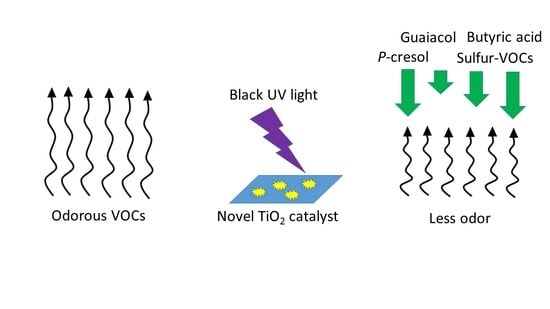
Mitigation of Livestock Odors Using Black Light and a New Titanium Dioxide-Based Catalyst: Proof-of-Concept
Abstract
:1. Introduction
2. Experiments
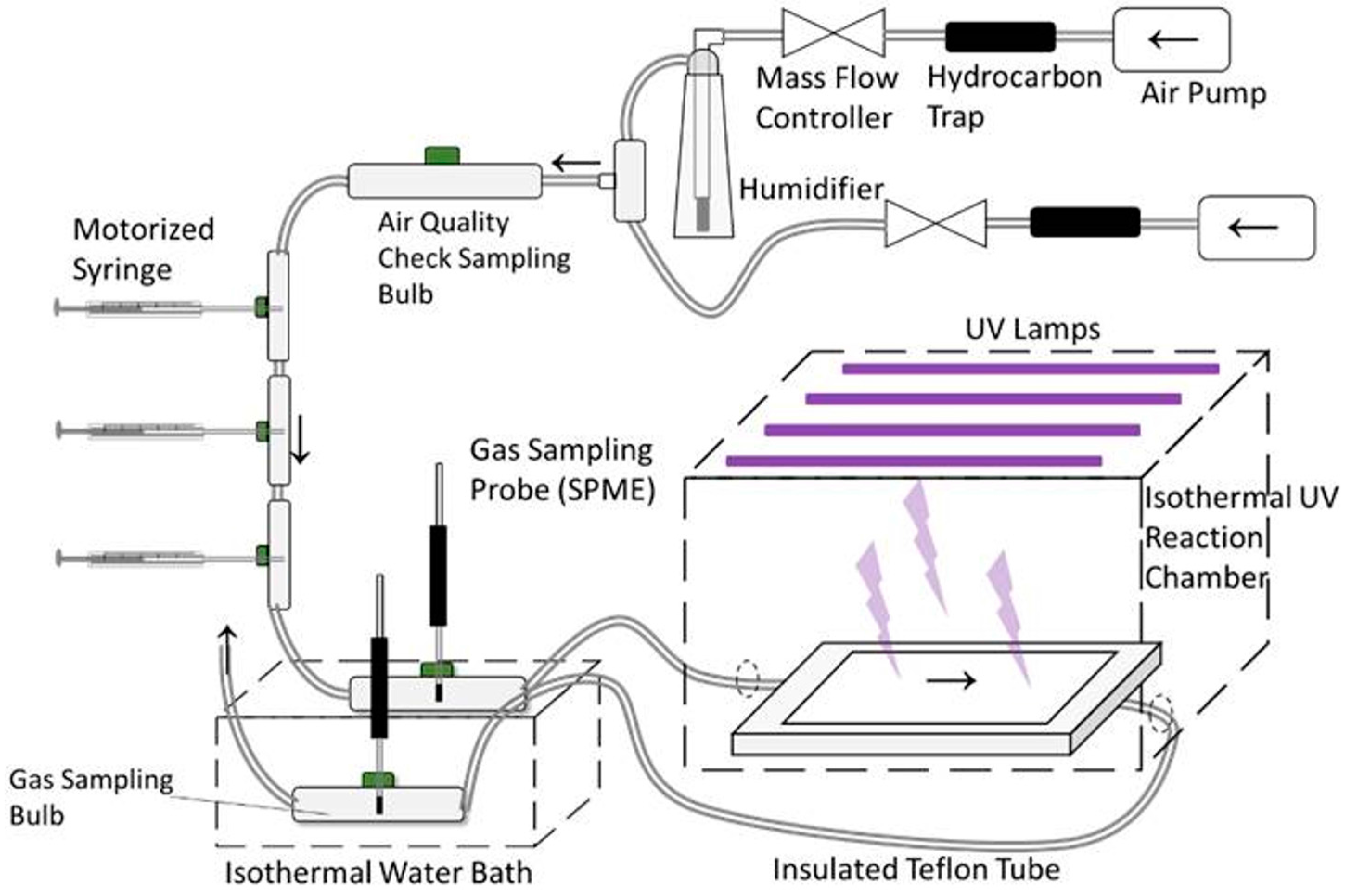
2.1. Standard Gas Generation and UV Treatment System
2.2. Testing the Effects of Environmental Parameters and Coating
2.3. Gas Sampling and Analysis
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. PureTi Coat Performance Variability
3.2. Effect of the Catalyst Loading Density
3.3. Effect of Relative Humidity and Temperature
3.4. Effect of Swine Barn Dust
3.5. Comparison of PureTi Coating Performance with Evonik P25
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- State agriculture overview, Iowa. National Agricultural Statistics Service, United States Department of Agriculture. Available online: https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=IOWA (accessed on 5 August 2016).
- Wright, D.; Eaton, D.; Nielsen, L.; Kuhrt, F.; Koziel, J.A.; Spinhirne, J.P.; Parker, D.B. Multidimensional GC-MS-olfactometry for identification and prioritization of malodors from confined animal feeding operations. J. Agric. Food Chem. 2005, 53, 8663–8672. [Google Scholar] [CrossRef] [PubMed]
- Bulliner, E.A.; Koziel, J.A.; Cai, L.; Wright, D. Characterization of livestock odors using steel plates, solid phase microextraction, and multidimensional—gas chromatography-mass spectrometry-olfactometry. J. Air Waste Manag. Assoc. 2006, 56, 1391–1403. [Google Scholar] [CrossRef]
- Cai, L.; Koziel, J.A.; Davis, J.; Lo, Y.C.; Xin, H. Characterization of VOCs and odors by in-vivo sampling of beef cattle rumen gas using SPME and GC-MS-olfactometry. Anal. Bioanal. Chem. 2006, 386, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Koziel, J.A.; Liang, Y.; Nguyen, A.T.; Xin, H. Evaluation of zeolite for control of odorants emissions from simulated poultry manure storage. J. Environ. Qual. 2007, 36, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Koziel, J.A.; Lo, Y.M.; Cai, L.; Wright, D.W. Simultaneous characterization of VOCs and livestock odors using solid-phase microextraction—multidimensional gas chromatography-mass spectrometry-olfactometry. Chem. Eng. Trans. 2010, 23, 73–78. [Google Scholar]
- Lo, Y.C.; Koziel, J.A.; Cai, L.; Hoff, S.J.; Jenks, W.S.; Xin, H. Simultaneous chemical and sensory characterization of VOCs and semi-VOCs emitted from swine manure using SPME and multidimensional gas chromatography-mass spectrometry-olfactometry system. J. Environ. Qual. 2008, 37, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Melse, R.W.; van der Werf, A.W. Biofiltration for mitigation of methane emission from animal husbandry. Environ. Sci. Technol. 2005, 39, 5460–5468. [Google Scholar] [CrossRef] [PubMed]
