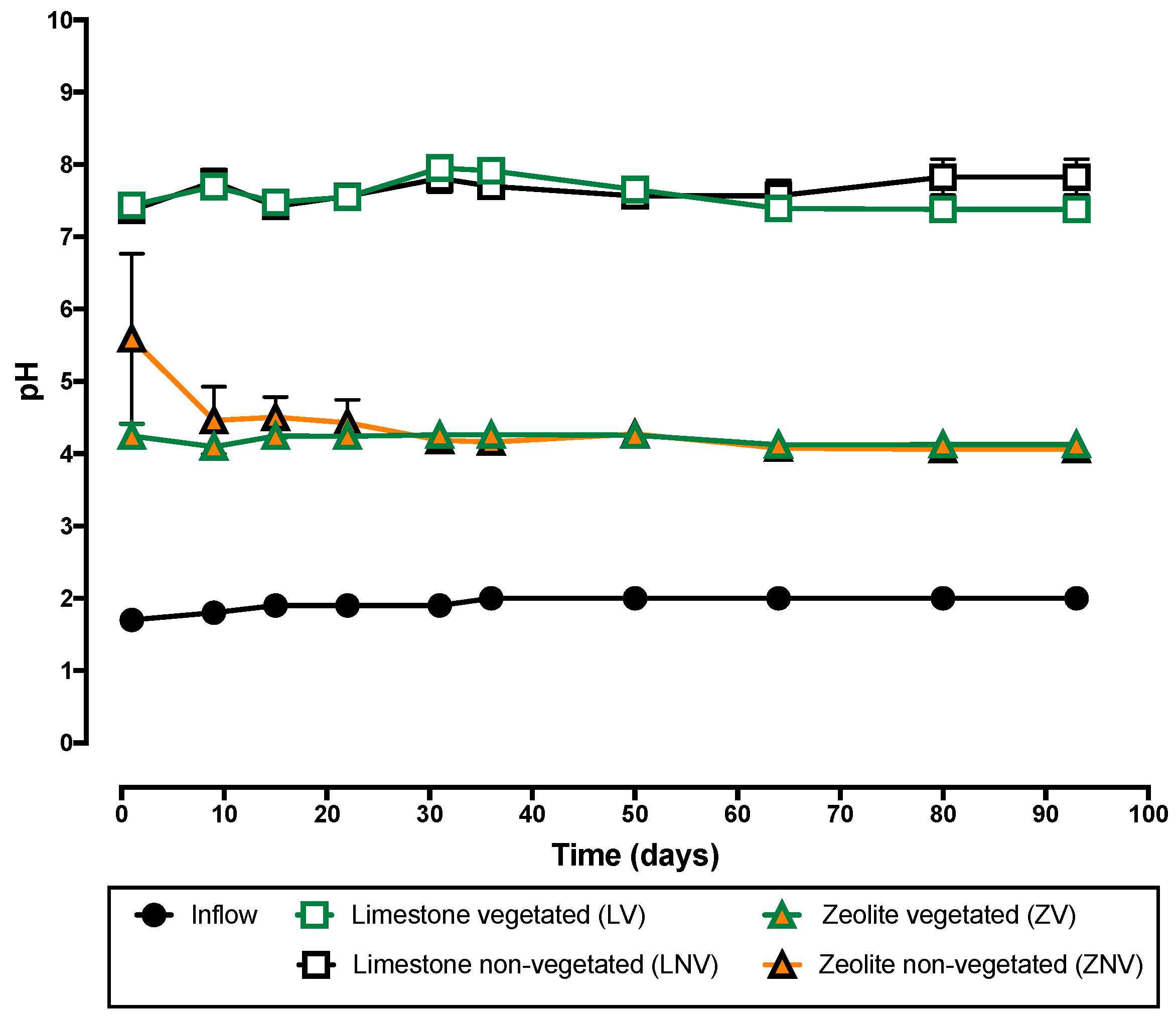
3.1. Neutralization Capacity of the System
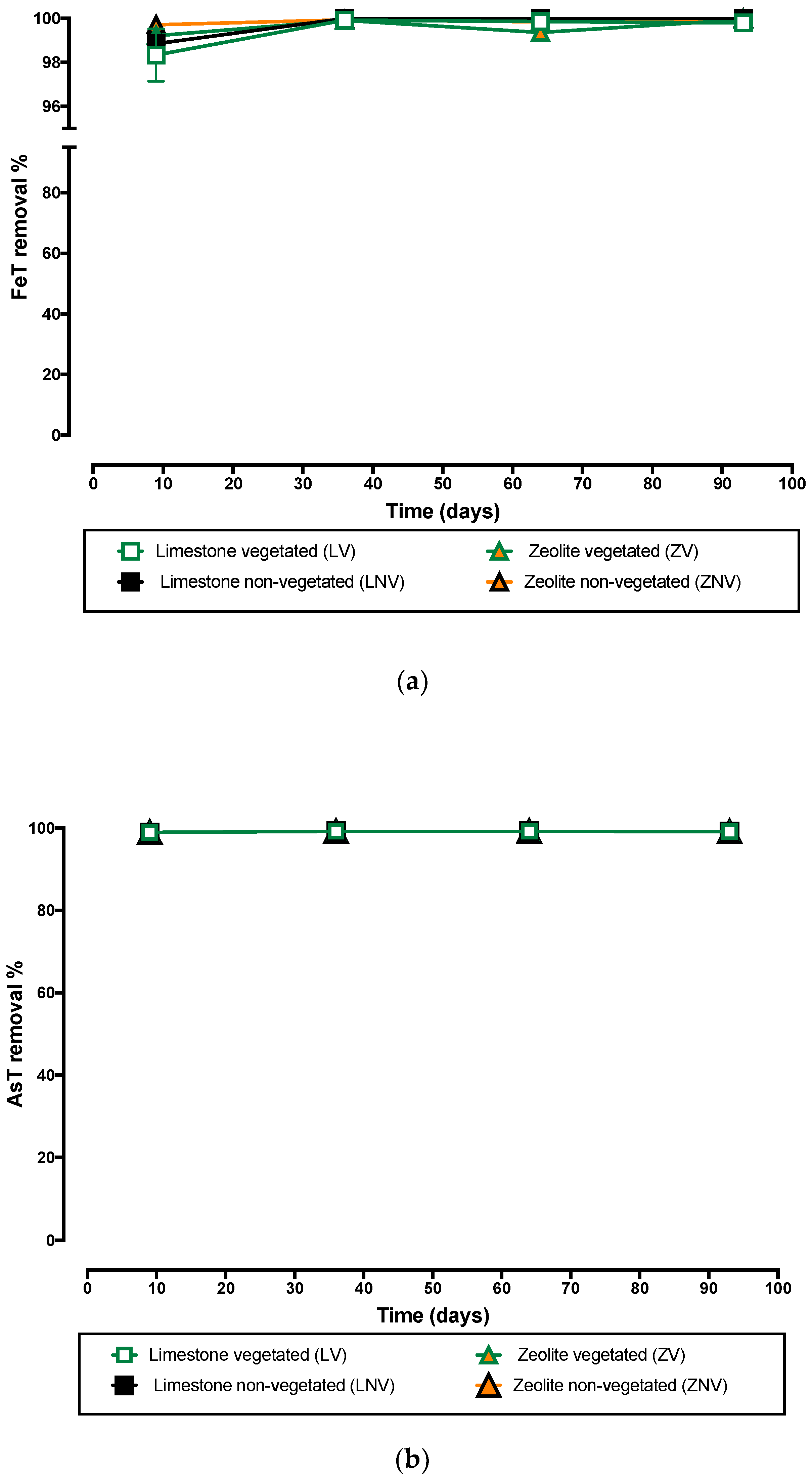
3.2. Iron and Arsenic Removal
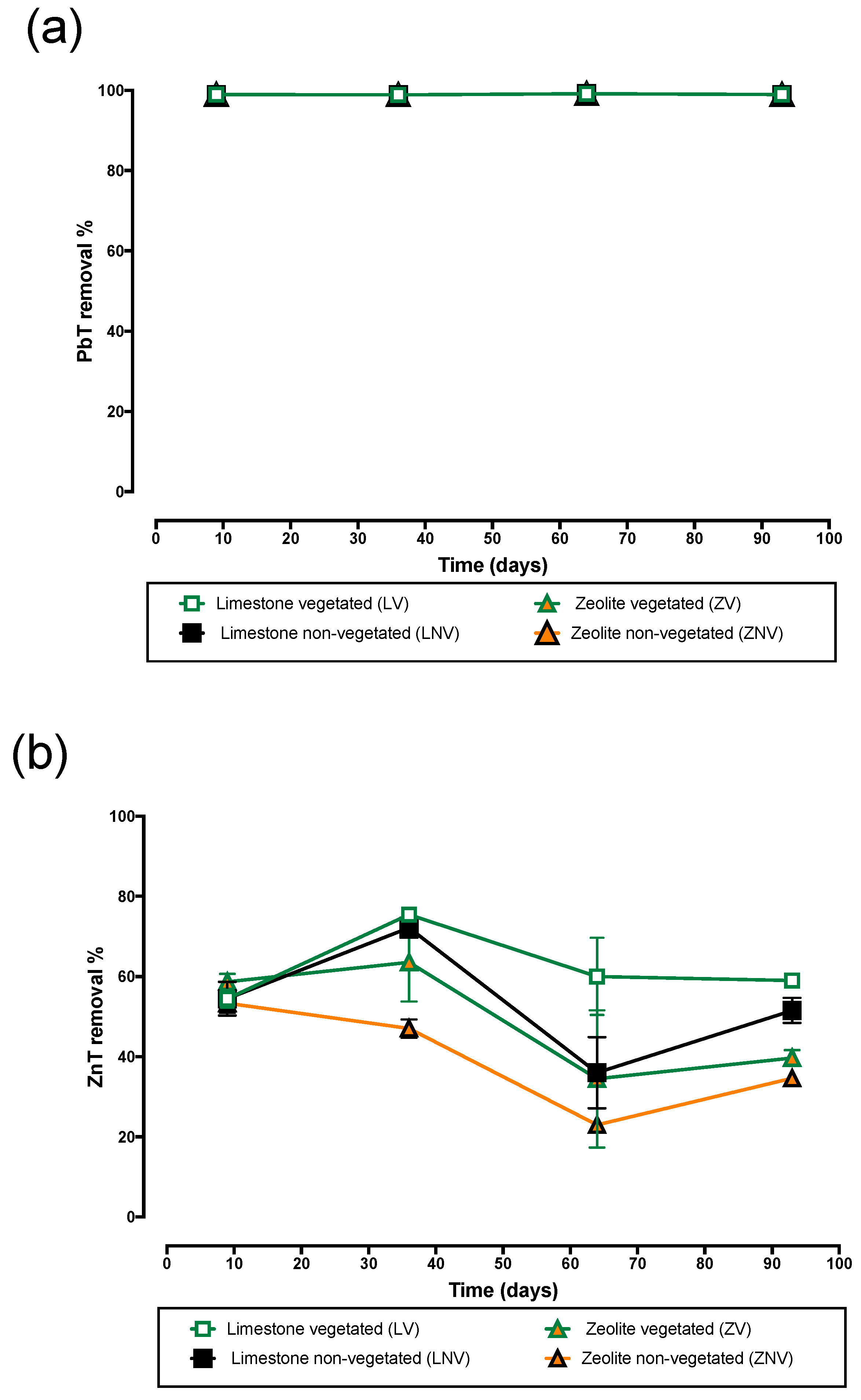
3.3. Lead and Zinc Removal
3.4. Water Losses and Geochemical Modeling
3.5. Role of Vegetation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marshall, G.; Ferreccio, C.; Yuan, Y.; Bates, M.N.; Steinmaus, C.; Selvin, S.; Liaw, J.; Smith, A.H. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J. Natl. Cancer Inst. 2007, 99, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.Y. Removal of metals in constructed wetlands: Review. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2008, 12, 96–101. [Google Scholar] [CrossRef]
- Marchand, L.; Mench, M.; Jacob, D.L.; Otte, M.L. Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: A review. Environ. Pollut. 2010, 158, 3447–3461. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hu, Y.; Luo, J.; Xu, B.; Zhao, J. Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems. J. Hazard. Mater. 2009, 165, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Márquez, M.A.; Alarcón-Herrera, M.T.; Martín-Domínguez, I.R. Performance of eleocharis macrostachya and its importance for arsenic retention in constructed wetlands. Environ. Sci. Pollut. Res. 2012, 19, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Valles-Aragón, M.C.; Olmos-Márquez, M.A.; Llorens, E.; Alarcón-Herrera, M.T. Redox potential and ph behavior effect on arsenic removal from water in a constructed wetland mesocosm. Environ. Progr. Sustain. Energy 2014, 33, 1332–1339. [Google Scholar] [CrossRef]
- Zurita, F.; Del Toro-Sánchez, C.L.; Gutierrez-Lomelí, M.; Rodriguez-Sahagún, A.; Castellanos-Hernandez, O.A.; Ramírez-Martínez, G.; White, J.R. Preliminary study on the potential of arsenic removal by subsurface flow constructed mesocosms. Ecol. Eng. 2012, 47, 101–104. [Google Scholar] [CrossRef]
