Fossil Diesel, Soybean Biodiesel and Hydrotreated Vegetable Oil: A Numerical Analysis of Emissions Using Detailed Chemical Kinetics at Diesel Engine Like Conditions
Abstract
1. Introduction
2. Literature Review on Fuel Surrogates, Experiments and Simulations
2.1. Fossil Diesel Surrogates
2.2. Biodiesel Surrogates
2.3. HVO Surrogates
3. Materials and Methods
3.1. The In-House Cantera-Python Computational Tool for Analysis
3.2. Fuel Surrogates Composition for Numerical Simulations
- Fossil diesel (diesel A): 81% n-docecane, 14% toluene, 5% cyclohexane, with cetane number of CN = 44.3, pointed out as the best surrogates mixture for engine performance and emissions in the work of Liu et al. [42].
- Soybean diesel (biodiesel): 11% methyl-decanoate, 11% methyl-palmitate, 78% methyl-linoleate, with C/H ratio of 1.82, and reported as soybean biodiesel composition in the work of Rinaldi et al. [65].
- Mixture M1 ☞ diesel A:biodiesel:HVO (90:10:0)
- Mixture M2 ☞ diesel A:biodiesel:HVO (85:15:0)
- Mixture M3 ☞ diesel A:biodiesel:HVO (80:15:5)
3.3. The Detailed Kinetics Model for Fossil Diesel, Soybean Biodiesel and HVO
3.3.1. Simulation Parameters and Conditions Used in This Work
4. Results and Discussion
4.1. Number of Cycles Simulated for Engine Stable Operation
4.2. Predicted Engine Expansion Power
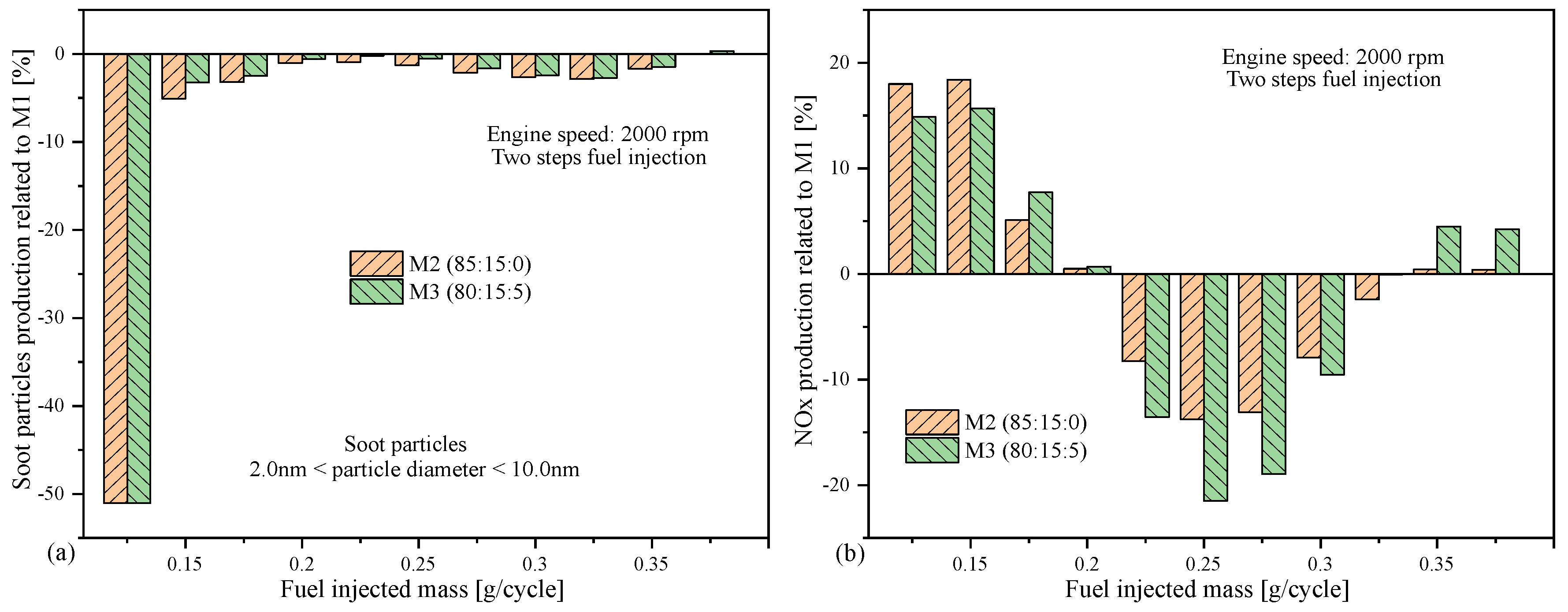
