Particle Size as a Key Driver of Black Carbon Wet Removal: Advances and Insights
Abstract
1. Introduction
2. Theoretical Mechanisms: Particle Size-Dependent Activation and Wet Removal of Black Carbon
2.1. Key Role of Particle Size in Cloud Droplet Activation
2.2. The Predominant Influence of Particle Size Distribution on Wet Removal Efficiency
3. Observational Evidence: Empirical Support for Size-Dependent Effects
3.1. Lab and Cloud Chamber Evidence of Particle Size Effects on BC
3.2. Field Evidence on BC Size and Mixing State from SP2–CCN Measurements
4. Modeling Studies: Sensitivity of BC Scavenging to Particle Size Distributions
5. Discussion
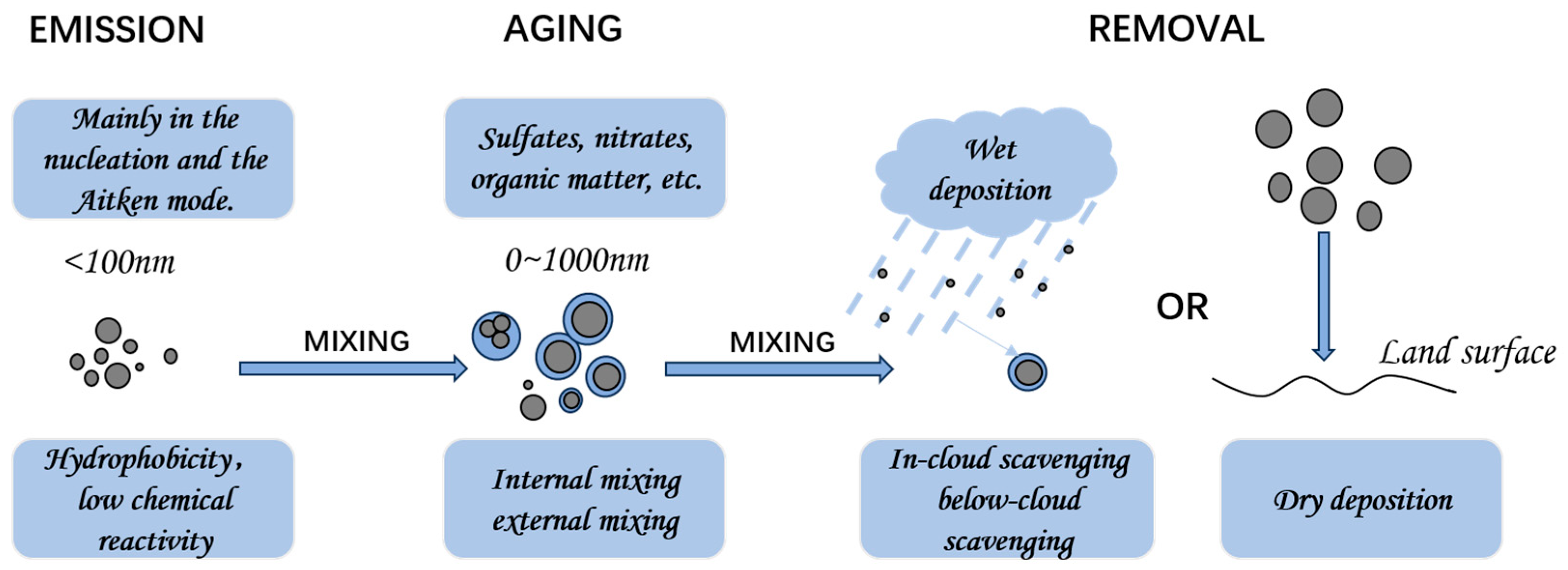
5.1. Aerosol Size Dynamics Driven by Aging: From Formation to Atmospheric Removal
5.2. Regional Differences
6. Conclusions and Outlook
6.1. Summary of Main Conclusions
6.2. Sources of Uncertainty and Future Research Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Black carbon |
| CCN | Cloud condensation nuclei |
| IN | Ice nuclei |
| Sc | Critical supersaturation |
| VOCs | Volatile organic compounds |
| SOAs | Secondary organic aerosols |
| GMD | Geometric mean diameter |
| T | Temperatures |
| SP2 | Single-particle soot photometer |
| LII | Laser-induced incandescence |
| rBC | Refractory black carbon |
| HTDMA | Humidified tandem differential mobility analyzer |
| APM/CPMA | Aerosol or centrifugal particle mass analyzers |
| TOMAS | Two-moment aerosol sectional |
References
- Kleeman, M.J.; Cass, G.R. Source contributions to the size and composition distribution of urban particulate air pollution. Atmos. Environ. 1998, 32, 2803–2816. [Google Scholar] [CrossRef]
- Novakov, T.; Andreae, M.O.; Gabriel, R.; Kirchstetter, T.W.; Mayol-Bracero, O.L.; Ramanathan, V. Origin of carbonaceous aerosols over the tropical Indian Ocean: Biomass burning or fossil fuels? Geophys. Res. Lett. 2000, 27, 4061–4064. [Google Scholar] [CrossRef]
- Madhavi Latha, K.; Badarinath, K.V.S. Black carbon aerosols over tropical urban environment—A case study. Atmos. Res. 2003, 69, 125–133. [Google Scholar] [CrossRef]
- Penner, J.E.; Chuang, C.C.; Grant, K. Climate forcing by carbonaceous and sulfate aerosols. Clim. Dyn. 1998, 14, 839–851. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Strong radiative heating due to the mixing state of black carbon in atmospheric aerosols. Nature 2001, 409, 695–697. [Google Scholar] [CrossRef]
- Koch, D. Transport and direct radiative forcing of carbonaceous and sulfate aerosols in the GISS GCM. J. Geophys. Res. Atmos. 2001, 106, 20311–20332. [Google Scholar] [CrossRef]
- Quinn, P.K.; Bates, T.S.; Baum, E.; Doubleday, N.; Fiore, A.M.; Flanner, M.; Fridlind, A.; Garrett, T.J.; Koch, D.; Menon, S.; et al. Short-lived pollutants in the Arctic: Their climate impact and possible mitigation strategies. Atmos. Chem. Phys. 2008, 8, 1723–1735. [Google Scholar] [CrossRef]
- Ramanathan, V.; Carmichael, G. Global and regional climate changes due to black carbon. Nat. Geosci. 2008, 1, 221–227. [Google Scholar] [CrossRef]
- Twomey, S. The Influence of Pollution on the Shortwave Albedo of Clouds. J. Atmos. Sci. 1977, 34, 1149–1152. [Google Scholar] [CrossRef]
- Albrecht, B.A. Aerosols, Cloud Microphysics, and Fractional Cloudiness. Science 1989, 245, 1227–1230. [Google Scholar] [CrossRef]
- Ackerman, A.S.; Toon, O.B.; Stevens, D.E.; Heymsfield, A.J.; Ramanathan, V.; Welton, E.J. Reduction of Tropical Cloudiness by Soot. Science 2000, 288, 1042–1047. [Google Scholar] [CrossRef]
- Norris, J.R. Has northern Indian Ocean Cloud cover changed due to increasing anthropogenic aerosol? Geophys. Res. Lett. 2001, 28, 3271–3274. [Google Scholar] [CrossRef]
- Bond, T.C.; Bergstrom, R.W. Light Absorption by Carbonaceous Particles: An Investigative Review. Aerosol Sci. Technol. 2006, 40, 27–67. [Google Scholar] [CrossRef]
- Chung, S.H.; Seinfeld, J.H. Climate response of direct radiative forcing of anthropogenic black carbon. J. Geophys. Res. Atmos. 2005, 110, D11102. [Google Scholar] [CrossRef]
- Allen, R.J.; Sherwood, S.C. Aerosol-cloud semi-direct effect and land-sea temperature contrast in a GCM. Geophys. Res. Lett. 2010, 37, L07702. [Google Scholar] [CrossRef]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Kärcher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Gustafsson, Ö.; Ramanathan, V. Convergence on climate warming by black carbon aerosols. Proc. Natl. Acad. Sci. USA 2016, 113, 4243–4245. [Google Scholar] [CrossRef]
- Textor, C.; Schulz, M.; Guibert, S.; Kinne, S.; Balkanski, Y.; Bauer, S.; Berntsen, T.; Berglen, T.; Boucher, O.; Chin, M.; et al. Analysis and quantification of the diversities of aerosol life cycles within AeroCom. Atmos. Chem. Phys. 2006, 6, 1777–1813. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Investigating cloud absorption effects: Global absorption properties of black carbon, tar balls, and soil dust in clouds and aerosols. J. Geophys. Res. Atmos. 2012, 117, D06205. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N.; Noone, K.J. Atmospheric chemistry and physics: From air pollution to climate change. Phys. Today 1998, 51, 88–90. [Google Scholar] [CrossRef]
- Adams, P.J.; Seinfeld, J.H. Disproportionate impact of particulate emissions on global cloud condensation nuclei concentrations. Geophys. Res. Lett. 2003, 30, 1239. [Google Scholar] [CrossRef]
- Taylor, J.W.; Allan, J.D.; Allen, G.; Coe, H.; Williams, P.I.; Flynn, M.J.; Le Breton, M.; Muller, J.B.A.; Percival, C.J.; Oram, D.; et al. Size-dependent wet removal of black carbon in Canadian biomass burning plumes. Atmos. Chem. Phys. 2014, 14, 13755–13771. [Google Scholar] [CrossRef]
- Begam, G.R.; Vachaspati, C.V.; Ahammed, Y.N.; Kumar, K.R.; Babu, S.S.; Reddy, R.R. Measurement and analysis of black carbon aerosols over a tropical semi-arid station in Kadapa, India. Atmos. Res. 2016, 171, 77–91. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, Y.; Lin, Q.; Jiang, F.; Lian, X.; Li, L.; Wang, Z.; Zhang, G.; Bi, X.; Wang, X.; et al. Recent Advances in Quantifying Wet Scavenging Efficiency of Black Carbon Aerosol. Atmosphere 2019, 10, 175. [Google Scholar] [CrossRef]
- Pruppacher, H.R.; Klett, J.D. Microphysics of Clouds and Precipitation. Nature 1980, 284, 88. [Google Scholar] [CrossRef]
- Zhang, R.; Khalizov, A.F.; Pagels, J.; Zhang, D.; Xue, H.; McMurry, P.H. Variability in morphology, hygroscopicity, and optical properties of soot aerosols during atmospheric processing. Proc. Natl. Acad. Sci. USA 2008, 105, 10291–10296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, P.-L.; Peng, J.; Zhang, R.; Jiang, J.H.; Easter, R.C.; Yung, Y.L. Constraining Aging Processes of Black Carbon in the Community Atmosphere Model Using Environmental Chamber Measurements. J. Adv. Model. Earth Syst. 2018, 10, 2514–2526. [Google Scholar] [CrossRef]
- Park, R.J.; Jacob, D.J.; Palmer, P.I.; Clarke, A.D.; Weber, R.J.; Zondlo, M.A.; Eisele, F.L.; Bandy, A.R.; Thornton, D.C.; Sachse, G.W.; et al. Export efficiency of black carbon aerosol in continental outflow: Global implications. J. Geophys. Res. Atmos. 2005, 110, D11205. [Google Scholar] [CrossRef]
- Park, S.; Gong, S.; Bouchet, V.; Gong, W.; Makar, P.; Moran, M.; Stroud, C.A.; Zhang, J. Effects of black carbon aging on air quality predictions direct radiative forcing estimation. Tellus B 2011, 63, 1026–1039. [Google Scholar] [CrossRef]
- Dusek, U.; Frank, G.P.; Hildebrandt, L.; Curtius, J.; Schneider, J.; Walter, S.; Chand, D.; Drewnick, F.; Hings, S.; Jung, D.; et al. Size Matters More Than Chemistry for Cloud-Nucleating Ability of Aerosol Particles. Science 2006, 312, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.Z.; Zhao, C.S.; Ma, N.; Liu, P.F.; Ran, L.; Xu, W.Y.; Chen, J.; Liang, Z.; Liang, S.; Huang, M.Y.; et al. Size-resolved and bulk activation properties of aerosols in the North China Plain. Atmos. Chem. Phys. 2011, 11, 3835–3846. [Google Scholar] [CrossRef]
- Peng, J.; Hu, M.; Guo, S.; Du, Z.; Zheng, J.; Shang, D.; Levy Zamora, M.; Zeng, L.; Shao, M.; Wu, Y.-S.; et al. Markedly enhanced absorption and direct radiative forcing of black carbon under polluted urban environments. Proc. Natl. Acad. Sci. USA 2016, 113, 4266–4271. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Zhang, Y.; Du, W.; Zhang, F.; Tan, H.; Xu, H.; Fan, T.; Jin, X.; Fan, X.; et al. Characterization of aerosol hygroscopicity, mixing state, and CCN activity at a suburban site in the central North China Plain. Atmos. Chem. Phys. 2018, 18, 11739–11752. [Google Scholar] [CrossRef]
- Tian, P.; Liu, D.; Hu, K.; Wu, Y.; Huang, M.; He, H.; Sheng, J.; Yu, C.; Hu, D.; Ding, D. Efficient droplet activation of ambient black carbon particles in a suburban environment. Atmos. Chem. Phys. 2024, 24, 5149–5164. [Google Scholar] [CrossRef]