- Hoff, S.J.; Harmon, J.; Chen, L.; Janni, K.; Schmidt, D.; Nicolai, R.; Jacobson, L. Practical Partial Biofiltration of Swine Exhaust Ventilation Air. In Proceedings of the Mitigating Air Emissions from Animal Feeding Operations Conference, Des Moines, IA, USA, 19–21 May 2008; Iowa State University, College of Agriculture and Life Sciences: Ames, IA, USA, 2008; pp. 144–149. [Google Scholar]
- Nicolai, D.; Janni, J.; Schmidt, D. Biofiltration-Mitigating Odor and Gas Emissions from Animal Operations. In Proceedings of the Mitigating Air Emissions from Animal Feeding Operations Conference, Des Moines, IA, USA, 19–21 May 2008; Iowa State University, College of Agriculture and Life Sciences: Ames, IA, USA, 2008; pp. 150–154. [Google Scholar]
- Robert, R.; Treloar, R. Significant odor reduction from a highly efficient microecosystem based on Biofiltration. In Proceedings of the Mitigating Air Emissions from Animal Feeding Operations Conference, Des Moines, IA, USA, 19–21 May 2008; Iowa State University, College of Agriculture and Life Sciences: Ames, IA, USA, 2008; pp. 155–161. [Google Scholar]
- Melse, R.W.; Ogink, N.; Bosma, B. Mutli-pollutant scrubbers for removal of ammonia, odor and particulate matter from animal house exhaust air. In Proceedings of the Mitigating Air Emissions from Animal Feeding Operations Conference, Des Moines, IA, USA, 19–21 May 2008; Iowa State University, College of Agriculture and Life Sciences: Ames, IA, USA, 2008; pp. 162–168. [Google Scholar]
- Snell, H.G.J.; Schwarz, A. Development of an efficient bioscrubber system for the reduction of emissions. In Proceedings of the 2003 ASABE Annual Meeting, Las Vegas, NV, USA, 27–30 July 2003; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2013. [Google Scholar]
- Ullman, J.L. Remedial activities to reduce atmospheric pollutants from animal feeding operations. Agric. Eng. Int. 2005. Invited Overview Paper No. 9. [Google Scholar]
- Ford, S.E.; Riskowski, G.L. Effect of windbreak wall location on ventilation fan performance. Appl. Eng. Agric. 2003, 19, 343–346. [Google Scholar]
- Sweeten, J.M. Odor and dust from livestock feedlots. Texas Agricultural Extension Service: College Station, TX, USA, 1991. [Google Scholar]
- Nonnenmann, M.W.; Donham, K.J.; Rautiainen, R.H.; O’Shaughnessy, P.T.; Burmeister, L.F.; Reynolds, S.J. Vegetable oil sprinkling as a dust reduction method in swine confinement. J. Agric. Saf. Health 2004, 10, 7–15. [Google Scholar] [CrossRef]
