Concentrations and Size Distributions of Bacteria-Containing Particles over Oceans from China to the Arctic Ocean
Abstract
:1. Introduction
2. Experimental Methods
2.1. Sampling Data
2.2. Bacterial Cultivation
2.3. Ancillary Data
3. Results and Discussion
3.1. Concentrations of Bacteria-Containing Particles
3.2. Size Distributions
3.3. Comparison with Literature Data
3.4. Potential Meteorological Factors Influencing the Variation of Concentrations of Airborne Bacteria
3.4.1. Temperature
3.4.2. Relative Humidity and Precipitation
3.4.3. Wind Speed
3.5. The Role of Sea Ice in the Arctic Ocean
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Després, V.R.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.S.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.O.; Pöschl, U.; et al. Primary biological aerosol particles in the atmosphere: A review. Tullus B 2012, 64, 15598. [Google Scholar] [CrossRef]
- Burrows, S.M.; Butler, T.; Jöckel, P.; Tost, H.; Kerkweg, A.; Pöschl, U.; Lawrence, M.G. Bacteria in the global atmosphere—Part 2: Modeling of emissions and transport between different ecosystems. Atmos. Chem. Phys. 2009, 9, 9281–9297. [Google Scholar] [CrossRef]
- Blanchard, D.C. The ejection of drops from the sea and their enrichment with bacteria and other materials: A review. Estuaries 1989, 12, 127–137. [Google Scholar] [CrossRef]
- Christner, B.C.; Morris, C.E.; Foreman, C.M.; Cai, R.; Sands, D.C. Ubiquity of biological ice nucleators in snowfall. Science 2008, 319, 1214. [Google Scholar] [CrossRef] [PubMed]
- Pöschl, U.; Martin, S.; Sinha, B.; Chen, Q.; Gunthe, S.; Huffman, J.; Borrmann, S.; Farmer, D.; Garland, R.; Helas, G. Rainforest aerosols as biogenic nuclei of clouds and precipitation in the amazon. Science 2010, 329, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Lighthart, B. The ecology of bacteria in the alfresco atmosphere. FEMS Microbiol. Ecol. 1997, 23, 263–274. [Google Scholar] [CrossRef]
- Bauer, H.; Kasper-Giebl, A.; Löflund, M.; Giebl, H.; Hitzenberger, R.; Zibuschka, F.; Puxbaum, H. The contribution of bacteria and fungal spores to the organic carbon content of cloud water, precipitation and aerosols. Atmos. Res. 2002, 64, 109–119. [Google Scholar] [CrossRef]
- Amato, P.; Ménager, M.; Sancelme, M.; Laj, P.; Mailhot, G.; Delort, A.-M. Microbial population in cloud water at the puy de dôme: Implications for the chemistry of clouds. Atmos. Environ. 2005, 39, 4143–4153. [Google Scholar] [CrossRef]
- Dueker, M.E.; Weathers, K.C.; O’Mullan, G.D.; Juhl, A.R.; Uriarte, M. Environmental controls on coastal coarse aerosols: Implications for microbial content and deposition in the near-shore environment. Environ. Sci. Technol. 2011, 45, 3386–3392. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qi, J.; Zhang, H.; Huang, S.; Li, L.; Gao, D. Concentration and size distribution of bioaerosols in an outdoor environment in the qingdao coastal region. Sci. Total Environ. 2011, 409, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- Montero, A.; Dueker, M.E.; O’Mullan, G.D. Culturable bioaerosols along an urban waterfront are primarily associated with coarse particles. PeerJ 2016, 4, e2827. [Google Scholar] [CrossRef] [PubMed]