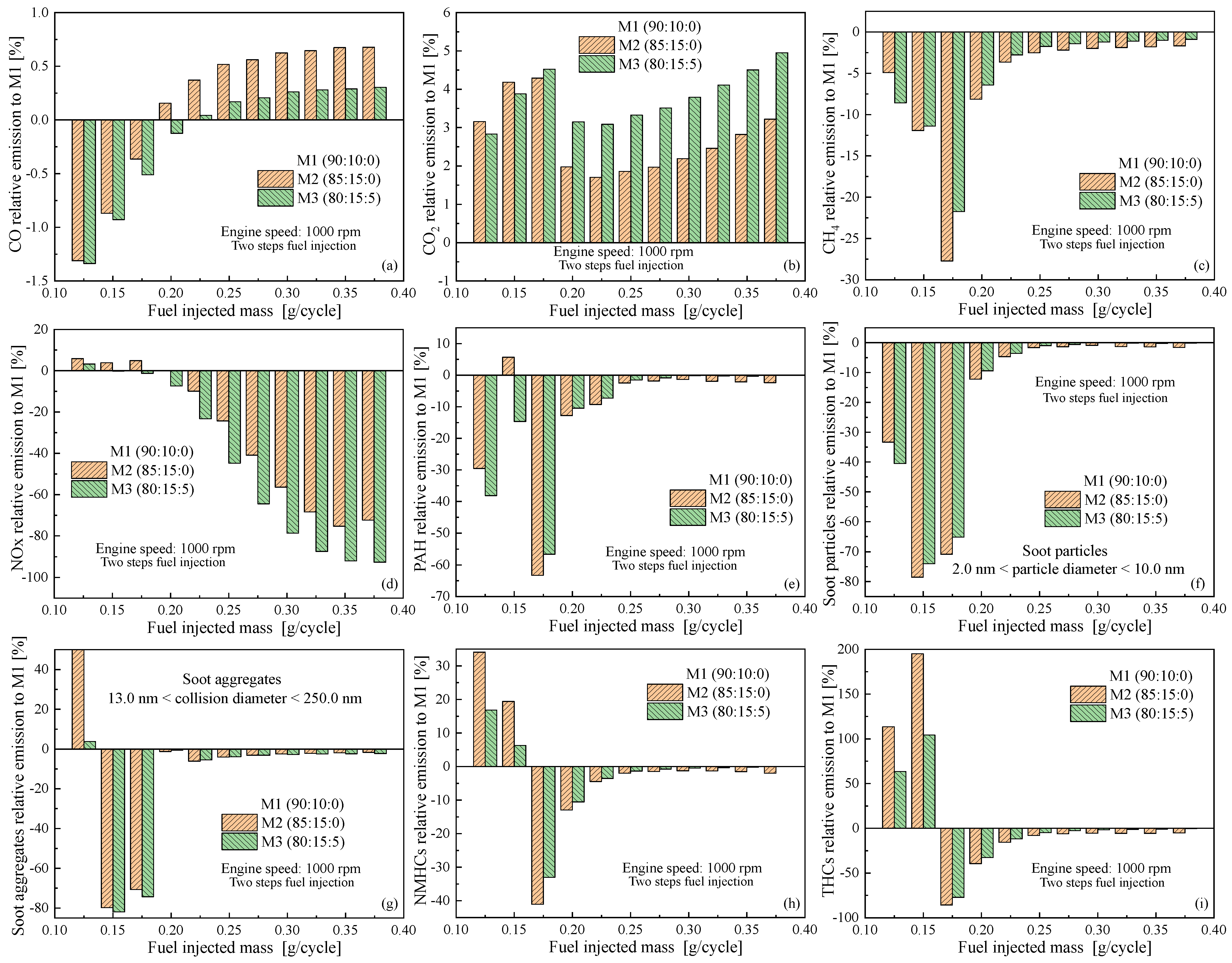
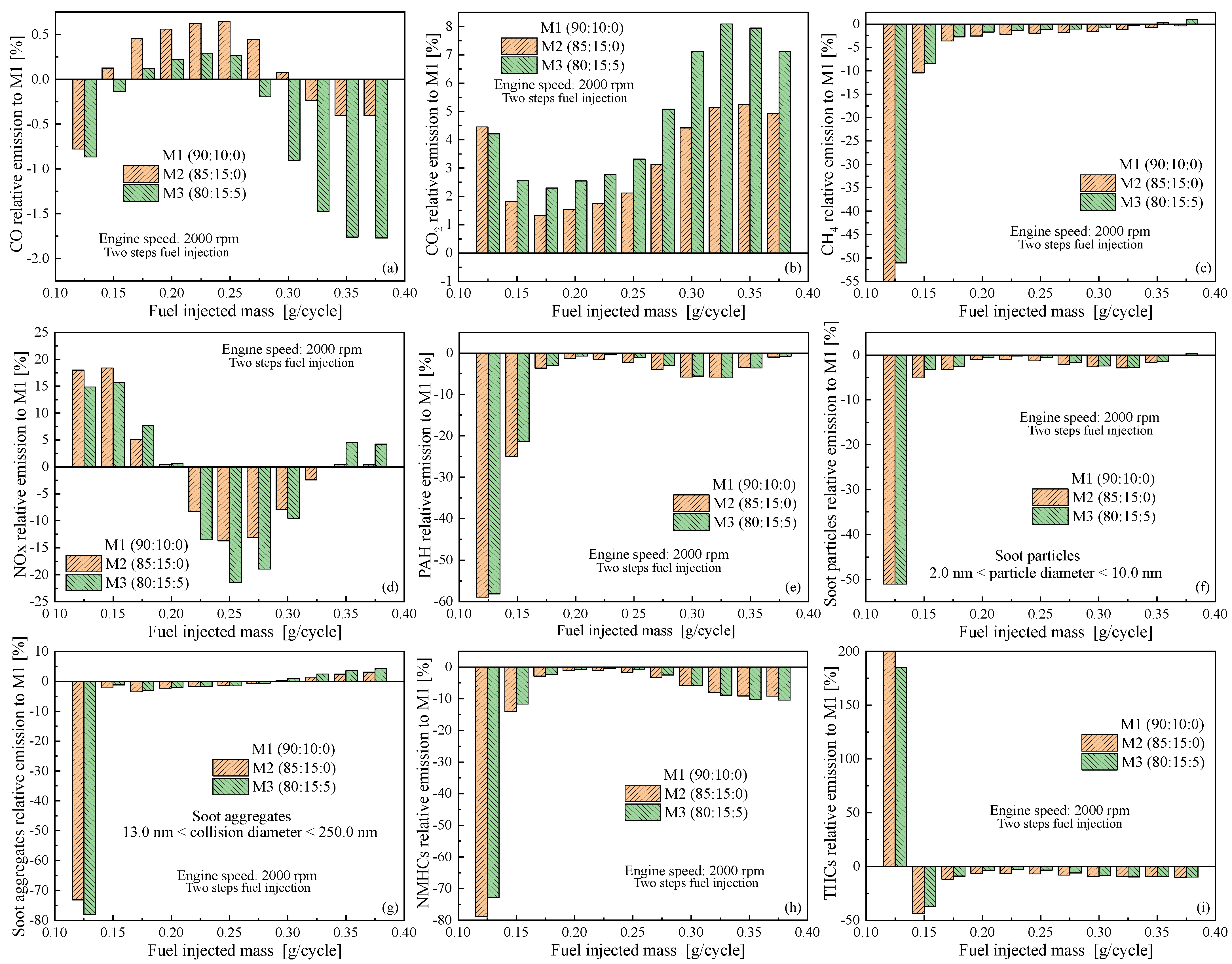
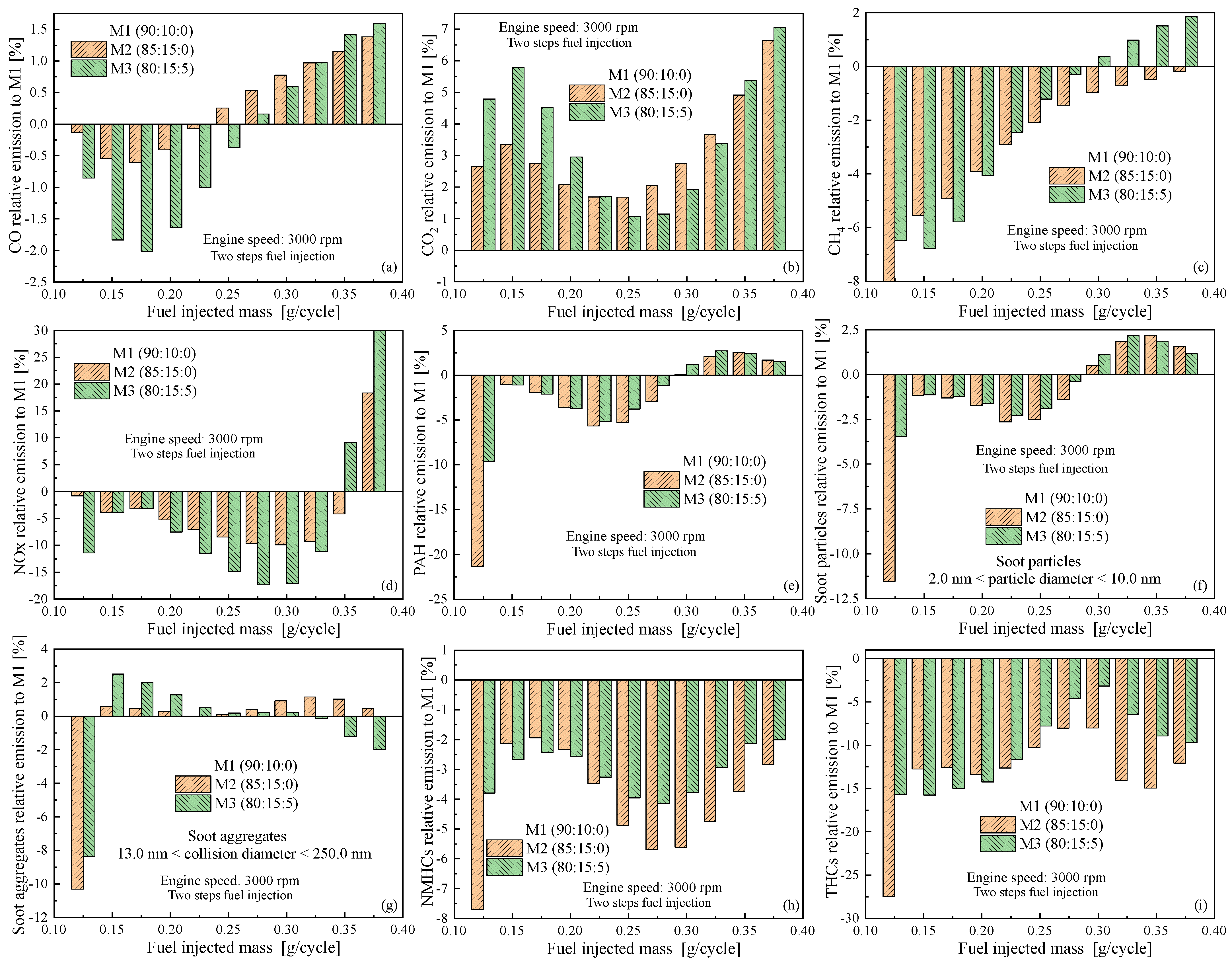
4.3. Emissions as Function of Engine Speed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NMHCs | Nonmethane hydrocarbons |
| THCs | Total hydrocarbons |
| NOx | Total nitrogen oxides (NO, NO2, NO3) |
| HCCI | Homogeneous charge compression ignition |
| Mixture M1 | diesel A:biodiesel:HVO (90:10:0) |
| Mixture M2 | diesel A:biodiesel:HVO (85:15:0) |
| Mixture M3 | diesel A:biodiesel:HVO (80:15:5) |
| IC | Internal combustion engines |
| CO2e | tonnes of carbon dioxide equivalents |
| Mtoe | Megatonne of oil equivalents |
References
- Temizer, I.; Cihan, O. Experimental and numerical evaluation of combustion analysis of a DI diesel engine. Energy Rep. 2021, 7, 5549–5561. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, P.; Mahendran, R.; Huang, W.; Gao, Y.; Yang, Z.; Ye, T.; Wen, B.; Wu, Y.; Li, S.; et al. Global climate change and human health: Pathways and possible solutions. Eco-Environ. Health 2022, 1, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change. Climate Change 2022: Impacts, Adaptation and Vulnerability; Technical Report, WMO—UNEP, 2022. Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Available online: https://www.ipcc.ch/report/ar6/wg2/ (accessed on 1 April 2023).
