1. Introduction
Atmospheric particulate matter (PM) is one of the most monitored air pollutants due to the negative effects it can have on human health. It is estimated that PM is the thirteenth leading cause of mortality worldwide, causing approximately 800,000 premature deaths each year [
1]. Moreover, chronic exposure to PM has been identified as one of the causes of the increase in hospital admissions for respiratory problems and cardiovascular diseases. According to most epidemiological studies conducted to date, 3% of cardiopulmonary deaths and 5% of lung cancer deaths are attributable to PM exposure [
2]. PM is also one of the major indoor air pollutants, due to its ability to accumulate in confined spaces and to its numerous indoor sources [
3]. In the absence of adequate ventilation and/or air purification systems, concentration levels exceeding the WHO health alert thresholds can easily be reached [
4]. Given that, on average, people spend more than 80% of their time indoors, this represents a major health concern for the population.
Until recent years, the concentration of airborne particles was considered the only metric to assess the health hazard they pose; however, chemical composition may also play an important role [
5]. Indeed, studies suggest that the adverse effects caused by atmospheric PM can be attributed to the oxidative stress that PM causes in our bodies. These studies highlight how certain redox active species present in PM, such as heavy metals and quinones, can catalyze the formation of ROS (reactive oxygen species): highly reactive molecules that can react indiscriminately with the body’s cells [
6]. The overall oxidative capacity of PM has been proposed as a metric that is more closely related to the biological responses caused by PM exposure and thus may be more informative than particulate mass alone in assessing the health hazard of PM [
7].
Currently, oxidative potential (OP) is being proposed as a parameter with which to measure the oxidative stress directly caused by PM. This is defined as the ability of PM to oxidize target molecules that have similar functional groups and reactivity to endogenous molecules [
8]. Based on this definition, various methods have been developed to measure this parameter, including both cellular and acellular assays. The former are more widespread since they offer a lower degree of complexity and have the advantages of being fast, inexpensive, and allowing for the rapid development of large data sets [
9]. Most of these assays are based on oxidation reactions promoted by components of PM, which generate ROS. The only method that allows direct determination of these radical species employs electron spin resonance (ESR) [
10] but is seldom used due to several drawbacks such as high costs, high complexity, and low sensitivity. Instead, particle-bound methods measure ROS within and on PM particles with fluorescent techniques, such as the dichlorofluorescein (DCFH) test. However, recent studies are questioning their relevance and therefore research is shifting mainly towards OP measurements [
9].
Indirect methods evaluate the progress of specific reactions by assessing the depletion rate of the reagent (target molecule) or the formation rate of the products with different analytical techniques. Numerous alternatives are currently being proposed in the literature for the determination of OP, such as ascorbic acid (AA), dithiothreitol (DTT), reduced glutathione (GSH), and chemiluminescence reductive acridinium triggering (CRAT) tests [
11]. Each assay has different strengths and weaknesses, but most importantly every test has a different sensitivity towards the oxidizing components found in PM [
12]. To date, there is still no acellular assay that can respond to all PM components that generate ROS [
9]. Moreover, in some cases the application procedures of the various protocols vary from author to author, complicating data comparison [
6]. This is a critical issue if OP is to be regarded as the main parameter assessing the toxicity of PM.
Many different protocols can be found in the literature, including varying extraction solvents, filter types, incubation times, metal chelators, and other parameters [
9]. For instance, Teflon filters are associated with higher OP values if compared to quartz fiber [
13]. Also, extraction with methanol, as opposed to ultrapure water or buffer solutions, can result in better efficiencies and therefore higher OP readings [
14]. However, since OP readings should reproduce real-life conditions as faithfully as possible, methanol may not be the best option to evaluate OP in relation to potential adverse health effects. Another parameter that varies between studies is the filter area [
12,
15]. This may also potentially be an issue because linearity with increasing filter area has not been yet studied.