- Matrai, P.A.; Tranvik, L.; Leck, C.; Knulst, J.C. Are high arctic surface microlayers a potential source of aerosol organic precursors? Mar. Chem. 2008, 108, 109–122. [Google Scholar] [CrossRef]
- Orellana, M.V.; Matrai, P.A.; Leck, C.; Rauschenberg, C.D.; Lee, A.M.; Coz, E. Marine microgels as a source of cloud condensation nuclei in the high arctic. PNAS 2011, 108, 13612–13617. [Google Scholar] [CrossRef] [PubMed]
- Zuraimi, M.S.; Fang, L.; Tan, T.K.; Chew, F.T.; Tham, K.W. Airborne fungi in low and high allergic prevalence child care centers. Atmos. Environ. 2009, 43, 2391–2400. [Google Scholar] [CrossRef]
- Harrison, R.M.; Jones, A.M.; Biggins, P.D.E.; Pomeroy, N.; Cox, C.S.; Kidd, S.P.; Hobman, J.L.; Brown, N.L.; Beswick, A. Climate factors influencing bacterial count in background air samples. Int. J. Biometeorol. 2005, 49, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, B.T.; Lighthart, B. Survey of culturable airborne bacteria at four diverse locations in oregon: Urban, rural, forest, and coastal. Microb. Ecol. 1997, 34, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Vladavets, V.; Mats, L. The influence of meteorological factors on the microflora. Microbiologya 1958, 59, 539–544. [Google Scholar]
- Kelly, C.D.; Pady, S.M. Microbiological studies of air masses over montreal during 1950 and 1951. Can. J. Bot. 1954, 32, 591–600. [Google Scholar] [CrossRef]
- Prospero, J.M.; Blades, E.; Mathison, G.; Naidu, R. Interhemispheric transport of viable fungi and bacteria from africa to the caribbean with soil dust. Aerobiologia 2005, 21, 1–19. [Google Scholar] [CrossRef]
- Marks, R.; Kruczalak, K.; Jankowska, K.; Michalska, M. Bacteria and fungi in air over the gulf of gdańsk and baltic sea. J. Aerosol Sci. 2001, 32, 237–250. [Google Scholar] [CrossRef]
- Baylor, E.; Baylor, M.; Blanchard, D.; Syzdek, L.; Appel, C. Virus transfer from surf to wind. Science 1977, 198, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Aller, J.Y.; Kuznetsova, M.R.; Jahns, C.J.; Kemp, P.F. The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. J. Aerosol Sci. 2005, 36, 801–812. [Google Scholar] [CrossRef]
- Cho, B.C.; Hwang, C.Y. Prokaryotic abundance and 16s rrna gene sequences detected in marine aerosols on the east sea (Korea). FEMS Microbiol. Ecol. 2011, 76, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Dueker, M.E.; O‘Mullan, G.D.; Weathers, K.C.; Juhl, A.R.; Uriarte, M. Coupling of fog and marine microbial content in the near-shore coastal environment. Biogeosciences 2012, 9, 803–813. [Google Scholar] [CrossRef]
- Urbano, R.; Palenik, B.; Gaston, C.; Prather, K. Detection and phylogenetic analysis of coastal bioaerosols using culture dependent and independent techniques. Biogeosciences 2011, 8, 301. [Google Scholar] [CrossRef]