- British Petroleum. bp Statistical Review of World Energy; Technical Report; British Petroleum: London, UK, 2022. [Google Scholar]
- Reitz, R.D.; Ogawa, H.; Payri, R.; Fansler, T.; Kokjohn, S.; Moriyoshi, Y.; Agarwal, A.; Arcoumanis, D.; Assanis, D.; Bae, C.; et al. IJER editorial: The future of the internal combustion engine. Int. J. Engine Res. 2020, 21, 3–10. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. Greenhouse Gas Emissions by Sector. 2023. Available online: https://ourworldindata.org/grapher/ghg-emissions-by-sector-stacked (accessed on 1 April 2023).
- Vignesh, P.; Kumar, A.R.P.; Ganesh, N.S.; Jayaseelan, V.; Sudhakar, K. Biodiesel and green diesel generation: An overview. Oil Gas Sci. Technol.—Revue d’IFP Energies Nouv. 2021, 76, 6. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z. Structure Evolution of Synthetic Amino Acids-Derived Basic Ionic Liquids for Catalytic Production of Biodiesel. ACS Sustain. Chem. Eng. 2017, 5, 1237–1247. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Agarwal, R.A.; Gupta, T.; Gurjar, B.R. (Eds.) Biofuels; Green Energy and Technology; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Hamidi, R.; Damizia, M.; De Filippis, P.; Patrizi, D.; Verdone, N.; Vilardi, G.; de Caprariis, B. Recent developments and future outlooks of hydrodynamic cavitation as an intensification technology for renewable biofuels production. J. Environ. Chem. Eng. 2023, 11, 110819. [Google Scholar] [CrossRef]
- Elkelawy, M.; El Shenawy, E.A.; Alm-Eldin Bastawissi, H.; Shams, M.M.; Panchal, H. A comprehensive review on the effects of diesel/biofuel blends with nanofluid additives on compression ignition engine by response surface methodology. Energy Convers. Manag. X 2022, 14, 100177. [Google Scholar] [CrossRef]
- Tamilselvan, P.; Nallusamy, N.; Rajkumar, S. A comprehensive review on performance, combustion and emission characteristics of biodiesel fuelled diesel engines. Renew. Sustain. Energy Rev. 2017, 79, 1134–1159. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Y.; Lu, S.; Zhang, Z.; Tan, D. A Comprehensive Review of the Properties, Performance, Combustion, and Emissions of the Diesel Engine Fueled with Different Generations of Biodiesel. Processes 2022, 10, 1178. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals 2E, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Campbell, M.F.; Davidson, D.F.; Hanson, R.K. Ignition delay times of very-low-vapor-pressure biodiesel surrogates behind reflected shock waves. Fuel 2014, 126, 271–281. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, F.; Zhang, H.; Wang, S.; Zhao, Z.; Wang, W.; Chen, R. Fishhook characteristics of biodiesel lubricity during autoxidation. Fuel 2023, 331, 125897. [Google Scholar] [CrossRef]
- Bays, J.T.; Gieleciak, R.; Viola, M.B.; Lewis, R.P.; Cort, J.R.; Campbell, K.B.; Coffey, G.W.; Linehan, J.C.; Kusinski, M. Detailed Compositional Comparison of Hydrogenated Vegetable Oil with Several Diesel Fuels and Their Effects on Engine-Out Emissions. SAE Int. J. Fuels Lubr. 2022, 16, 193–220. [Google Scholar] [CrossRef]
- Alkhayat, S.A. Surrogate Fuels for Hydrotreated Vegetable Oil (HVO): Development, Experimental Validation, and 3D-CFD Simulation. Ph.D. Thesis, Wayne State University, Detroit, MI, USA, 2023. [Google Scholar]
- Cancino, L.R. Development and Application of Detailed Chemical Kinetics Mechanisms for Ethanol and Ethanol Containing Hydrocarbon Fuels. Ph.D. Thesis, Graduate Program in Mechanical Engineering—POSMEC, Federal University of Santa Catarina, Florianópolis, Brazil, 2009. [Google Scholar]
- Kundu, P.; Xu, C.; Som, S.; Temme, J.; Kweon, C.B.M.; Lapointe, S.; Kukkadapu, G.; Pitz, W.J. Implementation of multi-component diesel fuel surrogates and chemical kinetic mechanisms for engine combustion simulations. Transp. Eng. 2021, 3, 100042. [Google Scholar] [CrossRef]
- Luning, D.J.; Cowart, J.S.; Hamilton, L.J.; Hoang, D.T.; Brown, E.K.; Trulove, P.C. Development of a Surrogate Mixture for Algal-Based Hydrotreated Renewable Diesel. Energy Fuels 2013, 27, 954–961. [Google Scholar] [CrossRef]
- Kerras, H.; Outili, N.; Loubar, K.; Meniai, A.H. Optimization of formulation for surrogate fuels for diesel - biodiesel mixtures. Comptes Rendus. Chim. 2021, 24, 119–129. [Google Scholar] [CrossRef]
- Kukkadapu, G.; Whitesides, R.; Wang, M.; Wagnon, S.W.; Mehl, M.; Westbrook, C.K.; McCormick, R.; Sung, C.J.; Pitz, W.J. Development of a diesel surrogate for improved autoignition prediction: Methodology and detailed chemical kinetic modeling. Appl. Energy Combust. Sci. 2023, 16, 100216. [Google Scholar] [CrossRef]
- Cancino, L.R.; Silva, A.d.; Toni, A.R.D.; Fikri, M.; Oliveira, A.A.M.; Schulz, C.; Curran, H.J. A six-compound, high performance gasoline surrogate for internal combustion engines: Experimental and numerical study of autoignition using high-pressure shock tubes. Fuel 2020, 261, 116439. [Google Scholar] [CrossRef]
- Curran, H.J.; Gaffuri, P.; Pitz, W.J.; Westbrook, C.K. A comprehensive modeling study of iso-octane oxidation. Combust. Flame 2002, 129, 253–280. [Google Scholar] [CrossRef]
- De Toni, A.R.; Werler, M.; Hartmann, R.M.; Cancino, L.R.; Schießl, R.; Fikri, M.; Schulz, C.; Oliveira, A.A.M.; Oliveira, E.J.; Rocha, M.I. Ignition delay times of Jet A-1 fuel: Measurements in a high-pressure shock tube and a rapid compression machine. Proc. Combust. Inst. 2017, 36, 3695–3703. [Google Scholar] [CrossRef]
- Al-Esawi, N.; Al Qubeissi, M. A new approach to formulation of complex fuel surrogates. Fuel 2021, 283, 118923. [Google Scholar] [CrossRef]
- Pei, Y.; Mehl, M.; Liu, W.; Lu, T.; Pitz, W.J.; Som, S. A Multicomponent Blend as a Diesel Fuel Surrogate for Compression Ignition Engine Applications. J. Eng. Gas Turbines Power 2015, 137. [Google Scholar] [CrossRef]
- Mueller, C.J.; Cannella, W.J.; Bays, J.T.; Bruno, T.J.; DeFabio, K.; Dettman, H.D.; Gieleciak, R.M.; Huber, M.L.; Kweon, C.B.; McConnell, S.S.; et al. Diesel Surrogate Fuels for Engine Testing and Chemical-Kinetic Modeling: Compositions and Properties. Energy Fuels 2016, 30, 1445–1461. [Google Scholar] [CrossRef] [PubMed]
- Cancino, L.R.; Fikri, M.; Oliveira, A.A.M.; Schulz, C. Ignition delay times of ethanol-containing multi-component gasoline surrogates: Shock-tube experiments and detailed modeling. Fuel 2011, 90, 1238–1244. [Google Scholar] [CrossRef]
- Merker, G.P.; Schwarz, C.; Teichmann, R. (Eds.) Combustion Engines Development—Mixture Formation, Combustion, Emissions and Simulation; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sánchez, Y.O. Modelagem e Análise Numérica da Combustão de óleo Vegetal in-Natura em Motores a Combustão Interna de Ignição por Compressão. Ph.D. Thesis, Graduate Program in Mechanical Engineering—POSMEC, Federal University of Santa Catarina, Florianópolis, Brasil, 2017. [Google Scholar]
- Balestrin, K.; Vicente, J.; Bonini, V.; de Sousa, M.; Cancino, L. Reduced Kinetics Models For Gasoline Surrogates. In Proceedings of the COBEM 2021—26th International Congress of Mechanical Engineering, Online, 22–26 November 2021; p. COB–2021–1251. [Google Scholar]
- Çengel, Y.; Boles, M.; Kanoglu, M. Thermodynamics: An Engineering Approach, 9th ed.; McGraw Hill: New York, NY, USA, 2018. [Google Scholar]
- Brunetti, F. Motores de Combustõo Interna (Volume 1); Blucher: São Paulo, Brazil, 2012. [Google Scholar]
- Romano, A.; Cancino, L. A simplified model for compression-ignition internal combustion engines analysis by using detailed chemical kinetics. In Proceedings of the 18th Brazilian Congress of Thermal Sciences and Engineering, Online, 16–20 November 2020; p. ENC–2020–0069. [Google Scholar]
- Westbrook, C.K.; Dryer, F.L. Simplified Reaction Mechanisms for the Oxidation of Hydrocarbon Fuels in Flames. Combust. Sci. Technol. 1981, 27, 31–43. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr.; et al. GRIMech 3.0. 1999. Available online: http://www.me.berkeley.edu/gri_mech (accessed on 1 January 2023).