- Reddington, C.L.; McMeeking, G.; Mann, G.W.; Coe, H.; Frontoso, M.G.; Liu, D.; Flynn, M.; Spracklen, D.V.; Carslaw, K.S. The mass and number size distributions of black carbon aerosol over Europe. Atmos. Chem. Phys. 2013, 13, 4917–4939. [Google Scholar] [CrossRef]
- Dournaux, M.; Tulet, P.; Pianezze, J.; Brioude, J.; Metzger, J.-M.; Thyssen, M. Origin, size distribution and hygroscopic properties of marine aerosols in the south-western Indian Ocean: Report of 6 campaigns of shipborne observations. EGUsphere 2025, 2025, 1–38. [Google Scholar] [CrossRef]
- Moteki, N. Climate-relevant properties of black carbon aerosols revealed by in situ measurements: A review. Prog. Earth Planet. Sci. 2023, 10, 12. [Google Scholar] [CrossRef]
- Hilario, M.R.A.; Arellano, A.F.; Behrangi, A.; Crosbie, E.C.; DiGangi, J.P.; Diskin, G.S.; Shook, M.A.; Ziemba, L.D.; Sorooshian, A. Assessing potential indicators of aerosol wet scavenging during long-range transport. Atmos. Meas. Tech. 2024, 17, 37–55. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Wang, J.; Riemer, N.; Liu, C.; Jin, Y.; Tian, Z.; Cai, J.; Cheng, Y.; Chen, G.; et al. Steady-state mixing state of black carbon aerosols from a particle-resolved model. Atmos. Chem. Phys. 2025, 25, 1869–1881. [Google Scholar] [CrossRef]
- Lei, S.; Ge, B.; Liu, H.; Quan, J.; Xu, D.; Zhang, Y.; Yao, W.; Lei, L.; Tian, Y.; Liao, Q.; et al. Refractory black carbon aerosols in rainwater in the summer of 2019 in Beijing: Mass concentration, size distribution and wet scavenging ratio. J. Environ. Sci. 2023, 132, 31–42. [Google Scholar] [CrossRef]
- Ching, J.; Riemer, N.; West, M. Impacts of black carbon mixing state on black carbon nucleation scavenging: Insights from a particle-resolved model. J. Geophys. Res. Atmos. 2012, 117, D23209. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, J.; Wang, H.; Cui, S.; Zhang, Y.; Li, H.; Wu, Y.; Wang, M.; Aruffo, E.; Ge, X. Measurement report: Characteristics of airborne black-carbon-containing particles during the 2021 summer COVID-19 lockdown in a typical Yangtze River Delta city, China. Atmos. Chem. Phys. 2024, 24, 9733–9748. [Google Scholar] [CrossRef]
- Sricharoenvech, P.; Edwards, R.; Yaşar, M.; Gay, D.; Schauer, J.J. Investigation of Black Carbon Wet Deposition to the United States from National Atmospheric Deposition Network Samples. Aerosol Air Qual. Res. 2023, 24, 230089. [Google Scholar] [CrossRef]
- Tuccella, P.; Di Antonio, L.; Di Muzio, A.; Colaiuda, V.; Lidori, R.; Menut, L.; Pitari, G.; Raparelli, E. Modeling the Black and Brown Carbon Absorption and Their Radiative Impact: The June 2023 Intense Canadian Boreal Wildfires Case Study. J. Geophys. Res. Atmos. 2025, 130, e2024JD042674. [Google Scholar] [CrossRef]
- Brown, H.; Liu, X.; Feng, Y.; Jiang, Y.; Wu, M.; Lu, Z.; Wu, C.; Murphy, S.; Pokhrel, R. Radiative effect and climate impacts of brown carbon with the Community Atmosphere Model (CAM5). Atmos. Chem. Phys. 2018, 18, 17745–17768. [Google Scholar] [CrossRef]
- Curci, G.; Alyuz, U.; Barò, R.; Bianconi, R.; Bieser, J.; Christensen, J.H.; Colette, A.; Farrow, A.; Francis, X.; Jiménez-Guerrero, P.; et al. Modelling black carbon absorption of solar radiation: Combining external and internal mixing assumptions. Atmos. Chem. Phys. 2019, 19, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Esteve, A.R.; Highwood, E.J.; Morgan, W.T.; Allen, G.; Coe, H.; Grainger, R.G.; Brown, P.; Szpek, K. A study on the sensitivities of simulated aerosol optical properties to composition and size distribution using airborne measurements. Atmos. Environ. 2014, 89, 517–524. [Google Scholar] [CrossRef]
- Long, C.M.; Nascarella, M.A.; Valberg, P.A. Carbon black vs. Black carbon and other airborne materials containing elemental carbon: Physical and chemical distinctions. Environ. Pollut. 2013, 181, 271–286. [Google Scholar] [CrossRef]
- Lahaye, J.; Prado, G. Morphology and Internal Structure of Soot and Carbon Blacks. In Particulate Carbon: Formation During Combustion; Siegla, D.C., Smith, G.W., Eds.; Springer: New York, NY, USA, 1981; pp. 33–55. [Google Scholar] [CrossRef]
- Zhang, R.; Khalizov, A.; Wang, L.; Hu, M.; Xu, W. Nucleation and Growth of Nanoparticles in the Atmosphere. Chem. Rev. 2012, 112, 1957–2011. [Google Scholar] [CrossRef]
- Köhler, H. The nucleus in and the growth of hygroscopic droplets. Trans. Faraday Soc. 1936, 32, 1152–1161. [Google Scholar] [CrossRef]
- Sorjamaa, R.; Laaksonen, A. The effect of H2O adsorption on cloud drop activation of insoluble particles: A theoretical framework. Atmos. Chem. Phys. 2007, 7, 6175–6180. [Google Scholar] [CrossRef]
- Dalirian, M.; Ylisirniö, A.; Buchholz, A.; Schlesinger, D.; Ström, J.; Virtanen, A.; Riipinen, I. Cloud droplet activation of black carbon particles coated with organic compounds of varying solubility. Atmos. Chem. Phys. 2018, 18, 12477–12489. [Google Scholar] [CrossRef]
- Henning, S.; Wex, H.; Hennig, T.; Kiselev, A.; Snider, J.R.; Rose, D.; Dusek, U.; Frank, G.P.; Pöschl, U.; Kristensson, A.; et al. Soluble mass, hygroscopic growth, and droplet activation of coated soot particles during LACIS Experiment in November (LExNo). J. Geophys. Res. Atmos. 2010, 115, D11206. [Google Scholar] [CrossRef]
- Henning, S.; Ziese, M.; Kiselev, A.; Saathoff, H.; Möhler, O.; Mentel, T.F.; Buchholz, A.; Spindler, C.; Michaud, V.; Monier, M.; et al. Hygroscopic growth and droplet activation of soot particles: Uncoated, succinic or sulfuric acid coated. Atmos. Chem. Phys. 2012, 12, 4525–4537. [Google Scholar] [CrossRef]
- Maskey, S.; Chong, K.Y.; Seo, A.; Park, M.; Lee, K.; Park, K. Cloud Condensation Nuclei Activation of Internally Mixed Black Carbon Particles. Aerosol Air Qual. Res. 2017, 17, 867–877. [Google Scholar] [CrossRef]
- Petters, M.D.; Kreidenweis, S.M.; Ziemann, P.J. Prediction of cloud condensation nuclei activity for organic compounds using functional group contribution methods. Geosci. Model Dev. 2016, 9, 111–124. [Google Scholar] [CrossRef]
- Tritscher, T.; Jurányi, Z.; Martin, M.; Chirico, R.; Gysel, M.; Heringa, M.F.; DeCarlo, P.F.; Sierau, B.; Prévôt, A.S.H.; Weingartner, E.; et al. Changes of hygroscopicity and morphology during ageing of diesel soot. Environ. Res. Lett. 2011, 6, 034026. [Google Scholar] [CrossRef]
- Li, W.; Riemer, N.; Xu, L.; Wang, Y.; Adachi, K.; Shi, Z.; Zhang, D.; Zheng, Z.; Laskin, A. Microphysical properties of atmospheric soot and organic particles: Measurements, modeling, and impacts. Npj Clim. Atmos. Sci. 2024, 7, 65. [Google Scholar] [CrossRef]
- Cheng, Z.; Shrivastava, M.; Ijaz, A.; Veghte, D.; Vandergrift, G.W.; Tseng, K.-P.; Lata, N.N.; Kew, W.; Suski, K.; Weis, J.; et al. Enhanced light absorption for solid-state brown carbon from wildfires due to organic and water coatings. Nat. Commun. 2024, 15, 10326. [Google Scholar] [CrossRef]
- Chakrabarty, R.K.; Shetty, N.J.; Thind, A.S.; Beeler, P.; Sumlin, B.J.; Zhang, C.; Liu, P.; Idrobo, J.C.; Adachi, K.; Wagner, N.L.; et al. Shortwave absorption by wildfire smoke dominated by dark brown carbon. Nat. Geosci. 2023, 16, 683–688. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Huang, J.; Liu, L.; Pang, Y.; He, C.; Liu, F.; Liu, D.; Bi, L.; Zhang, X.; et al. Nonlinear Enhancement of Radiative Absorption by Black Carbon in Response to Particle Mixing Structure. Geophys. Res. Lett. 2021, 48, e2021GL096437. [Google Scholar] [CrossRef]
- Cappa, C.D.; Zhang, X.; Russell, L.M.; Collier, S.; Lee, A.K.Y.; Chen, C.-L.; Betha, R.; Chen, S.; Liu, J.; Price, D.J.; et al. Light Absorption by Ambient Black and Brown Carbon and its Dependence on Black Carbon Coating State for Two California, USA, Cities in Winter and Summer. J. Geophys. Res. Atmos. 2019, 124, 1550–1577. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, H.; Zhou, C.; Wei, X.; Liu, Y. Optical properties of mixed black and brown carbon aerosols. Opt. Express 2022, 30, 33588–33602. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.H.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of Organic Aerosols in the Atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Pfrang, C.; Koop, T.; Pöschl, U. Kinetic multi-layer model of gas-particle interactions in aerosols and clouds (KM-GAP): Linking condensation, evaporation and chemical reactions of organics, oxidants and water. Atmos. Chem. Phys. 2012, 12, 2777–2794. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Su, H.; Rose, D.; Gunthe, S.S.; Berghof, M.; Wehner, B.; Achtert, P.; Nowak, A.; Takegawa, N.; Kondo, Y.; et al. Size-resolved measurement of the mixing state of soot in the megacity Beijing, China: Diurnal cycle, aging and parameterization. Atmos. Chem. Phys. 2012, 12, 4477–4491. [Google Scholar] [CrossRef]