- Griffin, D.W. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Hallar, A.; Chirokova, G.; McCubbin, I.; Painter, T.H.; Wiedinmyer, C.; Dodson, C. Atmospheric bioaerosols transported via dust storms in the western united states. Geophys. Res. Lett. 2011. [Google Scholar] [CrossRef]
- Burrows, S.M.; Elbert, W.; Lawrence, M.G.; Pöschl, U. Bacteria in the global atmosphere—Part 1: Review and synthesis of literature data for different ecosystems. Atmos. Chem. Phys. 2009, 9, 9263–9280. [Google Scholar] [CrossRef]
- Clark, S.; Rylander, R.; Larsson, L. Airborne bacteria, endotoxin and fungi in dust in poultry and swine confinement buildings. Am. Ind. Hyg. Assoc. J. 1983, 44, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, J.-X.; Huang, H.-Y.; Zhu, B. Development and evaluation of 16s rdna microarray for detecting bacterial pathogens in cerebrospinal fluid. Exp. Biol. Med. 2005, 230, 587–591. [Google Scholar] [CrossRef]
- Slabova, O.I.; Nikitin, D.I. Influence of the incubation temperature on the reaction of oligotrophic bacteria to stress. Microbiology 2004, 73, 650–653. [Google Scholar] [CrossRef]
- Li, E.; Wu, J.; Wang, P.; Zhang, D. D-Phenylalanine inhibits biofilm development of a marine microbe, Pseudoalteromonas sp. SC2014. FEMS Microbiol. Lett. 2016, 363, fnw198. [Google Scholar] [CrossRef] [PubMed]
- Boreson, J.; Dillner, A.M.; Peccia, J. Correlating bioaerosol load with PM2.5 and PM10cf concentrations: A comparison between natural desert and urban-fringe aerosols. Atmos. Environ. 2004, 38, 6029–6041. [Google Scholar] [CrossRef]
- Kuske, C.R.; Barns, S.M.; Grow, C.C.; Merrill, L.; Dunbar, J. Environmental survey for four pathogenic bacteria and closely related species using phylogenetic and functional genes. J. Forensic Sci. 2006, 51, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.W.; Westphal, D.L.; Gray, M.A. Airborne microorganisms in the african desert dust corridor over the mid-atlantic ridge, Ocean Drilling Program, Leg 209. Aerobiologia 2006, 22, 211–226. [Google Scholar] [CrossRef]
- Sobek, A.; Olli, K.; Gustafsson, Ö. On the relative significance of bacteria for the distribution of polychlorinated biphenyls in arctic ocean surface waters. Environ. Sci. Technol. 2006, 40, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Deguillaume, L.; Leriche, M.; Amato, P.; Ariya, P.A.; Delort, A.M.; Pöschl, U.; Chaumerliac, N.; Bauer, H.; Flossmann, A.I.; Morris, C.E. Microbiology and atmospheric processes: Chemical interactions of primary biological aerosols. Biogeosciences 2008, 5, 1073–1084. [Google Scholar] [CrossRef]
- Lighthart, B.; Shaffer, B.T. Bacterial flux from chaparral into the atmosphere in mid-summer at a high desert location. Atmos. Environ. 1994, 28, 1267–1274. [Google Scholar] [CrossRef]
- Morris, C.; Georgakopoulos, D.; Sands, D. Ice nucleation active bacteria and their potential role in precipitation. J. Phys. 2004, 121, 87–103. [Google Scholar] [CrossRef]
- Jones, A.M.; Harrison, R.M. The effects of meteorological factors on atmospheric bioaerosol concentrations—A review. Sci. Total Environ. 2004, 326, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, J.; Upper, C.D. Aerial dispersal of epiphytic bacteria over bean plants. Appl. Environ. Microbiol. 1985, 50, 1229–1232. [Google Scholar] [PubMed]