- Goodwin, D.G.; Speth, R.L.; Moffat, H.K.; Weber, B.W. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes. Version 3.0. 2021. Available online: https://www.cantera.org (accessed on 1 February 2023). [CrossRef]
- Yao, T.; Pei, Y.; Zhong, B.J.; Som, S.; Lu, T.; Luo, K.H. A compact skeletal mechanism for n-dodecane with optimized semi-global low-temperature chemistry for diesel engine simulations. Fuel 2017, 191, 339–349. [Google Scholar] [CrossRef]
- Ramirez L., H.P.; Hadj-Ali, K.; Diévart, P.; Moréac, G.; Dagaut, P. Kinetics of Oxidation of Commercial and Surrogate Diesel Fuels in a Jet-Stirred Reactor: Experimental and Modeling Studies. Energy Fuels 2010, 24, 1668–1676. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Wang, X.; Zheng, Z.; Yao, M. Experimental and modelling investigations of the diesel surrogate fuels in direct injection compression ignition combustion. Appl. Energy 2017, 189, 187–200. [Google Scholar] [CrossRef]
- Chuahy, F.D.F.; Sluder, C.S.; Curran, S.J.; Kukkadapu, G.; Wagnon, S.W.; Whitesides, R. Numerical assessment of fuel physical properties on high-dilution diesel advanced compression ignition combustion. Appl. Energy Combust. Sci. 2023, 13, 100102. [Google Scholar] [CrossRef]
- Frassoldati, A.; Cuoci, A.; Stagni, A.; Faravelli, T.; Ranzi, E. Skeletal kinetic mechanism for diesel combustion. Combust. Theory Model. 2017, 21, 79–92. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, L.; Li, Z.; Zhang, Y.; Xu, L.; Zhou, Q.; Han, D.; Lu, X. A new methodology for diesel surrogate fuel formulation: Bridging fuel fundamental properties and real engine combustion characteristics. Energy 2018, 148, 424–447. [Google Scholar] [CrossRef]
- Yu, L.; Mao, Y.; Li, A.; Wang, S.; Qiu, Y.; Qian, Y.; Han, D.; Zhu, L.; Lu, X. Experimental and modeling validation of a large diesel surrogate: Autoignition in heated rapid compression machine and oxidation in flow reactor. Combust. Flame 2019, 202, 195–207. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Wang, X.; Wang, P. Development of a skeletal mechanism for tri-component diesel surrogate fuel: N-hexadecane/iso-cetane/1-methylnaphthalene. Fuel 2020, 259, 116217. [Google Scholar] [CrossRef]
- Mueller, C.J.; Cannella, W.J.; Bruno, T.J.; Bunting, B.; Dettman, H.D.; Franz, J.A.; Huber, M.L.; Natarajan, M.; Pitz, W.J.; Ratcliff, M.A.; et al. Methodology for Formulating Diesel Surrogate Fuels with Accurate Compositional, Ignition-Quality, and Volatility Characteristics. Energy Fuels 2012, 26, 3284–3303. [Google Scholar] [CrossRef]
- Xing, Z.; Chen, C.; Jiang, X. A molecular investigation on the mechanism of co-pyrolysis of ammonia and biodiesel surrogates. Energy Convers. Manag. 2023, 289, 117164. [Google Scholar] [CrossRef]
- De Bortoli, A.; Pereira, F. Modeling and simulation of a turbulent jet diffusion flame of a biodiesel surrogate composed of MD, n-Hept, MC and EtOH. Fuel 2022, 313, 122649. [Google Scholar] [CrossRef]
- Kholghy, M.R.; Weingarten, J.; Thomson, M.J. A study of the effects of the ester moiety on soot formation and species concentrations in a laminar coflow diffusion flame of a surrogate for B100 biodiesel. Proc. Combust. Inst. 2015, 35, 905–912. [Google Scholar] [CrossRef]
- Da Costa, S.; Salkar, A.; Krishnasamy, A.; Fernandes, R.; Morajkar, P. Investigating the oxidative reactivity and nanostructural characteristics of diffusion flame generated soot using methyl crotonate and methyl butyrate blended diesel fuels. Fuel 2022, 309, 122141. [Google Scholar] [CrossRef]
- Sula, C.; Grosshans, H.; Papalexandris, M.V. Numerical study of spray combustion of a biodiesel surrogate fuel using the LES-FGM approach. Combust. Flame 2023, 249, 112611. [Google Scholar] [CrossRef]
- Ramirez L., H.P.; Fikri, M.; Cancino, L.R.; Moréac, G.; Schulz, C.; Dagaut, P. Autoignition of surrogate biodiesel fuel (B30) at high pressures: Experimental and modeling kinetic study. Combust. Flame 2012, 159, 996–1008. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Li, Y.; Zhang, Y.; Xie, M.; Wang, H.; Reitz, R.D. Development of a skeletal oxidation mechanism for biodiesel surrogate. Proc. Combust. Inst. 2015, 35, 3037–3044. [Google Scholar] [CrossRef]
- Li, A.; Zhu, L.; Mao, Y.; Zhai, J.; Han, D.; Lu, X.; Huang, Z. Surrogate formulation methodology for biodiesel based on chemical deconstruction in consideration of molecular structure and engine combustion factors. Combust. Flame 2019, 199, 152–167. [Google Scholar] [CrossRef]
- He, Y.; Liang, H.; Liang, M.; Liu, C.; Liao, S. Effects of methanol addition on the combustion characteristics of bio-diesel based counterflow nonpremixed flames. Fuel 2022, 323, 124297. [Google Scholar] [CrossRef]
- Lee, C.C.; Tran, M.V.; Nurmukan, D.; Tan, B.T.; Chong, C.T.; Scribano, G. Investigation of flame structure and stabilisation characteristics of palm methyl esters diffusion flames. Fuel 2022, 313, 123034. [Google Scholar] [CrossRef]
- Lin, K.C.; Dahiya, A.; Tao, H.; Kao, F.H. Combustion mechanism and CFD investigation of methyl isobutanoate as a component of biodiesel surrogate. Energy 2022, 249, 123589. [Google Scholar] [CrossRef]
- Al-Gharibeh, E.; Kumar, K. Oxidation kinetics of methyl decanoate in a motored engine. Fuel 2022, 308, 121912. [Google Scholar] [CrossRef]
- Sui, M.; Li, F.; Wang, S.; Wang, H. Molecular dynamics simulation and experimental research on the oxidation reaction of methyl linoleate at low oxygen and high temperature. Fuel 2021, 305, 121478. [Google Scholar] [CrossRef]
- Lele, A.D.; Vallabhuni, S.K.; Moshammer, K.; Fernandes, R.X.; Krishnasamy, A.; Narayanaswamy, K. Experimental and chemical kinetic modeling investigation of methyl butanoate as a component of biodiesel surrogate. Combust. Flame 2018, 197, 49–64. [Google Scholar] [CrossRef]
- Lee, C.Y.; Cheng, X.; Mun Poon, H.; Reddy Yelugoti, S.; Wang, W.C. The spray ignition characteristics of ethanol blended with hydro-processed renewable diesel in a constant volume combustion chamber. Fuel 2022, 314, 123089. [Google Scholar] [CrossRef]
- Lanfaloni, S. Surrogate Fuel Definition and Spray Calibration for Diesel Engines. Laurea, Politecnico di Torino. 2020. Available online: https://webthesis.biblio.polito.it/15394/ (accessed on 1 February 2023).
