Effect of Werner-Type Complex Formation of Cu2+ and Fe2+ on Oxidative Potentials Assessed Using Ascorbic Acid Assay
Abstract
1. Introduction
2. Method
2.1. Reagents
2.2. Determination of Metal Ion Concentration Used for Testing
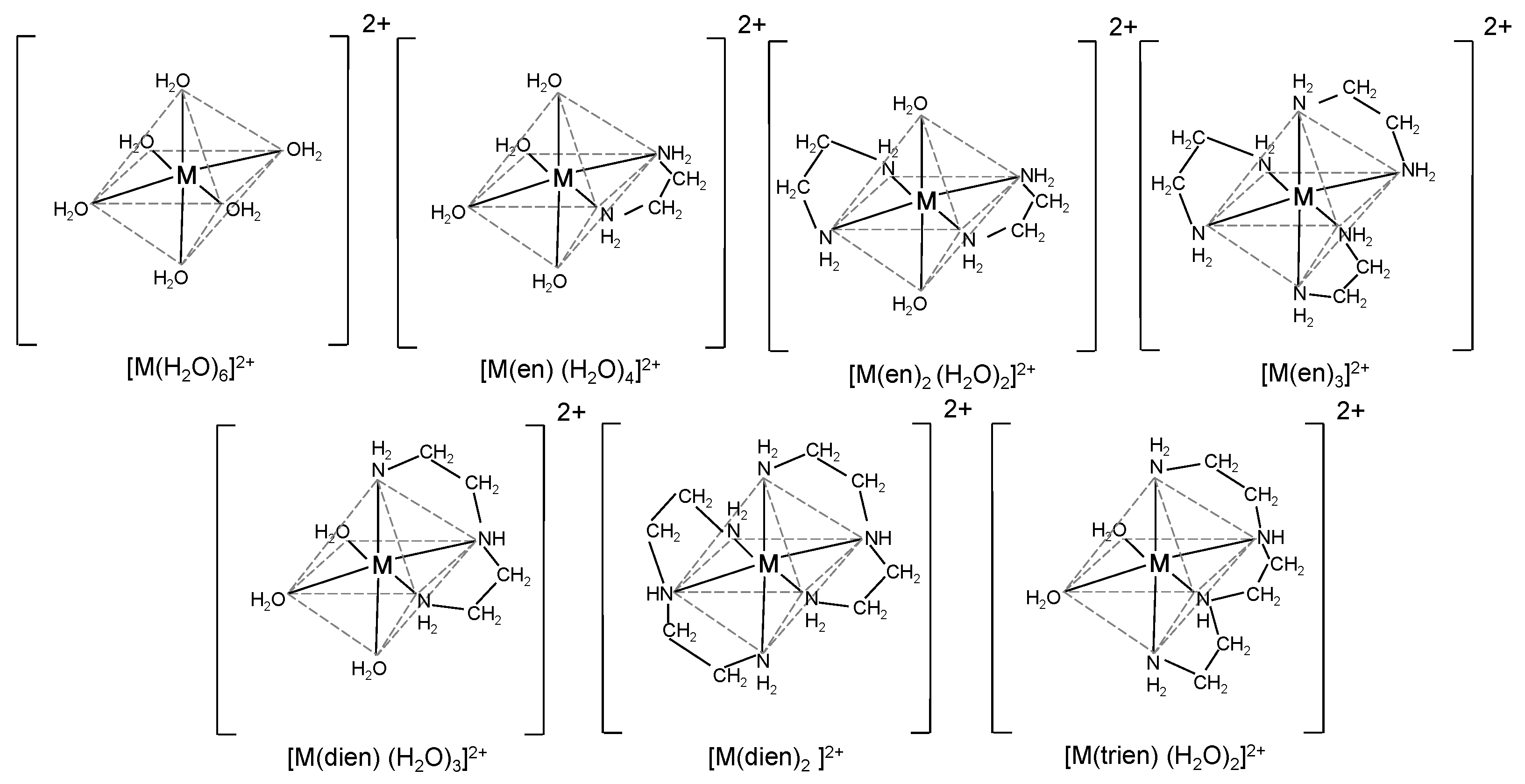
2.3. Preparation of Metal Complexes with Different Coordination Structures
