Abstract
In recent years, the greenhouse effect has emerged as a major environmental concern. As a result, the utilization and capture of greenhouse gases have become urgent tasks. The dry reforming of methane (DRM) reaction is a technology that directly converts greenhouse gases (CH4 and CO2) into valuable products. Ni-based catalysts have gained significant attention and industrial prospects in DRM reactions due to their excellent performance and cost-effectiveness compared to noble metals. However, challenges such as metal sintering and carbon deposition hinder its industrialization. This paper provides a review of the recent advancements in catalyst design for DRM reactions, with a focus on the impact of additives, supports, and preparation methods on Ni-based catalysts. It also describes the reaction and deactivation mechanisms, as well as the thermodynamics and kinetics of DRM reactions. Furthermore, this paper envisions the main challenges and opportunities associated with Ni-based catalysts in DRM research.
1. Introduction
The reliance on fossil fuels to meet the growing energy demand has resulted in numerous adverse effects. CO2 emissions have been steadily increasing over the past few decades, and it has been widely recognized that CO2 poses a significant threat to the planet [1,2]. The immediate priority for addressing the global warming problem and energy crisis is to effectively utilize CO2 and convert it into value-added products [3]. Scientists are dedicated to researching technologies aimed at mitigating the greenhouse effect that encompass the capture, storage, and utilization of these gases. Among these technologies, DRM holds the most promise [4]. This process allows for the utilization of both CH4 and CO2 as feedstock, leading to the conversion of these two greenhouse gases into syngas (H2 and CO) with a molar ratio close to 1:1 (Equation (1)). The DRM reaction is highly endothermic and requires operation at elevated temperatures. Based on thermodynamic calculations, the reaction temperature should exceed 643 °C to promote spontaneous reaction, and at temperatures above 850 °C, the conversion of CO2 and CH4 exceeds 95%. However, several side effects arise as the reaction proceeds. These include the CH4 cracking reaction (Equation (2)), the CO disproportionation reaction (Equation (3)), and the reverse water–gas shift reaction (Equation (4)). The formation of carbon deposits resulting from these side reactions can lead to catalyst bed blockage, increased pressure drop, and metal sintering, thereby reducing the activity of the catalyst [5]. Hence, it is critical to develop highly active and thermally stable catalysts.
The catalysts commonly employed for DRM reactions can be classified into two categories: precious metal catalysts (such as Rh, Pt, Pd, and Au) and non-precious metal catalysts (such as Ni and Co). Precious metals generally exhibit higher catalytic activity compared to non-precious metals. Additionally, they tend to generate less coke formation on the catalyst surface. This is because the intermediate coke species are more readily gasified in the presence of CO2 [6]. However, due to the scarcity and high cost of precious metals, there is a growing interest among researchers to explore non-precious metals (e.g., Ni, Co) that offer both high catalytic performance and cost-effectiveness. Among non-precious metals, Ni has emerged as a favored catalyst for DRM applications due to its abundance and affordability. Ni-based catalysts demonstrate relatively high DRM activity when compared to other non-precious metals [7,8]. However, Ni metal is prone to agglomeration. During the DRM process, filamentary carbon, also known as whisker carbon, tends to form on the metal surface, which is the primary cause of catalyst deactivation [9].
This paper provides an overview of the current research on Ni-based catalysts for dry reforming of methane reactions. The introduction section covers the thermodynamics and kinetics associated with DRM reactions. It then delves into the surface reaction mechanism and deactivation mechanisms specific to Ni-based catalysts. Furthermore, the paper discusses the influence of catalyst supports, additives, active components, and preparation methods on the catalytic performance of Ni-based catalysts in the reforming process. Finally, the design principles of Ni-based catalysts are outlined, along with suggestions for the future research directions in this field.
2. Thermodynamic Study of DRM Reactions
Indeed, thermodynamic studies of the DRM reaction play a crucial role in determining the optimal conditions for efficient syngas production. The DRM reaction consists of four main reactions, as described in Equations (1)–(4). The primary reaction, as shown in Equation (1), is a highly endothermic reversible reaction. This reaction is favored by high temperatures and low pressures, ultimately leading to an H2/CO ratio that is close to 1 [10]. By utilizing catalysts, the energy required for syngas generation can be reduced while minimizing the formation of undesirable byproducts [11]. The occurrence of side reactions can be determined based on the Gibbs free energy and the reaction conditions. The reverse water–gas shift reaction (Equation (4)), for instance, occurs at temperatures below 820 °C, leading to an H2/CO ratio less than 1 and CO2 conversion surpassing CH4 conversion [11]. Although an H2/CO ratio less than 1 may seem unfavorable, this syngas can still be utilized in the Fischer–Tropsch synthesis process to produce additional hydrocarbons [12]. Aside from the reverse water–gas shift reaction, other side reactions such as coke formation can take place. Coke tends to deactivate active metals and block pore structures, thus, impeding catalyst activity and unfavorably affecting the DRM reaction. Many researchers attribute coke formation to CO disproportionation (Equation (3)) and CH4 cracking (Equation (2)). The CO disproportionation reaction is not favorable at high temperatures and should be conducted below 700 °C. On the other hand, CH4 cleavage requires temperatures above 557 °C. Consequently, carbon deposition is more likely to occur within the temperature range of 557 °C to 700 °C. Additionally, two other reactions, CO2 hydrogenation (Equation (5)) and CO hydrogenation (Equation (6)), were proposed as contributing factors to coke formation. Hence, the reaction temperature, pressure, and feed ratio all influence the occurrence of the side reactions.
DRM reactions are highly heat-absorbing reactions. Therefore, the reaction rate increases as the temperature rises. Nikoo and Amin’s thermodynamic calculations indicate that at reaction temperatures above 900 °C and a CH4/CO2 feed ratio of 1:1, coke generation is reduced, and CO2 conversion exceeds 98%. As the temperature increases, CH4 conversion and H2 yield also increase, as shown in Figure 1a. Figure 1b illustrates the impact of the feed ratio on the conversion of CH4 and CO2 at 850 °C and 1 atm. Lower feed ratios result in higher CH4 conversion but lower H2/CO ratios lead to CH4 cracking and more coke generation. A higher CH4/CO2 feed ratio (2:1) leads to lower CO generation and a closer H2/CO ratio to unity. Larger feed ratios reduce carbon generation but also decrease carbon deposition. Cao et al. [13] demonstrated that according to Le Chatelier’s principle, high pressure inhibits DRM and CH4 cracking reactions. At a certain temperature, the conversion of CH4 and CO2 decreases with the increasing pressure, resulting in higher conversion rates. Thus, achieving high conversion requires depressurization at low temperatures, and high pressures are not conducive to DRM reactions.
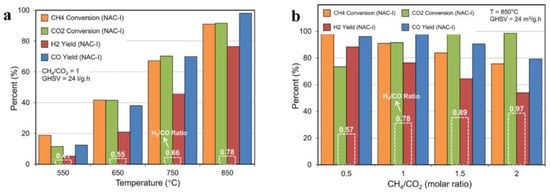
Figure 1.
Influence of (a) temperature and (b) feed ratio on product yield, feed conversion, as well as H2/CO molar ratio over NAC-I nanocatalyst [13].