- Rinaldi, R.; Garcia, C.; Marciniuk, L.L.; Rossi, A.V.; Schuchardt, U. Síntese de biodiesel: Uma proposta contextualizada de experimento para laboratório de química geral. Química Nova 2007, 30, 1374–1380. [Google Scholar] [CrossRef]
- Pejpichestakul, W.; Cuoci, A.; Frassoldati, A.; Pelucchi, M.; Parente, A.; Faravelli, T. Buoyancy effect in sooting laminar premixed ethylene flame. Combust. Flame 2019, 205, 135–146. [Google Scholar] [CrossRef]
- Saggese, C.; Cuoci, A.; Frassoldati, A.; Ferrario, S.; Camacho, J.; Wang, H.; Faravelli, T. Probe effects in soot sampling from a burner-stabilized stagnation flame. Combust. Flame 2016, 167, 184–197. [Google Scholar] [CrossRef]
- Saggese, C.; Ferrario, S.; Camacho, J.; Cuoci, A.; Frassoldati, A.; Ranzi, E.; Wang, H.; Faravelli, T. Kinetic modeling of particle size distribution of soot in a premixed burner-stabilized stagnation ethylene flame. Combust. Flame 2015, 162, 3356–3369. [Google Scholar] [CrossRef]
- Faravelli, T.; Frassoldati, A.; Ranzi, E. Kinetic modeling of the interactions between NO and hydrocarbons in the oxidation of hydrocarbons at low temperatures. Combust. Flame 2003, 132, 188–207. [Google Scholar] [CrossRef]
- Frassoldati, A.; Faravelli, T.; Ranzi, E. Kinetic modeling of the interactions between NO and hydrocarbons at high temperature. Combust. Flame 2003, 135, 97–112. [Google Scholar] [CrossRef]
- Shukla, P.C.; Gupta, T.; Labhsetwar, N.K.; Agarwal, A.K. Physico-chemical speciation of particulates emanating from Karanja biodiesel fuelled automotive engine. Fuel 2015, 162, 84–90. [Google Scholar] [CrossRef]
- Abed, K.A.; Gad, M.S.; El Morsi, A.K.; Sayed, M.M.; Elyazeed, S.A. Effect of biodiesel fuels on diesel engine emissions. Egypt. J. Pet. 2019, 28, 183–188. [Google Scholar] [CrossRef]
- Werncke, I.; de Souza, S.N.M.; Bassegio, D.; Secco, D. Comparison of Emissions and Engine Performance of Crambe Biodiesel and Biogas. Eng. Agrícola 2023, 43, e20220104. [Google Scholar] [CrossRef]
- Ergen, G. Comprehensive analysis of the effects of alternative fuels on diesel engine performance combustion and exhaust emissions: Role of biodiesel, diethyl ether, and EGR. Therm. Sci. Eng. Prog. 2024, 47, 102307. [Google Scholar] [CrossRef]
- Kathumbi, L.K.; Home, P.G.; Raude, J.M.; Gathitu, B.B. Performance and emission characteristics of a diesel engine fuelled by biodiesel from black soldier fly larvae: Effects of synthesizing catalysts with citric acid. Heliyon 2023, 9, e21354. [Google Scholar] [CrossRef]
- Kumar, A.; Subramanian, K.A. Control of greenhouse gas emissions (CO2, CH4 and N2O) of a biodiesel (B100) fueled automotive diesel engine using increased compression ratio. Appl. Therm. Eng. 2017, 127, 95–105. [Google Scholar] [CrossRef]
- Subramanian, K.A.; Chintala, V. Reduction of GHGs Emissions in a Biodiesel Fueled Diesel Engine Using Hydrogen. In American Society of Mechanical Engineers Digital Collection; American Society of Mechanical Engineers: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Karavalakis, G.; Jiang, Y.; Yang, J.; Durbin, T.; Nuottimäki, J.; Lehto, K. Emissions and Fuel Economy Evaluation from Two Current Technology Heavy-Duty Trucks Operated on HVO and FAME Blends. SAE Int. J. Fuels Lubr. 2016, 9, 177–190. [Google Scholar] [CrossRef]