- Mouli, P.; Mohan, S.; Reddy, S. Assessment of microbial(bacteria) concentrations of ambient air at semi-arid urban region: Influence of meteorological factors. Appl. Ecol. Environ. Res. 2005, 3, 139–149. [Google Scholar] [CrossRef]
- Tong, Y.; Lighthart, B. Diurnal distribution of total and culturable atmospheric bacteria at a rural site. Aerosol Sci. Technol. 1999, 30, 246–254. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Thomas, D.N. Large scale importance of sea ice biology in the southern ocean. Antarct. Sci. 2004, 16, 471–486. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Lin, S.; Capone, D.G. Bacterial activity in south pole snow. Appl. Environ. Microbiol. 2000, 66, 4514–4517. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Li, Z.; Cheng, B.; Lei, R.; Zhang, R. Sea ice surface features in arctic summer 2008: Aerial observations. Remote Sens. Environ. 2010, 114, 693–699. [Google Scholar] [CrossRef]
- Harding, T.; Jungblut, A.D.; Lovejoy, C.; Vincent, W.F. Microbes in high arctic snow and implications for the cold biosphere. Appl. Environ. Microbiol. 2011, 77, 3234–3243. [Google Scholar] [CrossRef] [PubMed]
- Ariya, P.A.; Domine, F.; Kos, G.; Amyot, M.; Côté, V.; Vali, H.; Lauzier, T.; Kuhs, W.F.; Techmer, K.; Heinrichs, T.; et al. Snow–A photobiochemical exchange platform for volatile and semi-volatile organic compounds with the atmosphere. Environ. Chem. 2011, 8, 62–73. [Google Scholar] [CrossRef]
- Møller, A.K.; Barkay, T.; Al-Soud, W.A.; Sorensen, S.J.; Skov, H.; Kroer, N. Diversity and characterization of mercury-resistant bacteria in snow, freshwater and sea-ice brine from the high arctic. FEMS Microbiol. Ecol. 2011, 75, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.; Deming, J. Elevated bacterial abundance and exopolymers in saline frost flowers and implications for atmospheric chemistry and microbial dispersal. Geophys. Res. Lett. 2010. [Google Scholar] [CrossRef]
- Leck, C.; Bigg, E.K. Biogenic particles in the surface microlayer and overlaying atmosphere in the central arctic ocean during summer. Tellus B 2005, 57, 305–316. [Google Scholar] [CrossRef]
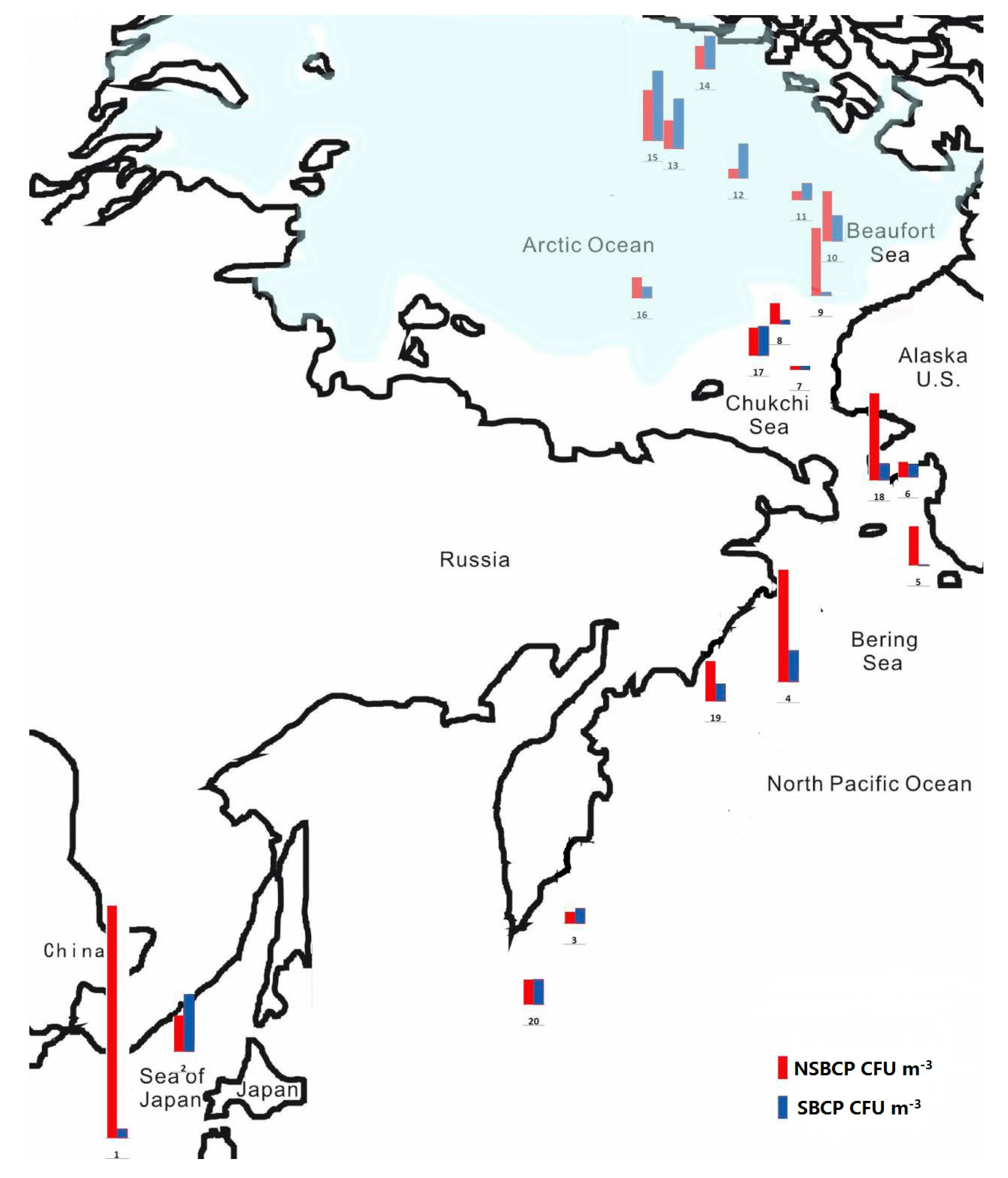



| Site | Date | UTC Time | Sampling Duration (min) | Coordinate | Location | Wind Speed (m/s) | Wind Direction (°) | RH (%) | T (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 14 July | 1:40 | 10 | 133° E 38° N | East China Sea | 13 | 36 | 89 | 22.7 |
| 2 | 17 July | 23:30 | 10 | 139° E 45° N | Sea of Japan | 11.3 | 66 | 99 | 17.5 |
| 3 | 22 July | 22:30 | 15 | 161° E 51° N | North Pacific Ocean | 4 | 195 | 99 | 11.2 |
| 4 | 25 July | 22:30 | 15 | 179° E 59° N | North Pacific Ocean | 14 | 282 | 90 | 7 |
| 5 | 31 July | 1:00 | 15 | 169° W 62° N | Bering Sea | 15.1 | 131 | 86 | 5.5 |
| 6 | 2 August | 23:00 | 15 | 165° W 64° N | Bering Sea | 5.6 | 237 | 100 | 1.2 |
| 7 | 5 August | 21:30 | 18 | 168° W 72° N | Chukchi Sea | 7.8 | 128 | 99 | 0 |