3. CH4-CO2 Reforming Reactions and Deactivation Mechanisms
3.1. Reaction Mechanism
CH4-CO2 reforming to syngas is a complex reaction process accompanied by many side reactions. Different reaction mechanisms may be exhibited due to factors such as differences in supports, additives, preparation methods, and the use of different characterization techniques. Therefore, the reaction mechanism of the DRM reaction remains widely debated [14]. The rate-controlling steps in the DRM reaction are CH4 adsorption and activation [15]. The first step in the DRM reaction is CH4 adsorption. At low temperatures, CH4 molecules first adsorb on the metal surface to form an intermediate state before undergoing gradual dissociation, whereas CH4 molecules at high temperatures undergo direct dissociation. The total energy required for CHx-H bond dissociation depends on the catalytic system, and selecting a suitable catalyst helps in the dissociation of the CHx-H bond. Li et al. [16] proposed a catalyst model and reaction mechanism, as shown in Figure 2. Both catalysts, 5Ni/La2O3-C, and 5Ni/La2O3-LOC, undergo CH4 activation and dissociation on the Ni surface to produce activated carbon intermediates and H2. CO2 is adsorbed on La2O3 to form La2O2CO3, which then combines with an activated carbon intermediate to form CO and regenerate La2O3. This completes the cycle of La2O3 and La2O2CO3. In addition, La2O2CO3 can reflect the degree of dispersion of the active metal. The formed La2O2CO3 can react with nearby Ni particles and coke on the carrier surface. If the Ni metal is well dispersed, La2O2CO3 eliminates the coke in time. If the Ni metal is poorly dispersed, La2O2CO3 accumulates. Wang et al. [17] proposed that the DRM reaction pathway is divided into four parts: CH4 dissociation, CO2 dissociation, C* oxidation pathway, and CH* oxidation pathway. The results are shown in Figure 3. CH4 cleavage involves four basic steps of dehydrogenation reactions, while CO2 cleavage contains only one basic step, CO2 → CO* + O*. For the oxidation of carbon-containing substances, the C* oxidation pathway and the CH* oxidation pathway are included. In the C* oxidation pathway, C* decomposed by CH* couples with adsorbed O* to form CO*. In the CH* oxidation pathway, CH* is directly oxidized by O* to CHO*, and then CHO* is decomposed to CO* and H*. Ussa et al. [18] have studied the DRM reaction mechanism over a Ni-CZ sol-gel catalyst. They propose that H2 molecules dissociate at the Ni center while CO2 is activated on CeO2. CO2 adsorption at weakly basic sites forms carbonates and bicarbonates. It is then further reduced by H atoms to form formate. Finally, methoxyl substances are formed, releasing CH4, as shown in Figure 4. DFT can reveal the reaction pathways as well as the structure of intermediates and their stability during the reaction process by calculating adsorption energies, reaction energies, and trapping transition states. Based on the stable adsorption configurations of the possible species, and in order to obtain the effect of particle size on the catalytic activity, Xu et al. [19] comprehensively described the dry reforming mechanism of CH4 on the Ni2Fe overlayer on the Ni(111) surface using density functional theory (DFT) calculations. The results showed that the introduction of Fe increased the adsorption energies of O, OH, H2O, COOH, and CHO, while weakening the adsorption energies of CO and CHO, compared to the pure Ni(111) surface. The introduction of Fe significantly enhances the energy barrier of CH* → C* + H* compared to the Ni(111) surface. As a result, the h-induced CO2 activation pathway is facilitated such that the oxidation of CH and C on the Ni2Fe overlayer is not only affected by surface oxygen atoms but also by OH radicals. The most efficient oxidation pathway for CH and C is the CH* + OH* → CHOH* → CHO* + H* → CO* + 2H* reaction, which has a limiting energy barrier of 1.12 eV. Dou et al. [20] prepared SiO2@Ni and SiO2@Ni@ZrO2 catalysts for DRM reactions. The experimental results showed that the reactivity of the SiO2@Ni@ZrO2 catalyst was 5–7 times higher than that of the SiO2@Ni catalyst at temperatures ranging from 500 to 700 °C. Additionally, the SiO2@Ni@ZrO2 catalyst exhibited good anti-coking properties. The reaction pathways of the two catalysts were investigated using DFT calculations. The calculation results are consistent with the experimental findings, and the highest dissociation potential for the sequential dissociation of CH4 into C on Ni15/ZrO2 relative to gas-phase CH4 is only 0.03 eV, which is 1.37 eV lower than that on the Ni(111) surface (1.40 eV). The significant decrease in the dissociation barrier indicates that the catalytic activity of the Ni15/ZrO2 catalyst for CH4 dissociation is much higher than that of the Ni(111) surface. This explains the observed 5–7 fold increase in drying reforming activity with ZrO2 coating. On the other hand, for CO2 dissociation to CO, the highest potential barrier energy decreased by 2.56 eV from Ni15/ZrO2 to Ni(111), which improved the dry reforming activity of the SiO2@Ni@ZrO2 catalyst. Furthermore, the binding energy of CO2 is higher than that of CH4, which leads to CO2 enrichment on the catalyst surface and reduces coke generation. Figure 5 illustrates the basic steps of dissociation and the potential energy changes of CH4 and CO2 on Ni(111) and Ni15/ZrO2.
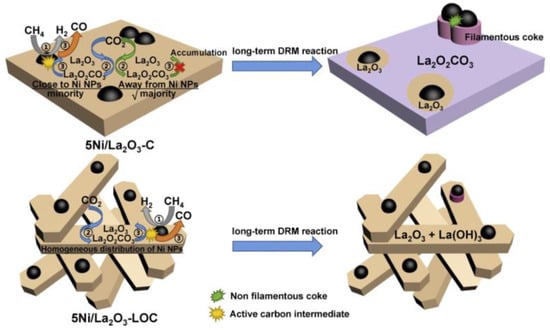
Figure 2.
Scheme of the catalyst models and reaction mechanisms in the DRM process [16].
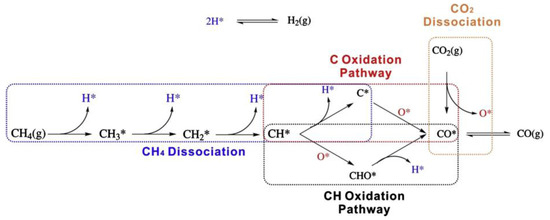
Figure 3.
A schematic diagram of the whole reaction network of dry reforming. The adsorbed hydrogen and oxygen atoms are marked in blue and red, respectively. The whole network is divided into four parts: CH4 dissociation (in dashed blue rectangle), C oxidation pathway (in dashed red rectangle), CH oxidation pathway (in dashed black rectangle), and CO2 dissociation (in dashed orange rectangle). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) [17].
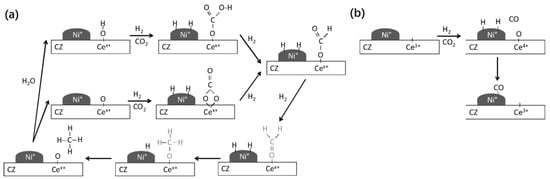
Figure 4.
Reaction mechanism proposed on Ni-CZ sol-gel sample for: (a) CO2 methanation and (b) CO formation [18].
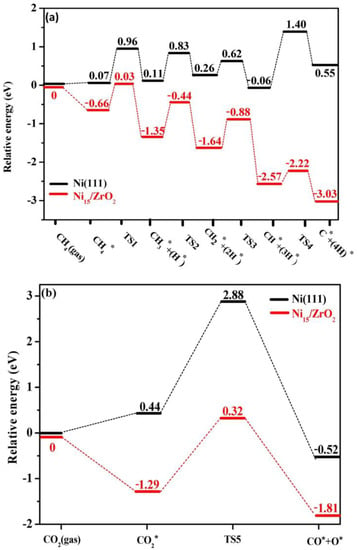
Figure 5.
The potential energy profiles for (a) CH4 sequential dissociation and (b) CO2 dissociation on Ni(111) and Ni15/ZrO2, respectively [20].
3.2. Inactivation Mechanism
Carbon buildup and sintering of active metals are the main causes of catalyst deactivation during DRM reactions. The formation of carbon deposits is mainly due to CH4 cracking and CO disproportionation reactions [21,22]. The carbon species generated covers the active sites, resulting in a decrease in the catalyst’s activity. However, at the same time, there is a carbon elimination reaction (Equation (7)), so the extent of carbon deposition depends on the carbon balance between the two [22,23]. Pakhare et al. [12] concluded that both CH4 cleavage and CO disproportionation reactions are affected by temperature and pressure during DRM reactions. The low-temperature conditions favor CO disproportionation reactions, while high temperatures are more favorable for CH4 cleavage reactions. Sintering is a process in which active crystals on the catalyst’s surface undergo agglomeration at high temperatures. This reduces the number of active sites and leads to catalyst deactivation. The mechanisms of metal sintering have been summarized: the particle migration mechanism and the Ostwald maturation mechanism. The particle migration mechanism means that particles undergo similar Brownian motion, leading to migration, collision between particles, and aggregation to form large particles. The Ostwald ripening mechanism refers to the migration of atomic or molecular species from the surface of one particle to the surface of other particles through surface diffusion or gas-phase diffusion, resulting in particle growth due to differences in surface free energy and the concentration of atoms loaded with the metal [24]. Hansen et al. [25] suggested that sintering can be divided into three stages. The first stage is the rapid deactivation of the catalyst, the second stage is the slowing down of catalyst deactivation, and the third stage is the stabilization of catalyst activity, as shown in Figure 6.
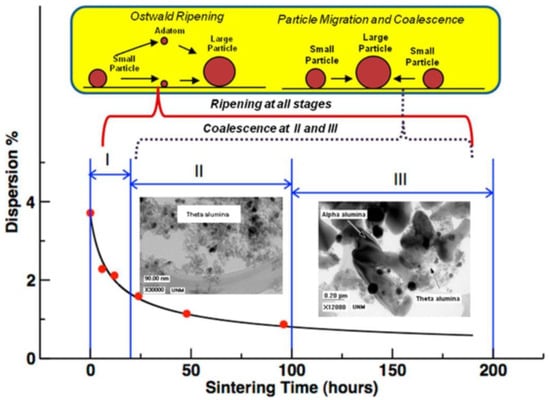
Figure 6.
Two mechanisms of sintering. Phase I involves rapid loss in catalyst activity (or surface area), phase II is where sintering slows down, and phase III is where the catalyst may reach a stable performance [25].