- Sutton, A.L.; Kephart, K.B.; Verstegen, M.W.; Canh, T.T.; Hobbs, P.J. Potential for reduction of odorous compounds in swine manure through diet modification. J. Anim. Sci. 1999, 77, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Stowell, R.R.; Koppolu, L.; Schulte, D.D.; Koelsch, R.K. Applications of using the odor footprint tool. In Livestock Environment VII, Proceedings of the Seventh International Symposium, Beijing, China, 18–20 May 2005; American Society of Agricultural Engineers: St. Joseph, MI, USA, 2005; Number 701P0205. [Google Scholar]
- Maurer, D.; Koziel, J.A.; Harmon, J.D.; Hoff, S.J.; Rieck-Hinz, A.M.; Andersen, D.S. Summary of performance data for technologies to control gaseous, odor, and particulate emissions from livestock operations: Air Management Practices Assessment Tool (AMPAT). Data Brief. 2016, 7, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Pichat, P. A brief survery of the potential health risks of TiO2 particles and TiO2-containing photocatalytic or non-photocatalytic materials. J. Adv. Oxid. Technol. 2010, 13, 238–246. [Google Scholar]
- Ollis, D.F. Photocatalysis purification and remediation of contaminated air and water. Acad. Sci. Paris Ser. IIc Chem. 2000, 3, 405–411. [Google Scholar]
- Scheider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: mechanisms and material. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.M. Heterogeneous photocatalysis: state of the art and present applications. Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Sunada, K.; Kikuchi, Y.; Hashimoto, K.; Fujishima, A. Bactericidal and detoxification effects of TiO2 thin film photocatalysts. Environ. Sci. Technol. 1998, 32, 726–728. [Google Scholar] [CrossRef]
- Armon, R.; Narkis, N.; Neeman, I. Photocatalytic inactivation of different bacteria and bacteriophages in drinking water at different TiO2 concentration with or without exposure to O2. 2nd Int. TiO2 Conference on Photocatalytic Purification and Treatment of Water and Air, Cincinnati, OH. J. Adv. Oxid. Technol. 1998, 3, 145–150. [Google Scholar]
- Yang, X.; Koziel, J.A.; Cutler, T.; van Leeuwen, J.; Zhang, S.; Hoff, S.J.; Jenks, W.; Zimmerman, J. Treatment of livestock odor and pathogens with ultraviolet light. In Proceedings of the 2008 ASABE Annual Meeting, Rhode Island Convention Center, RI, USA, June 29 – July 2 2008; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2008. Paper No. 085198. [Google Scholar]
- Costa, A.; Chiarello, G.L.; Selli, E.; Guarino, M. Effects of TiO2 based photocatalytic paint on concentrations and emissions of pollutants and on animal performance in a swine weaning unit. J. Environ. Manag. 2012, 96, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Murphy, P.; Maghirang, R.; DeRouchey, J. Mitigation of air emissions from swine buildings through the photocatalytic technology using UV/TiO2. In Proceedings of the 2015 ASABE Annual Meeting, St. Joseph, MI, USA, 27 June 2008; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2008. Paper No. 152189332. [Google Scholar]