| 8 | 8 August | 21:30 | 18 | 157° W 73° N | Chukchi Sea | 3.3 | 245 | 94 | 3.5 |
| 9 | 11 August | 21:30 | 20 | 152° W 75° N | Arctic Ocean | 10.1 | 7 | 89 | 0.2 |
| 10 | 14 August | 21:30 | 20 | 147° W 76° N | Beaufort Sea | 18.4 | 298 | 100 | 0.8 |
| 11 | 17 August | 21:30 | 20 | 147° W 81° N | Arctic Ocean | 9 | 329 | 92 | 0.2 |
| 12 | 21 August | 22:00 | 20 | 144° W 84° N | Arctic Ocean | 1 | 176 | 99 | 0.6 |
| 13 | 26 August | 22:00 | 20 | 147° W 85° N | Arctic Ocean | 14.4 | 28 | 98 | −0.5 |
| 14 | 27 August | 22:40 | 20 | 102° W 85° N | Arctic Ocean | 11.6 | 125 | 100 | −1.7 |
| 15 | 31 August | 21:40 | 20 | 148° W 84° N | Arctic Ocean | 0.9 | 203 | 100 | −3.6 |
| 16 | 2 September | 21:40 | 20 | 170° W 80° N | Arctic Ocean | 2 | 47 | 100 | −4.7 |
| 17 | 6 September | 21:50 | 20 | 169° W 73° N | Chukchi Sea | 15.5 | 131 | 97 | 1.7 |
| 18 | 9 September | 22:10 | 20 | 165° W 64° N | Bering Sea | 6.3 | 47 | 63 | 15 |
| 19 | 12 September | 22:00 | 20 | 170° E 59° N | North Pacific Ocean | 9.9 | 216 | 91 | 12.5 |
| 20 | 15 September | 23:10 | 20 | 151° E 44° N | Sea of Okhotsk | 4 | 222 | 92 | 12.8 |
| Species | Whole Cruise | Group I | Group II | Group III | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WS | RH | T | WS | RH | T | WS | RH | T | WS | RH | T | ||
| TBCP | Pearson Correlation | 296 | −291 | 516 * | −063 | −171 | −365 | 101 | −858 * | 878 * | 028 | 236 | −242 |
| Sig. (2-tailed) | 205 | 214 | 020 | 906 | 746 | 477 | 848 | 029 | 022 | 947 | 574 | 563 | |
| N | 20 | 20 | 20 | 6 | 6 | 6 | 6 | 6 | 6 | 8 | 8 | 8 | |
| NSBCP | Pearson Correlation | 312 | −414 | 564 ** | 112 | −322 | −212 | 006 | −965 ** | 976 ** | 216 | −240 | −099 |
| Sig. (2-tailed) | 180 | 069 | 010 | 832 | 534 | 686 | 991 | 002 | 001 | 607 | 568 | 816 | |
| N | 20 | 20 | 20 | 6 | 6 | 6 | 6 | 6 | 6 | 8 | 8 | 8 | |
| SBCP | Pearson Correlation | −019 | 316 | −087 | −180 | −653 | −660 | 302 | −083 | 115 | −174 | 559 | −234 |
| Sig. (2-tailed) | 937 | 175 | 714 | 732 | 160 | 153 | 561 | 875 | 829 | 680 | 150 | 577 | |
| N | 20 | 20 | 20 | 6 | 6 | 6 | 6 | 6 | 6 | 8 | 8 | 8 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Yu, X.; Kang, H.; Xie, Z.; Zhang, P. Concentrations and Size Distributions of Bacteria-Containing Particles over Oceans from China to the Arctic Ocean. Atmosphere 2017, 8, 82. https://doi.org/10.3390/atmos8050082
Li M, Yu X, Kang H, Xie Z, Zhang P. Concentrations and Size Distributions of Bacteria-Containing Particles over Oceans from China to the Arctic Ocean. Atmosphere. 2017; 8(5):82. https://doi.org/10.3390/atmos8050082
Chicago/Turabian StyleLi, Ming, Xiawei Yu, Hui Kang, Zhouqing Xie, and Pengfei Zhang. 2017. "Concentrations and Size Distributions of Bacteria-Containing Particles over Oceans from China to the Arctic Ocean" Atmosphere 8, no. 5: 82. https://doi.org/10.3390/atmos8050082
APA StyleLi, M., Yu, X., Kang, H., Xie, Z., & Zhang, P. (2017). Concentrations and Size Distributions of Bacteria-Containing Particles over Oceans from China to the Arctic Ocean. Atmosphere, 8(5), 82. https://doi.org/10.3390/atmos8050082