Indeed, regulatory bodies and agencies have started to express their interest in implementing OP measurements to the other routine analyses carried out on PM samples. The EU is currently proposing a draft of the new air quality directive [
16], in substitution of the old 2008/50/CE, which includes OP measurements in PM filters without specifying the methods of analysis. The new directive states that OP measurements will need to be carried out in both rural and urban sites in at least 45% of monitored fixed sites, highlighting the importance of this parameter to comprehend the effect of PM on health and the environment. However, to do so it is necessary to select a test with a common procedure to be universally applied.
In this study, two acellular tests (AA and DTT) were applied in parallel to a series of PM filters collected during a one-year sampling conducted by ARPA Lombardia, a local technical agency that supports the implementation of environmental policy, at an urban background site in the city of Milan. The same tests were also applied to another set of samples collected in two different indoor sites. Literature protocols that best simulated endogenous conditions were implemented for both assays and the DTT test was also optimized to ensure reproducibility of the measurements and improve accuracy of the results. The choice of the AA and DTT assays was made based on their widespread applications in literature, but mostly their speed and affordable costs, which make them good candidates for routine applications of OP measurements. Moreover, having different sensitivities towards the two main categories of oxidizing species in PM: metals and quinones; the two tests provide complementary information on the oxidative properties of PM.
Initially, optimization of the DTT assay was performed to obtain a reliable and reproducible protocol with which to carry out the tests. Eventually, calibration and limit of quantification (LOQ) determination were performed to establish the working ranges and sensitivity of the two methods. Before carrying out the assays on the PM samples, standard solutions of metals and quinones were tested and results were compared with similar studies to evaluate instrumental response. Finally, both outdoor and indoor PM samples were assessed using the AA and optimized DTT assays.
2. Materials and Methods
OP measurements were carried out on all PM samples with the AA and DTT assays following the procedures described in Visentin et al. 2016 [
7]. These protocols were chosen because they are the ones that best simulate physiological conditions. This must be one of the main factors when selecting the type of test to carry out since the aim of the measurement is to assess the oxidative capacity of PM in the human body.
2.1. Reagents and Materials
The AA assay was carried out with a UV-VIS spectrophotometer (DU 800, Beckman Coulter, Fullerton, CA, USA) using quartz cuvettes. Ascorbic acid (Fluka Chemika, 99.5%, Buchs, Switzerland) was used to prepare the starting reagent solution in ultrapure water (milli-Q, Millipore, Darmstadt, Germany). The pH 7.4 buffer used to extract PM was prepared starting from Na2HPO4 (Sigma Aldrich, 99%, Saint Louis, MO, USA) and NaH2PO4 (Carlo Erba Reagents, 99%, Milan, Italy) salts and purified with a cationic exchange resin (Chelex 100, sodic form, Sigma Aldrich) to remove trace metals. Quarters of 47-mm diameter PM filters were extracted using an ultrasonic bath (Branson 2510, Branson Ultrasonics Corp., Danbury, CT, USA) for 30 min and filtered with 0.45 μm filters before analysis.
The DTT assay was performed with a UV-VIS spectrophotometer (Jasco V-730) using quartz cuvettes. The reaction was carried out in amber vials covered in tin foil using a stirring and heating plate (RCT basic, IKA Werke, Staufen, Germany) and digital thermoregulation (vertex, Velp scientifica). Ultrapure water (milli-Q, Millipore) was used to prepare all solutions: dithiothreitol (Sigma Aldrich, 99%), trichloroacetic acid (Fluka BioChemika, 99.5%), DTNB (Sigma Aldrich). The same pH 7.4 buffer was used to extract PM, whereas the pH 8.9 buffer was prepared from TRIS (Sigma Aldrich, 99%) and HCl (Sigma Aldrich, 37% w/w) with the addition of EDTA (Sigma Aldrich, 98%).
To verify the correct response of the two tests, standard solutions of metals and quinones were tested before applying the methods to the samples: Cu2+ (CuSO4·H2O; Sigma Aldrich; 98%) Cr3+ (CrO3, Carlo Erba Reagents, 99%, Milan, Italy), Mn2+ (MnO2, Carlo Erba Reagents, 90%), 1,2-naphthoquinone (1,2-NPQ), 9,10-phenatrenechinone (9,10-PNQ), and 1,4-naphthoquinone (1,4-NPQ). Quinone standard solutions were prepared in acetonitrile, whereas milli-Q water was selected as the solvent for the metal solutions.