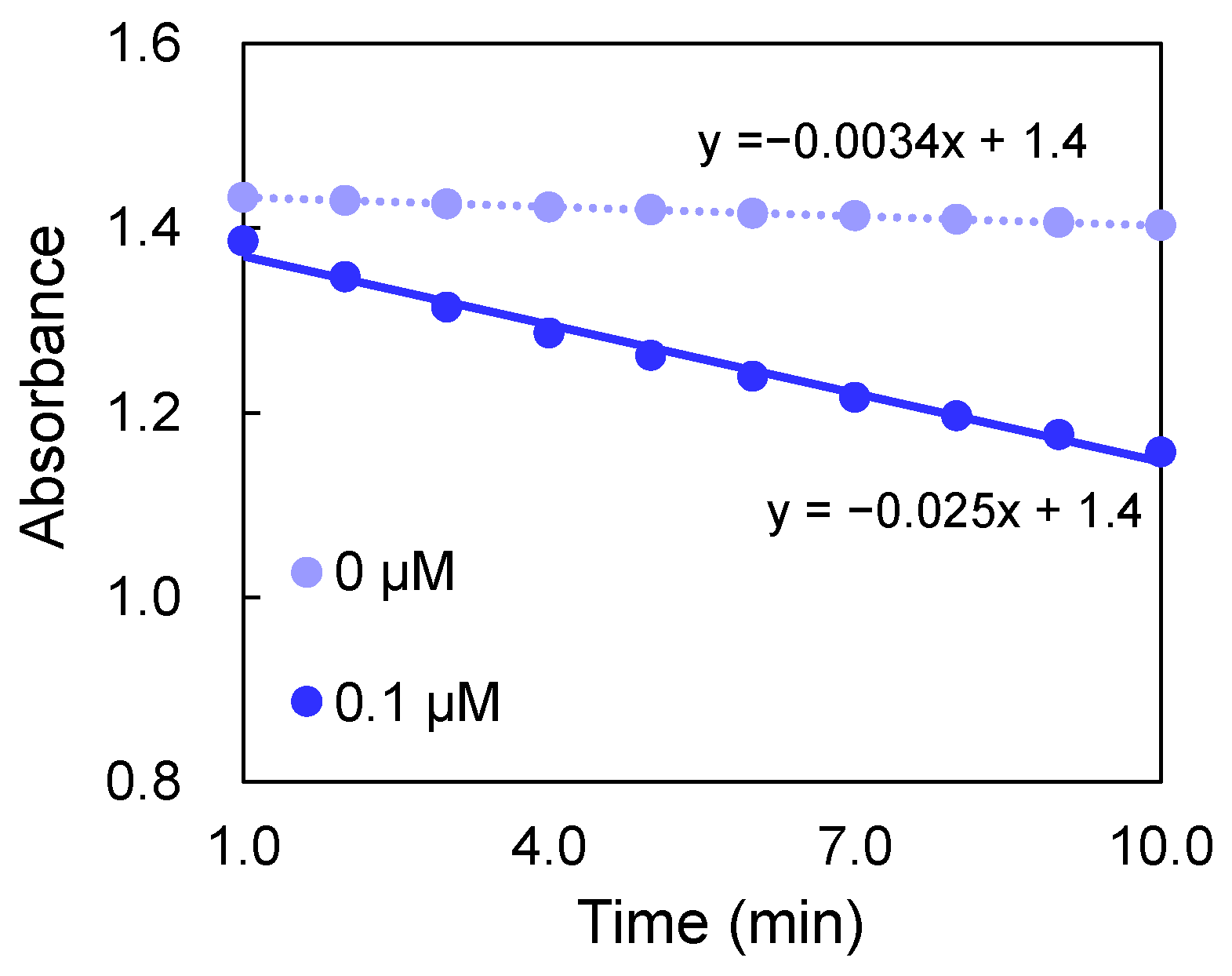
2.4. Measurement of AA Depletion Rate
2.5. Measurement of ORP
2.6. Estimation of Coordination Structures in Sample Solutions
3. Results
3.1. Rate of AA Depletion by Metal Ions
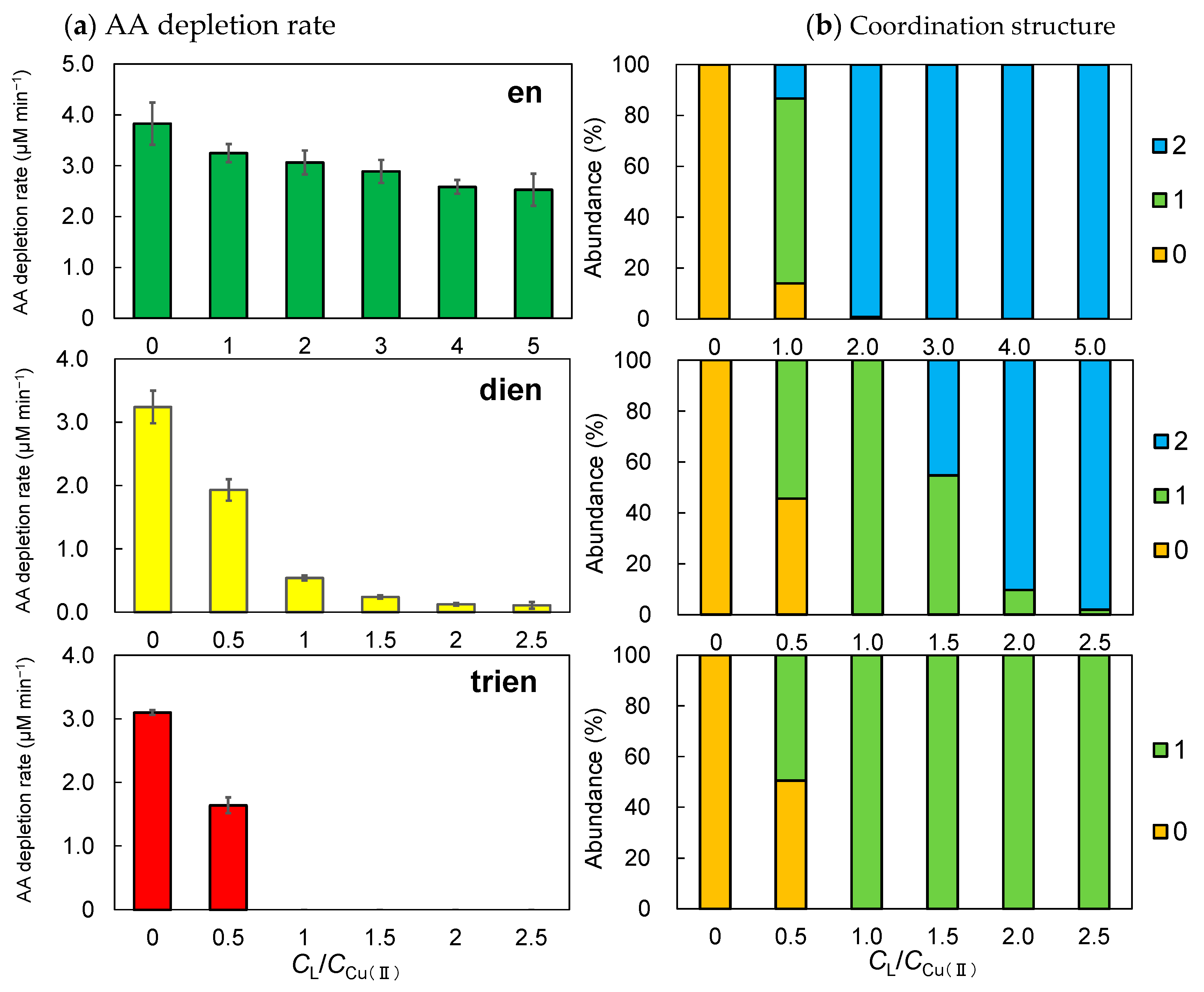