4. DRM Reaction Kinetics Study
Kinetic studies are an important aspect of catalytic reaction processes as they help describe reaction rates, define the chemistry involved, and enable calculations on theoretical reaction rate models [26]. From a theoretical chemistry perspective, it is also possible to optimize the DRM reaction, which aids in reactor design and catalyst selection. There are three types of kinetic models for DRM reactions: the power law model, the Eley–Rideal model (E-R model), and the Langmuir–Hinshelwood model (L-H model) or the Langmuir–Hinshelwood–Hougen–Watson model (LHHW model). The kinetic equations for the DRM reaction take different forms depending on the catalyst used. The power law model can be expressed as (Equation (8)), where r represents the CH4 reaction rate, P denotes the partial pressure of CO2 or CH4, k is the apparent rate constant, and m and n represent the number of reaction stages. The power law model is widely studied because it is easy to apply and estimate parameters, can be used for initial estimation before computation, and is useful for solving complex models with large amounts of data [23,27]. However, it is not applicable to the reaction kinetics of all catalysts, cannot explain the reaction mechanism on all catalysts, and has limitations. The power law model assumes a specific functional form for the relationship between variables, typically based on empirical observations. It implies a power law dependence between the rate of reaction and the concentrations of reactants or other relevant variables. However, different catalysts and reaction systems may exhibit more complex behavior that cannot be accurately described solely by a simple power law relationship. It can only provide rough estimates of the required parameters and is not applicable to the overall reaction kinetics of various catalysts [28]. It can be used for initial guess estimation to solve complex models that require large amounts of data. However, it does not fully explain the various steps of the reaction mechanism occurring on the catalyst surface. It is important to consider the specific characteristics of the catalyst and the reaction system when selecting an appropriate kinetic model. Experimental data and theoretical insights should be combined to develop a comprehensive understanding of the reaction mechanism and accurately describe its kinetics [29].
However, there are fewer reports in the literature on E-R modeling of Ni-based catalysts, and there is even a lack of experimental validation for this approach. This may be attributed to the lack of clarity regarding the gas-phase reactions of the surface adsorption reactants. In contrast, the LHHW model has a wider application for Ni-based catalysts compared to the power law model and the E-R model. Therefore, this paper will specifically focus on the LHHW model.
Wang et al. [30] analyzed the reaction kinetics, as well as the bifunctional mechanism of Ni-CaO-ZrO2 catalysts, under different conditions and proposed the following DRM reaction mechanism.
where m is a metal site and s an oxide support site. Based on the above mechanism, the authors developed a Langmuir–Hinshelwood type kinetic rate equation. The conversion of CH4 can be expressed as:
H2 + 2m ↔ 2H − m
CO2 − s+ 2H − m ↔ CO + H2O + s + m
Bobrova et al. [31] proposed kinetic equations for Pt/PrSmCeZrO oxide catalysts with the reaction route going through the following steps:
CO2 + [PtO] ↔ [PtCO3]
CH4 + [PtCO3] → 2CO + 2H2 + [PtO]
CH4 + [PtO] → CO + 2H2 + [Pt]
[Pt] + [Os] ↔ [PtO] + [Vs]
CO2 + [Vs] → CO + [Os]
H2 + [PtO] → H2O + [Pt]
The kinetic equation for CH4 conversion in the DRM reaction was derived from the basic steps:
From the above analysis, it can be observed that the DRM reaction involves multiple reaction processes, and the different catalysts exhibit different kinetic models. Despite the varying mechanisms in kinetic studies for different catalysts, such studies remain crucial in order to unveil the catalytic activity. Efficient and stable catalysts can be designed more effectively, thereby promoting the industrialization of DRM reaction catalysts.
5. Effect of Parameters on Catalyst Performance
5.1. Effect of Supports on Ni-Based Catalysts
Supports play a crucial role in determining the catalytic performance of catalysts. They not only promote the dispersion of Ni metal but also provide a metal–oxide interface for interaction with the active metal. This interaction impacts the catalyst’s structure, particle size, and the dispersion of the active components, subsequently influencing the catalyst’s activity, stability, and resistance to carbon accumulation [28]. Commonly used supports include Al2O3, molecular sieves, rare earth metal oxides, and bimetallic composite oxides.
Al2O3 has been widely used as one of the most popular carriers for a long time [32,33,34,35]. Talkhoncheh et al. [36] prepared different support-loaded Ni-based catalysts for the DRM reaction using the impregnation method. The Ni/Al2O3 catalyst offers several advantages, including a larger specific surface area and better dispersion, leading to enhanced activity and stability.
Furthermore, magnesium aluminate spinel (MgAl2O4) is a promising Ni-based catalyst support with high thermal stability and favorable surface properties. It aids in enhancing the dispersion of Ni metal, thereby preventing metal sintering. Hadian et al. suggested that Ni-based catalysts loaded with MgAl2O4 demonstrated improved activity and stability due to the support’s refractoriness, low acidity, high mechanical strength, and excellent resistance to thermal shock, which promoted the conversion of CH4 and CO2 [37]. Khoja et al. [38] utilized La2O3 as a co-support for the DRM reaction alongside the Ni/MgAl2O4 catalyst. The introduction of La2O3 increased the alkalinity of the catalyst, resulting in the enhanced conversion of CH4 and CO2, as well as an improved metal–support interaction. Additionally, the authors concluded that La2O2CO3 formed on the catalyst surface effectively inhibited coke generation.
Makri et al. [39] investigated the carbon dioxide reforming of methane to synthesis gas over solid catalysts consisting of 5 wt% Ni supported on Ce1−xMxO2−δ (M = Zr4+, Pr3+) carriers. The study revealed that the composition of the support and reaction temperature strongly influenced the amount and reactivity of carbon formed via different activation routes. The 5%Ni/Ce0.8Pr0.2O2 catalyst exhibited good performance with high CO2 conversion, H2 yield, and low carbon accumulation. The nickel particle size was found to be influenced by the support composition, affecting the origin, kinetics, and reactivity of carbon deposition.
Lorber et al. [40] conducted a study on Ni loading on CeO2 rods, cubes, and spheres with loadings ranging from 1 to 4 wt.%. The results revealed that the catalysts exhibited distinct catalytic properties in the DRM reaction. The CO2 activation pathways of 2Ni/CeO2 nanorod catalysts were analyzed in the presence and absence of hydrogen using temperature-programmed DRIFT-MS and DFT. Usman et al. [41] synthesized Ni-based catalysts using MgO as a carrier via microemulsion synthesis. The results demonstrated that the catalysts exhibited optimal catalytic performance under the conditions of calcination at 800 °C and reduction at 550 °C. This can be attributed to the presence of a high number of active sites on the support surface and the formation of stronger interactions between NiO-MgO solid solutions. Löfberg et al. [42] prepared Ni/CeO2 catalysts for the methane chemical cycle dry reforming reaction. The results indicated that Ni played a vital role in reactant activation and solid oxygenation. Supports with unique redox properties and oxygen storage capacity were found to be crucial. The storage of O2 facilitated the oxidation of carbon deposits and generated oxygen vacancies that activated CO2 [39] (Figure 7).
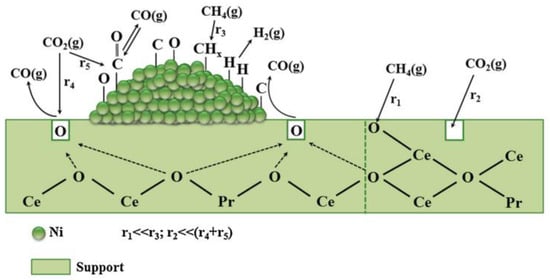
Figure 7.
Representation of the main chemical reaction steps of the CH4 and CO2 activation routes on the 5 wt%Ni/Ce0.8Pr0.2O2- catalyst towards dry reforming of methane for syngas (CO/H2) production [39].
Carbon materials have several advantages, including low cost, high mechanical resistance, a large specific surface area, variable pore size distribution, and good reducibility [43]. Activated carbon, which exhibits neutral surface properties and excellent structural properties, is commonly used as a catalyst carrier for DRM reactions, with scientists extracting it from biomass [44,45].
Natural mineral clay offers the advantages of affordability and excellent physical and chemical properties, making it a viable alternative to traditional supports. Chen et al. [46] utilized natural mineral clay as a support for DRM reactions, and characterization techniques revealed that the catalysts exhibited good activity and stability. Biomass charcoal surfaces contain numerous functional groups, such as carboxyl, hydroxyl, carbonyl, phenolic hydroxyl, lactone group, ether bond, and other polar or non-polar functional groups. Notably, phenolic hydroxyl and lactone groups, due to their oxygen-containing functional groups, can form H-bonds with CH4, generating a dipole force that enhances CH4 activation and significantly improves the catalytic performance of biomass char [47,48,49]. Pal, a natural fibrous silicate mineral, serves as an ideal catalyst carrier due to its large specific surface area, surface hydroxyl groups, and impressive chemisorption capacity [50]. Figure 8 illustrates a model of porous biochar containing various oxygenated functional groups [51].
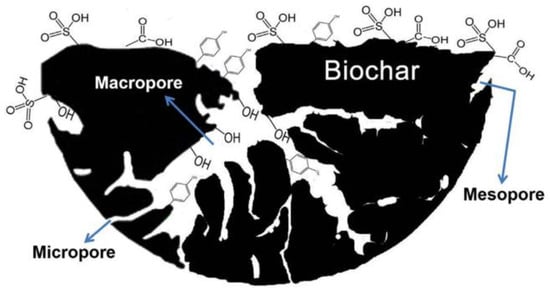
Figure 8.
A model of porous biochar containing different functional group [51].