2.2. Optimization of the DTT Assay
Two modifications were made to the DTT method described in Visentin et al. 2016 [
7] to ensure the reproducibility of the data and linearity of the results. First, the time between two successive withdrawals (data points) was set to 10 min. This allowed us to observe a statistically significant difference between data points, improving linearity and therefore trueness of the final OP result. Second, the time between the last step of the method (addition of the pH 8.9 buffer) and the spectrophotometric analysis was set at a minimum of five minutes. This guaranteed the stability of the absorbance of the solution being tested.
2.3. Calibration and LOD, LOQ Determination
Calibration curves were constructed for both methods (AA and DTT) to verify linearity in the working range. For the AA assay, standard solutions of ascorbic acid at different concentrations between 10 µM and 100 µM were prepared and analyzed. Instead, a different approach was taken for the DTT assay since the molecule that is determined spectrophometrically, namely 2-nitro-5-tiobenzoic acid (TNB), is not commercially available. In this case, the calibration curve was constructed by running several tests following the optimized procedure with different starting concentrations of DTT in pH 7.4 buffer (6.7 µM–33.3 µM).
Ten blank measurements were carried out to determine the limit of detection (LOD) and LOQ of both methods:
where: µ
b is the mean average absorbance of blank measurements, σ
b is the standard deviation of blank measurements and m is the slope of the calibration curve.
2.4. Outdoor Particulate Matter Samples
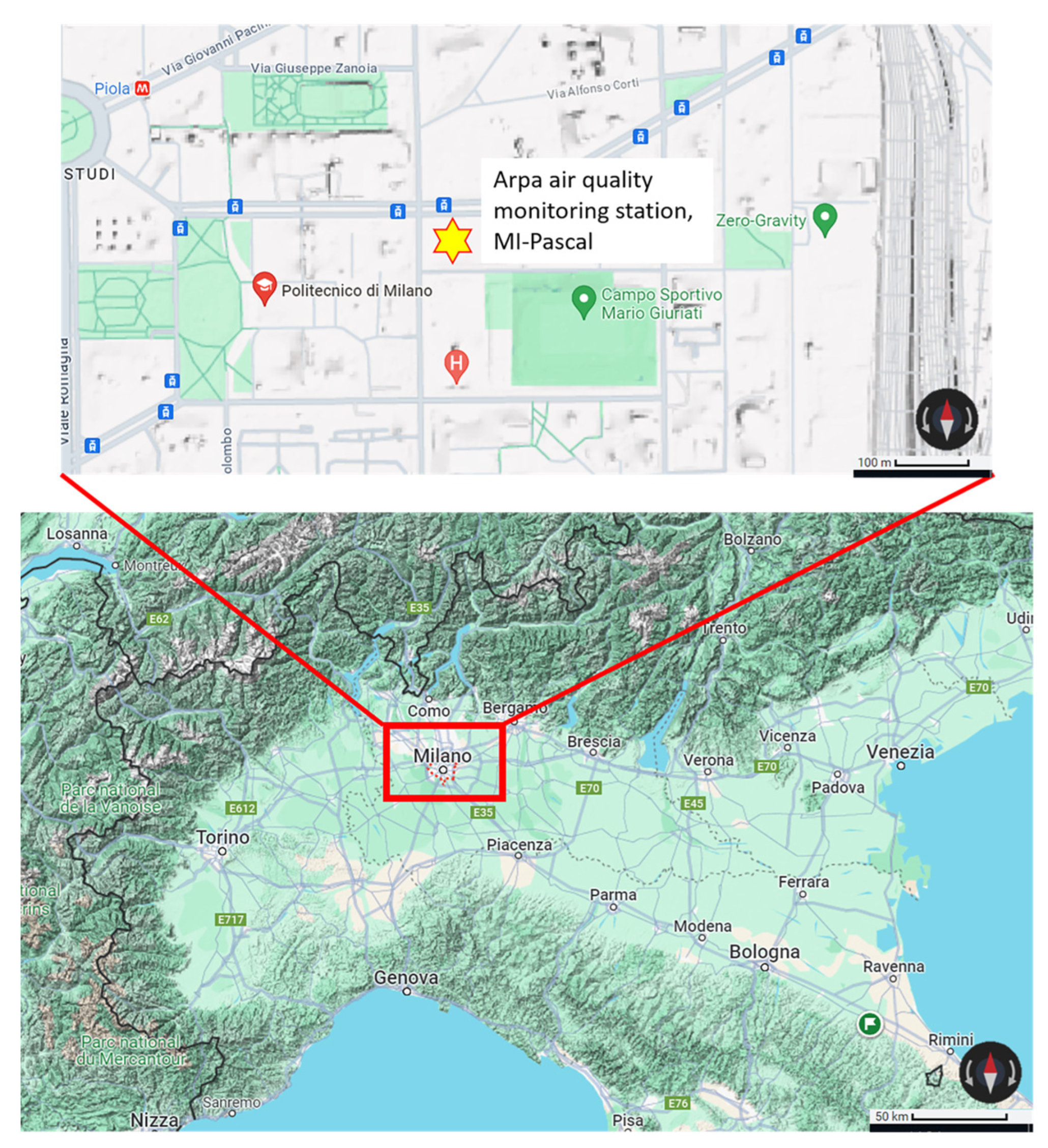
Twenty-four particulate matter (PM10) filters were selected from a one-year sampling campaign conducted at an urban background site in the city of Milan in the year 2022 (