3.2. Rate of AA Depletion by Ethyleneamines
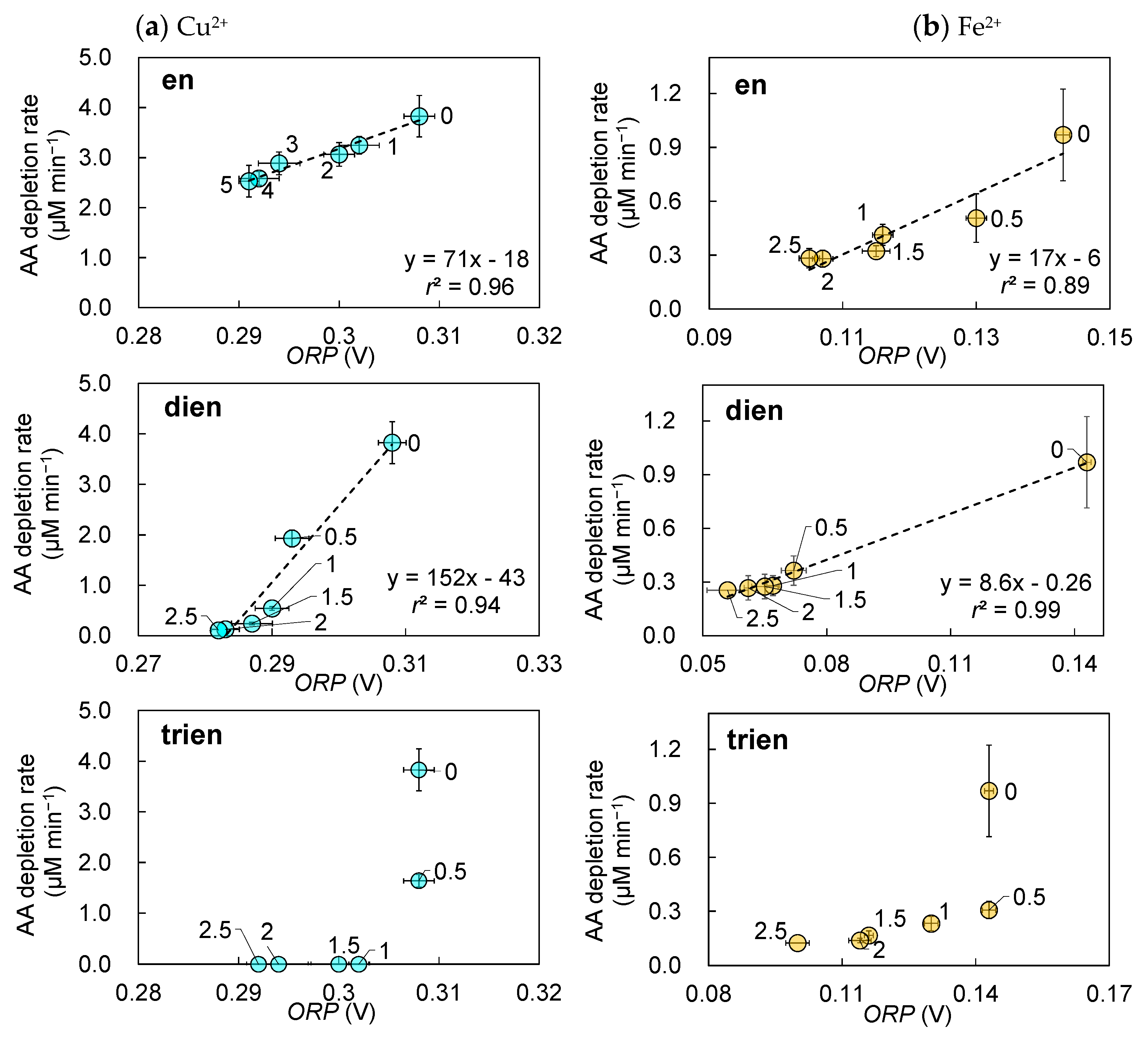
3.3. Changes in the AA Depletion Rate Owing to the Formation of Cu2+ Complexes
3.4. Changes in the AA Depletion Rate Owing to the Formation of Fe2+ Complexes
4. Discussion
- Stability constants: The stability constants of the Fe2+- and Cu2+-trien complexes, log β1, are 7.69 and 20.32, respectively (Table 2). The complexes with higher stability constants tended to exhibit lower oxidative activity. This observation aligns with the results of studies examining the oxidative activity of Cu2+ complexes with ethyleneamine derivatives [39].