- Przybyla, G.; Hadavi, S.; Li, H.; Andrews, G.E. Real World Diesel Engine Greenhouse Gas Emissions for Diesel Fuel and B100; SAE Technical Paper 2013-01-1514; SAE International: Warrendale, PA, USA, 2013. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Guan, C.; Cheung, C.S.; Huang, Z. Effect of biodiesel on PAH, OPAH, and NPAH emissions from a direct injection diesel engine. Environ. Sci. Pollut. Res. Int. 2018, 25, 34131–34138. [Google Scholar] [CrossRef] [PubMed]
- Devan, P.K.; Mahalakshmi, N.V. Utilization of unattended methyl ester of paradise oil as fuel in diesel engine. Fuel 2009, 88, 1828–1833. [Google Scholar] [CrossRef]
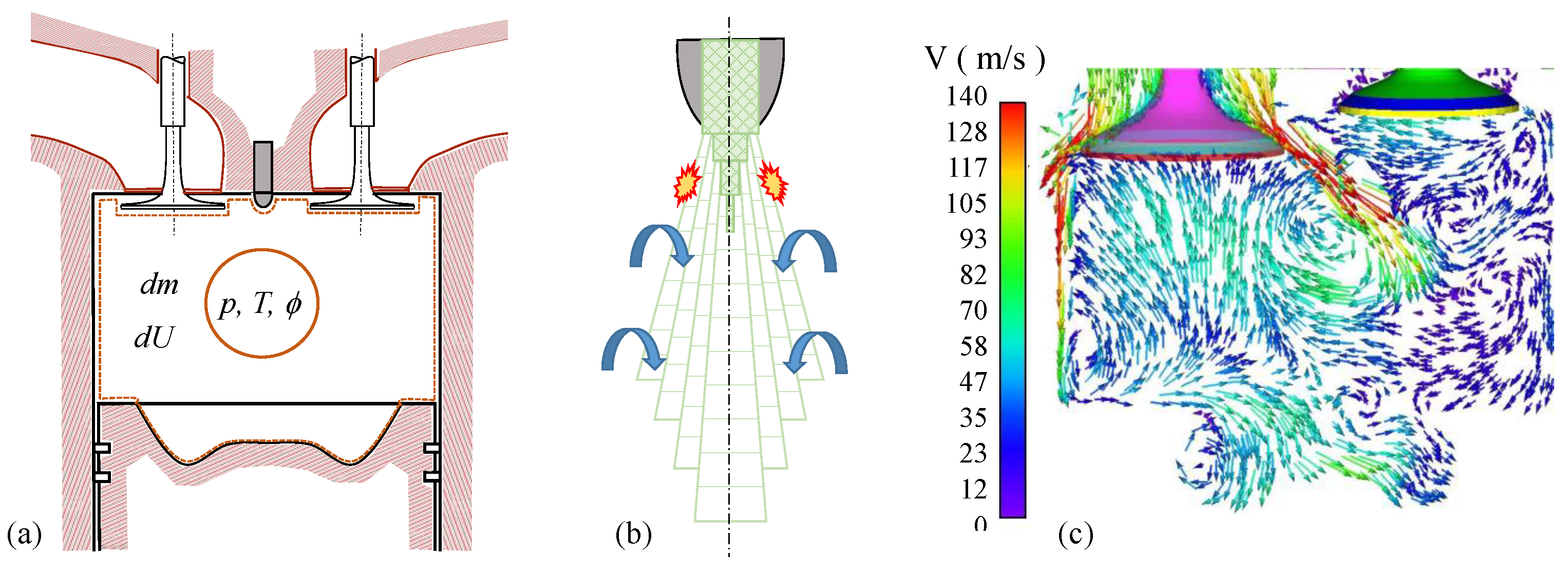

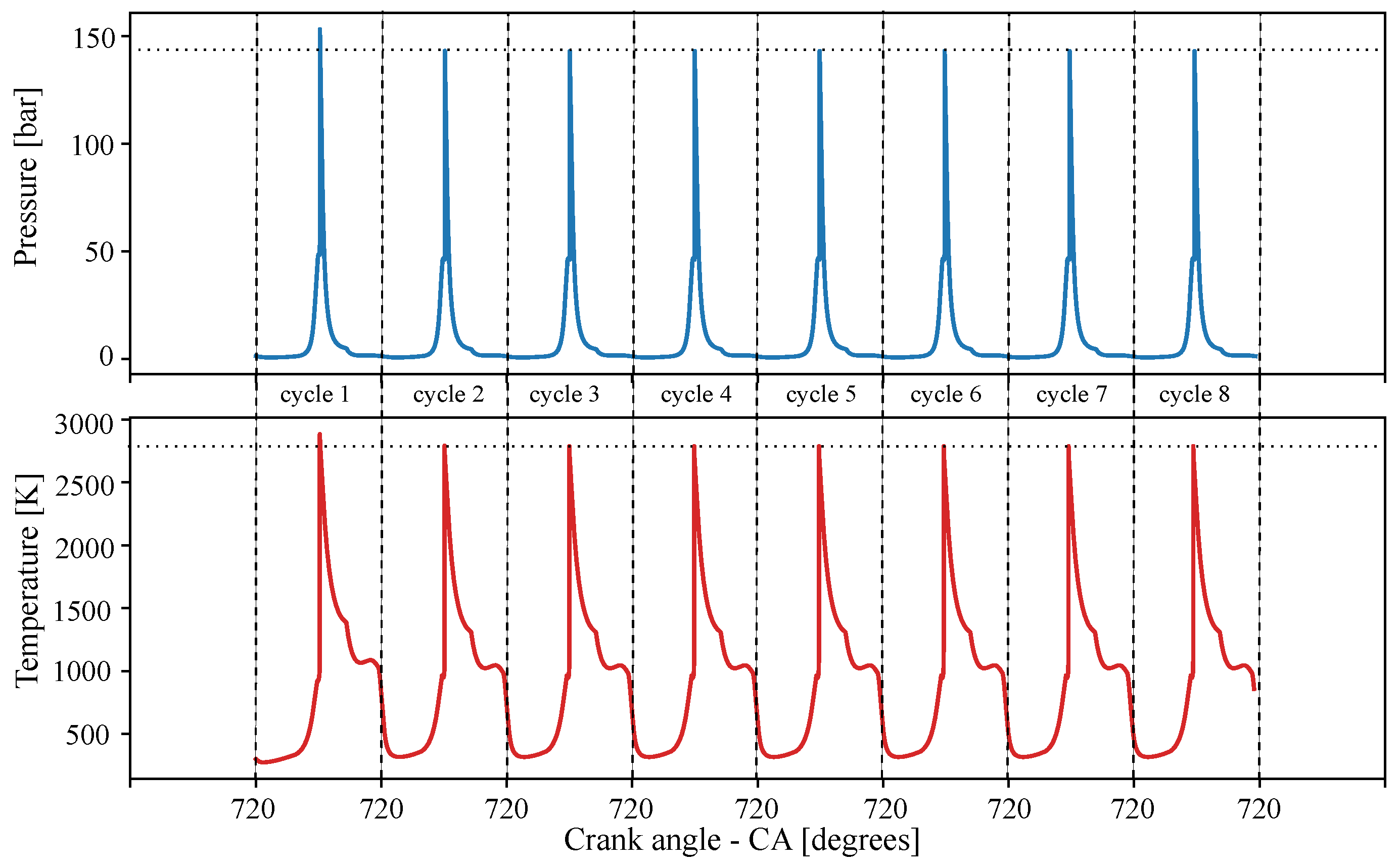
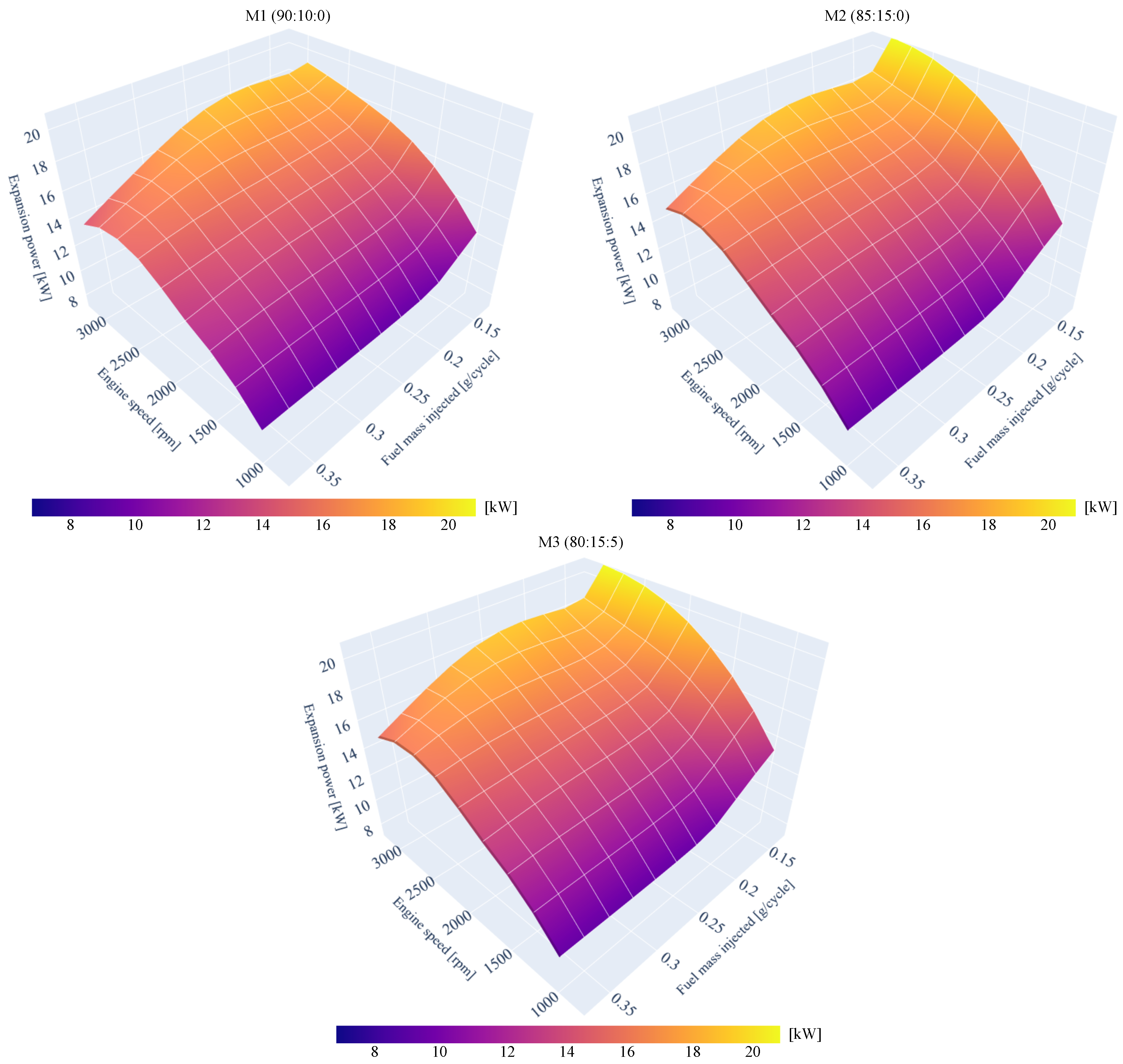

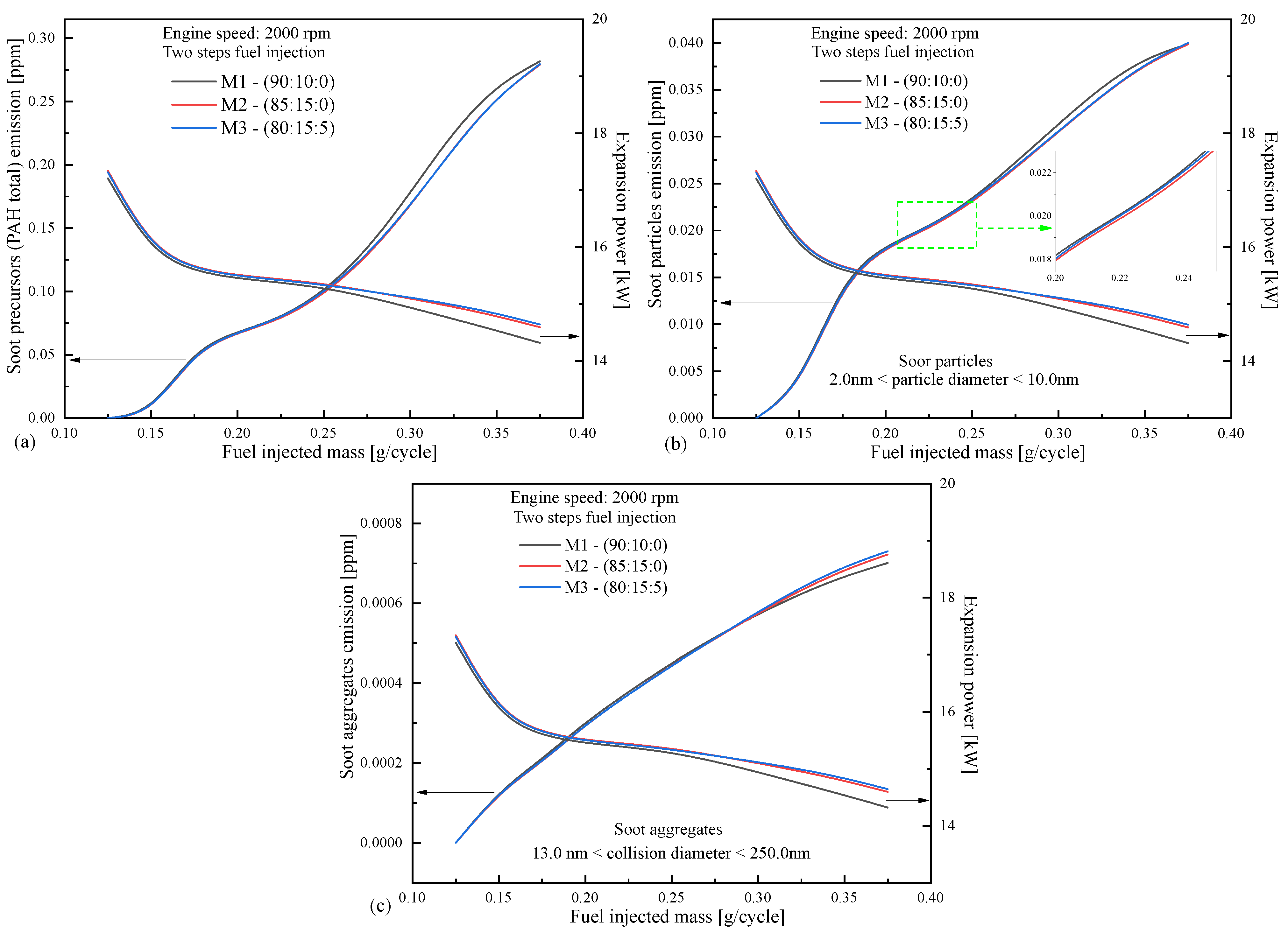

| Blends of Surrogates | Year-Ref. |
|---|---|
| 70% n-decane/30% 1-methylnaphthalene | (2010)-[41] |
| 27.8% n-hexadecane/36.3% i-cetane/14.8% trans-decalin/ | (2016)-[29] |
| 21.1% 1-methylnapthalene | |
| 23.5% n-octadecane/27.0% i-cetane/12.5% 1,2,4-trimethylbenzene/ | |
| 20.9% tetralin/16.1% 1-methylnapthalene | |
| 2.7% n-hexadecane/20.2% n-octadecane/29.2% i-cetane/ | |
| 5.1% n-butylcyclohexane/5.5% trans-decalin/7.5% 1,2,4-trimethylbenzene | |
| 15.4% tetralin/14.4% 1-methylnapthalene | |
| 10.8% n-octadecane/13.9% 1-methylnapthalene/7.3% 2-methylheptadecane/ | |
| 19.1% n-butylcyclohexane/11.0% 1,3,5-triisopropylcyclohexane/16.4% tetralin | |
| 6.0% perhydrophenanthrene/14.7% 1,3,5-triisopropylbenzene/0.8% n-eicosane | |
| 100% n-dodecane | (2017)-[40] |
| 63.1% n-decane/36.9% 1-methylnaphthalene | (2017)-[44] |
| 85% n-heptane/15% toluene | (2017)-[42] |
| 81% n-heptane/14% toluene/5% cyclohexane | |
| 80% n-heptane/20% toluene | |
| 81% n-dodecane/14% toluene/5% cyclohexane | |
| 41.3% n-hexadecane/36.8% i-cetane/21.9% 1-methylnaphthalene | (2018)-[45] |
| 21.6% n-hexadecane/15.5% n-octadecane/26.0% i-cetane/ | |
| 20.7% 1-methylnaphthalene/16.2% decalin | |
| 21.5% n-hexadecane/15.4% n-octadecane/25.8% i-cetane/ | |
| 13.7% 1-methylnaphthalene/8.1% trans-decalin/8.1% n-butylbenzene/ | |
| 7.4% n-butylcyclohexane | |
| 41.3% n-cetane/36.8% i-cetane/21.9% 1-methylnaphthalene | (2019)-[46] |
| 41.3% n-hexadecane/36.8% i-cetane/21.9% 1-methylnaphthalene | (2020)-[47] |
| 8.6% n-hexadecane/52% i-cetane/15.7% 1-methylnaphtalene/23.6%n-eicosane | (2021)-[22] |
| 7.2% 1-methylnaphthalene/46.4% trans-decalin/30% i-cetane/16.4% n-eicosane | (2023)-[43] |
| 20.5% i-cetane/3.5% n-octadecane/3.47% n-eicosane | (2023)-[23] |
| 7.24% n-butylcyclohexane/17.7% 1-methylnaphthalene/13.9% tetralin | |
| 13.7% n-hexadecane/20% trans-decalin | |
| 11% n-hexadecane/2.8% n-octadecane/2.6% n-eicosane | |
| 26.1% 1-methylnaphthalene/6.7% tetralin/7.24% n-butylcyclohexane | |
| 17.8% trans-decalin/1.7% n-butylbenzene |
| Blends of Surrogates | Year-Ref. |
|---|---|
| B30: 49% n-decane/21% 1-methylnaphthalene/30% methyl-octanoate | (2012)-[54] |
| B100: 100% methyl decanoate (MD, C11H22O2) | (2014)-[15] |
| B100: 100% methyl laurate (MLA, C13H26O2) | |
| B100: 100% methyl myristate (MM, C15H30O2) | |
| B100: 50% n-decane/50% methyl-octanoate | (2015)-[51] |
| B100: 41.18% n-decane/9.41% methyl decenoate/49.41% methyl 5-decenoate | (2015)-[55] |