Figure 1). The filters were collected following the specifications of the UNI EN 12341:2014 technical standard [
17] on quartz fiber filters (Ø = 47 mm) and each one corresponds to 24 h of sampling (
Table 1).
2.5. Indoor Particulate Matter Samples
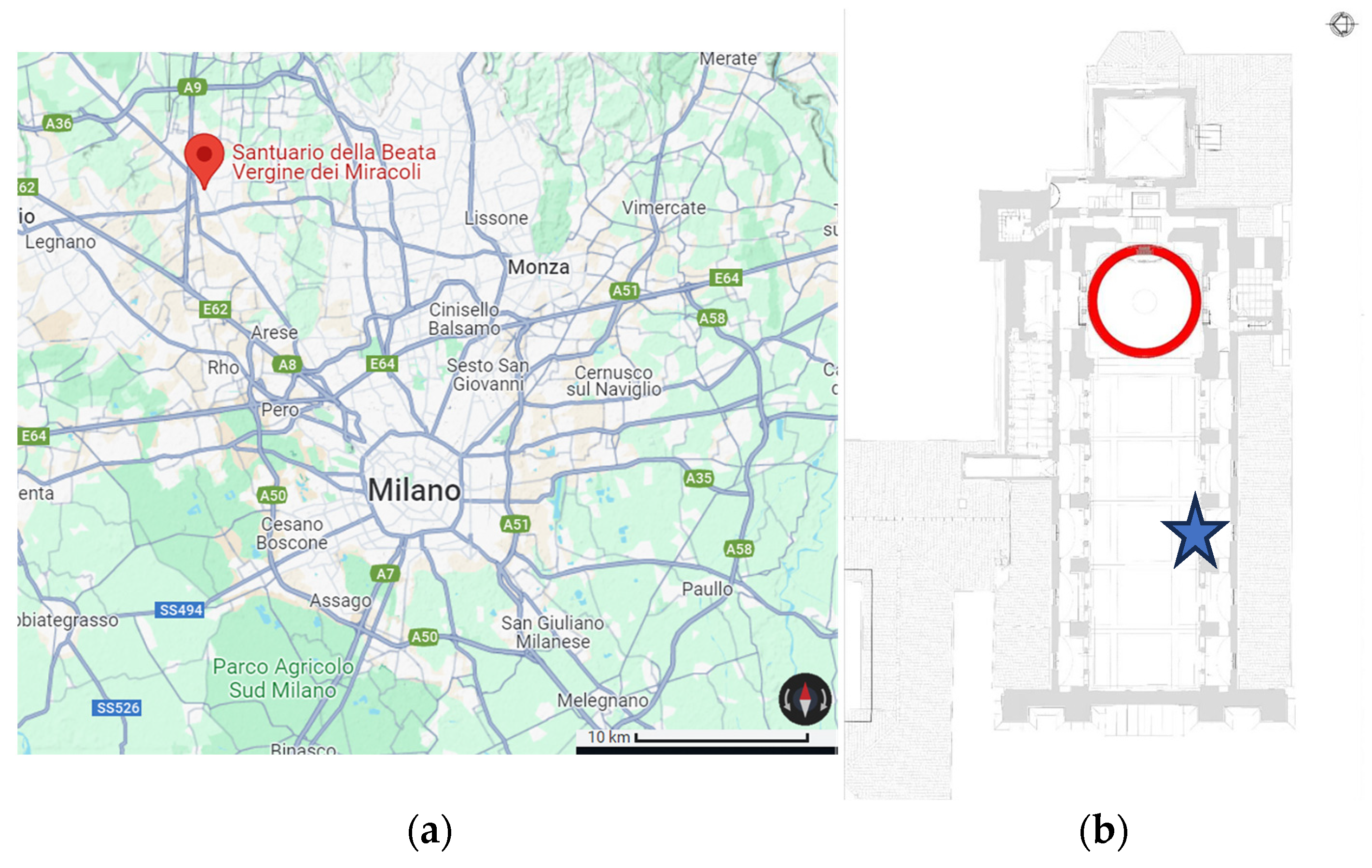
PM filters from a previous sampling campaign conducted in the
Santuario della Beata Vergine dei Miracoli in Saronno (VA) [
18] were selected for this study as indoor samples (
Figure 2). One filter collected in a laboratory of the University of Milan was also analyzed as a reference.
Sampling was carried out using a high-volume sampler (ECHO Emergency, Tecora, Cogliate, Italy) on quartz fiber filters (Ø = 101.6 mm) and each one corresponds to multiple days of sampling (
Table 2).
4. Discussion
PM has a very complex chemical composition which depends on the type of pollutant sources, their intensity, and atmospheric conditions. Therefore, the composition can vary greatly depending on location, season, and other contributing factors. Even though the negative effects that PM has on the human body are known, the complexity of the chemical composition makes it difficult to establish a direct relationship between toxicity and its different components. However, toxicological studies are trying to shed light on this important issue and significant steps forward have been made. OP is currently being proposed as an alternative metric to particulate mass alone, able to assess the toxicity of airborne particles. Several methods have been proposed in the literature, but a universally accepted one is lacking. This makes it difficult to compare data from different studies worldwide, even more so considering the great variations in terms of the chemical composition of PM.