- The Jahn–Teller effect: Cu2+, with its 3d9 electron configuration, experiences significant electrostatic repulsion with the negatively charged ligands in an octahedral complex. To stabilize the structure, it undergoes distortion from an ideal octahedral shape owing to the Jahn–Teller effect [40]. This effect causes the bond distance along one axis to become longer than that along the other two axes, resulting in a unique coordination structure. This specific structural characteristic may explain the differences in the AA depletion rates compared with those of Fe2+ complexes.
- Catalytic activity of Cu2+: Cu2+ acts catalytically, being reduced to Cu+ by AA. The Cu+ reacts with O2 to produce hydrogen peroxide, which is subsequently re-oxidized to Cu2+ [41]. Similarly, the Cu+ species generates highly reactive •OH radicals through Fenton-like reactions with hydrogen peroxide [42]. The complexation of Cu2+ by trien possibly prevented its reduction by AA, suppressing AA oxidation. Furthermore, the antioxidant properties of trien may have inhibited AA oxidation.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dockery, D.W.; Pope, D.C.A., III; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An association between Air Pollution and Mortality in Six U.S. Cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Burnett, R.T.; Goldberg, M.S.; Hoover, K.; Siemiatycki, J.; Abrahamowicz, M.; White, W.H. Validation of the Harvard Six Cities Study of particulate air pollution and mortality. N. Engl. J. Med. 2004, 350, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Laden, F.; Schwartz, J.; Speizer, F.E.; Dockery, D.W. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am. J. Respir. Crit. CareMed. 2006, 173, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Pope, D.C.A., III; Thun, M.J.; Namboodiri, M.M.; Dockery, D.W.; Evans, J.S.; Speizer, F.E.; Heath, C.W., Jr. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am. J. Respir. Crit. CareMed. 1995, 151, 669–674. [Google Scholar] [CrossRef]
- Pope, D.C.A., III; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J. Am. Med. Assoc. 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Pope, D.C.A., III; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef]
- Krewski, D.; Jerrett, M.; Burnett, R.T.; Ma, R.; Hughes, E.; Shi, Y.; Turner, M.C.; Pope, D.C.A., III; Thurston, G.; Calle, E.E.; et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res. Rep. Health Eff. Inst. 2009, 140, 5–136. Available online: https://www.researchgate.net/publication/26690365_Extended_Follow-Up_and_Spatial_Analysis_of_the_American_Cancer_Society_Study_Linking_Particulate_Air_Pollution_and_Mortality (accessed on 23 January 2025).