Table 1 summarizes the effects of different supports on the performance of Ni-based catalysts [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. The table illustrates that various supports have distinct roles. Al2O3 is a commonly used catalyst support carrier. CeO2 and La2O3 exhibit strong capabilities in adsorbing and dissociating oxygen, leading to excellent catalytic properties when used as supports due to their strong metal–support interaction. Moreover, natural materials can serve as alternatives to conventional supports, providing advantages such as widespread availability and low cost. This makes them a promising option for future support research.

Table 1.
Influence of different supports on Ni-based catalysts.
5.2. Effect of Additives on Ni-Based Catalysts
The addition of suitable additives to the catalyst can effectively adjust the electron density of the metal atoms, thereby enhancing the dissociation of CH4. Additionally, these additives have the ability to modulate the acidity and alkalinity of the catalyst surface to some extent, reduce the size of Ni metal particles, and improve the dispersion of the active components, as well as their interaction with the support [24]. Table 2 presents the impact of different promoters on the catalytic performance of Ni-based catalysts [24,52,53,54,55,56,57,58,59,60,61]. Generally, alkali metals (K), alkaline earth metal oxides (MgO), certain rare earth metals (Sc, Zr), and noble metals (Pd, Ag, Ru) are commonly used as promoters. The addition of alkali and alkaline earth metal oxides helps neutralize the surface acidity of the catalyst, enhances CO2 adsorption on the catalyst surface, and improves carbon removal efficiency. Incorporating rare earth metals primarily enhances oxygen adsorption and dissociation. Furthermore, the addition of Pd, Ag, and Ru results in a stronger Lewis acidity of the support, better metal dispersion, and improved catalyst performance.

Table 2.
Influence of promoters on Ni-based catalysts.
It is widely recognized that the addition of alkaline accelerators can enhance the coking resistance, stability, and catalytic activity of catalysts. Azancot et al. [52] conducted a study on the effect of K addition in the DRM reaction, focusing on carbon generation and the relationship between the promotion of basicity in the MgAl2O4 support and the activity, selectivity, and stability of Ni sites with varying K loadings. The FTIR method was employed to determine CO2 adsorption at room temperature, and the alkalinity of the catalyst was evaluated. The results indicated that the inverse water–gas reaction and CO disproportionation reaction were favored when the K content exceeded 3%. The strongly basic Mg-O-K sites inhibited carbon deposition and improved catalyst stability. Alipour et al. [53] used the impregnation method to prepare Ni/Al2O3 catalysts containing alkaline earth metal oxides. The results demonstrated that the introduction of MgO reduced the reduction temperature of NiO species, increased CH4 conversion, and reduced carbon generation. On the other hand, CaO and BaO decreased CH4 conversion but also reduced coke formation.
Rare earth metals are also excellent choices as additives. GI Siakavelas et al. demonstrated that the introduction of Sm3+ or Pr3+ as additives can modify CeO2 supports, leading to the formation of more surface oxygen vacancies, thus, enhancing the DRM rate at lower temperatures. Furthermore, the inclusion of La3+ facilitates the stabilization of Ni active sites and restricts the agglomeration of Ni metal particles [54]. In another study conducted by Al-Fatesh et al. [55], the reforming performance of the Ni/SBA-15 catalyst with Sc as an additive was explored. Through characterization techniques such as BET, XRD, and TPR, it was found that the introduction of Sc increased the strength and reduction temperature of the catalysts compared to those without additives. Notably, the catalysts loaded with 0.5 wt% Sc exhibited the highest conversions and lowest deactivation rates.
Precious metals exhibit favorable activity and stability, and their incorporation as additives in Ni-based catalysts can enhance the catalytic performance. Luisetto et al. [56] discovered that the introduction of Ru significantly improved the activity and stability of the catalysts. Ni-based catalysts are prone to rapid deactivation due to carbon deposition, whereas Ni-Ru catalysts exhibit notably higher activity compared to monometallic Ni-based catalysts. The positive effect of Ru on Ni reduction was demonstrated. Ma et al. [57] investigated the impact of Pd introduction on catalyst performance. The results showed that the bimetallic nano-alloy catalyst displayed higher activity than the Ni-based catalyst, attributed to the synergistic effect between Pd and Ni. Yu et al. [58] prepared Ag-loaded Ni/CeO2 catalysts and observed that Ag effectively promoted carbon gasification while altering the coke type formed on the surface of Ni, thereby forming easily gasifiable amorphous carbon. Jin et al. [2] utilized atomic layer deposition (ALD) to coat Ni/Al2O3 catalyst with ZrO2. During high-temperature H2 reduction, interfacial oxygen vacancies formed on the surface of the ZrO2 coating, enhancing CO2 activation by dissociating CO2 and releasing reactive oxygen. This process limits carbon generation and improves the catalytic performance of Ni/Al2O3 catalysts. ALD is a thin-film coating technology that enables the precise control of self-limiting reactions at the metal–oxide interface at the atomic level [59,60]. Chatla et al. [61] investigated the effect of Zr as an additive on the performance of Ni/MgO catalysts. Zr doping significantly improved the dispersion of Ni compared to Ni/MgO catalysts. Shakir et al. [62] utilized NaBH4 reduction for the DRM reaction to synthesize B(x)-Ni/MgAl2O4 catalysts (x = 1 wt.%, 3 wt.%, and 5 wt.%). The results demonstrated that B promoted the formation of , Ni-B, and smaller-sized Ni particles. B(3)-Ni/MgAl2O4 exhibited improved conversion of CH4 and CO2. In contrast, Deng et al. [63] applied BN coatings on Ni/ZrO2 catalysts to enhance their anti-coking performance by reducing carbon crystallinity and accelerating carbon oxidation.
5.3. Effect of Active Components on Ni-Based Catalysts
The active component plays a crucial role in the catalytic process and is also the primary substance in the catalyst. In recent years, noble metals [64] such as Pt, Ru, Ir, and Rh, as well as group VIII transition elements [65] including Fe, Co, and Ni, have been commonly used as active components. Although precious metals exhibit excellent activity and catalytic properties, their high cost limits their wide-scale application in production practice. Non-precious metals, on the other hand, have the advantage of being inexpensive, with Ni-based catalysts having the closest activity to that of precious metals. Therefore, Ni is the preferred choice as the active component. However, it is also prone to more severe issues such as metal sintering and carbon buildup. Bimetals, however, possess varying physicochemical properties, electronic and geometric effects, and synergistic intermetallic interactions. As a result, they exhibit unique properties and demonstrate excellent catalytic performance, different from monometallic catalysts [66,67,68,69,70,71].
Many of the literature reports claim that Co is the best second metal, and Ni-Co bimetallic catalysts have better catalytic performance [72,73,74]. The presence of Co alleviates the reduction in Ni metal and reduces the size of Ni metal particles, thus, improving the stability of the catalyst. Additionally, the Ni/Co ratio affects the catalyst’s performance. If the Ni content is large, it tends to lead to metal sintering. On the other hand, if the Co content is large, an oxidation reaction occurs, forming a CoAl2O4 spinel structure, both of which result in catalyst deactivation. The experimental results indicate that the optimal molar ratio is 70Ni/30Co-80Ni/20Co. Sengupta et al. [75] prepared identical Ni/Al2O3, Ni-Co/Al2O3, and Co/Al2O3 catalysts and tested them for DRM and CH4 cracking reactions. The results showed that the initial activities of the bimetallic catalysts with different Co/Ni ratios were in the following order: 1Co3Ni/Al2O3 > 1Co1Ni/Al2O3 > 3Co1Ni/Al2O3. Feng et al. [76] prepared a series of Ni-Co/Si3N4 catalysts and monometallic catalysts with different Ni/Co ratios. The results demonstrated that the bimetallic catalysts were more active than the monometallic ones and exhibited good stability over a reaction time of 50 h. Furthermore, the results indicated that the bimetallic catalysts were more active than the monometallic ones. In the DRM reaction, different Ni/Co ratios have different effects on the catalytic performance. The effects of different active components on the reforming performance of Ni-based catalysts are listed in Table 3 [72,73,74,75,76,77,78]. In summary, Co can be used as the best second metal in addition to precious metals. Ni-Co bimetallic catalysts are widely utilized in DRM reactions. The introduction of Co reduces the reducing properties of Ni. Alloying between Ni-Co creates a synergistic effect and promotes metal–support interactions.

Table 3.
Influence of active components on Ni-based catalysts.
Lyu et al. [77] prepared Fe-doped Ni/Al2O3 catalysts with a two-pore structure using a one-pot evaporation-induced self-assembly (EISA) method. After a reaction time of 100 h, the catalyst with Fe/Ni = 0.5 showed the best catalytic performance and resistance to carbon accumulation. During the DRM reaction, CO2 is oxidized and partially converts Fe to FeOx, which, in turn, promotes the interaction of Fe and Ni to form an alloy. Consequently, the activation of CH4 leads to the production of H2 and C species, ensuring a cycle of redox reactions. Sarkar et al. [78] prepared a Gd-Ni/ZSM-5 catalyst. The doping of Gd reduced the agglomeration of Ni nanoparticles. In the Gd-Ni/ZSM-5 catalyst, Gd reduces CO2 to CO through activated O and facilitates the release of nascent O, which further oxidizes C. Therefore, Gd inhibits carbon buildup from the CO disproportionation reaction and CH4 cracking reaction. The reaction mechanism is shown in Figure 9. Wang et al. [79] prepared a series of Ni-In/γ-Al2O3 catalysts with different Ni contents using the “two-solvent” method. The experimental results demonstrated that the Ni-In/γ-Al2O3 catalyst exhibited better stability. When the Ni/In ratio was 3/2, the conversion of CH4 reached 91.1% and CO2 conversion reached 97.1%. The addition of In increased the number of adsorbed oxygen species and oxygen vacancies on the catalyst surface compared to the Ni/γ-Al2O3 catalyst. This improvement enhanced the CO2 adsorption capacity and the catalyst’s resistance to carbon accumulation.