Some agencies and regulatory bodies have started to express their interest in adding OP measurements to the other routine analyses aimed at the chemical characterization of PM. In the draft of the new air quality directive, the EU introduced OP as an additional parameter to be monitored, covering a minimum of 45% of yearly PM samples. However, the draft does not indicate measurement methods or protocols, leaving the choice to the member states. Given the large number of methods and protocols in the literature, it is crucial to have a single reference methodology with an accepted protocol for results to be comparable. For such large-scale applications, measurement methods need to be fast and easily applicable. Two of the most widespread methods in literature that display these characteristics are the AA and the DTT assay.
In this study, these two methods were optimized and validated to assess their potential applicability for routine PM analysis carried out by regulatory bodies and agencies. With regards to the integrations made, fixing a minimum time before analysis was important because in several samples the differences in absorbance values between two successive data points were comparable to the difference in absorbance between the analysis carried out after 1 and 5 min following the addition of the pH 8.9 buffer. This was especially true for samples with a low OP, in which the absorbance difference between successive data points is very small. Moreover, setting a time interval between withdrawals also helped to improve the quality of the measurement, especially for these types of samples, in which it was often difficult to measure observable differences between data points taken separated by only five minutes. Both integrations have a direct impact on the linearity of the data points, and therefore on the accuracy and reliability of OP values.