- Serrano, J.; Leiva, E. Removal of arsenic using acid/metal-tolerant sulfate reducing bacteria: A new approach for bioremediation of high-arsenic acid mine waters. Water 2017, 9, 994. [Google Scholar] [CrossRef]
- Lizama Allende, K.; McCarthy, D.T.; Fletcher, T.D. The influence of media type on removal of arsenic, iron and boron from acidic wastewater in horizontal flow wetland microcosms planted with phragmites australis. Chem. Eng. J. 2014, 246, 217–228. [Google Scholar] [CrossRef]
- Lizama A., K.; Fletcher, T.D.; Sun, G. Removal processes for arsenic in constructed wetlands. Chemosphere 2011, 84, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Lizama-Allende, K.; Henry-Pinilla, D.; Diaz-Droguett, D.E. Removal of arsenic and iron from acidic water using zeolite and limestone: Batch and column studies. Water Air Soil Pollut. 2017, 228, 275. [Google Scholar] [CrossRef]
- Guerra, P.; Gonzalez, C.; Escauriaza, C.; Pizarro, G.; Pasten, P. Incomplete mixing in the fate and transport of arsenic at a river affected by acid drainage. Water Air Soil Pollut. 2016, 227. [Google Scholar] [CrossRef]
- Kröpfelová, L.; Vymazal, J.; Švehla, J.; Štíchová, J. Removal of trace elements in three horizontal sub-surface flow constructed wetlands in the Czech republic. Environ. Pollut. 2009, 157, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association; American Water Works Association and Water Environmental Federation. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Parkhurst, D. Phreeqc (Version 3)—A COMPUTER Program for Speciation, Batch-reaction, One-dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Reston, VA, USA, 2016.