- Li, W.; Shao, L.; Zhang, D.; Ro, C.-U.; Hu, M.; Bi, X.; Geng, H.; Matsuki, A.; Niu, H.; Chen, J. A review of single aerosol particle studies in the atmosphere of East Asia: Morphology, mixing state, source, and heterogeneous reactions. Prev. Smog Cris. 2016, 112, 1330–1349. [Google Scholar] [CrossRef]
- Riemer, R.; Vogel, H.; Vogel, B. Soot aging time scales in polluted regions during day and night. Atmos. Chem. Phys. 2004, 4, 1885–1893. [Google Scholar] [CrossRef]
- Ma, M.; Rivellini, L.-H.; Zong, Y.; Kraft, M.; Yu, L.E.; Lee, A.K.Y. Advances in characterization of black carbon particles and their associated coatings using the soot-particle aerosol mass spectrometer in Singapore, a complex city environment. Atmos. Chem. Phys. 2025, 25, 8185–8211. [Google Scholar] [CrossRef]
- Lorenzo, G.R.; Ziemba, L.D.; Arellano, A.F.; Barth, M.C.; Crosbie, E.C.; DiGangi, J.P.; Diskin, G.S.; Ferrare, R.; Hilario, M.R.A.; Shook, M.A.; et al. Measurement report: Characterization of aerosol hygroscopicity over southeast asia during the NASA camp2ex campaign. Atmos. Chem. Phys. 2025, 25, 5469–5495. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, J.; Horowitz, L.W.; Henze, D.K.; Fan, S.; Levy, H., II; Mauzerall, D.L.; Lin, J.-T.; Tao, S. Analysis of transpacific transport of black carbon during HIPPO-3: Implications for black carbon aging. Atmos. Chem. Phys. 2014, 14, 6315–6327. [Google Scholar] [CrossRef]
- Li, K.; Chen, L.; Han, K.; Lv, B.; Bao, K.; Wu, X.; Gao, X.; Cen, K. Smog chamber study on aging of combustion soot in isoprene/SO2/NOx system: Changes of mass, size, effective density, morphology and mixing state. Atmos. Res. 2017, 184, 139–148. [Google Scholar] [CrossRef]
- McMeeking, G.R.; Good, N.; Petters, M.D.; McFiggans, G.; Coe, H. Influences on the fraction of hydrophobic and hydrophilic black carbon in the atmosphere. Atmos. Chem. Phys. 2011, 11, 5099–5112. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, D.; Niu, Z.; Zhao, G.; Ding, D.; Chen, Y.; Liu, H. Aircraft observations of aerosol and BC during the East Asian dust storm event: Vertical profiles, size distribution and mixing state. Atmos. Environ. 2024, 327, 120492. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Sosedova, Y.; Rouvière, A.; Yang, H.; Zhang, Y.; Abbatt, J.P.D.; Ammann, M.; Pöschl, U. The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles. Nat. Chem. 2011, 3, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Ammann, M.; Pöschl, U. Kinetic model framework for aerosol and cloud surface chemistry and gas-particle interactions—Part 2: Exemplary practical applications and numerical simulations. Atmos. Chem. Phys. 2007, 7, 6025–6045. [Google Scholar] [CrossRef]
- Pöschl, U.; Rudich, Y.; Ammann, M. Kinetic model framework for aerosol and cloud surface chemistry and gas-particle interactions—Part 1: General equations, parameters, and terminology. Atmos. Chem. Phys. 2007, 7, 5989–6023. [Google Scholar] [CrossRef]
- Lennard-Jones, J.E. Processes of adsorption and diffusion on solid surfaces. Trans. Faraday Soc. 1932, 28, 333–359. [Google Scholar] [CrossRef]
- Cape, J.N.; Coyle, M.; Dumitrean, P. The atmospheric lifetime of black carbon. Atmos. Environ. 2012, 59, 256–263. [Google Scholar] [CrossRef]
- Liu, D.; Allan, J.; Whitehead, J.; Young, D.; Flynn, M.; Coe, H.; McFiggans, G.; Fleming, Z.L.; Bandy, B. Ambient black carbon particle hygroscopic properties controlled by mixing state and composition. Atmos. Chem. Phys. 2013, 13, 2015–2029. [Google Scholar] [CrossRef]
- Fierce, L.; Onasch, T.B.; Cappa, C.D.; Mazzoleni, C.; China, S.; Bhandari, J.; Davidovits, P.; Fischer, D.A.; Helgestad, T.; Lambe, A.T.; et al. Radiative absorption enhancements by black carbon controlled by particle-to-particle heterogeneity in composition. Proc. Natl. Acad. Sci. USA 2020, 117, 5196–5203. [Google Scholar] [CrossRef]
- Hu, D.; Liu, D.; Kong, S.; Zhao, D.; Wu, Y.; Li, S.; Ding, S.; Zheng, S.; Cheng, Y.; Hu, K.; et al. Direct Quantification of Droplet Activation of Ambient Black Carbon Under Water Supersaturation. J. Geophys. Res. Atmos. 2021, 126, e2021JD034649. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, J.; Li, C.; Huang, X.; Wang, J.; Nie, W.; Liu, Y.; Wang, J.; Tian, Z.; Liu, C.; et al. Simulation of the Steady Mixing State of Black Carbon With a Two-Dimensional Sectional Model. J. Geophys. Res. Atmos. 2024, 129, e2024JD041851. [Google Scholar] [CrossRef]
- Matsui, H.; Koike, M.; Kondo, Y.; Moteki, N.; Fast, J.D.; Zaveri, R.A. Development and validation of a black carbon mixing state resolved three-dimensional model: Aging processes and radiative impact. J. Geophys. Res. Atmos. 2013, 118, 2304–2326. [Google Scholar] [CrossRef]
- Leskinen, J.; Hartikainen, A.; Väätäinen, S.; Ihalainen, M.; Virkkula, A.; Mesceriakovas, A.; Tiitta, P.; Miettinen, M.; Lamberg, H.; Czech, H.; et al. Photochemical Aging Induces Changes in the Effective Densities, Morphologies, and Optical Properties of Combustion Aerosol Particles. Environ. Sci. Technol. 2023, 57, 5137–5148. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ye, X.; Pang, H.; Lu, X.; Chen, H.; Wang, X.; Yang, X.; Chen, J.; Chen, Y. Temporal variations in the hygroscopicity and mixing state of black carbon aerosols in a polluted megacity area. Atmos. Chem. Phys. 2018, 18, 15201–15218. [Google Scholar] [CrossRef]
- Lu, J.; Gong, S.; Zhang, J.; Chen, J.; Zhang, L.; Zhou, C. Assessment of the impacts of cloud chemistry on surface SO2 and sulfate levels in typical regions of China. Atmos. Chem. Phys. 2023, 23, 8021–8037. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, C.; Chen, H.; Nizkorodov, S.A.; Chen, J.; Yang, X. Size distribution and mixing state of black carbon particles during a heavy air pollution episode in Shanghai. Atmos. Chem. Phys. 2016, 16, 5399–5411. [Google Scholar] [CrossRef]
- Healy, R.M.; Sciare, J.; Poulain, L.; Kamili, K.; Merkel, M.; Müller, T.; Wiedensohler, A.; Eckhardt, S.; Stohl, A.; Sarda-Estève, R.; et al. Sources and mixing state of size-resolved elemental carbon particles in a European megacity: Paris. Atmos. Chem. Phys. 2012, 12, 1681–1700. [Google Scholar] [CrossRef]
- Hu, W.; Hu, M.; Hu, W.; Jimenez, J.L.; Yuan, B.; Chen, W.; Wang, M.; Wu, Y.; Chen, C.; Wang, Z.; et al. Chemical composition, sources, and aging process of submicron aerosols in Beijing: Contrast between summer and winter. J. Geophys. Res. Atmos. 2016, 121, 1955–1977. [Google Scholar] [CrossRef]
- Ge, X.; Setyan, A.; Sun, Y.; Zhang, Q. Primary and secondary organic aerosols in Fresno, California during wintertime: Results from high resolution aerosol mass spectrometry. J. Geophys. Res. Atmos. 2012, 117, D19301. [Google Scholar] [CrossRef]
- Sorooshian, A.; Murphy, S.M.; Hersey, S.; Gates, H.; Padro, L.T.; Nenes, A.; Brechtel, F.J.; Jonsson, H.; Flagan, R.C.; Seinfeld, J.H. Comprehensive airborne characterization of aerosol from a major bovine source. Atmos. Chem. Phys. 2008, 8, 5489–5520. [Google Scholar] [CrossRef]
- DeMott, P.J.; Prenni, A.J.; Liu, X.; Kreidenweis, S.M.; Petters, M.D.; Twohy, C.H.; Richardson, M.S.; Eidhammer, T.; Rogers, D.C. Predicting global atmospheric ice nuclei distributions and their impacts on climate. Proc. Natl. Acad. Sci. USA 2010, 107, 11217–11222. [Google Scholar] [CrossRef]
- Knopf, D.A.; Alpert, P.A.; Wang, B. The Role of Organic Aerosol in Atmospheric Ice Nucleation: A Review. ACS Earth Space Chem. 2018, 2, 168–202. [Google Scholar] [CrossRef]
- Knopf, D.A.; Alpert, P.A. Atmospheric ice nucleation. Nat. Rev. Phys. 2023, 5, 203–217. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, Z.; Cheng, M.; Huang, X.; Liu, K.; Wang, Y.; Sun, H.; Liao, L.; Xu, Z.; Chen, J.; et al. Molecularly resolved mapping of heterogeneous ice nucleation and crystallization pathways using in-situ cryo-TEM. Nat. Commun. 2025, 16, 7349. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, W.; Heymsfield, A. Production of Ice in Tropospheric Clouds: A Review. Bull. Am. Meteorol. Soc. 2005, 86, 795–808. [Google Scholar] [CrossRef]
- David, R.O.; Marcolli, C.; Fahrni, J.; Qiu, Y.; Perez Sirkin, Y.A.; Molinero, V.; Mahrt, F.; Brühwiler, D.; Lohmann, U.; Kanji, Z.A. Pore condensation and freezing is responsible for ice formation below water saturation for porous particles. Proc. Natl. Acad. Sci. USA 2019, 116, 8184–8189. [Google Scholar] [CrossRef]
- Ullrich, R.; Hoose, C.; Möhler, O.; Niemand, M.; Wagner, R.; Höhler, K.; Hiranuma, N.; Saathoff, H.; Leisner, T. A New Ice Nucleation Active Site Parameterization for Desert Dust and Soot. J. Atmos. Sci. 2017, 74, 699–717. [Google Scholar] [CrossRef]