| Soybean biodiesel: 62.9% methyl decanoate/15.0% n-hexadecane/ | (2019)-[56] |
| 9.4% methyl trans-3-hexenoate/12.7% 1,4-hexadiene | |
| B5: 11.44% 1-methylnaphtalene/58.39% i-cetane/30.16% n-eicosane | (2021)-[22] |
| B10: 16.24% 1-methylnaphtalene/54.22% i-cetane/29.52% n-eicosane | |
| B20: 17.93% 1-methylnaphtalene/51.15% i-cetane/30.90% n-eicosane | |
| B50: 17.02% 1-methylnaphtalene/54.93% i-cetane/28.04% n-eicosane | |
| B80: 35.28% 1-methylnaphtalene/38.85% i-cetane/25.85% n-eicosane | |
| B100: 56.83% 1-methylnaphtalene/33.25% i-cetane/30.08% n-eicosane | |
| B100: 50% methyl decanoate/40% n-heptane/ | (2022)-[50] |
| 9% methyl crotonate/1% ethanol | |
| B100: 100% methyl decanoate | (2022)-[57] |
| B100: 47.5% methyl palmitate/4.5% methyl stearate/ | (2022)-[58] |
| 39.6% methyl oleate/8.4% methyl linoleate | |
| B25: 25% methyl butyrate/75% fossil diesel | (2022)-[52] |
| B25: 25% methyl crotonate/75% fossil diesel | |
| B100: 100% methyl isobutanoate | (2022)-[59] |
| B100: 35.68% methyl butanoate/64.32% n-dodecane | (2023)-[53] |
| B100: 100% methyl butanoate (MB, C5H10O2) | |
| B100: 100% ethyl propionate (EP, C5H10O2) | (2023)-[49] |
| B100: 100% methyl crotonate (MC, C5H8O2) | |
| B100: 100% methyl decanoate (MDN, C11H10O2) | |
| B100: 100% n-dodecane (DDC, C12H26) |
| Blends of Surrogates | Year-Ref. |
|---|---|
| 57% n-tetradecane/24% n-hexadecane/9% i-cetane | (2023)-[18] |
| 22% n-dodecane/55% n-hexadecane/23% i-cetane | |
| 16% n-decano/61% n-hexadecane/23% i-cetane | |
| 8% n-heptane/67% n-hexadecane/25% i-cetane | |
| 70% n-hexadecane/19% i-cetane/11% cyclohexane | |
| 69% n-hexadecane/17% i-cetane/14% methyl-cyclohexane | |
| 70% n-hexadecane/30% i-cetane | |
| Mix # 1: 81% i-cetane/19% 2-methyl-pentadecane | (2020)-[64] |
| Mix # 2: 68% i-cetane/16% 2-methyl-pentadecane/16% 2-methyl-heptane | |
| Mix # 3: 9% n-pentadecane/75% i-cetane/16% 2-methyl-pentadecane | |
| Mix # 1: 9.3% n-pentadecane/13.8% n-hexadecane/26.5% n-heptadecane/ | (2013)-[21] |
| 19.3% n-octadecane/31.1% i-dodecane | |
| Mix # 2: 9.8% n-pentadecane/14.9% n-hexadecane/27.3% n-heptadecane/ | |
| 20.7% n-octadecane/27.2% 2-methyl nonane | |
| Mix # 3: 12.2% n-pentadecane/18.2% n-hexadecane/34.7% n-heptadecane/ | |
| and 26.3% n-octadecane/8.6% 2-methyl octane | |
| Mix # 4: 13.13% n-pentadecane/19.54% n-hexadecane/37.70% n-heptadecane/ | |
| and 27.40% n-octadecane/.23% i-octane | |
| Mix # 5: 13.17% n-pentadecane/19.59% n-hexadecane/37.81% n-heptadecane/ | |
| and 27.49% n-octadecane/1.94% i-octane |
| Surrogate | Formula | Real Fuel | ||
|---|---|---|---|---|
| Diesel A | Soybean Diesel | HVO | ||
| cyclohexane | C6H12 | ✓ | ||
| toluene | C6H5CH3 | ✓ | ||
| n-dodecane | C12H26 | ✓ | ||
| n-hexadecane (n-cetane) | C16H34 | ✓ | ✓ | |
| i-cetane (2,2,4,4,6,8,8-heptamethylnonane) | C16H34 | ✓ | ✓ | |
| methyl-decanoate | C11H22O2 | ✓ | ||
| methyl-palmitate | C17H34O2 | ✓ | ||
| methyl-linoleate | C19H34O2 | ✓ | ||
| Parameter | Description | Value |
|---|---|---|
| Engine speed and geometric | ||
| # disp_vol [m*3] | Displaced volume | 0.00075 |
| # comp_ratio [-] | Compression ratio | 17.5 |
| # piston_diam [cm] | Piston diameter | 9.58 |
| # rpm | Engine speed | 1000–3000 ( = 250) |