Calibration curves were also constructed for both assays to evaluate the linearity of the absorbance instrumental response within the working range. To the knowledge of the authors, most literature studies that employed the AA and/or DTT assays calculated analyte concentrations from absorbance values through a direct application of the Lambert-Beer law using tabulated molar absorption coefficients. Some studies use an alternative approach for the calculation of OP [
20] and employ calibration curves to obtain initial analyte concentration. However, analyte rate decay is calculated without considering the linearity of the response, which is a distinctive element of AA and DTT OP measurements. While it is true that the low concentrations involved in these assays are within the range of application of the Lambert-Beer law, it is good practice to check the linearity of the response with a calibration curve due to possible instrumental deviations which may be non-negligible. Once again, this is even more important given the very small differences in absorbance between data points, especially for the DTT assay. Moreover, the determination of the LOQ was also important to establish a working range and discard any outlying data points that would otherwise affect linearity and therefore the reliability of the OP value.
Method validation and protocol optimization (DTT) carried out in this study confirmed the potential large-scale applicability of both assays for routine PM analysis, which is crucial for regional and international regulation agencies looking to introduce OP as an alternative parameter to be monitored. For this reason, a first-ever application of OP measurements was carried out on PM filters from a yearly sampling campaign conducted by the regional protection agency of Lombardy, Italy (ARPA Lombardia). Once the new EU air quality directive is adopted, ARPA Lombardia amongst the bodies responsible for carrying out OP measurements. It is therefore in their interest to evaluate the applicability of different methods for routine PM analysis.
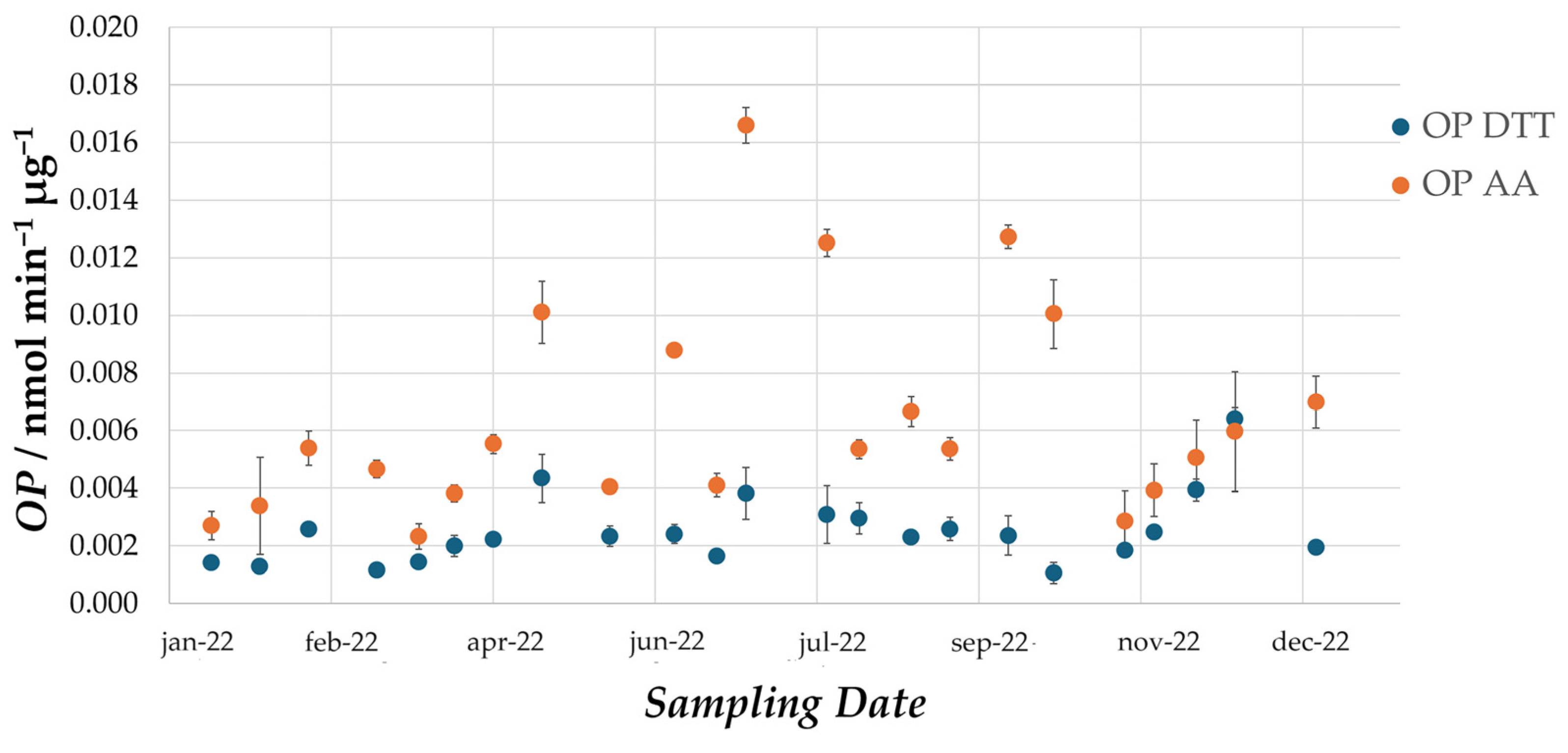
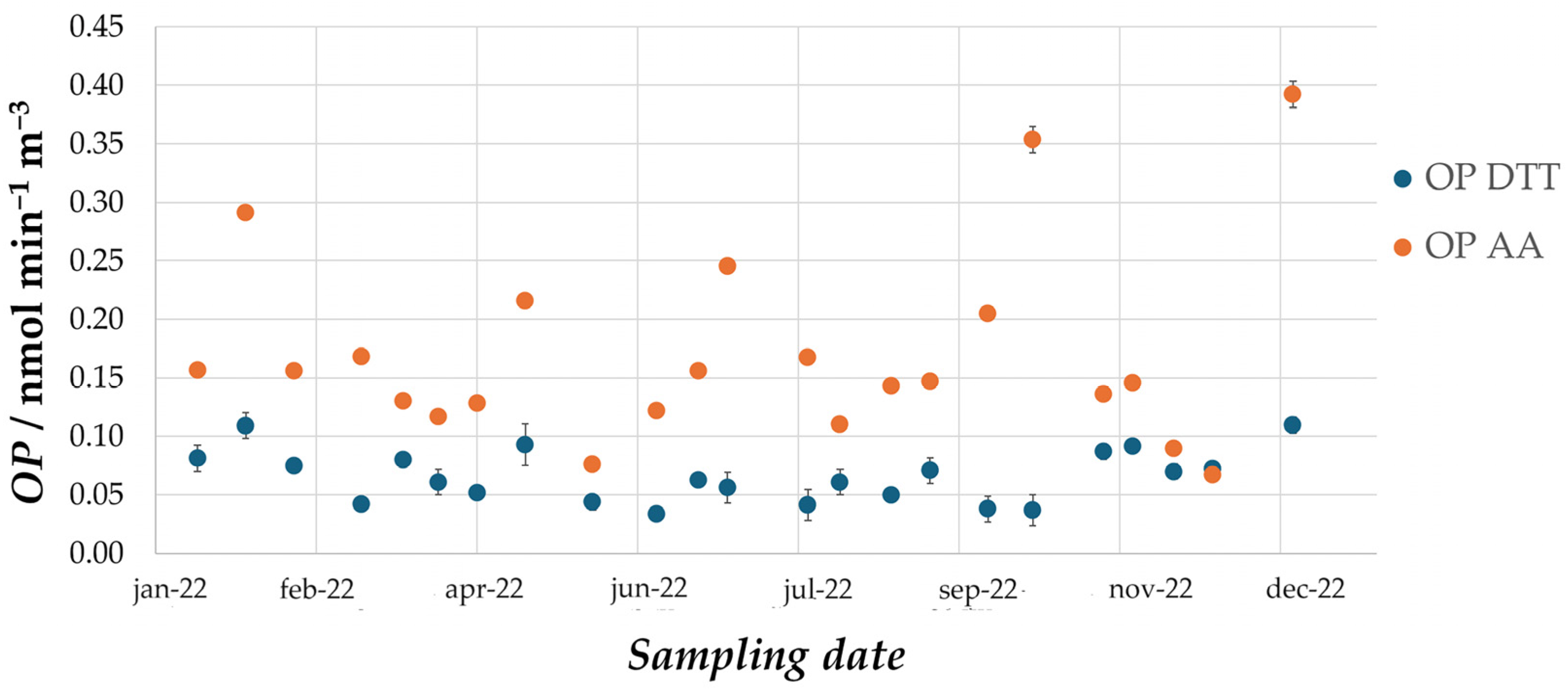
The results of the campaign show that OP values fall within the wide range of values observed in literature for urban sites [
5,
20,