- Guarino, M.; Costa, A.; Porro, M. Photocatalytic TiO2 coating to reduce ammonia and greenhouse gases concentration and emission from animal husbandries. Bioresour. Technol. 2008, 99, 2650–2658. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, A.C.; Predicala, B.Z. Effect of nanoparticles on gas emissions and growth and transmission of microorganisms in swine production facilities. In Proceedings of the CSBE/SCGAB Annual International Meeting, Montreal, QC, Canada, 13–16 July 2014; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2014. Paper No. 141913472. [Google Scholar]
- Longstreth, J.; de Gruijl, F.R.; Kripke, M.L.; Abseck, S.; Arnold, F.; Slaper, H.I.; Velders, G.; Takizawa, Y.; van der Leun, J.C. Health risks. J. Photochem. Photobiol. B. 1998, 46, 20–39. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: present situation and future approaches. C.R. Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Yang, X.; Koziel, J.A.; Cai, L.; Hoff, S.J.; Harmon, J.D.; van Leeuwen, J.; Jenks, W.S.; Zimmerman, J.J.; Cutler, T.D. Novel treatment of odor and VOCs using photolysis. In Proceedings of the ASABE Annual Meeting, St. Joseph, MI, USA, 17–20 June 2007; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2007. Paper No. 074139. [Google Scholar]
- Rockafellow, E.M.; Koziel, J.A.; Jenks, W.S. Laboratory-scale investigation of UV treatment of ammonia for livestock and poultry barn exhaust applications. J. Environ. Qual. 2012, 41, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Feilberg, A. Characterisation of photocatalytic degradation of odorous compounds associated with livestock facilities by means of PTR-MS. Chem. Eng. J. 2015, 277, 341–351. [Google Scholar] [CrossRef]
- Akdeniz, N.; Koziel, J.A.; Ahn, H.K.; Glanville, T.; Crawford, B.; Raman, R.D. Laboratory scale evaluation of VOC emissions as indication of swine carcass degradation inside biosecure composting units. Bioresour. Technol. 2010, 101, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, W.; Koziel, J.A.; Cai, L.; Jenks, W.S.; Laor, Y.; van Leeuwen, J.; Hoff, S.J. Improved quantification of livestock associated odorous volatile organic compounds in a standard flow-through system using solid phase microextraction and gas chromatography-mass spectrometry. J. Chromatogr. A. 2015, 1414, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bereznicki, S.D.; Heber, A.J.; Akdeniz, N.; Jacobson, L.D.; Hetchler, B.P.; Heathcote, K.Y.; Hoff, S.J.; Koziel, J.A.; Cai, L.; Zhang, S.; et al. Odor and odorous chemical emissions from animal buildings: Part 1–project overview, collection methods and quality control. Trans. ASABE 2012, 55, 2325–2334. [Google Scholar] [CrossRef]