- Brook, R.D.; Rajagopalan, S.; Pope, D.C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Squadrito, G.L.; Cueto, R.; Dellinger, B.; Pryor, W.A. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radic. Bio. Med. 2001, 31, 1132–1138. [Google Scholar] [CrossRef]
- Cho, A.K.; Sioutas, C.; Miguel, A.H.; Kumagai, Y.; Schmitz, D.A.; Singh, M.; Eiguren-Fernandez, A.; Froines, J.R. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ. Res. 2005, 99, 40–47. [Google Scholar] [CrossRef]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012, 12, 11317–11350. [Google Scholar] [CrossRef] [PubMed]
- Kishikawa, N.; Ohkubo, N.; Ohyama, K.; Nakashima, K.; Kuroda, N. Chemiluminescence assay for quinones based on generation of reactive oxygen species through the redox cycle of quinone. Anal. Bioanal. Chem. 2008, 393, 1337–1343. [Google Scholar] [CrossRef]
- Sugimoto, R.; Kumagai, Y.; Nakai, Y.; Ishii, T. 9,10-Phenanthraquinone in diesel exhaust particles downregulates Cu, Zn-SOD and HO-1 in human pulmonary epithelial cells: Intracellular iron scavenger 1,10-phenanthroline affords protection against apoptosis. Free Radic. Biol. Med. 2005, 38, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, Y.; Bekki, K.; Chung, S.W.; Tang, N.; Kameda, T.; Toriba, A.; Taguchi, K.; Hayakawa, K. Oxidative Stress More Strongly Induced by ortho- Than para-quinoid Polycyclic Aromatic Hydrocarbons in A549 Cells. J. Health Sci. 2009, 55, 845–850. [Google Scholar] [CrossRef]
- Visentin, M.; Pagnoni, A.; Sarti, E.; Pietrogrande, M.C. Urban PM2.5 oxidative potential: Importance of chemical species and comparison of two spectrophotometric cell-free assays. Environ. Pollut. 2016, 19, 72–79. [Google Scholar] [CrossRef]
- Mudway, I.S.; Stenfors, N.; Duggan, S.T.; Roxborough, H.; Zielinski, H.; Marklund, S.L.; Blomberg, A.; Frew, A.J.; Sandström, T.; Kelly, F.J. An in vitro and in vivo investigation of the effects of diesel exhaust on human airway lining fluid antioxidants. Arch. Biochem. Biophys. 2004, 423, 200–212. [Google Scholar] [CrossRef]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Tolbert, P.E.; et al. Oxidative potential of ambient water-soluble PM2:5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 2016, 16, 3865–3879. [Google Scholar] [CrossRef]
- Sugimoto, K.; Okuda, T.; Hasegawa, S.; Nishita, C.; Hara, K.; Hayashi, M. Examination of the Oxidation Mechanism of Ascorbic Acid When Measuring Oxidative Potential Using the Ascorbic Acid Assay. J. Jpn. Soc. Atmos. Environ. 2021, 56, 96–107. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Bertoli, I.; Manarini, F.; Russo, M. Ascorbate assay as a measure of oxidative potential for ambient particles: Evidence for the importance of cell-free surrogate lung fluid composition. Atmos. Environ. 2019, 211, 103–112. [Google Scholar] [CrossRef]
- Dubey, S.; Vijay, P.; Raparthi, N.; Phuleria1, H.C. Investigating PM2.5 Oxidative Potential and Its Association with Chemical Constituents Measured outside of Urban Residences in Three Metropolitan Cities of India. J. Health Pollut. 2024, 12, 1–4. [Google Scholar] [CrossRef]
- Misawa, K.; Sohara, K.; Kumai, Y.; Sekine, Y. Photocatalytic reduction of oxidative potential of particulate matter 2.5 (PM2.5). Indoor Environ. 2019, 22, 15–22. [Google Scholar] [CrossRef]
- Guo, H.; Jin, L.; Huang, S. Effect of PM characterization on PM oxidative potential by acellular assays: A review. Rev. Environ. Health 2020, 35, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Sohara, K.; Yamauchi, K.; Sekine, Y. Evaluation of ·OH Production Potential of Particulate Matter (PM2.5) Collected on TiO2-Supporting Quartz Filters. Catalysts 2022, 12, 1016. [Google Scholar] [CrossRef]