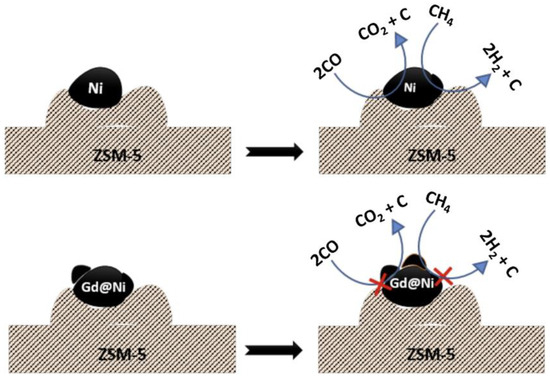
Figure 9.
Schematic illustration of the catalytic process over Gd-Ni/ZSM-5 catalyst [78].
5.4. Effect of Preparation Method on Ni-Based Catalysts
The method of catalyst preparation has a crucial influence on the catalytic performance of catalysts. The differences in preparation methods result in variations in catalyst composition, structure, size, and chemistry, ultimately affecting catalytic performance, resistance to carbon buildup, and selectivity. In DRM reactions, catalysts are commonly prepared using impregnation, co-precipitation, and sol-gel methods [80]. Among these, the conventional impregnation method is the most frequently used for catalyst preparation, but it has several shortcomings. For instance, the active metal Ni is not uniformly distributed on the carrier surface, which may impact the catalyst’s performance. Liu et al. [81] employed alkali-induced adsorption to prepare Ni/Al2O3 catalysts. Through characterization techniques such as TEM and HRTEM, the results indicate that under alkaline conditions, Al2O3 forms a unique lamellar stacking structure. This facilitates the uniform distribution of Ni on the catalyst surface and enhances dispersion. The catalyst exhibited better low-temperature reforming performance compared to the conventional impregnation method. Chen et al. [82] prepared three Ni/SiO2 catalysts using impregnation, sol-gel, and ammonia evaporation methods, respectively, to investigate the relationship between catalyst preparation methods and catalytic performance. The catalyst obtained by the ammonia evaporation method demonstrated good catalytic performance. These catalysts had smaller Ni particles, stronger metal–support interactions, and better resistance to sintering. In contrast, the catalysts prepared by the impregnation method showed uneven Ni distribution, weak metal–carrier interaction, and poor stability and activity. The catalysts prepared by the sol-gel method had fewer Ni active sites on the surface, resulting in poorer reforming performance. Fang et al. [83] prepared Ni-Al2O3 catalysts using the EISA method. Compared with the catalysts prepared by the conventional impregnation method, metal sintering and carbon accumulation were significantly reduced, leading to enhanced reforming performance. On the other hand, in Ni/Al2O3-IMP catalysts, CH4 is prone to dissociate around Ni and form a large amount of coke, which wraps around the Ni active sites, diminishing catalyst activity and eventually deactivating it. The Ni-Al2O3-EISA catalyst, with its mesoporous structure, restricts the aggregation of Ni particles. Ni grains exhibit strong interactions with Al2O3, making it less likely for coke to form and wrap around the active sites on the catalyst surface (as shown in Figure 10). Therefore, the EISA method can improve the catalytic performance of the catalyst.
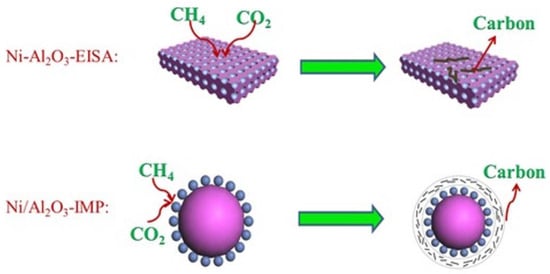
Figure 10.
Schematic coke formation for methane dry reforming on the catalysts prepared by different methods [83].
Zhang et al. [84] prepared Ni-based catalysts by homogeneous precipitation (Ni-HP) and impregnation (Ni-IM). The effect of different preparation methods on metal dispersion was explored. The correlation results show that the interaction between metal Ni and the carrier is stronger compared to the Ni-IM catalyst. This led to an increase in the reduction temperature of the Ni-HP catalyst and improved the dispersion of Ni. The CH4 conversion of the Ni-HP catalyst decreased slightly from 74.5% to 73.8% as the reaction progressed, while the conversion of CH4 with the Ni-IM catalyst decreased from 61.7% to 37.3%. Dekkar et al. [85] performed a comparison between catalysts prepared using two different methods: microemulsion and impregnation. The catalysts prepared via the microemulsion method exhibited smaller particle sizes and a better dispersion of Ni particles, in contrast to those prepared using the impregnation method. This favorable characteristic resulted in reduced metal sintering and carbon buildup, leading to improved catalyst activity and stability.
Table 4 summarizes the effects of different preparation methods on the catalytic performance of Ni-based catalysts [80,81,82,83,84]. The impregnation method is commonly used for catalyst preparation. However, this method generally exhibits poor reforming performance and often leads to an uneven distribution of Ni. In alkali-induced adsorption, alkali metals interact with the catalyst material or surface. These alkali metals have low ionization energies and readily donate their outermost valence electron to form positive ions. This property makes them highly reactive and capable of inducing changes in adsorption behavior. It is important to note that the specific mechanism and effects of alkali-induced adsorption can vary depending on the materials and systems involved. Therefore, comprehensive experimental studies and theoretical modeling are required to fully understand this phenomenon and its applications. The EISA method is characterized by the ability to combine all necessary components for self-assembly in a single reaction vessel, simplifying the experimental process and minimizing the risk of contamination. However, it is important to consider that conditions such as temperature and humidity during the evaporation stage can also impact the formation of self-assembled structures. Homogeneous precipitation offers several advantages over other techniques in nanoparticle synthesis. It provides better control over particle size and distribution. Additionally, it is a relatively simple and scalable process that can be easily modified. However, it is important to note that the homogeneous precipitation method requires careful optimization of the reaction parameters. Therefore, challenges remain in improving the synthesis technology of catalysts and enhancing their catalytic performance.

Table 4.
Influence of preparation method on Ni-based catalysts.
6. Summary and Outlook
In recent years, the research on Ni-based catalysts for CH4-CO2 reforming to produce syngas has made significant progress. These catalysts, serving as alternatives to precious metal catalysts, have demonstrated comparable performances. However, two major challenges associated with Ni-based catalysts are the buildup of carbon and the sintering of metal particles. This paper presents a brief overview of the research on Ni-based catalysts, with a specific focus on their application in CH4-CO2 reforming to syngas. The kinetics, thermodynamics, reaction mechanism, and deactivation mechanism of these catalysts are discussed. Additionally, a summary of strategies aimed at improving coking resistance and enhancing catalyst stability was provided.
The formation of carbon deposits is a complex process influenced by various factors such as operating conditions, preparation methods, raw material composition, and the content of active components. Avoiding conditions that promote carbon deposition ensures the catalyst’s activity and stability during the DRM reaction. Furthermore, several methods were found to effectively enhance the reforming performance of Ni-based catalysts and increase the conversion of CH4 and CO2. For example, the use of suitable supports facilitates the formation of strong metal–support interactions. Doping additives can regulate the acid–base and oxygen mobility of the catalyst surface. Additionally, the application of bimetallic catalysts promotes intermetallic synergism. Finally, modifying the preparation method can impact the physicochemical structure of the catalyst, resulting in improved performance. Addressing these aspects aims to advance the understanding and development of Ni-based catalysts for CH4-CO2 reforming, creating opportunities for sustainable syngas production.
To date, an increasing number of scientists have invested in research on CH4-CO2 reforming to syngas and have made significant progress. However, the understanding of the reaction and deactivation mechanisms remains controversial, which greatly limits the further industrialization of Ni-based catalysts. Despite these challenges, there is no doubt that DRM responses have a promising future and can lead to more economic benefits. In order to address the existing problems and challenges, the following concerns and recommendations for research in the field of DRM are presented.
First, it is recommended to develop new dry reforming catalysts by adjusting their components and structure. The biggest challenge faced by Ni-based catalysts is deactivation, unlike noble metal catalysts that exhibit excellent activity and stability. Therefore, a focus on developing bimetallic catalysts is warranted, as they exhibit a synergistic effect that enhances catalytic performance and enables the high conversion of CH4 and CO2. When considering bimetallic catalysts, the choice of the second metal and the content ratio between the second metal and Ni become critical factors. The addition of precious metals can improve the dispersion of Ni, but having too high a content ratio can lead to crystal aggregation and a decrease in activity. Apart from precious metals, Co metals can also be utilized as secondary metals. The literature suggests that Co has a strong carbon adsorption capacity, which regulates the formation rate of C* and reduces coke generation. Consequently, Co-based catalysts exhibit better activity and resistance to carbon buildup. Second, alloying between Co-Ni reduces the particle size of Ni, thus, improving the catalyst’s stability.