- Kanji, Z.A.; Ladino, L.A.; Wex, H.; Boose, Y.; Burkert-Kohn, M.; Cziczo, D.J.; Krämer, M. Overview of Ice Nucleating Particles. Meteorol. Monogr. 2017, 58, 1.1–1.33. [Google Scholar] [CrossRef]
- Cziczo, D.J.; Ladino, L.; Boose, Y.; Kanji, Z.A.; Kupiszewski, P.; Lance, S.; Mertes, S.; Wex, H. Measurements of Ice Nucleating Particles and Ice Residuals. Meteorol. Monogr. 2017, 58, 8.1–8.13. [Google Scholar] [CrossRef]
- Riemer, N.; West, M.; Zaveri, R.A.; Easter, R.C. Simulating the evolution of soot mixing state with a particle-resolved aerosol model. J. Geophys. Res. Atmos. 2009, 114, D09202. [Google Scholar] [CrossRef]
- Croft, B.; Lohmann, U.; Martin, R.V.; Stier, P.; Wurzler, S.; Feichter, J.; Posselt, R.; Ferrachat, S. Aerosol size-dependent below-cloud scavenging by rain and snow in the ECHAM5-HAM. Atmos. Chem. Phys. 2009, 9, 4653–4675. [Google Scholar] [CrossRef]
- Lemaitre, P.; Quérel, A.; Dépée, A.; Guerra Devigne, A.; Monier, M.; Hiron, T.; Soto Minguez, C.; Hardy, D.; Flossmann, A. Microphysical modelling of aerosol scavenging by different types of clouds: Description and validation of the approach. Atmos. Chem. Phys. 2024, 24, 9713–9732. [Google Scholar] [CrossRef]
- Moteki, N.; Kondo, Y.; Oshima, N.; Takegawa, N.; Koike, M.; Kita, K.; Matsui, H.; Kajino, M. Size dependence of wet removal of black carbon aerosols during transport from the boundary layer to the free troposphere. Geophys. Res. Lett. 2012, 39, L13802. [Google Scholar] [CrossRef]
- Schwarz, J.P.; Spackman, J.R.; Gao, R.S.; Watts, L.A.; Stier, P.; Schulz, M.; Davis, S.M.; Wofsy, S.C.; Fahey, D.W. Global-scale black carbon profiles observed in the remote atmosphere and compared to models. Geophys. Res. Lett. 2010, 37, L18812. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, H.-M.; Park, D.; Yoon, Y.J.; Choi, Y.; Um, J.; Lee, S.S.; Lee, J.Y.; Kim, Y.P. Parameterization of below-cloud scavenging for polydisperse fine mode aerosols as a function of rain intensity. J. Environ. Sci. 2023, 132, 43–55. [Google Scholar] [CrossRef]
- Yao, L.; Kong, S.; Nemitz, E.; Vieno, M.; Cheng, Y.; Zheng, H.; Wang, Y.; Chen, N.; Hu, Y.; Liu, D.; et al. Improving Below-Cloud Scavenging Coefficients of Sulfate, Nitrate, and Ammonium in PM2.5 and Implications for Numerical Simulation and Air Pollution Control. J. Geophys. Res. Atmos. 2024, 129, e2023JD039487. [Google Scholar] [CrossRef]
- Reid, J.; Maring, H.; Narisma, G.; van den Heever, S.; Girolamo, L.; Ferrare, R.; Holz, R.; Lawson, P.; Mace, G.; Simpas, J.; et al. The Coupling Between Tropical Meteorology, Aerosol Lifecycle, Convection, and Radiation during the Cloud, Aerosol and Monsoon Processes Philippines Experiment (CAMP2Ex). Bull. Am. Meteorol. Soc. 2023, 104, E1179–E1205. [Google Scholar] [CrossRef]
- Hilario, M.R.; Barth, M.; Bennett, R.; Crosbie, E.; DiGangi, J.; Diskin, G.; Lorenzo, G.; Rutledge, S.; Martin, M.; Ziemba, L.; et al. Quantifying Scavenging Efficiencies of Different Aerosol Species and Size-Resolved Volume Concentrations in Tropical Convective Clouds over the West Pacific. J. Atmos. Sci. 2025, 82, 267–282. [Google Scholar] [CrossRef]
- Ryu, Y.; Min, S.-K. Improving Wet and Dry Deposition of Aerosols in WRF--Chem: Updates to Below--Cloud Scavenging and Coarse--Particle Dry Deposition. J. Adv. Model. Earth Syst. 2022, 14, e2021MS002792. [Google Scholar] [CrossRef]
- Croft, B.; Lohmann, U.; Martin, R.V.; Stier, P.; Wurzler, S.; Feichter, J.; Hoose, C.; Heikkilä, U.; van Donkelaar, A.; Ferrachat, S. Influences of in-cloud aerosol scavenging parameterizations on aerosol concentrations and wet deposition in ECHAM5-HAM. Atmos. Chem. Phys. 2010, 10, 1511–1543. [Google Scholar] [CrossRef]
- Liu, M.; Matsui, H. Improved Simulations of Global Black Carbon Distributions by Modifying Wet Scavenging Processes in Convective and Mixed-Phase Clouds. J. Geophys. Res. Atmos. 2021, 126, e2020JD033890. [Google Scholar] [CrossRef]
- Flossmann, A.I. The effect of the impaction scavenging efficiency on the wet deposition by a convective warm cloud. Tellus B 1993, 45, 34–39. [Google Scholar] [CrossRef]
- Choi, Y.; Kanaya, Y.; Takigawa, M.; Zhu, C.; Park, S.-M.; Matsuki, A.; Sadanaga, Y.; Kim, S.-W.; Pan, X.; Pisso, I. Investigation of the wet removal rate of black carbon in East Asia: Validation of a below- and in-cloud wet removal scheme in FLEXPART v10.4. Atmos. Chem. Phys. 2020, 20, 13655–13670. [Google Scholar] [CrossRef]
- Liu, D.; Ding, S.; Zhao, D.; Hu, K.; Yu, C.; Hu, D.; Wu, Y.; Zhou, C.; Tian, P.; Liu, Q.; et al. Black Carbon Emission and Wet Scavenging from Surface to the Top of Boundary Layer Over Beijing Region. J. Geophys. Res. Atmos. 2020, 125, e2020JD033096. [Google Scholar] [CrossRef]
- Badarinath, K.V.S.; Kumar Kharol, S.; Kiran Chand, T.R.; Parvathi, Y.G.; Anasuya, T.; Jyothsna, A.N. Variations in black carbon aerosol, carbon monoxide and ozone over an urban area of Hyderabad, India, during the forest fire season. Atmos. Res. 2007, 85, 18–26. [Google Scholar] [CrossRef]
- Oshima, N.; Kondo, Y.; Moteki, N.; Takegawa, N.; Koike, M.; Kita, K.; Matsui, H.; Kajino, M.; Nakamura, H.; Jung, J.S.; et al. Wet removal of black carbon in Asian outflow: Aerosol Radiative Forcing in East Asia (A-FORCE) aircraft campaign. J. Geophys. Res. Atmos. 2012, 117, D03204. [Google Scholar] [CrossRef]
- Vignati, E.; Karl, M.; Krol, M.; Wilson, J.; Stier, P.; Cavalli, F. Sources of uncertainties in modelling black carbon at the global scale. Atmos. Chem. Phys. 2010, 10, 2595–2611. [Google Scholar] [CrossRef]
- Liu, J.; Fan, S.; Horowitz, L.W.; Levy, H., II. Evaluation of factors controlling long-range transport of black carbon to the Arctic. J. Geophys. Res. Atmos. 2011, 116, D04307. [Google Scholar] [CrossRef]
- Kipling, Z.; Stier, P.; Schwarz, J.P.; Perring, A.E.; Spackman, J.R.; Mann, G.W.; Johnson, C.E.; Telford, P.J. Constraints on aerosol processes in climate models from vertically-resolved aircraft observations of black carbon. Atmos. Chem. Phys. 2013, 13, 5969–5986. [Google Scholar] [CrossRef]
- Kipling, Z.; Stier, P.; Johnson, C.E.; Mann, G.W.; Bellouin, N.; Bauer, S.E.; Bergman, T.; Chin, M.; Diehl, T.; Ghan, S.J.; et al. What controls the vertical distribution of aerosol? Relationships between process sensitivity in HadGEM3–UKCA and inter-model variation from AeroCom Phase II. Atmos. Chem. Phys. 2016, 16, 2221–2241. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, R. Laboratory Investigation of Heterogeneous Interaction of Sulfuric Acid with Soot. Environ. Sci. Technol. 2005, 39, 5722–5728. [Google Scholar] [CrossRef]
- Zuberi, B.; Johnson, K.S.; Aleks, G.K.; Molina, L.T.; Molina, M.J.; Laskin, A. Hydrophilic properties of aged soot. Geophys. Res. Lett. 2005, 32, L01807. [Google Scholar] [CrossRef]
- Schnaiter, M.; Linke, C.; Möhler, O.; Naumann, K.-H.; Saathoff, H.; Wagner, R.; Schurath, U.; Wehner, B. Absorption amplification of black carbon internally mixed with secondary organic aerosol. J. Geophys. Res. Atmos. 2005, 110, D19204. [Google Scholar] [CrossRef]
- Khalizov, A.F.; Ivanova, E.V.; Demidov, E.V.; Hasani, A.; Curtis, J.H.; Riemer, N.; Gor, G.Y. Capillary Condensation: An Unaccounted Pathway for Rapid Aging of Atmospheric Soot. Environ. Sci. Technol. 2025, 59, 14564–14571. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hu, J.; Liu, C.; Xie, X.; Yin, J.; Guo, S.; Hu, M.; Peng, J.; Wang, H. Advanced modeling of the absorption enhancement of black carbon particles in chamber experiments by considering the morphology and coating thickness. Front. Environ. Sci. Eng. 2024, 18, 116. [Google Scholar] [CrossRef]
- Chang, K.; Bench, J.; Brege, M.; Cantrell, W.; Chandrakar, K.; Ciochetto, D.; Mazzoleni, C.; Mazzoleni, L.R.; Niedermeier, D.; Shaw, R.A. A Laboratory Facility to Study Gas–Aerosol–Cloud Interactions in a Turbulent Environment: The Π Chamber. Bull. Am. Meteorol. Soc. 2016, 97, 2343–2358. [Google Scholar] [CrossRef]
- Kirkby, J.; Curtius, J.; Almeida, J.; Dunne, E.; Duplissy, J.; Ehrhart, S.; Franchin, A.; Gagné, S.; Ickes, L.; Kürten, A.; et al. Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Nature 2011, 476, 429–433. [Google Scholar] [CrossRef]
- Krüger, M.L.; Mertes, S.; Klimach, T.; Cheng, Y.F.; Su, H.; Schneider, J.; Andreae, M.O.; Pöschl, U.; Rose, D. Assessment of cloud supersaturation by size-resolved aerosol particle and cloud condensation nuclei (CCN) measurements. Atmos. Meas. Tech. 2014, 7, 2615–2629. [Google Scholar] [CrossRef]
- Ditas, F.; Shaw, R.A.; Siebert, H.; Simmel, M.; Wehner, B.; Wiedensohler, A. Aerosols-cloud microphysics-thermodynamics-turbulence: Evaluating supersaturation in a marine stratocumulus cloud. Atmos. Chem. Phys. 2012, 12, 2459–2468. [Google Scholar] [CrossRef]