- Peral, J.; Domènech, X.; Ollis, D.F. Heterogeneous photocatalysis for purification, decontamination and deodorization of air. J. Chem. Technol. Biotechnol. 1997, 70, 117–140. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X. Photocatalytic oxidation for indoor air purification: a literature review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Yang, X.; Koziel, J.A.; Laor, Y.; Zhu, W.; van Leeuwen, H.; Jenks, W.; Hoff, S.J.; Zimmerman, J.; Zhang, S.; Saadi, I.; et al. UV light treatment of gaseous emissions tested on synthetic and real livestock manure atmosphere. Atmos. Environ. 2017. in preparation. [Google Scholar]
- Cai, L.; Koziel, J.A.; Zhang, S.; Heber, A.J.; Cortus, E.L.; Parker, D.B.; Hoff, S.J.; Sun, G.; Heathcote, K.Y.; Jacobson, L.D.; et al. Odor and Odorous Chemical Emissions from Animal Buildings: Part 3–Chemical Emissions. Trans. ASABE 2015, 58, 1333–1347. [Google Scholar]
- Koziel, J.A.; Martos, P.; Pawliszyn, J. System for generation of standard gas mixtures of volatile and semivolatile organic compounds for calibrations of solid phase microextraction and other sampling devices. J. Chromatogr. A 2004, 1025, 3–9. [Google Scholar] [CrossRef] [PubMed]
| References | Catalyst/Support Material | Light Source | Reactant | Environment | Results | Comments |
|---|---|---|---|---|---|---|
| [29] | Evonik P25 TiO2 5-stage honeycomb filter | UV-C (254 nm) | H2S, NH3, CH4 in exhaust air | Two swine nursery houses at Kansas State Universities Segregated Early Wean Unit. Each house is 1.3 × 0.7 × 0.2 m, with 200 head capacity | Reduction of H2S at 5.3–22.8 ppb/sec; NH3 at 0.3–1.7 ppm/sec | To achieve sufficient reduction rate, suggested solutions are: using 10-stage honeycomb with 1 m2 TiO2-coated filter; or locate the filter at low air velocity position; or modify geometry of honeycomb |
| [28] | Active PPS TiO2 70 g/m2 on wall | Full-spectrum light (solar spectrum lamp) | Particulate matter, NH3, greenhouse gases in barn indoor air | Two identical weaning units in northern Italy, with 391 pigs in each unit | Significant reduction of CH4 (27%, p < 0.05), PM10 (17%, p < 0.01) | - |
| [30] | Active PPS 70 g/m2 on wall | UV-A | NH3, CH4, CO2 in barn indoor air | Two identical farrowing rooms. Each room is 10.9 × 17.3 × 2.4 m, with 30 sows and 10 piglets with each sow | Significant reduction of NH3 (30.37%, p < 0.001), CH4 (15%, p < 0.001), CO2 (10.52%, p < 0.001), N2O (3.92%, p < 0.001) | Ventilation rate has high impact on pollutant abatement |
| [31] | ZnO nanoparticle Ventilation air recirculation system | Not specified | CH4, CO2, bioaerosol in exhaust air | Two identical chambers in Prairie Swine Center barn in Canada. Each chamber is 4.2 × 3.6 × 2.7 m, with 6 grower pigs | No significant impact on GHGs. Filter with ZnO effectively reduced bioaerosol at 5 days after installation, but increased after 5 days. | No significant impact on swine production performance |