Second, there is still a lack of understanding regarding the reaction mechanism on the catalyst surface in the DRM reaction. Therefore, it is important to study the DRM reaction from a mechanistic perspective by combining its reaction thermodynamics and kinetics. Modern in situ dynamic characterization techniques can be employed to detect real-time changes in the surface reactions of Ni-based catalysts during DRM reactions. This will aid in gaining a deeper understanding of the conversion of CH4 and CO2 on the catalyst surface, the reactions between Ni and other components of the catalyst (such as supports and additives), as well as principles related to carbon formation and carbon elimination. Additionally, simulations using density functional theory (DFT) can be employed to construct models prior to the actual reactions. DFT can elucidate the fundamental steps and reaction mechanisms of many non-homogeneous catalytic processes. It can also serve to confirm experimental results. By utilizing simulations, not only can time and resources be saved, but it can also contribute to a stronger grasp of reaction mechanisms and kinetics. First, DFT calculations play a crucial role in accurately determining the electronic structure of nickel-based oxides. These calculations provide valuable insights into the distribution of electrons, energy levels, and bonding interactions within the material. Such information is essential for comprehending and predicting various characteristics of these oxides. Second, DFT calculations aid in predicting and rationalizing the reactivity of these materials by offering a detailed understanding of reaction mechanisms, active sites, and energetics. This knowledge is instrumental in the design and optimization of nickel-based catalysts for DRM reactions. Furthermore, DFT calculations facilitate the study of defects and doping in nickel-based oxides by simulating the incorporation of different elements and analyzing their effects on the electronic structure.
Third, when it comes to the reactor concept, there are several emerging technologies that could surpass the traditional limitations and conditions of DRM reactions. It is crucial to dedicate more attention to finding a breakthrough that can establish DRM as a major pathway for CO2 utilization. It is worth noting that the utilization of plasma-driven catalytic reactors, photocatalytic reactors, and microwave-assisted reactors at lower temperatures can enhance the efficiency of DRM’s solar thermochemical reactors, induction-heated reactors, and catalytic membrane reactors. Additionally, the implementation of a molten metal bubble reactor and an oxygen permeable membrane reactor minimizes carbon deposition issues.
Fourth, while scientists have made significant progress in synthesizing anticarbon catalysts and developing novel catalytic systems, there remains a dearth of studies to evaluate the economic viability of catalyst and catalytic system industrialization. The future research should not only focus on developing DRM catalysts with superior catalytic performance but also on demonstrating their economic feasibility for industrial applications.
In conclusion, despite the controversial understanding of the reaction and deactivation mechanisms, the research on CH4-CO2 reforming to syngas shows great potential for the future of DRM responses. Through the development of new catalysts, the study of reaction mechanisms from a mechanistic perspective, the exploration of emerging reactor technologies, and conducting techno-economic studies, the industrialization of Ni-based catalysts can be advanced in the field of DRM.
Author Contributions
Conceptualization, Y.C. and Y.Z. (Yunfei Zhang); methodology, Y.Z. (Yuqiong Zhao); validation, X.Z., Y.Z. (Yunfei Zhang) and G.Z.; resources, G.Z. and Y.Z. (Yuqiong Zhao); writing—original draft preparation, Y.C. and X.Z.; writing—review and editing, Y.C., X.Z., G.L. and G.Z.; visualization, Y.W.; supervision, G.Z.; funding acquisition, G.Z. and Y.Z. (Yuqiong Zhao). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China grant number (No. 21878200) and the Postdoctoral Science Foundation of China (No. 2022M712339).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Torrez-Herrera, J.J.; Korili, S.A.; Gil, A. Recent progress in the application of Ni-based catalysts for the dry reforming of methane. Catal. Rev. 2021, 63, 1–58. [Google Scholar] [CrossRef]
- Jin, B.; Li, S.; Liu, Y.; Liang, X. Engineering metal-oxide interface by depositing ZrO2 overcoating on Ni/Al2O3 for dry reforming of methane. Chem. Eng. J. 2022, 436, 135195. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, T.; Yu, J.; Zhan, W.; Wang, L.; Guo, Y.; Guo, Y. Robust nanosheet-assembled Al2O3-supported Ni catalysts for the dry reforming of methane: The effect of nickel content on the catalytic performance and carbon formation. New J. Chem. 2021, 45, 21750–21762. [Google Scholar] [CrossRef]
- Pham, C.Q.; Cao, A.N.T.; Phuong, P.T.T.; Hoang Pham, L.K.; Vi Tran, T.T.; Trinh, T.H.; Vo, D.V.N.; Bui, T.P.T.; Nguyen, T.M. Enhancement of syngas production from dry reforming of methane over Co/Al2O3 catalyst: Insight into the promotional effects of europium and neodymium. J. Energy Inst. 2022, 105, 314–322. [Google Scholar] [CrossRef]
- Estephane, J.; Aouad, S.; Hany, S.; El Khoury, B.; Gennequin, C.; El Zakhem, H.; El Nakat, J.; Aboukaïs, A.; Abi Aad, E. CO2 reforming of methane over Ni–Co/ZSM5 catalysts. Aging and carbon deposition study. Int. J. Hydrogen Energy 2015, 40, 9201–9208. [Google Scholar] [CrossRef]
- Fujitsuka, H.; Kobayashi, T.; Tago, T. Development of Silicalite-1-encapsulated Ni nanoparticle catalyst from amorphous silica-coated Ni for dry reforming of methane: Achieving coke formation suppression and high thermal stability. J. CO2 Util. 2021, 53, 101707. [Google Scholar] [CrossRef]
- Zhou, R.; Mohamedali, M.; Ren, Y.; Lu, Q.; Mahinpey, N. Facile synthesis of multi-layered nanostructured Ni/CeO2 catalyst plus in-situ pre-treatment for efficient dry reforming of methane. Appl. Catal. B Environ. 2022, 316, 121696. [Google Scholar] [CrossRef]
- Ekeoma, B.C.; Yusuf, M.; Johari, K.; Abdullah, B. Mesoporous silica supported Ni-based catalysts for methane dry reforming: A review of recent studies. Int. J. Hydrogen Energy 2022, 47, 41596–41620. [Google Scholar] [CrossRef]
- Afzal, S.; Sengupta, D.; Sarkar, A.; El-Halwagi, M.; Elbashir, N. Optimization Approach to the Reduction of CO2 Emissions for Syngas Production Involving Dry Reforming. ACS Sustain. Chem. Eng. 2018, 6, 7532–7544. [Google Scholar] [CrossRef]
- Lavoie, J.M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, Y.; Wang, S.; Zhao, Q.; Mao, D.; Hu, C. Low-Temperature Catalytic CO2 Dry Reforming of Methane on Ni–Si/ZrO2 Catalyst. ACS Catal. 2018, 8, 6495–6506. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Adegbite, S.; Zhao, H.; Lester, E.; Wu, T. Tuning dry reforming of methane for F-T syntheses: A thermodynamic approach. Appl. Energy 2018, 227, 190–197. [Google Scholar] [CrossRef]
- Horlyck, J.; Lawrey, C.; Lovell, E.C.; Amal, R.; Scott, J. Elucidating the impact of Ni and Co loading on the selectivity of bimetallic NiCo catalysts for dry reforming of methane. Chem. Eng. J. 2018, 352, 572–580. [Google Scholar] [CrossRef]
- Anil, C.; Modak, J.M.; Madras, G. Syngas production via CO2 reforming of methane over noble metal (Ru, Pt, and Pd) doped LaAlO3 perovskite catalyst. Mol. Catal. 2020, 484, 110805. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Tian, H.; Zeng, L.; Zhao, Z.-J.; Gong, J. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B Environ. 2017, 202, 683–694. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, X.-M.; Zhu, J.; Hu, P. Activity and coke formation of nickel and nickel carbide in dry reforming: A deactivation scheme from density functional theory. J. Catal. 2014, 311, 469–480. [Google Scholar] [CrossRef]