- Hammer, E.; Bukowiecki, N.; Gysel, M.; Jurányi, Z.; Hoyle, C.R.; Vogt, R.; Baltensperger, U.; Weingartner, E. Investigation of the effective peak supersaturation for liquid-phase clouds at the high-alpine site Jungfraujoch, Switzerland (3580 m a.s.l.). Atmos. Chem. Phys. 2014, 14, 1123–1139. [Google Scholar] [CrossRef]
- Yu, C.; Liu, D.; Hu, K.; Tian, P.; Wu, Y.; Zhao, D.; Wu, H.; Hu, D.; Guo, W.; Li, Q.; et al. Aerodynamic size-resolved composition and cloud condensation nuclei properties of aerosols in a Beijing suburban region. Atmos. Chem. Phys. 2022, 22, 4375–4391. [Google Scholar] [CrossRef]
- Eriksson, A.C.; Wittbom, C.; Roldin, P.; Sporre, M.; Öström, E.; Nilsson, P.; Martinsson, J.; Rissler, J.; Nordin, E.Z.; Svenningsson, B.; et al. Diesel soot aging in urban plumes within hours under cold dark and humid conditions. Sci. Rep. 2017, 7, 12364. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, A.; Noone, K.J.; Ogren, J.A.; Svenningsson, I.B.; Flossmann, A.; Wiedensohler, A.; Hansson, H.-C.; Heintzenberg, J.; Anderson, T.L.; Arends, B.G.; et al. Phase partitioning of aerosol particles in clouds at Kleiner Feldberg. J. Atmos. Chem. 1994, 19, 107–127. [Google Scholar] [CrossRef]
- Heintzenberg, J.; Leck, C. Seasonal variation of the atmospheric aerosol near the top of the marine boundary layer over Spitsbergen related to the Arctic sulphur cycle. Tellus B Chem. Phys. Meteorol. 1994, 46, 52–67. [Google Scholar] [CrossRef]
- Benner, W.H.; Hansen, A.D.A.; Novakov, T. A Concurrent-Flow Cloud Chamber Study of Incorporation of Black Carbon into Droplets. Aerosol Sci. Technol. 1989, 10, 84–92. [Google Scholar] [CrossRef]
- Peng, J.; Hu, M.; Guo, S.; Du, Z.; Shang, D.; Zheng, J.; Zheng, J.; Zeng, L.; Shao, M.; Wu, Y.; et al. Ageing and hygroscopicity variation of black carbon particles in Beijing measured by a quasi-atmospheric aerosol evolution study (QUALITY) chamber. Atmos. Chem. Phys. 2017, 17, 10333–10348. [Google Scholar] [CrossRef]
- Hansen, A.D.A.; Novakov, T. Real-Time Measurements of the Size Fractionation of Ambient Black Carbon Aerosols at Elevated Humidities. Aerosol Sci. Technol. 1989, 10, 106–110. [Google Scholar] [CrossRef]
- Hoppel, W.A.; Frick, G.M.; Fitzgerald, J.W.; Wattle, B.J. A Cloud Chamber Study of the Effect That Nonprecipitating Water Clouds Have on the Aerosol Size Distribution. Aerosol Sci. Technol. 1994, 20, 1–30. [Google Scholar] [CrossRef]
- Lohmann, U.; Lüönd, F.; Mahrt, F. An Introduction to Clouds: From the Microscale to Climate; Cambridge University Press: Cambridge, UK, 2016; Cambridge Core. [Google Scholar] [CrossRef]
- Gordon, H.; Glassmeier, F.; McCoy, D.T. An Overview of Aerosol-Cloud Interactions. In Clouds and Their Climatic Impacts; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 13–45. [Google Scholar] [CrossRef]
- Möhler, O.; Field, P.R.; Connolly, P.; Benz, S.; Saathoff, H.; Schnaiter, M.; Wagner, R.; Cotton, R.; Krämer, M.; Mangold, A.; et al. Efficiency of the deposition mode ice nucleation on mineral dust particles. Atmos. Chem. Phys. 2006, 6, 3007–3021. [Google Scholar] [CrossRef]
- Cziczo, D.J.; Froyd, K.D.; Hoose, C.; Jensen, E.J.; Diao, M.; Zondlo, M.A.; Smith, J.B.; Twohy, C.H.; Murphy, D.M. Clarifying the Dominant Sources and Mechanisms of Cirrus Cloud Formation. Science 2013, 340, 1320–1324. [Google Scholar] [CrossRef]
- Hoose, C.; Möhler, O. Heterogeneous ice nucleation on atmospheric aerosols: A review of results from laboratory experiments. Atmos. Chem. Phys. 2012, 12, 9817–9854. [Google Scholar] [CrossRef]
- Kulkarni, G.; China, S.; Liu, S.; Nandasiri, M.; Sharma, N.; Wilson, J.; Aiken, A.C.; Chand, D.; Laskin, A.; Mazzoleni, C.; et al. Ice nucleation activity of diesel soot particles at cirrus relevant temperature conditions: Effects of hydration, secondary organics coating, soot morphology, and coagulation. Geophys. Res. Lett. 2016, 43, 3580–3588. [Google Scholar] [CrossRef]
- Schill, G.P.; Froyd, K.D.; Bian, H.; Kupc, A.; Williamson, C.; Brock, C.A.; Ray, E.; Hornbrook, R.S.; Hills, A.J.; Apel, E.C.; et al. Widespread biomass burning smoke throughout the remote troposphere. Nat. Geosci. 2020, 13, 422–427. [Google Scholar] [CrossRef]
- Fan, J.; Wang, Y.; Rosenfeld, D.; Liu, X. Review of Aerosol–Cloud Interactions: Mechanisms, Significance, and Challenges. J. Atmos. Sci. 2016, 73, 4221–4252. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Bretherton, C.; Carslaw, K.S.; Coe, H.; DeMott, P.J.; Dunlea, E.J.; Feingold, G.; Ghan, S.; Guenther, A.B.; Kahn, R.; et al. Improving our fundamental understanding of the role of aerosol−cloud interactions in the climate system. Proc. Natl. Acad. Sci. USA 2016, 113, 5781–5790. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, Y.; Wang, X.; Liu, X.; Mao, Q.; Yuan, Y. A review of aerosol-cloud interactions: Mechanisms, climate effects, and observation methods. Atmos. Res. 2025, 325, 108267. [Google Scholar] [CrossRef]
- Carslaw, K.S.; Lee, L.A.; Reddington, C.L.; Pringle, K.J.; Rap, A.; Forster, P.M.; Mann, G.W.; Spracklen, D.V.; Woodhouse, M.T.; Regayre, L.A.; et al. Large contribution of natural aerosols to uncertainty in indirect forcing. Nature 2013, 503, 67–71. [Google Scholar] [CrossRef]
- Kerminen, V.-M.; Paramonov, M.; Anttila, T.; Riipinen, I.; Fountoukis, C.; Korhonen, H.; Asmi, E.; Laakso, L.; Lihavainen, H.; Swietlicki, E.; et al. Cloud condensation nuclei production associated with atmospheric nucleation: A synthesis based on existing literature and new results. Atmos. Chem. Phys. 2012, 12, 12037–12059. [Google Scholar] [CrossRef]
- Andreae, M.O.; Afchine, A.; Albrecht, R.; Holanda, B.A.; Artaxo, P.; Barbosa, H.M.J.; Borrmann, S.; Cecchini, M.A.; Costa, A.; Dollner, M.; et al. Aerosol characteristics and particle production in the upper troposphere over the Amazon Basin. Atmos. Chem. Phys. 2018, 18, 921–961. [Google Scholar] [CrossRef]
- Mülmenstädt, J.; Feingold, G. The Radiative Forcing of Aerosol–Cloud Interactions in Liquid Clouds: Wrestling and Embracing Uncertainty. Curr. Clim. Change Rep. 2018, 4, 23–40. [Google Scholar] [CrossRef]
- Schwarz, J.P.; Gao, R.S.; Fahey, D.W.; Thomson, D.S.; Watts, L.A.; Wilson, J.C.; Reeves, J.M.; Darbeheshti, M.; Baumgardner, D.G.; Kok, G.L.; et al. Single-particle measurements of midlatitude black carbon and light-scattering aerosols from the boundary layer to the lower stratosphere. J. Geophys. Res. Atmos. 2006, 111, D16207. [Google Scholar] [CrossRef]
- Balestrini, R.; Galli, L.; Tartari, G. Wet and dry atmospheric deposition at prealpine and alpine sites in northern Italy. Atmos. Environ. 2000, 34, 1455–1470. [Google Scholar] [CrossRef]
- Lim, S.; Lee, M.; Yoo, H.-J. Size distributions, mixing state, and morphology of refractory black carbon in an urban atmosphere of northeast Asia during summer. Sci. Total Environ. 2023, 856, 158436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, R.-J.; Zhao, Z.; Cao, J.; Ni, H.; Tie, X.; Zhao, S.; Su, X.; Han, Y.; Shen, Z.; et al. Physicochemical characteristics of black carbon aerosol and its radiative impact in a polluted urban area of China. J. Geophys. Res. Atmos. 2016, 121, 12505–12519. [Google Scholar] [CrossRef]
- Gao, K.; Friebel, F.; Zhou, C.-W.; Kanji, Z.A. Enhanced soot particle ice nucleation ability induced by aggregate compaction and densification. Atmos. Chem. Phys. 2022, 22, 4985–5016. [Google Scholar] [CrossRef]
- Zanatta, M.; Mertes, S.; Jourdan, O.; Dupuy, R.; Järvinen, E.; Schnaiter, M.; Eppers, O.; Schneider, J.; Jurányi, Z.; Herber, A. Airborne investigation of black carbon interaction with low-level, persistent, mixed-phase clouds in the Arctic summer. Atmos. Chem. Phys. 2023, 23, 7955–7973. [Google Scholar] [CrossRef]
- Kasparoglu, S.; Cai, L.; Meskhidze, N.; Petters, M.D. Evolution of refractory black carbon mixing state in an urban environment. Atmos. Environ. 1994, 333, 120651. [Google Scholar] [CrossRef]
- Deng, Y.; Tanimoto, H.; Ikeda, K.; Kameyama, S.; Okamoto, S.; Jung, J.; Yoon, Y.J.; Yang, E.J.; Kang, S.-H. Shipborne observations of black carbon aerosols in the western Arctic Ocean during summer and autumn 2016–2020: Impact of boreal fires. Atmos. Chem. Phys. 2024, 24, 6339–6357. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Ge, X.; Shen, Y.; Ge, S.; Chen, M. Characteristics and sources of ambient refractory black carbon aerosols: Insights from soot particle aerosol mass spectrometer. Atmos. Environ. 2018, 185, 147–152. [Google Scholar] [CrossRef]
- Goto-Azuma, K.; Dallmayr, R.; Ogawa-Tsukagawa, Y.; Moteki, N.; Mori, T.; Ohata, S.; Kondo, Y.; Koike, M.; Hirabayashi, M.; Ogata, J.; et al. Technical note: High-resolution analyses of concentrations and sizes of refractory black carbon particles deposited in northwestern Greenland over the past 350 years—Part 1: Continuous flow analysis of the SIGMA-D ice core using the wide-range Single-Particle Soot Photometer and a high-efficiency nebulizer. Atmos. Chem. Phys. 2024, 24, 12985–13000. [Google Scholar] [CrossRef]