| [36] | TiO2-coated ceramic filter | UV-A (black light lamp) | H2S, volatile organic compounds (VOCs) | Laboratory | Low reduction of H2S Above 80% removal of VOCs at low flow and low concentration; low reduction rates at high flow rates | Humidity has no impact on photocatalysis efficiency at 40–80% |
| [27] | Evonik P25 TiO2 | UV-C (254 nm, 185 nm) | VOCs | Laboratory | Complete reduction of VOCs after 112 s photocatalysis reaction; over 50% reduction after 18.5 s reaction | - |
| Compound | Standard Solution Concentration (mg/mL) | Air Flow at 300 mL/min 40 s Treatment Time | Air Flow at 60 mL/min 200 s Treatment Time | ||
|---|---|---|---|---|---|
| Gas Concentration (ng/mL) * | Gas Concentration (ppb) * | Gas Concentration (ng/mL) * | Gas Concentration (ppb) * | ||
| DMDS | 0.678 | 0.038 | 9.84 | 0.113 | 29.3 |
| DEDS | 0.200 | 0.011 | 4.32 | 0.033 | 13.0 |
| DMTS | 0.678 | 0.038 | 7.34 | 0.113 | 21.8 |
| Butyric acid | 19.2 | 1.069 | 296 | 3.208 | 888 |
| Guaiacol | 0.444 | 0.025 | 4.91 | 0.074 | 14.5 |
| p-cresol | 6.598 | 0.367 | 82.8 | 1.100 | 248 |
| Compound | 40 s Treatment Time | 200 s Treatment Time | ||||||
|---|---|---|---|---|---|---|---|---|
| Rep 1 RSD | Rep 2 RSD | Average % Reduction | Total RSD | Rep 1 RSD | Rep 2 RSD | Average % Reduction | Total RSD | |
| DMDS | 21.6% | 8.6% | 35.0 ± 6.0 | 17.0% | 81.2% | 25.5% | 40.4 ± 24.8 | 61.4% |
| DEDS | 37.7% | 25.7% | 27.7 ± 9.4 | 34.1% | 24.7% | 1.9% | 81.0 ± 16.7 | 20.7% |
| DMTS | 34.3% | 24.2% | 37.1 ± 11.7 | 31.5% | 35.8% | 3.3% | 76.3 ± 22.1 | 29.0% |
| Butyric acid | 22.9% | 10.7% | 62.2 ± 7.5 | 12.0% | 19.6% | 0.7% | 86.9 ± 14.2 | 16.3% |
| Guaiacol | 47.6% | 36.7% | 37.4 ± 11.3 | 30.3% | 0.0% | 0.0% | 100.0 ± 0.0 | 0.0% |
| p-cresol | 42.7% | 34.0% | 27.4 ± 8.3 | 30.2% | 2.2% | 1.7% | 93.8 ± 1.8 | 1.9% |
| Compound | 10 µg/cm2 | 1.0 µg/cm2 | 0.1 µg/cm2 | p-value |
|---|---|---|---|---|
| DMDS | 33.9 ± 27.5 | −18.5 ± 2.4 | 5.7 ± 34.5 | 0.0518 |
| DEDS | 75.8 ± 18.7 | 8.2 ± 5.7 | 12.1 ± 30.3 | 0.0011 * |
| DMTS | 69.8 ± 25.0 | 12.1 ± 3.3 | 10.7 ± 41.6 | 0.0148 * |
| Butyric acid | 82.8 ± 16.2 | 20.7 ± 3.3 | 20.1 ± 40.7 | 0.0035 * |
| Guaiacol | 100.0 ± 0.0 | 77.5 ± 3.9 | 32.3 ± 34.6 | 0.0008 * |
| p-cresol | 93.9 ± 2.1 | 70.3 ± 3.1 | 24.6 ± 17.4 | 0.0000 * |
| Compound | RH = 40% | RH = 75% | RH = 90% | p-value |
|---|---|---|---|---|
| DMDS | 32.74 ± 9.07 | 37.73 ± 18.44 | −0.91 ± 6.44 | 0.0174 * |
| DEDS | 28.17 ± 15.20 | 28.41 ± 21.21 | 25.26 ± 7.09 | 0.9630 |
| DMTS | 49.79 ± 6.48 | 33.26 ± 11.83 | 29.85 ± 5.40 | 0.0557 |