- Xiong, Q.; Yu, H.; Wang, R.; Wei, J.; Verma, V. Rethinking Dithiothreitol-Based Particulate Matter Oxidative Potential: Measuring Dithiothreitol Consumption versus Reactive Oxygen Species Generation. Environ. Sci. Technol. 2017, 51, 6507–6514. [Google Scholar] [CrossRef]
- Shi, T.; Schins, R.P.F.; Knaapen, A.M.; Kuhlbusch, T.; Pitz, M.; Heinrich, J.; Borm, P.J.A. Hydroxyl radical generation by election paramagnetic resonance as a new method to monitor ambient particulate matter composition. J. Environ. Monit. 2003, 5, 550–556. [Google Scholar] [CrossRef]
- Zielinski, H.; Mudway, I.S.; Bérubé, K.A.; Murphy, S.; Richards, R.; Kelly, F.J. Modeling the interactions of particulates with epithelial lining fluid antioxidants. Am. J. Physiol. 1999, 27, L719–L726. [Google Scholar] [CrossRef]
- Ayres, J.G.; Borm, P.; Cassee, F.R.; Castranova, V.; Donaldson, K.; Ghio, A.; Harrison, R.M.; Hider, R.; Kelly, F.; Kooter, I.M.; et al. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential–a workshop report and consensus statement. Inhal. Toxicol. 2008, 20, 75–99. [Google Scholar] [CrossRef]
- Perronea, M.R.; Bertolib, I.; Romanoa, S.; Russob, M.; Rispolia, G.; Pietrograndeb, M.C. PM2.5 and PM10 oxidative potential at a Central Mediterranean Site: Contrasts between dithiothreitol- and ascorbic acid-measured values in relation with particle size and chemical composition. Atmos. Environ. 2019, 210, 143–155. [Google Scholar] [CrossRef]
- Ehnbom, A.; Ghosh, S.K.; Lewis, K.G.; Gladysz, J.A. Octahedral Werner complexes with substituted ethylenediamine ligands: A stereochemical primer for a historic series of compounds now emerging as a modern family of catalysts. Chem. Soc. Rev. 2016, 45, 6799. [Google Scholar] [CrossRef]
- Bates, J.T.; Ting, F.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.Y.; Sarnat, S.E.; Klein, M.; Mulholland, J.A.; et al. Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef]
- Copper Sulfate Pentahydrate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Copper-sulfate-pentahydrate (accessed on 23 January 2025).
- Iron Dichloride Tetrahydrate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Iron-dichloride-tetrahydrate (accessed on 23 January 2025).
- Van Gaal, H.L.M.; Van der Linden, J.G.M. Trends in redox potentials of transition metal complexes. Coord. Chem. Rev. 1982, 47, 41–45. [Google Scholar] [CrossRef]
- Hawkins, C.J.; Perrin, D.D. Oxidation–reduction potentials of metal complexes in water: Some copper complexes. J. Chem. Soc. 1962, 1351–1357. [Google Scholar] [CrossRef]
- Sekine, H.; Ikeda, S.; Sekine, Y. Effect of coordination structure of Werner-type metal complexes on bioluminescence intensity of marine luminescent bacterium vibrio fischeri. J. Environ. Chem. 2021, 31, 23–29. [Google Scholar] [CrossRef]
- Guenther, W.B. Chemical Equilibrium, 1st ed.; Springer: New York, NY, USA, 1975; pp. 119–139. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E. Critical Stability Constants Volume 2: Amines, 1st ed.; Springer: New York, NY, USA, 1975; pp. 36–111. [Google Scholar] [CrossRef]
- Khan, M.M.; Martell, A.E. Metal ion and metal chelate catalyzed oxidation of ascorbic acid by molecular oxygen. I. Cupric and ferric ion catalyzed oxidation. J. Am. Chem. Soc. 1967, 89, 4176–4185. [Google Scholar] [CrossRef]
- Kushioka, K. Autoxidation of Phenols catalysed by Copper-Amine Complexes (Part II)–Copper (II)–Ethylenediamine Complexes-. J. Food Sci. Kyoto Women’s Univ. 1981, 36, 29–34. Available online: http://hdl.handle.net/11173/1280 (accessed on 23 January 2025).