- Ohata, S.; Moteki, N.; Mori, T.; Koike, M.; Kondo, Y. A key process controlling the wet removal of aerosols: New observational evidence. Sci. Rep. 2016, 6, 34113. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Q.; Fu, Y.; Lian, X.; Jiang, F.; Peng, L.; Zhang, G.; Li, L.; Chen, D.; Li, M.; et al. Stage-resolved in-cloud scavenging of submicron and BC-containing particles: A case study. Atmos. Environ. 2021, 244, 117883. [Google Scholar] [CrossRef]
- Ding, S.; Zhao, D.; He, C.; Huang, M.; He, H.; Tian, P.; Liu, Q.; Bi, K.; Yu, C.; Pitt, J.; et al. Observed Interactions Between Black Carbon and Hydrometeor During Wet Scavenging in Mixed-Phase Clouds. Geophys. Res. Lett. 2019, 46, 8453–8463. [Google Scholar] [CrossRef]
- Schwarz, J.P.; Spackman, J.R.; Fahey, D.W.; Gao, R.S.; Lohmann, U.; Stier, P.; Watts, L.A.; Thomson, D.S.; Lack, D.A.; Pfister, L.; et al. Coatings and their enhancement of black carbon light absorption in the tropical atmosphere. J. Geophys. Res. Atmos. 2008, 113, D03203. [Google Scholar] [CrossRef]
- Ditas, J.; Ma, N.; Zhang, Y.; Assmann, D.; Neumaier, M.; Riede, H.; Karu, E.; Williams, J.; Scharffe, D.; Wang, Q.; et al. Strong impact of wildfires on the abundance and aging of black carbon in the lowermost stratosphere. Proc. Natl. Acad. Sci. USA 2018, 115, E11595–E11603. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xie, X.; Wei, X.; Zhang, H.; Ying, Q.; Hu, J. Source-specified atmospheric age distribution of black carbon and its impact on optical properties over the Yangtze River Delta. Sci. Total Environ. 2024, 923, 171353. [Google Scholar] [CrossRef]
- Sarkar, A.; Panda, J. Significance of anthropogenic black carbon in modulating atmospheric and cloud properties through aerosol-radiation interaction during a winter-time fog-haze. Atmos. Environ. 2024, 334, 120720. [Google Scholar] [CrossRef]
- Ching, J.; Kajino, M.; Matsui, H. Resolving aerosol mixing state increases accuracy of black carbon respiratory deposition estimates. One Earth 2020, 3, 763–776. [Google Scholar] [CrossRef]
- Lack, D.A.; Langridge, J.M.; Bahreini, R.; Cappa, C.D.; Middlebrook, A.M.; Schwarz, J.P. Brown carbon and internal mixing in biomass burning particles. Proc. Natl. Acad. Sci. USA 2012, 109, 14802–14807. [Google Scholar] [CrossRef] [PubMed]
- Roldin, P.; Ehn, M.; Kurtén, T.; Olenius, T.; Rissanen, M.P.; Sarnela, N.; Elm, J.; Rantala, P.; Hao, L.; Hyttinen, N.; et al. The role of highly oxygenated organic molecules in the Boreal aerosol-cloud-climate system. Nat. Commun. 2019, 10, 4370. [Google Scholar] [CrossRef]
- Koike, M.; Kawai, Y.; Adachi, K.; Aiki, H.; Kanaya, Y.; Kawai, H.; Kita, K.; Kondo, F.; Koshiro, T.; Matsui, H.; et al. Integrated aircraft and research vessel observational studies of aerosols and clouds in summer over the western North Pacific. Prog. Earth Planet. Sci. 2025, 12, 50. [Google Scholar] [CrossRef]
- Matsui, H.; Kawai, K.; Tobo, Y.; Iizuka, Y.; Matoba, S. Increasing Arctic dust suppresses the reduction of ice nucleation in the Arctic lower troposphere by warming. Npj Clim. Atmos. Sci. 2024, 7, 266. [Google Scholar] [CrossRef]
- Samset, B.H.; Myhre, G.; Schulz, M.; Balkanski, Y.; Bauer, S.; Berntsen, T.K.; Bian, H.; Bellouin, N.; Diehl, T.; Easter, R.C.; et al. Black carbon vertical profiles strongly affect its radiative forcing uncertainty. Atmos. Chem. Phys. 2013, 13, 2423–2434. [Google Scholar] [CrossRef]
- Matsui, H.; Hamilton, D.S.; Mahowald, N.M. Black carbon radiative effects highly sensitive to emitted particle size when resolving mixing-state diversity. Nat. Commun. 2018, 9, 3446. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Bi, L. Assessing direct radiative effect of aging black carbon using an advanced aerosol optics module AI-NAOS and the climate model CAM6. Npj Clim. Atmos. Sci. 2025, 8, 187. [Google Scholar] [CrossRef]
- Mann, G.W.; Carslaw, K.S.; Spracklen, D.V.; Ridley, D.A.; Manktelow, P.T.; Chipperfield, M.P.; Pickering, S.J.; Johnson, C.E. Description and evaluation of GLOMAP-mode: A modal global aerosol microphysics model for the UKCA composition-climate model. Geosci. Model Dev. 2010, 3, 519–551. [Google Scholar] [CrossRef]
- Korhonen, H.; Carslaw, K.S.; Spracklen, D.V.; Ridley, D.A.; Ström, J. A global model study of processes controlling aerosol size distributions in the Arctic spring and summer. J. Geophys. Res. Atmos. 2008, 113, D08211. [Google Scholar] [CrossRef]
- Lee, Y.H.; Pierce, J.R.; Adams, P.J. Representation of nucleation mode microphysics in a global aerosol model with sectional microphysics. Geosci. Model Dev. 2013, 6, 1221–1232. [Google Scholar] [CrossRef]
- Liu, X.; Easter, R.C.; Ghan, S.J.; Zaveri, R.; Rasch, P.; Shi, X.; Lamarque, J.-F.; Gettelman, A.; Morrison, H.; Vitt, F.; et al. Toward a minimal representation of aerosols in climate models: Description and evaluation in the Community Atmosphere Model CAM5. Geosci. Model Dev. 2012, 5, 709–739. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.-L.; Wang, H.; Tilmes, S.; Singh, B.; Easter, R.C.; Ghan, S.J.; Rasch, P.J. Description and evaluation of a new four-mode version of the modal aerosol module (MAM4) within version 5.3 of the community atmosphere model. Geosci. Model Dev. 2016, 9, 505–522. [Google Scholar] [CrossRef]
- Mills, M.J.; Schmidt, A.; Easter, R.; Solomon, S.; Kinnison, D.E.; Ghan, S.J.; Neely, R.R., III; Marsh, D.R.; Conley, A.; Bardeen, C.G.; et al. Global volcanic aerosol properties derived from emissions, 1990–2014, using CESM1(WACCM). J. Geophys. Res. Atmos. 2016, 121, 2332–2348. [Google Scholar] [CrossRef]
- Mori, T.; Morris, S.; Kondo, Y.; Sharma, S.; Eleftheriadis, K.; Klimont, Z.; Soulie, A.; Granier, C.; Quinn, P.K.; Pratt, K.A. Sensitivity of Wintertime Arctic Black Carbon to Removal Processes and Regional Alaskan Sources. J. Geophys. Res. Atmos. 2025, 130, e2024JD042885. [Google Scholar] [CrossRef]
- Stier, P.; Feichter, J.; Kinne, S.; Kloster, S.; Vignati, E.; Wilson, J.; Ganzeveld, L.; Tegen, I.; Werner, M.; Balkanski, Y.; et al. The aerosol-climate model ECHAM5-HAM. Atmos. Chem. Phys. 2005, 5, 1125–1156. [Google Scholar] [CrossRef]
- Bellouin, N.; Davies, W.; Shine, K.P.; Quaas, J.; Mülmenstädt, J.; Forster, P.M.; Smith, C.; Lee, L.; Regayre, L.; Brasseur, G.; et al. Radiative forcing of climate change from the Copernicus reanalysis of atmospheric composition. Earth Syst. Sci. Data 2020, 12, 1649–1677. [Google Scholar] [CrossRef]
- Mann, G.W.; Carslaw, K.S.; Ridley, D.A.; Spracklen, D.V.; Pringle, K.J.; Merikanto, J.; Korhonen, H.; Schwarz, J.P.; Lee, L.A.; Manktelow, P.T.; et al. Intercomparison of modal and sectional aerosol microphysics representations within the same 3-D global chemical transport model. Atmos. Chem. Phys. 2012, 12, 4449–4476. [Google Scholar] [CrossRef]
- Tegen, I.; Neubauer, D.; Ferrachat, S.; Siegenthaler-Le Drian, C.; Bey, I.; Schutgens, N.; Stier, P.; Watson-Parris, D.; Stanelle, T.; Schmidt, H.; et al. The global aerosol–climate model ECHAM6.3–HAM2.3—Part 1: Aerosol evaluation. Geosci. Model Dev. 2019, 12, 1643–1677. [Google Scholar] [CrossRef]
- Stolzenburg, D.; Fischer, L.; Vogel, A.L.; Heinritzi, M.; Schervish, M.; Simon, M.; Wagner, A.C.; Dada, L.; Ahonen, L.R.; Amorim, A.; et al. Rapid growth of organic aerosol nanoparticles over a wide tropospheric temperature range. Proc. Natl. Acad. Sci. USA 2018, 115, 9122–9127. [Google Scholar] [CrossRef]
- Samset, B.H.; Myhre, G.; Herber, A.; Kondo, Y.; Li, S.-M.; Moteki, N.; Koike, M.; Oshima, N.; Schwarz, J.P.; Balkanski, Y.; et al. Modelled black carbon radiative forcing and atmospheric lifetime in AeroCom Phase II constrained by aircraft observations. Atmos. Chem. Phys. 2014, 14, 12465–12477. [Google Scholar] [CrossRef]
- Chapman, E.G.; Gustafson Jr, W.I.; Easter, R.C.; Barnard, J.C.; Ghan, S.J.; Pekour, M.S.; Fast, J.D. Coupling aerosol-cloud-radiative processes in the WRF-Chem model: Investigating the radiative impact of elevated point sources. Atmos. Chem. Phys. 2009, 9, 945–964. [Google Scholar] [CrossRef]
- Adams, P.J.; Seinfeld, J.H. Predicting global aerosol size distributions in general circulation models. J. Geophys. Res. Atmos. 2002, 107, 4370. [Google Scholar] [CrossRef]
- Lee, Y.H.; Adams, P.J. A Fast and Efficient Version of the TwO-Moment Aerosol Sectional (TOMAS) Global Aerosol Microphysics Model. Aerosol Sci. Technol. 2012, 46, 678–689. [Google Scholar] [CrossRef]