21]. Mean values observed for PM10 in Milan are lower than mean values observed in most of the other similar studies carried out in the literature; however, it is difficult to compare absolute values when the reproducibility of OP measurements remains to be established. Indeed, intercomparisons between different laboratories still have to be carried out and differences in the protocols probably lead to differences in the final results. Instead, relevant conclusions can be drawn based on seasonal trends, which highlight that exposure to oxidative airborne particles is constant throughout the entire year. In fact, OP
DTT and volume-normalized OP
AA values do not show significant variations between the different months of sampling. On the other hand, mass-normalized OP
AA values are higher in the summer months. Given that the average mass concentration of PM is lower in summer than in winter, this is probably due to a greater percentage contribution of organic oxidizing species generated by photochemical aging. As reported by Antinolo et al. 2015 [
22], these molecules are part of secondary organic aerosol and promote the formation of ROS in the human body.
This suggests that the chemical composition of the particles plays a fundamental role in defining the oxidative properties of PM. Nevertheless, the fact that volume-normalized OPAA does not highlight a similar seasonal trend indicates that a different factor is driving the oxidative capacity of the particles collected in winter. Possibly, the high mass concentrations in the colder months are enough to counterbalance a lower percentage contribution of oxidizing species. Instead, some studies suggest that enhanced partitioning of semi-volatile organic compounds (SVOCs) in the particulate phase contributes to high OP in winter. Overall, the results of this study indicate that both the chemical composition and the mass concentrations play an important role in defining the OP of atmospheric PM, further highlighting the importance of carrying out such measurements on a yearly basis.
The results observed for the indoor samples also support this conclusion. On the one hand, reduced photochemical activity in indoor sites decreases secondary organic aerosol formation therefore reducing the oxidative capacity of the particles. On the other hand, average PM concentrations were lower than outdoors in the monitored site [
18]. Other factors that may have played a role are different sampling conditions and locations between indoors and outdoors. With regards to the former, studies suggest that the response of acellular assays tends to be low for particles with an aerodynamic diameter lower than 10 µm [