- Ussa, P.A.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyka, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar]
- Xu, L.L.; Wen, H.; Jin, X.; Bing, Q.M.; Liu, J.Y. DFT study on dry reforming of methane over Ni2Fe overlayer of Ni(111) surface. Appl. Surf. Sci. 2018, 443, 515–524. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, R.; Hao, X.; Bao, Z.; Wu, T.; Wang, B.; Yu, F. Sandwiched SiO2@Ni@ZrO2 as a coke resistant nanocatalyst for dry reforming of methane. Appl. Catal. B Environ. 2019, 254, 612–623. [Google Scholar] [CrossRef]
- Wang, D.; Littlewood, P.; Marks, T.J.; Stair, P.C.; Weitz, E. Coking Can Enhance Product Yields in the Dry Reforming of Methane. ACS Catal. 2022, 12, 8352–8362. [Google Scholar] [CrossRef]
- Gould, T.D.; Izar, A.; Weimer, A.W.; Falconer, J.L.; Medlin, J.W. Stabilizing Ni Catalysts by Molecular Layer Deposition for Harsh, Dry Reforming Conditions. ACS Catal. 2014, 4, 2714–2717. [Google Scholar] [CrossRef]
- Abdullah, B.; Ghani, N.A.A.; Vo, D.-V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, P.; Cao, Z.; Campbell, C.T.; Xia, Y. The physical chemistry and materials science behind sinter-resistant catalysts. Chem. Soc. Rev. 2018, 47, 4314–4331. [Google Scholar] [CrossRef]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or Ostwald ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lua, A.C. Deactivation and kinetic studies of unsupported Ni and Ni–Co–Cu alloy catalysts used for hydrogen production by methane decomposition. Chem. Eng. J. 2014, 243, 79–91. [Google Scholar] [CrossRef]
- Arman, A.; Hagos, F.Y.; Abdullah, A.A.; Mamat, R.; Aziz, A.R.A.; Cheng, C.K. Syngas production through steam and CO2 reforming of methane over Ni-based catalyst-A Review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 042032. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A review of CH4–CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Khan, M.R.; Lam, S.S.; Cheng, C.K. Production of CO-rich hydrogen from methane dry reforming over lanthania-supported cobalt catalyst: Kinetic and mechanistic studies. Int. J. Hydrogen Energy 2016, 41, 4603–4615. [Google Scholar] [CrossRef]
- Wang, C.; Sun, N.; Kang, M.; Wen, X.; Zhao, N.; Xiao, F.; Wei, W.; Zhao, T.; Sun, Y. The bi-functional mechanism of CH4 dry reforming over a Ni–CaO–ZrO2 catalyst: Further evidence via the identification of the active sites and kinetic studies. Catal. Sci. Technol. 2013, 3, 2435–2443. [Google Scholar] [CrossRef]
- Bobrova, L.; Bobin, A.; Mezentseva, N.; Sadykov, V.; Thybaut, J.; Marin, G. Kinetic assessment of dry reforming of methane on Pt + Ni containing composite of fluorite-like structure. Appl. Catal. B Environ. 2016, 182, 513–524. [Google Scholar] [CrossRef]
- Yu, L.; Song, M.; Williams, P.T.; Wei, Y. Optimized Reforming of Biomass Derived Gas Based on Thermodynamic and Kinetics Analysis with Activated Carbon Fibers Supported Ni–Al2O3. BioEnergy Res. 2020, 13, 581–590. [Google Scholar] [CrossRef]
- Yu, L.; Song, M.; Williams, P.T.; Wei, Y. Alumina-Supported Spinel NiAl2O4 as a Catalyst for Re-forming Pyrolysis Gas. Ind. Eng. Chem. Res. 2019, 58, 11770–11778. [Google Scholar] [CrossRef]
- Yu, L.; Song, M.; Wei, Y.; Xiao, J. Combining Carbon Fibers with Ni/γ–Al2O3 Used for Syngas Production: Part A: Preparation and Evaluation of Complex Carrier Catalysts. Catalysts 2018, 8, 658. [Google Scholar] [CrossRef]
- Chatla, A.; Ghouri, M.M.; El Hassan, O.W.; Mohamed, N.; Prakash, A.V.; Elbashir, N.O. An experimental and first principles DFT investigation on the effect of Cu addition to Ni/Al2O3 catalyst for the dry reforming of methane. Appl. Catal. A Gen. 2020, 602, 117699. [Google Scholar] [CrossRef]
- Talkhoncheh, S.K.; Haghighi, M. Syngas production via dry reforming of methane over Ni-based nanocatalyst over various supports of clinoptilolite, ceria and alumina. J. Nat. Gas Sci. Eng. 2015, 23, 16–25. [Google Scholar] [CrossRef]
- Jalali, R.; Nematollahi, B.; Rezaei, M.; Baghalha, M. Mesoporous nanostructured Ni/MgAl2O4 catalysts: Highly active and stable catalysts for syngas production in combined dry reforming and partial oxidation. Int. J. Hydrogen Energy 2019, 44, 10427–10442. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Saidina Amin, N.A. Evaluating the Performance of a Ni Catalyst Supported on La2O3-MgAl2O4 for Dry Reforming of Methane in a Packed Bed Dielectric Barrier Discharge Plasma Reactor. Energy Fuels 2019, 33, 11630–11647. [Google Scholar] [CrossRef]
- Makri, M.M.; Vasiliades, M.A.; Petallidou, K.C.; Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5 wt% Ni/Ce1−xMxO2−δ (M = Zr4+, Pr3+) catalysts. Catal. Today 2016, 259, 150–164. [Google Scholar] [CrossRef]
- Lorber, K.; Zavasnik, J.; Arcon, I.; Hus, M.; Terzan, J.; Likozar, B.; Djinovic, P. CO2 Activation over Nanoshaped CeO2 Decorated with Nickel for Low-Temperature Methane Dry Reforming. ACS Appl. Mater. Interfaces 2022, 14, 31862–31878. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A. An investigation on the influence of catalyst composition, calcination and reduction temperatures on Ni/MgO catalyst for dry reforming of methane. RSC Adv. 2016, 6, 91603–91616. [Google Scholar] [CrossRef]
- Löfberg, A.; Guerrero-Caballero, J.; Kane, T.; Rubbens, A.; Jalowiecki-Duhamel, L. Ni/CeO2 based catalysts as oxygen vectors for the chemical looping dry reforming of methane for syngas production. Appl. Catal. B Environ. 2017, 212, 159–174. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Zhang, G.; Liu, J.; Lv, Y.; Zhang, Y. Highly stable activity of cobalt based catalysts with tungsten carbide-activated carbon support for dry reforming of methane: Role of tungsten carbide. Fuel 2022, 311, 122512. [Google Scholar] [CrossRef]
- García, R.; Soto, G.; Escalona, N.; Sepúlveda, C.; Orellana, M.J.; Morales, N.; Radovic, L.R.; Buitrago-Sierra, R.; Rodriguez-Reinoso, F.; Sepúlveda-Escribano, A. Methane dry reforming over Ni support on pine sawdust activated carbon: Effects of support surface properties and metal loading. Química Nova 2015, 38, 506–509. [Google Scholar] [CrossRef]
- Izhab, I.; Amin, N.A.S.; Asmadi, M. Dry reforming of methane over oil palm shell activated carbon and ZSM-5 supported cobalt catalysts. Int. J. Green Energy 2017, 14, 831–838. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, T.; Liu, H.; Zhang, P.; Wang, C.; Dong, S.; Chen, D.; Xie, J.; Zou, X.; Suib, S.L.; et al. High catalytic performance of the Al-promoted Ni/Palygorskite catalysts for dry reforming of methane. Appl. Clay Sci. 2020, 188, 105498. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.-H.; Tsang, D.C.; et al. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into Multiple and Multilevel Structures of Biochars and Their Potential Environmental Applications: A Critical Review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Xie, A.; Zhou, X.; Zhou, W.; Luo, S.; Yao, C. Preparation and enhanced photocatalytic activity of S-doped TiO2/palygorskite composites. Mater. Technol. 2016, 32, 265–271. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Azancot, L.; Bobadilla, L.F.; Centeno, M.A.; Odriozola, J.A. Effect of potassium loading on basic properties of Ni/MgAl2O4 catalyst for CO2 reforming of methane. J. CO2 Util. 2021, 52, 101681. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of alkaline earth promoters (MgO, CaO, and BaO) on the activity and coke formation of Ni catalysts supported on nanocrystalline Al2O3 in dry reforming of methane. J. Ind. Eng. Chem. 2014, 20, 2858–2863. [Google Scholar] [CrossRef]