- Spracklen, D.V.; Pringle, K.J.; Carslaw, K.S.; Chipperfield, M.P.; Mann, G.W. A global off-line model of size-resolved aerosol microphysics: I. Model development and prediction of aerosol properties. Atmos. Chem. Phys. 2005, 5, 2227–2252. [Google Scholar] [CrossRef]
- Mann, G.W.; Carslaw, K.S.; Reddington, C.L.; Pringle, K.J.; Schulz, M.; Asmi, A.; Spracklen, D.V.; Ridley, D.A.; Woodhouse, M.T.; Lee, L.A.; et al. Intercomparison and evaluation of global aerosol microphysical properties among AeroCom models of a range of complexity. Atmos. Chem. Phys. 2014, 14, 4679–4713. [Google Scholar] [CrossRef]
- Kodros, J.K.; Pierce, J.R. Important global and regional differences in aerosol cloud-albedo effect estimates between simulations with and without prognostic aerosol microphysics. J. Geophys. Res. Atmos. 2017, 122, 4003–4018. [Google Scholar] [CrossRef]
- Morrison, H.; Gettelman, A. A New Two-Moment Bulk Stratiform Cloud Microphysics Scheme in the Community Atmosphere Model, Version 3 (CAM3). Part I. J. Clim. 2008, 21, 3642–3659, JSTOR. [Google Scholar] [CrossRef]
- Gettelman, A.; Morrison, H.; Santos, S.; Bogenschutz, P.; Caldwell, P.M. Advanced Two-Moment Bulk Microphysics for Global Models. Part II: Global Model Solutions and Aerosol–Cloud Interactions. J. Clim. 2015, 28, 1288–1307. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, G.; Guo, S.; Zamora, M.L.; Ying, Q.; Lin, Y.; Wang, W.; Hu, M.; Wang, Y. Formation of Urban Fine Particulate Matter. Chem. Rev. 2015, 115, 3803–3855. [Google Scholar] [CrossRef]
- Kuwata, M.; Kondo, Y.; Takegawa, N. Critical condensed mass for activation of black carbon as cloud condensation nuclei in Tokyo. J. Geophys. Res. Atmos. 2009, 114, D20202. [Google Scholar] [CrossRef]
- McMeeking, G.R.; Hamburger, T.; Liu, D.; Flynn, M.; Morgan, W.T.; Northway, M.; Highwood, E.J.; Krejci, R.; Allan, J.D.; Minikin, A.; et al. Black carbon measurements in the boundary layer over western and northern Europe. Atmos. Chem. Phys. 2010, 10, 9393–9414. [Google Scholar] [CrossRef]
- Lund, M.T.; Samset, B.H.; Skeie, R.B.; Watson-Parris, D.; Katich, J.M.; Schwarz, J.P.; Weinzierl, B. Short Black Carbon lifetime inferred from a global set of aircraft observations. Npj Clim. Atmos. Sci. 2018, 1, 31. [Google Scholar] [CrossRef]
- Coppola, A.I.; Wagner, S.; Lennartz, S.T.; Seidel, M.; Ward, N.D.; Dittmar, T.; Santín, C.; Jones, M.W. The black carbon cycle and its role in the Earth system. Nat. Rev. Earth Environ. 2022, 3, 516–532. [Google Scholar] [CrossRef]
- Matsui, H.; Mahowald, N. Development of a global aerosol model using a two-dimensional sectional method: 2. Evaluation and sensitivity simulations. J. Adv. Model. Earth Syst. 2017, 9, 1887–1920. [Google Scholar] [CrossRef]
- Tan, T.; Guo, S.; Wu, Z.; He, L.; Huang, X.; Hu, M. Impact of aging process on atmospheric black carbon aerosol properties and climate effects. Chin. Sci. Bull. 2020, 65, 4235–4250. [Google Scholar] [CrossRef]
- Kokkola, H.; Kühn, T.; Laakso, A.; Bergman, T.; Lehtinen, K.E.J.; Mielonen, T.; Arola, A.; Stadtler, S.; Korhonen, H.; Ferrachat, S.; et al. SALSA2.0: The sectional aerosol module of the aerosol–chemistry–climate model ECHAM6.3.0-HAM2.3-MOZ1.0. Geosci. Model Dev. 2018, 11, 3833–3863. [Google Scholar] [CrossRef]
- Dahlkötter, F.; Gysel, M.; Sauer, D.; Minikin, A.; Baumann, R.; Seifert, P.; Ansmann, A.; Fromm, M.; Voigt, C.; Weinzierl, B. The Pagami Creek smoke plume after long-range transport to the upper troposphere over Europe; aerosol properties and black carbon mixing state. Atmos. Chem. Phys. 2014, 14, 6111–6137. [Google Scholar] [CrossRef]
- Reddington, C.L.; Carslaw, K.S.; Stier, P.; Schutgens, N.; Coe, H.; Liu, D.; Allan, J.; Browse, J.; Pringle, K.J.; Lee, L.A.; et al. The Global Aerosol Synthesis and Science Project (GASSP): Measurements and Modeling to Reduce Uncertainty. Bull. Am. Meteorol. Soc. 2017, 98, 1857–1877. [Google Scholar] [CrossRef]
- Zaveri, R.A.; Barnard, J.C.; Easter, R.C.; Riemer, N.; West, M. Particle-resolved simulation of aerosol size, composition, mixing state, and the associated optical and cloud condensation nuclei activation properties in an evolving urban plume. J. Geophys. Res. Atmos. 2010, 115, D17210. [Google Scholar] [CrossRef]
- Zheng, Z.; West, M.; Zhao, L.; Ma, P.-L.; Liu, X.; Riemer, N. Quantifying the structural uncertainty of the aerosol mixing state representation in a modal model. Atmos. Chem. Phys. 2021, 21, 17727–17741. [Google Scholar] [CrossRef]
- Fanourgakis, G.S.; Kanakidou, M.; Nenes, A.; Bauer, S.E.; Bergman, T.; Carslaw, K.S.; Grini, A.; Hamilton, D.S.; Johnson, J.S.; Karydis, V.A.; et al. Evaluation of global simulations of aerosol particle and cloud condensation nuclei number, with implications for cloud droplet formation. Atmos. Chem. Phys. 2019, 19, 8591–8617. [Google Scholar] [CrossRef]
- Kulmala, M.; Kontkanen, J.; Junninen, H.; Lehtipalo, K.; Manninen, H.E.; Nieminen, T.; Petäjä, T.; Sipilä, M.; Schobesberger, S.; Rantala, P.; et al. Direct Observations of Atmospheric Aerosol Nucleation. Science 2013, 339, 943–946. [Google Scholar] [CrossRef]
- Stolzenburg, D.; Cai, R.; Blichner, S.M.; Kontkanen, J.; Zhou, P.; Makkonen, R.; Kerminen, V.-M.; Kulmala, M.; Riipinen, I.; Kangasluoma, J. Atmospheric nanoparticle growth. Rev. Mod. Phys. 2023, 95, 045002. [Google Scholar] [CrossRef]
- Nieminen, T.; Kerminen, V.-M.; Petäjä, T.; Aalto, P.P.; Arshinov, M.; Asmi, E.; Baltensperger, U.; Beddows, D.C.S.; Beukes, J.P.; Collins, D.; et al. Global analysis of continental boundary layer new particle formation based on long-term measurements. Atmos. Chem. Phys. 2018, 18, 14737–14756. [Google Scholar] [CrossRef]
- Zhang, Q.; Jimenez, J.L.; Canagaratna, M.R.; Ulbrich, I.M.; Ng, N.L.; Worsnop, D.R.; Sun, Y. Understanding atmospheric organic aerosols via factor analysis of aerosol mass spectrometry: A review. Anal. Bioanal. Chem. 2011, 401, 3045–3067. [Google Scholar] [CrossRef]
- Ackermann, I.J.; Hass, H.; Memmesheimer, M.; Ebel, A.; Binkowski, F.S.; Shankar, U. Modal aerosol dynamics model for Europe: Development and first applications. Atmos. Environ. 1998, 32, 2981–2999. [Google Scholar] [CrossRef]
- Curtius, J. Nucleation of atmospheric aerosol particles. Nucleation 2006, 7, 1027–1045. [Google Scholar] [CrossRef]
- Kulmala, M.; Petäjä, T.; Nieminen, T.; Sipilä, M.; Manninen, H.E.; Lehtipalo, K.; Dal Maso, M.; Aalto, P.P.; Junninen, H.; Paasonen, P.; et al. Measurement of the nucleation of atmospheric aerosol particles. Nat. Protoc. 2012, 7, 1651–1667. [Google Scholar] [CrossRef] [PubMed]
- Ehn, M.; Thornton, J.A.; Kleist, E.; Sipilä, M.; Junninen, H.; Pullinen, I.; Springer, M.; Rubach, F.; Tillmann, R.; Lee, B.; et al. A large source of low-volatility secondary organic aerosol. Nature 2014, 506, 476–479. [Google Scholar] [CrossRef]
- Grieshop, A.P.; Miracolo, M.A.; Donahue, N.M.; Robinson, A.L. Constraining the Volatility Distribution and Gas-Particle Partitioning of Combustion Aerosols Using Isothermal Dilution and Thermodenuder Measurements. Environ. Sci. Technol. 2009, 43, 4750–4756. [Google Scholar] [CrossRef]
- Petters, M.D.; Kreidenweis, S.M. A single parameter representation of hygroscopic growth and cloud condensation nucleus activity. Atmos. Chem. Phys. 2007, 7, 1961–1971. [Google Scholar] [CrossRef]
- McFiggans, G.; Artaxo, P.; Baltensperger, U.; Coe, H.; Facchini, M.C.; Feingold, G.; Fuzzi, S.; Gysel, M.; Laaksonen, A.; Lohmann, U.; et al. The effect of physical and chemical aerosol properties on warm cloud droplet activation. Atmos. Chem. Phys. 2006, 6, 2593–2649. [Google Scholar] [CrossRef]
- Rosenfeld, D.; Andreae, M.O.; Asmi, A.; Chin, M.; de Leeuw, G.; Donovan, D.P.; Kahn, R.; Kinne, S.; Kivekäs, N.; Kulmala, M.; et al. Global observations of aerosol-cloud-precipitation-climate interactions. Rev. Geophys. 2014, 52, 750–808. [Google Scholar] [CrossRef]
- Flossmann, A.I.; Hall, W.D.; Pruppacher, H.R. A Theoretical Study of the Wet Removal of Atmospheric Pollutants. Part I: The Redistribution of Aerosol Particles Captured through Nucleation and Impaction Scavenging by Growing Cloud Drops. J. Atmos. Sci. 1985, 42, 583–606. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, S.; Padro, J.; Barrie, L. A size-segregated particle dry deposition scheme for an atmospheric aerosol module. Atmos. Environ. 2001, 35, 549–560. [Google Scholar] [CrossRef]
- Shan, Y.; Liu, X.; Lin, L.; Ke, Z.; Lu, Z.; Tilmes, S.; Gao, L.; Yu, P. The Role of In-Cloud Wet Removal in Simulating Aerosol Vertical Profiles and Cloud Radiative Forcing. J. Geophys. Res. Atmos. 2023, 128, e2023JD038564. [Google Scholar] [CrossRef]
- Zheng, X.; Dong, X.; Xi, B.; Logan, T.; Wang, Y. Distinctive aerosol–cloud–precipitation interactions in marine boundary layer clouds from the ACE-ENA and SOCRATES aircraft field campaigns. Atmos. Chem. Phys. 2024, 24, 10323–10347. [Google Scholar] [CrossRef]