23], therefore sampling without a size-selective inlet in indoor areas may explain part of the difference. However, the number of particles greater than 10 µm is minimal compared to the smaller size ranges [
18]. Despite them having a greater impact on the total mass, it is unlikely that these particles are responsible for the large differences in OP observed. It is also unlikely that the different sampling locations may have played a significant role. First, the
Santuario della Beata Vergine dei Miracoli is located close to another urban background ARPA monitoring station (Saronno-Santuario) [
18], highlighting similar characteristics of the two sites. Moreover, the PM10 concentrations recorded by the monitoring stations do not highlight significant differences in the concentrations (
Table S1).
This also reinforces the conclusion that OP values were mainly influenced by the conditions within the indoor site. Therefore, chemical composition and mass concentration were the main factors explaining the differences between indoors and outdoors in this case study. Given the large variability of indoor PM chemical composition and the large number of sources and factors which are involved, this may not reflect the situation in different indoor environments. It is therefore important to promote and carry out these types of investigations on other sites. With the aim of further understanding the relationship between OP and the chemical composition of PM, future work will include a complete chemical characterization of the filters analyzed in this study. It is important to underline that scientific research on this topic is still in the early stages from a methodological point of view and the best methods and procedures to make OP a routine analysis are still being considered. In this regard, the proposed methods and protocols in this study represent valid candidates. As briefly mentioned in the discussion, the next step will be to carry out interlaboratory comparisons to test for reproducibility and robustness. The final goal is to obtain a unique method and established protocol that would enable full application of the new air quality directive whilst ensuring comparability of measurements carried out in unmonitored areas such as indoor spaces.