| Butyric acid | 56.57 ± 5.76 | 27.35 ± 21.01 | 43.09 ± 5.80 | 0.0864 |
| Guaiacol | 41.63 ± 7.35 | 23.26 ± 3.46 | 50.45 ± 6.30 | 0.0171 * |
| p-cresol | 31.47 ± 7.19 | 26.67 ± 3.46 | 46.22 ± 2.07 | 0.0058 * |
| Compound | 20 °C | 30 °C | 40 °C | p-value |
|---|---|---|---|---|
| DMDS | −17.26 ± 17.61 | 39.48 ± 6.47 | 32.74 ± 9.07 | 0.0023 * |
| DEDS | 17.05 ± 5.86 | 51.75 ± 0.17 | 28.17 ± 15.20 | 0.0106 * |
| DMTS | 30.91 ± 6.82 | 54.55 ± 5.43 | 49.79 ± 6.48 | 0.0081 * |
| Butyric acid | 66.91 ± 2.01 | 71.26 ± 2.68 | 56.57 ± 5.76 | 0.0088 * |
| Guaiacol | 66.34 ± 9.31 | 55.65 ± 5.14 | 41.63 ± 7.35 | 0.0188 * |
| p-cresol | 49.87 ± 5.75 | 45.12 ± 2.09 | 31.47 ± 7.19 | 0.0149 * |
| Dust Accumulation Time | Absorption | RSD |
|---|---|---|
| 1 week | 15.33 ± 2.33% | 15.18% |
| 2 weeks | 15.05 ± 4.49% | 29.81% |
| 3 weeks | 14.83 ± 10.88% | 73.39% |
| 4 weeks | 15.01 ± 0.22% | 1.46% |
| Average | 15.06 ± 5.30% | 35.17% |
| Compound | Reduction Rate (%) at a Treatment Time of 200 s with a 10-µg/cm2 Coating | ||
|---|---|---|---|
| Clean | Dusty | p-value | |
| DMDS | 40.4 ± 24.8 | 35.0 ± 11.5 | 0.6270 |
| DEDS | 81.0 ± 16.7 | 78.8 ± 8.3 | 0.7790 |
| DMTS | 76.3 ± 22.1 | 80.7 ± 7.3 | 0.6480 |
| Butyric acid | 86.9 ± 14.2 | 81.9 ± 10.3 | 0.4760 |
| Guaiacol | 100.0 ± 0.0 | 100.0 ± 0.0 | 0.4350 |
| p-cresol | 93.8 ± 1.8 | 91.6 ± 5.0 | 0.2300 |
| Compound | 10 µg /cm2 TiO2 in PureTi Coating | 250 µg/cm2 TiO2 in Evonik Coating | p-Value |
|---|---|---|---|
| DMDS | 33.89 ± 27.52% | 42.88 ± 23.93% | 0.6460 |
| DEDS | 75.80 ± 18.74% | 88.03 ± 5.20% | 0.3180 |
| DMTS | 69.76 ± 24.97% | 93.99 ± 2.29% | 0.4190 |
| butyric acid | 82.81 ± 16.20% | 97.82 ± 2.27% | 0.1660 |
| guaiacol | 100.00 ± 0.00% | 94.09 ± 10.24% | 0.1700 |
| p-cresol | 93.85 ± 2.07% | 90.23 ± 9.56% | 0.3750 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Koziel, J.A.; Maurer, D.L. Mitigation of Livestock Odors Using Black Light and a New Titanium Dioxide-Based Catalyst: Proof-of-Concept. Atmosphere 2017, 8, 103. https://doi.org/10.3390/atmos8060103
Zhu W, Koziel JA, Maurer DL. Mitigation of Livestock Odors Using Black Light and a New Titanium Dioxide-Based Catalyst: Proof-of-Concept. Atmosphere. 2017; 8(6):103. https://doi.org/10.3390/atmos8060103
Chicago/Turabian StyleZhu, Wenda, Jacek A. Koziel, and Devin L. Maurer. 2017. "Mitigation of Livestock Odors Using Black Light and a New Titanium Dioxide-Based Catalyst: Proof-of-Concept" Atmosphere 8, no. 6: 103. https://doi.org/10.3390/atmos8060103
APA StyleZhu, W., Koziel, J. A., & Maurer, D. L. (2017). Mitigation of Livestock Odors Using Black Light and a New Titanium Dioxide-Based Catalyst: Proof-of-Concept. Atmosphere, 8(6), 103. https://doi.org/10.3390/atmos8060103