- Appelo, C.A.J.; Van Der Weiden, M.J.J.; Tournassat, C.; Charlet, L. Surface complexation of ferrous iron and carbonate on ferrihydrite and the mobilization of arsenic. Environ. Sci. Technol. 2002, 36, 3096–3103. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Vitre, R.D.; Belzile, N.; Tessier, A. Speciation and adsorption of arsenic on diagenetic iron oxyhydroxides. Limnol. Oceanogr. 1991, 36, 1480–1485. [Google Scholar] [CrossRef]
- Dixit, S.; Hering, J.G. Comparison of arsenic(v) and arsenic(iii) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Technol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.A.; Fendorf, S.E.; Goldberg, S. Surface structures and stability of arsenic(III) on goethite: Spectroscopic evidence for inner-sphere complexes. Environ. Sci. Technol. 1998, 32, 2383–2388. [Google Scholar] [CrossRef]
- Pierce, M.L.; Moore, C.B. Adsorption of arsenite and arsenate on amorphous iron hydroxide. Water Res. 1982, 16, 1247–1253. [Google Scholar] [CrossRef]
- Shevade, S.; Ford, R.G. Use of synthetic zeolites for arsenate removal from pollutant water. Water Res. 2004, 38, 3197–3204. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.S.; Baek, K.; Park, J.K.; Oh, Y.K.; Lee, S.D. Adsorption characteristics of as(v) on iron-coated zeolite. J. Hazard. Mater. 2009, 163, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Pang, W.; Yu, J.; Huo, Q.; Chen, J. Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Singhakant, C.; Koottatep, T.; Satayavivad, J. Fractional analysis of arsenic in subsurface-flow constructed wetlands with different length to depth ratios. Water Sci. Technol. 2009, 60, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Singhakant, C.; Koottatep, T.; Satayavivad, J. Enhanced arsenic removals through plant interactions in subsurface-flow constructed wetlands. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2009, 44, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lizama Allende, K.; Fletcher, T.D.; Sun, G. The effect of substrate media on the removal of arsenic, boron and iron from an acidic wastewater in planted column reactors. Chem. Eng. J. 2012, 179, 119–130. [Google Scholar] [CrossRef]