- Hathaway, B.J.; Billing, D.E. The electronic properties and stereochemistry of mono-nuclear complexes of the copper (II) ion. Coord. Chem. Rev. 1970, 5, 143–207. [Google Scholar] [CrossRef]
- Ito, S.; Yamamoto, T.; Tokushige, Y. Valence change of copper in catalytic oxidation of l-ascorbic acid by molecular oxygen. Chem. Lett. 1980, 9, 1411–1414. [Google Scholar] [CrossRef]
- Moffettt, J.W.; Zika, R.G. Reaction kinetics of hydrogen peroxide with copper and iron in seawater. Environ. Sci. Technol. 1987, 21, 804–810. [Google Scholar] [CrossRef]
- Khan, M.M.; Martell, A.E. Metal ion and metal chelate catalyzed oxidation of ascorbic acid by molecular oxygen. II. Cupric and ferric chelate catalyzed oxidation. J. Am. Chem. Soc. 1967, 26, 7104–7111. [Google Scholar] [CrossRef]
- Balcerczyk, A.; Sowa, K.; Bartosz, G. Metal chelators react also with reactive oxygen and nitrogen species. Biochem. Biophys. Res. Commun. 2007, 352, 522–525. [Google Scholar] [CrossRef]
- Paris, R.; Desboeufs, K.V. Effect of atmospheric organic complexation on iron-bearing dust solubility. Atmos. Chem. Phys. 2023, 13, 4895–4905. [Google Scholar] [CrossRef]



| Test Tube # | 0.010 mM Cu2+ (mL) | 0.010 mM en (mL) | Cen/CCu(II) |
|---|---|---|---|
| C-1 | 1.0 | 0 | 0 |
| C-2 | 1.0 | 1.0 | 1.0 |
| C-3 | 1.0 | 2.0 | 2.0 |
| C-4 | 1.0 | 3.0 | 3.0 |
| C-5 | 1.0 | 4.0 | 4.0 |
| C-6 | 1.0 | 5.0 | 5.0 |
| Test Tube # | 1.0 mM Fe2+ (mL) | 1.0 mM en (mL) | Cen/CFe(II) |
| F-1 | 1.0 | 0 | 0 |
| F-2 | 1.0 | 0.5 | 0.5 |
| F-3 | 1.0 | 1.0 | 1.0 |
| F-4 | 1.0 | 1.5 | 1.5 |
| F-5 | 1.0 | 2.0 | 2.0 |
| F-6 | 1.0 | 2.5 | 2.5 |
| Ligands | Metal Ions | |||||
|---|---|---|---|---|---|---|
| Cu2+ | Fe2+ | |||||
| logβ1 | logβ2 | logβ3 | logβ1 | logβ2 | logβ3 | |
| en | 10.6 | 19.8 | - | 4.34 | 7.65 | 9.7 |
| dien | 15.9 | 20.9 | - | 6.23 | 10.36 | - |
| trien | 20.32 | - | - | 7.69 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekine, H.; Ito, H.; Sekine, Y. Effect of Werner-Type Complex Formation of Cu2+ and Fe2+ on Oxidative Potentials Assessed Using Ascorbic Acid Assay. Atmosphere 2025, 16, 192. https://doi.org/10.3390/atmos16020192
Sekine H, Ito H, Sekine Y. Effect of Werner-Type Complex Formation of Cu2+ and Fe2+ on Oxidative Potentials Assessed Using Ascorbic Acid Assay. Atmosphere. 2025; 16(2):192. https://doi.org/10.3390/atmos16020192
Chicago/Turabian StyleSekine, Hideaki, Hikaru Ito, and Yoshika Sekine. 2025. "Effect of Werner-Type Complex Formation of Cu2+ and Fe2+ on Oxidative Potentials Assessed Using Ascorbic Acid Assay" Atmosphere 16, no. 2: 192. https://doi.org/10.3390/atmos16020192
APA StyleSekine, H., Ito, H., & Sekine, Y. (2025). Effect of Werner-Type Complex Formation of Cu2+ and Fe2+ on Oxidative Potentials Assessed Using Ascorbic Acid Assay. Atmosphere, 16(2), 192. https://doi.org/10.3390/atmos16020192