- Kerminen, V.-M.; Chen, X.; Vakkari, V.; Petäjä, T.; Kulmala, M.; Bianchi, F. Atmospheric new particle formation and growth: Review of field observations. Environ. Res. Lett. 2018, 13, 103003. [Google Scholar] [CrossRef]
- Xu, B.; Cao, J.; Hansen, J.; Yao, T.; Joswia, D.R.; Wang, N.; Wu, G.; Wang, M.; Zhao, H.; Yang, W.; et al. Black soot and the survival of Tibetan glaciers. Proc. Natl. Acad. Sci. USA 2009, 106, 22114–22118. [Google Scholar] [CrossRef]
- Kurokawa, J.; Ohara, T. Long-term historical trends in air pollutant emissions in Asia: Regional Emission inventory in ASia (REAS) version 3. Atmos. Chem. Phys. 2020, 20, 12761–12793. [Google Scholar] [CrossRef]
- Liu, Z.; Bollasina, M.A.; Wilcox, L.J. Impact of Asian aerosols on the summer monsoon strongly modulated by regional precipitation biases. Atmos. Chem. Phys. 2024, 24, 7227–7252. [Google Scholar] [CrossRef]
- Zheng, B.; Tong, D.; Li, M.; Liu, F.; Hong, C.; Geng, G.; Li, H.; Li, X.; Peng, L.; Qi, J.; et al. Trends in China’s anthropogenic emissions since 2010 as the consequence of clean air actions. Atmos. Chem. Phys. 2018, 18, 14095–14111. [Google Scholar] [CrossRef]
- Chen, Y.; Ebenstein, A.; Greenstone, M.; Li, H. Evidence on the impact of sustained exposure to air pollution on life expectancy from China’s Huai River policy. Proc. Natl. Acad. Sci. USA 2013, 110, 12936–12941. [Google Scholar] [CrossRef]
- Ghude, S.D.; Chate, D.M.; Jena, C.; Beig, G.; Kumar, R.; Barth, M.C.; Pfister, G.G.; Fadnavis, S.; Pithani, P. Premature mortality in India due to PM2.5 and ozone exposure. Geophys. Res. Lett. 2016, 43, 4650–4658. [Google Scholar] [CrossRef]
- Hodzic, A.; Kasibhatla, P.S.; Jo, D.S.; Cappa, C.D.; Jimenez, J.L.; Madronich, S.; Park, R.J. Rethinking the global secondary organic aerosol (SOA) budget: Stronger production, faster removal, shorter lifetime. Atmos. Chem. Phys. 2016, 16, 7917–7941. [Google Scholar] [CrossRef]
- Ding, A.J.; Huang, X.; Nie, W.; Sun, J.N.; Kerminen, V.-M.; Petäjä, T.; Su, H.; Cheng, Y.F.; Yang, X.-Q.; Wang, M.H.; et al. Enhanced haze pollution by black carbon in megacities in China. Geophys. Res. Lett. 2016, 43, 2873–2879. [Google Scholar] [CrossRef]
- Guo, S.; Hu, M.; Zamora, M.L.; Peng, J.; Shang, D.; Zheng, J.; Du, Z.; Wu, Z.; Shao, M.; Zeng, L.; et al. Elucidating severe urban haze formation in China. Proc. Natl. Acad. Sci. USA 2014, 111, 17373–17378. [Google Scholar] [CrossRef]
- Lee, S.-H.; Gordon, H.; Yu, H.; Lehtipalo, K.; Haley, R.; Li, Y.; Zhang, R. New Particle Formation in the Atmosphere: From Molecular Clusters to Global Climate. J. Geophys. Res. Atmos. 2019, 124, 7098–7146. [Google Scholar] [CrossRef]
- Maso, M.D.; Kulmala, M.; Riipinen, I.; Wagner, R.; Hussein, T.; Aalto, P.P.; Lehtinen, K. Formation and growth of fresh atmospheric aerosols: Eight years of aerosol size distribution data from SMEAR II, Hyytiälä, Finland. Boreal Environ. Res. 2005, 10, 323–336. [Google Scholar]
- Huang, R.-J.; Zhang, Y.; Bozzetti, C.; Ho, K.-F.; Cao, J.-J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef]
- Wang, M.; Kong, W.; Marten, R.; He, X.-C.; Chen, D.; Pfeifer, J.; Heitto, A.; Kontkanen, J.; Dada, L.; Kürten, A.; et al. Rapid growth of new atmospheric particles by nitric acid and ammonia condensation. Nature 2020, 581, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.C.; Chou, C.C.-K.; Lee, C.S.L.; Kuo, W.-C.; Chang, S.-C. Hygroscopic properties and cloud condensation nuclei activity of atmospheric aerosols under the influences of Asian continental outflow and new particle formation at a coastal site in eastern Asia. Atmos. Chem. Phys. 2020, 20, 5911–5922. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Kang, L.; Yuan, T.; Luo, Y.; Alam, K.; Li, J.; He, Y.; Bi, H.; Zhao, D. Direct Radiative Forcing Induced by Light-Absorbing Aerosols in Different Climate Regions Over East Asia. J. Geophys. Res. Atmos. 2020, 125, e2019JD032228. [Google Scholar] [CrossRef]
- Riemer, N.; Ault, A.P.; West, M.; Craig, R.L.; Curtis, J.H. Aerosol Mixing State: Measurements, Modeling, and Impacts. Rev. Geophys. 2019, 57, 187–249. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Effects of biomass burning on climate, accounting for heat and moisture fluxes, black and brown carbon, and cloud absorption effects. J. Geophys. Res. Atmos. 2014, 119, 8980–9002. [Google Scholar] [CrossRef]
- Engelhart, G.J.; Asa-Awuku, A.; Nenes, A.; Pandis, S.N. CCN activity and droplet growth kinetics of fresh and aged monoterpene secondary organic aerosol. Atmos. Chem. Phys. 2008, 8, 3937–3949. [Google Scholar] [CrossRef]
- Chang, D.Y.; Lelieveld, J.; Tost, H.; Steil, B.; Pozzer, A.; Yoon, J. Aerosol physicochemical effects on CCN activation simulated with the chemistry-climate model EMAC. Atmos. Environ. 2017, 162, 127–140. [Google Scholar] [CrossRef]
- Akinyoola, J.A.; Oluleye, A.; Gbode, I.E. A Review of Atmospheric Aerosol Impacts on Regional Extreme Weather and Climate Events. Aerosol Sci. Eng. 2024, 8, 249–274. [Google Scholar] [CrossRef]
- Shrivastava, M.; Cappa, C.D.; Fan, J.; Goldstein, A.H.; Guenther, A.B.; Jimenez, J.L.; Kuang, C.; Laskin, A.; Martin, S.T.; Ng, N.L.; et al. Recent advances in understanding secondary organic aerosol: Implications for global climate forcing. Rev. Geophys. 2017, 55, 509–559. [Google Scholar] [CrossRef]
- Fan, J.; Rosenfeld, D.; Zhang, Y.; Giangrande, S.E.; Li, Z.; Machado, L.A.T.; Martin, S.T.; Yang, Y.; Wang, J.; Artaxo, P.; et al. Substantial convection and precipitation enhancements by ultrafine aerosol particles. Science 2018, 359, 411–418. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Y.; Beirle, S.; Xie, P.; Wagner, T.; Xu, J.; Li, A.; Dörner, S.; Ren, B.; Li, X. Technical note: Evaluation of profile retrievals of aerosols and trace gases for MAX-DOAS measurements under different aerosol scenarios based on radiative transfer simulations. Atmos. Chem. Phys. 2021, 21, 12867–12894. [Google Scholar] [CrossRef]
- Matsui, H.; Kondo, Y.; Moteki, N.; Takegawa, N.; Sahu, L.K.; Zhao, Y.; Fuelberg, H.E.; Sessions, W.R.; Diskin, G.; Blake, D.R.; et al. Seasonal variation of the transport of black carbon aerosol from the Asian continent to the Arctic during the ARCTAS aircraft campaign. J. Geophys. Res. Atmos. 2011, 116, D05202. [Google Scholar] [CrossRef]

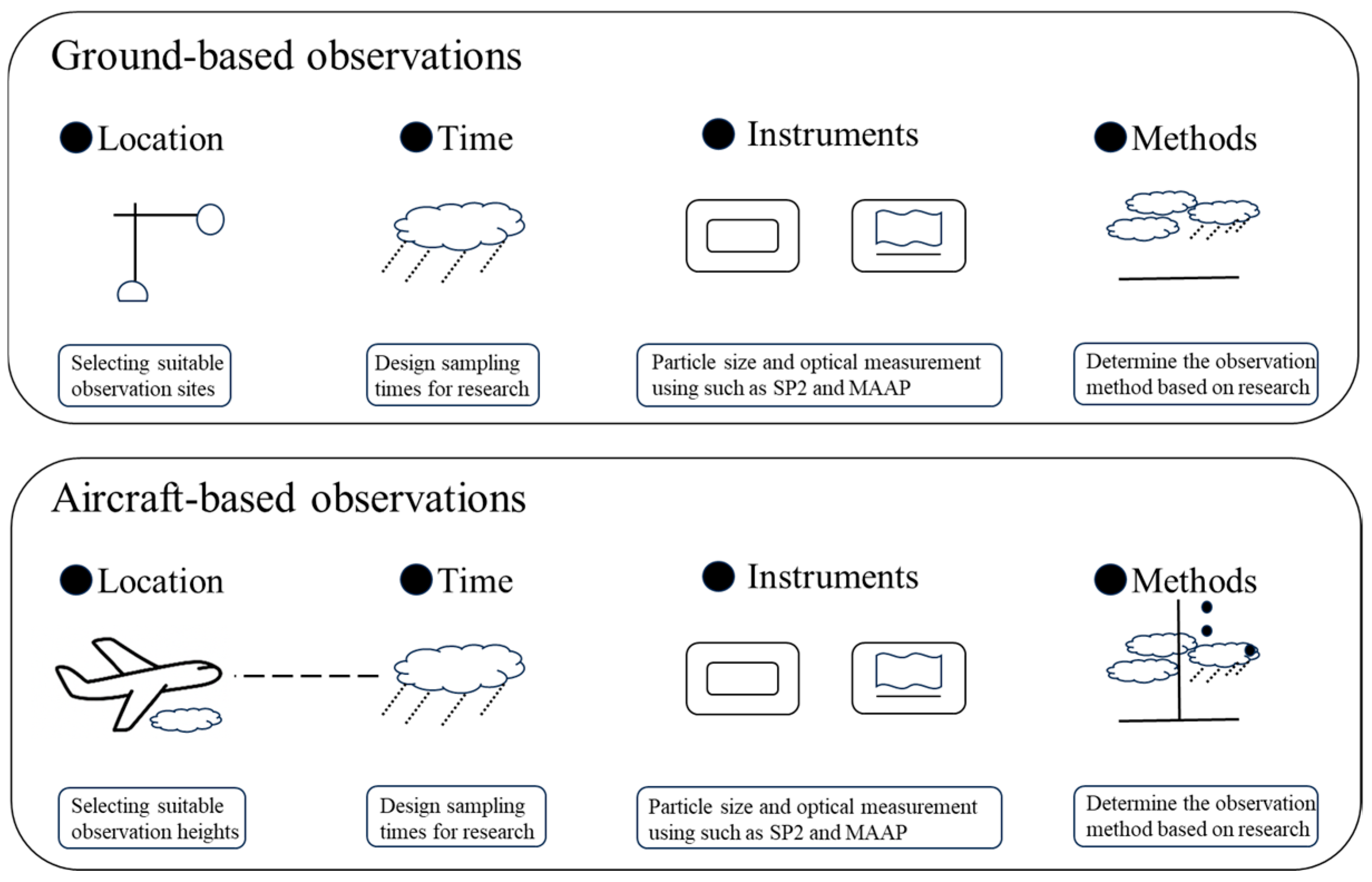
| Model/Tool | Type | Main Function | Features & Applications | References |
|---|---|---|---|---|
| GEOS-Chem | Global 3D chemical transport model | Simulates in-cloud scavenging in large-scale mixed-phase clouds | Supports multiple representations of scavenging efficiency, reflecting different research perspectives and mathematical descriptions | [187] |
| ECHAM-HAM | Numerical climate model system | High-resolution simulation of aerosol–cloud interactions | Couples aerosols as cloud condensation nuclei/ice nuclei affecting cloud microphysics (e.g., droplet spectra, cloud phase, albedo, and lifetime), and quantifies anthropogenic and natural aerosol impacts on radiation and hydrological cycles | [98,104,142,186,188,189,190,191] |
| GISS ModelE | Coupled climate system model | Simulates the radiative forcing of aerosols | Integrates atmospheric circulation, ocean circulation, and land surface modules; quantifies effects of sulfate, black carbon, etc.; ModelE2.1/2.2 extends carbon cycle processes, applicable to paleoclimate reconstruction and future climate projections | [6,14,178,192,193] |
| WRF-Chem | Regional-scale online coupled meteorology–chemistry model | Captures local circulation features and simulates black carbon transport and deposition | Kilometre-scale nested grids suitable for complex highland terrains; studies black carbon effects on glacier albedo and water resources; ongoing improvements in wet deposition parameterization | [112,194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Wang, J.; Wang, L.; Xu, B. Particle Size as a Key Driver of Black Carbon Wet Removal: Advances and Insights. Atmosphere 2025, 16, 1309. https://doi.org/10.3390/atmos16111309
Qiao Y, Wang J, Wang L, Xu B. Particle Size as a Key Driver of Black Carbon Wet Removal: Advances and Insights. Atmosphere. 2025; 16(11):1309. https://doi.org/10.3390/atmos16111309
Chicago/Turabian StyleQiao, Yumeng, Jiajia Wang, Li Wang, and Baiqing Xu. 2025. "Particle Size as a Key Driver of Black Carbon Wet Removal: Advances and Insights" Atmosphere 16, no. 11: 1309. https://doi.org/10.3390/atmos16111309
APA StyleQiao, Y., Wang, J., Wang, L., & Xu, B. (2025). Particle Size as a Key Driver of Black Carbon Wet Removal: Advances and Insights. Atmosphere, 16(11), 1309. https://doi.org/10.3390/atmos16111309