- Younger, P.L. The adoption and adaptation of passive treatment technologies for mine waters in the United Kingdom. Mine Water Environ. 2000, 19, 84–97. [Google Scholar] [CrossRef]
- Sarafraz, S.; Mohammad, T.A.; Noor, M.J.M.M.; Liaghat, A. Wastewater treatment using horizontal subsurface flow constructed wetland. Am. J. Environ. Sci. 2009, 5, 99–105. [Google Scholar]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; p. 1016. [Google Scholar]
- Sjöblom, Å. Mechanisms of metal immobilisation in mine drainage treatment wetlands—A sustainability perspective. In Proceedings of the 6th International Conference on Acid Rock Drainage, Victoria, Australia, 14–17 July 2003; pp. 817–823. [Google Scholar]
- Sheoran, A.S.; Sheoran, V. Heavy metal removal mechanism of acid mine drainage in wetlands: A critical review. Miner. Eng. 2006, 19, 105–116. [Google Scholar] [CrossRef]
- Seshadri, B.; Bolan, N.S.; Naidu, R. Rhizosphere-induced heavy metal(loid) transformation in relation to bioavailability and remediation. J. Soil Sci. Plant Nutr. 2015, 15, 524–548. [Google Scholar] [CrossRef]
- Ye, Z.H.; Baker, A.J.M.; Wong, M.H.; Willis, A.J. Zinc, lead and cadmium tolerance, uptake and accumulation by the common reed, phragmites australis (CAV.) trin. Ex steudel. Ann. Bot. 1997, 80, 363–370. [Google Scholar] [CrossRef]
- Deng, H.; Ye, Z.H.; Wong, M.H. Lead and zinc accumulation and tolerance in populations of six wetland plants. Environ. Pollut. 2006, 141, 69–80. [Google Scholar] [CrossRef] [PubMed]
- García, J.; Rousseau, D.P.L.; Morató, J.; Lesage, E.; Matamoros, V.; Bayona, J.M. Contaminant removal processes in subsurface-flow constructed wetlands: A review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 561–661. [Google Scholar] [CrossRef]