| Power boosting system | ||
| # turbo_T [K] | Air temperature (after turbo/intercooler) | 300 |
| # turbo_p [Pa] | Boost pressure | 1.35 × |
| Fuel injection system | ||
| # T_injector [K] | Fuel temperature at injector | 300 |
| # p_injector [Pa] | Fuel injection pressure | 1.80 × |
| # comp_fuel | Fuel composition (surrogates) | Mixtures M1, M2 and M3 |
| # injector_mass [kg] | Fuel injected mass (per cylinder, per cycle) | (12.5–37.5) × |
| = 2.5 × | ||
| # perc_injector_pre [%] | Fuel mass percentage at first injection stage | 7 |
| # perc_injector_post [%] | Fuel mass percentage at last injection stage | 0 |
| # injector_pre_open [CA] | SOI (at first injection stage) | 340 |
| # injector_pre_close [CA] | EOI (at first injection stage) | 348 |
| # injector_main_open [CA] | SOI (at main injection stage) | 350 |
| # injector_main_close [CA] | EOI (at main injection stage) | 365 |
| # injector_post_open [CA] | SOI (at last injection stage) | 395 |
| # injector_post_close [CA] | EOI (at last injection stage) | 415 |
| Room conditions | ||
| # T_room [K] | Temperature | 3.00 × |
| # p_room [Pa] | Pressure | 1.00 × |
| Valve timing | ||
| # inlet_open [CA] BTDC | IVO | 18 |
| # inlet_close [CA] | IVC | 198 |
| # outlet_open [CA] | EVO | 522 |
| # outlet_close [CA] | EVC | 18 |
| Kinetics model for combustion process | ||
| # reaction_mechanism | Kinetics and thermo database | CRECK Modeling Group |
| # comp_air | Intake air composition | 21% O2, 79% N2 |
| Simulation controls | ||
| # atol | Absolute tolerance for solution values | 1.00 × |
| # rtol | Relative tolerance for solution values | 1.00 × |
| # sim_n_revolutions | Cycles simulated (for each run condition) | 12 |
| # delta_T_max [K] | Maximum increase on temperature | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cancino, L.R.; Rebelo, J.F.; Kraus, F.d.C.; Cavalcanti, E.H.d.S.; Pimentel, V.S.d.B.; Maia, D.M.; Sá, R.A.B.d. Fossil Diesel, Soybean Biodiesel and Hydrotreated Vegetable Oil: A Numerical Analysis of Emissions Using Detailed Chemical Kinetics at Diesel Engine Like Conditions. Atmosphere 2024, 15, 1224. https://doi.org/10.3390/atmos15101224
Cancino LR, Rebelo JF, Kraus FdC, Cavalcanti EHdS, Pimentel VSdB, Maia DM, Sá RABd. Fossil Diesel, Soybean Biodiesel and Hydrotreated Vegetable Oil: A Numerical Analysis of Emissions Using Detailed Chemical Kinetics at Diesel Engine Like Conditions. Atmosphere. 2024; 15(10):1224. https://doi.org/10.3390/atmos15101224
Chicago/Turabian StyleCancino, Leonel R., Jessica F. Rebelo, Felipe da C. Kraus, Eduardo H. de S. Cavalcanti, Valéria S. de B. Pimentel, Decio M. Maia, and Ricardo A. B. de Sá. 2024. "Fossil Diesel, Soybean Biodiesel and Hydrotreated Vegetable Oil: A Numerical Analysis of Emissions Using Detailed Chemical Kinetics at Diesel Engine Like Conditions" Atmosphere 15, no. 10: 1224. https://doi.org/10.3390/atmos15101224
APA StyleCancino, L. R., Rebelo, J. F., Kraus, F. d. C., Cavalcanti, E. H. d. S., Pimentel, V. S. d. B., Maia, D. M., & Sá, R. A. B. d. (2024). Fossil Diesel, Soybean Biodiesel and Hydrotreated Vegetable Oil: A Numerical Analysis of Emissions Using Detailed Chemical Kinetics at Diesel Engine Like Conditions. Atmosphere, 15(10), 1224. https://doi.org/10.3390/atmos15101224