- Liang, L.; Miao, C.; Chen, S.; Zheng, X.; Ouyang, J. Effective CO2 methanation at ambient pressure over Lanthanides (La/Ce/Pr/Sm) modified cobalt-palygorskite composites. J. CO2 Util. 2022, 63, 102114. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kasim, S.O.; Ibrahim, A.A.; Osman, A.I.; Abasaeed, A.E.; Atia, H.; Armbruster, U.; Frusteri, L.; bin Jumah, A.; Alanazi, Y.M.; et al. Greenhouse gases utilization via catalytic reforming with Sc promoted Ni/SBA-15. Fuel 2022, 330, 125523. [Google Scholar] [CrossRef]
- Luisetto, I.; Sarno, C.; De Felicis, D.; Basoli, F.; Battocchio, C.; Tuti, S.; Licoccia, S.; Di Bartolomeo, E. Ni supported on γ-Al2O3 promoted by Ru for the dry reforming of methane in packed and monolithic reactors. Fuel Process. Technol. 2017, 158, 130–140. [Google Scholar] [CrossRef]
- Ma, Q.; Sun, J.; Gao, X.; Zhang, J.; Zhao, T.; Yoneyama, Y.; Tsubaki, N. Ordered mesoporous alumina-supported bimetallic Pd–Ni catalysts for methane dry reforming reaction. Catal. Sci. Technol. 2016, 6, 6542–6550. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, Y.; Lu, Y.; Tong, G.; Zhu, K.; Zhou, X. The promoting role of Ag in Ni-CeO2 catalyzed CH4-CO2 dry reforming reaction. Appl. Catal. B Environ. 2015, 165, 43–56. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, B.; Gu, X.; Liang, H.; Yang, H.; Gao, Z.; Wang, J.; Qin, Y. A Tandem Catalyst with Multiple Metal Oxide Interfaces Produced by Atomic Layer Deposition. Angew. Chem. Int. Ed. Engl. 2016, 55, 7197–7208. [Google Scholar] [CrossRef]
- Yan, H.; He, K.; Samek, I.A.; Jing, D. Tandem In2O3-Pt/Al2O3 catalyst for coupling of propane dehydrogenation to selective H2 combustion. Science 2021, 371, 1257–1260. [Google Scholar] [CrossRef]
- Chatla, A.; Almanassra, I.W.; Kallem, P.; Atieh, M.A.; Alawadhi, H.; Akula, V.; Banat, F. Dry (CO2) reforming of methane over zirconium promoted Ni-MgO mixed oxide catalyst: Effect of Zr addition. J. CO2 Util. 2022, 62, 102082. [Google Scholar] [CrossRef]
- Shakir, M.D.; Sengupta, S.; Sinhamahapatra, A.; Liu, S.; Vuthaluru, H. B-Ni/MgAl2O4 catalyzed dry reforming of methane: The role of boron to resist the formation of graphitic carbon. Fuel 2022, 320, 123950. [Google Scholar] [CrossRef]
- Deng, J.; Bu, K.; Shen, Y.; Zhang, X.; Zhang, J.; Faungnawakij, K.; Zhang, D. Cooperatively enhanced coking resistance via boron nitride coating over Ni-based catalysts for dry reforming of methane. Appl. Catal. B Environ. 2022, 302, 120859. [Google Scholar] [CrossRef]
- Pakhare, D.; Shaw, C.; Haynes, D.; Shekhawat, D.; Spivey, J. Effect of reaction temperature on activity of Pt- and Ru-substituted lanthanum zirconate pyrochlores (La2Zr2O7) for dry (CO2) reforming of methane (DRM). J. CO2 Util. 2013, 1, 37–42. [Google Scholar] [CrossRef]
- Bilal, M.; Jackson, S.D. Ethanol steam reforming over Rh and Pt catalysts: Effect of temperature and catalyst deactivation. Catal. Sci. Technol. 2013, 3, 754–766. [Google Scholar] [CrossRef]
- Al-Fatesh, A.; Singh, S.K.; Kanade, G.S.; Atia, H.; Fakeeha, A.H.; Ibrahim, A.A.; El-Toni, A.M.; Labhasetwar, N.K. Rh promoted and ZrO2/Al2O3 supported Ni/Co based catalysts: High activity for CO2 reforming, steam–CO2 reforming and oxy–CO2 reforming of CH4. Int. J. Hydrogen Energy 2018, 43, 12069–12080. [Google Scholar] [CrossRef]
- Palanichamy, K.; Umasankar, S.; Ganesh, S.; Sasirekha, N. Highly coke resistant Ni–Co/KCC-1 catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2023, 48, 11727–11745. [Google Scholar] [CrossRef]
- Odedairo, T.; Ma, J.; Chen, J.; Wang, S.; Zhu, Z. Influences of doping Cr/Fe/Ta on the performance of Ni/CeO2 catalyst under microwave irradiation in dry reforming of CH4. J. Solid State Chem. 2016, 233, 166–177. [Google Scholar] [CrossRef]
- Summa, P.; Świrk Da Costa, K.; Gopakumar, J.; Samojeden, B.; Motak, M.; Rønning, M.; Van Beek, W.; Da Costa, P. Optimization of Co–Ni–Mg–Al mixed-oxides CO2 methanation catalysts with solution combustion synthesis: On the importance of Co incorporation and basicity. Appl. Mater. Today 2023, 32, 101795. [Google Scholar] [CrossRef]
- Movasati, A.; Alavi, S.M.; Mazloom, G. Dry reforming of methane over CeO2-ZnAl2O4 supported Ni and Ni-Co nano-catalysts. Fuel 2019, 236, 1254–1262. [Google Scholar] [CrossRef]
- Arbag, H.; Tasdemir, H.M.; Yagizatli, Y.; Kucuker, M.; Yasyerli, S. Effect of Preparation Technique on the Performance of Ni and Ce Incorporated Modified Alumina Catalysts in CO2 Reforming of Methane. Catal. Lett. 2020, 150, 3256–3268. [Google Scholar] [CrossRef]
- Mohamedali, M.; Henni, A.; Ibrahim, H. Recent Advances in Supported Metal Catalysts for Syngas Production from Methane. ChemEngineering 2018, 2, 9. [Google Scholar] [CrossRef]
- Hasnan, N.S.N.; Timmiati, S.N.; Lim, K.L.; Yaakob, Z.; Kamaruddin, N.H.N.; Teh, L.P. Recent developments in methane decomposition over heterogeneous catalysts: An overview. Mater. Renew. Sustain. Energy 2020, 9, 8. [Google Scholar] [CrossRef]
- Zagaynov, I.V.; Loktev, A.S.; Mukhin, I.E.; Dedov, A.G.; Moiseev, I.I. Influence of the Ni/Co ratio in bimetallic NiCo catalysts on methane conversion into synthesis gas. Mendeleev Commun. 2017, 27, 509–511. [Google Scholar] [CrossRef]
- Sengupta, S.; Ray, K.; Deo, G. Effects of modifying Ni/Al2O3 catalyst with cobalt on the reforming of CH4 with CO2 and cracking of CH4 reactions. Int. J. Hydrogen Energy 2014, 39, 11462–11472. [Google Scholar] [CrossRef]
- Feng, T.C.; Zheng, W.T.; Sun, K.Q.; Xu, B.Q. CO2 reforming of methane over coke-resistant Ni–Co/Si3N4 catalyst prepared via reactions between silicon nitride and metal halides. Catal. Commun. 2016, 73, 54–57. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, J.; Ma, Q.; Makpal, S.; Gao, X.; Fan, H.; Zhang, J.; Sun, J.; Zhao, T. Fe Doped Bimodal Macro/Mesoporous Nickel-Based Catalysts for CO2–CH4 Reforming. Ind. Eng. Chem. Res. 2022, 61, 10347–10356. [Google Scholar] [CrossRef]
- Sarkar, B.; Goyal, R.; Pendem, C.; Sasaki, T.; Bal, R. Highly nanodispersed Gd-doped Ni/ZSM-5 catalyst for enhanced carbon-resistant dry reforming of methane. J. Mol. Catal. A Chem. 2016, 424, 17–26. [Google Scholar] [CrossRef]
- Wang, C.; Su, T.; Qin, Z.Z.; Ji, H. Coke-resistant Ni-based bimetallic catalysts for the dry reforming of methane: Effects of indium on the Ni/Al2O3 catalyst. Catal. Sci. Technol. 2022, 12, 4826–4836. [Google Scholar] [CrossRef]
- Rad, S.J.H.; Haghighi, M.; Eslami, A.A.; Rahmani, F.; Rahemi, N. Sol–gel vs. impregnation preparation of MgO and CeO2 doped Ni/Al2O3 nanocatalysts used in dry reforming of methane: Effect of process conditions, synthesis method and support composition. Int. J. Hydrogen Energy 2016, 41, 5335–5350. [Google Scholar]
- Liu, X.; Zhang, L.; Zheng, X.; Zhang, Y.; He, D.; Luo, Y. Highly dispersed Ni/Al2O3 catalysts for dry reforming of methane prepared by alkaline-induced adsorption process. Int. J. Hydrogen Energy 2022, 47, 30937–30949. [Google Scholar] [CrossRef]
- Chen, C.; Wang, W.; Ren, Q.; Ye, R.; Nie, N.; Liu, Z.; Zhang, L.; Xiao, J. Impact of preparation method on nickel speciation and methane dry reforming performance of Ni/SiO2 catalysts. Front. Chem. 2022, 10, 1071. [Google Scholar] [CrossRef]
- Fang, X.; Peng, C.; Peng, H.; Liu, W.; Xu, X.; Wang, X.; Li, C.; Zhou, W. Methane Dry Reforming over Coke-Resistant Mesoporous Ni-Al2O3 Catalysts Prepared by Evaporation-Induced Self-Assembly Method. ChemCatChem 2015, 7, 3753–3762. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Ning, P.; Zhang, T.; Wang, M.; Long, K.; Huang, J. Dry reforming of methane over Ni/SBA-15 catalysts prepared by homogeneous precipitation method. Korean J. Chem. Eng. 2017, 34, 2823–2831. [Google Scholar] [CrossRef]
- Dekkar, S.; Tezkratt, S.; Sellam, D.; Ikkour, K.; Parkhomenko, K.; Martinez-Martin, A.; Roger, A.C. Dry Reforming of Methane over Ni–Al2O3 and Ni–SiO2 Catalysts: Role of Preparation Methods. Catal. Lett. 2020, 150, 2180–2199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).