- Bavandpour, F.; Zou, Y.; He, Y.; Saeed, T.; Sun, Y.; Sun, G. Removal of dissolved metals in wetland columns filled with shell grits and plant biomass. Chem. Eng. J. 2018, 331, 234–241. [Google Scholar] [CrossRef]

| Parameter | Unit | Average ± SD |
|---|---|---|
| pH | - | 1.9 ± 0.1 |
| T | °C | 21.8 ± 1.5 |
| TDS | g/L | 4.15 ± 0.26 |
| Hardness | mg/L CaCO3 | 1554 ± 179 |
| ORP | mV | 6472 ± 23 |
| SO4 | mg/L | 2092 ± 509 |
| As | mg/L | 2.03 ± 0.19 |
| Fe | mg/L | 61.54 ± 7.79 |
| Pb | mg/L | 0.89 ± 0.10 |
| Zn | mg/L | 11.92 ± 1.30 |
| Mn | mg/L | 10.9 ± 0.46 |
| Al | mg/L | 58 ± 4.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizama-Allende, K.; Jaque, I.; Ayala, J.; Montes-Atenas, G.; Leiva, E. Arsenic Removal Using Horizontal Subsurface Flow Constructed Wetlands: A Sustainable Alternative for Arsenic-Rich Acidic Waters. Water 2018, 10, 1447. https://doi.org/10.3390/w10101447
Lizama-Allende K, Jaque I, Ayala J, Montes-Atenas G, Leiva E. Arsenic Removal Using Horizontal Subsurface Flow Constructed Wetlands: A Sustainable Alternative for Arsenic-Rich Acidic Waters. Water. 2018; 10(10):1447. https://doi.org/10.3390/w10101447
Chicago/Turabian StyleLizama-Allende, Katherine, Ignacio Jaque, José Ayala, Gonzalo Montes-Atenas, and Eduardo Leiva. 2018. "Arsenic Removal Using Horizontal Subsurface Flow Constructed Wetlands: A Sustainable Alternative for Arsenic-Rich Acidic Waters" Water 10, no. 10: 1447. https://doi.org/10.3390/w10101447
APA StyleLizama-Allende, K., Jaque, I., Ayala, J., Montes-Atenas, G., & Leiva, E. (2018). Arsenic Removal Using Horizontal Subsurface Flow Constructed Wetlands: A Sustainable Alternative for Arsenic-Rich Acidic Waters. Water, 10(10), 1447. https://doi.org/10.3390/w10101447





