Temporal Evolution of PM2.5 Levels and COVID-19 Mortality in Europe for the 2020–2022 Period
Abstract
1. Introduction
2. Materials and Methods
2.1. PM2.5 and COVID-19 Data
2.2. Data Analysis Procedure
3. Results
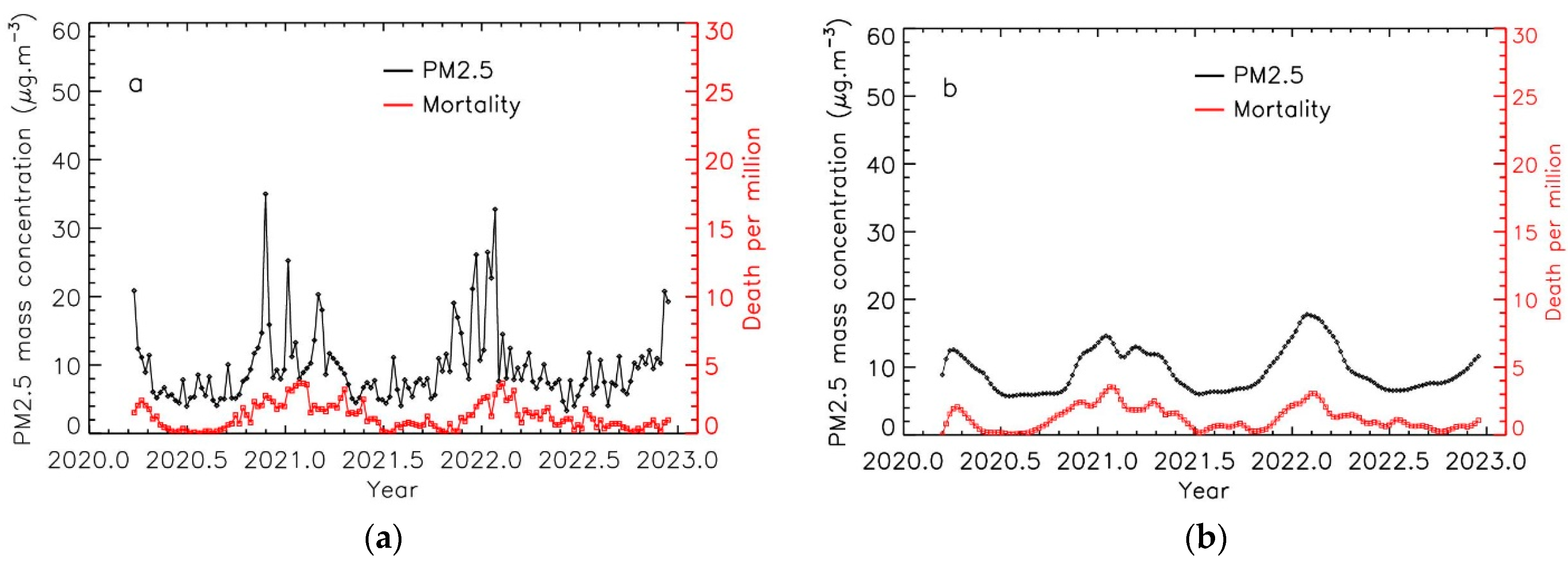
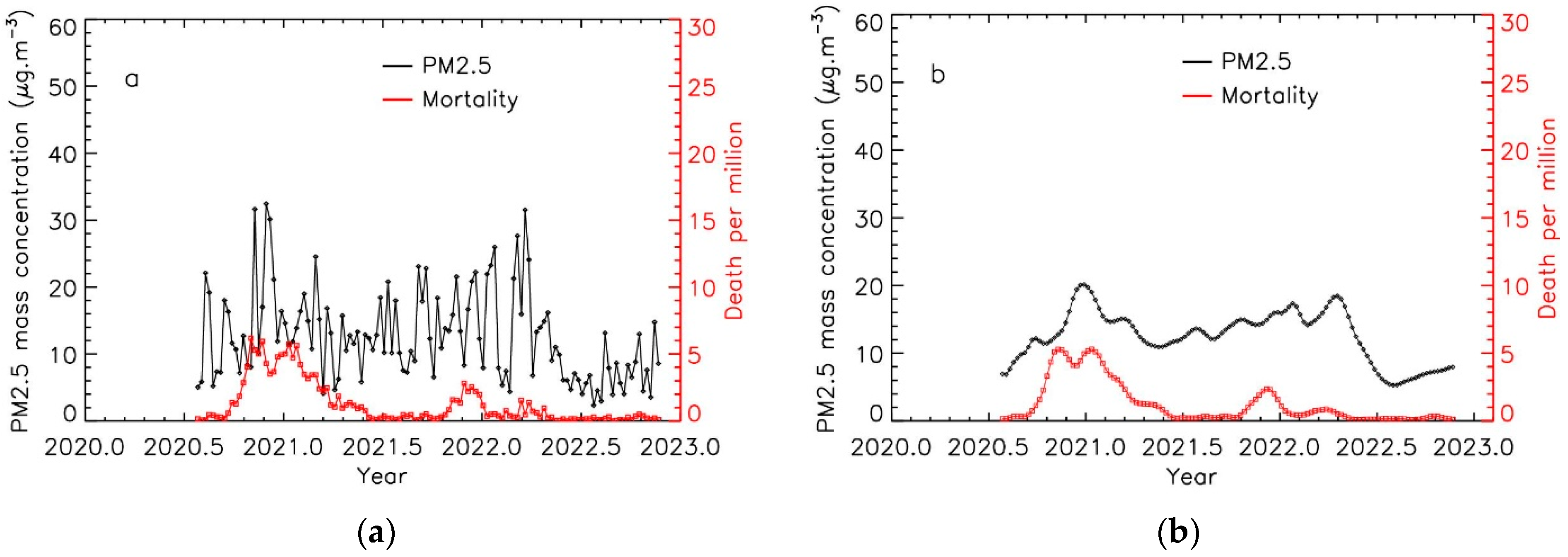
3.1. Time Evolution of PM2.5 Levels and COVID-19 Mortality
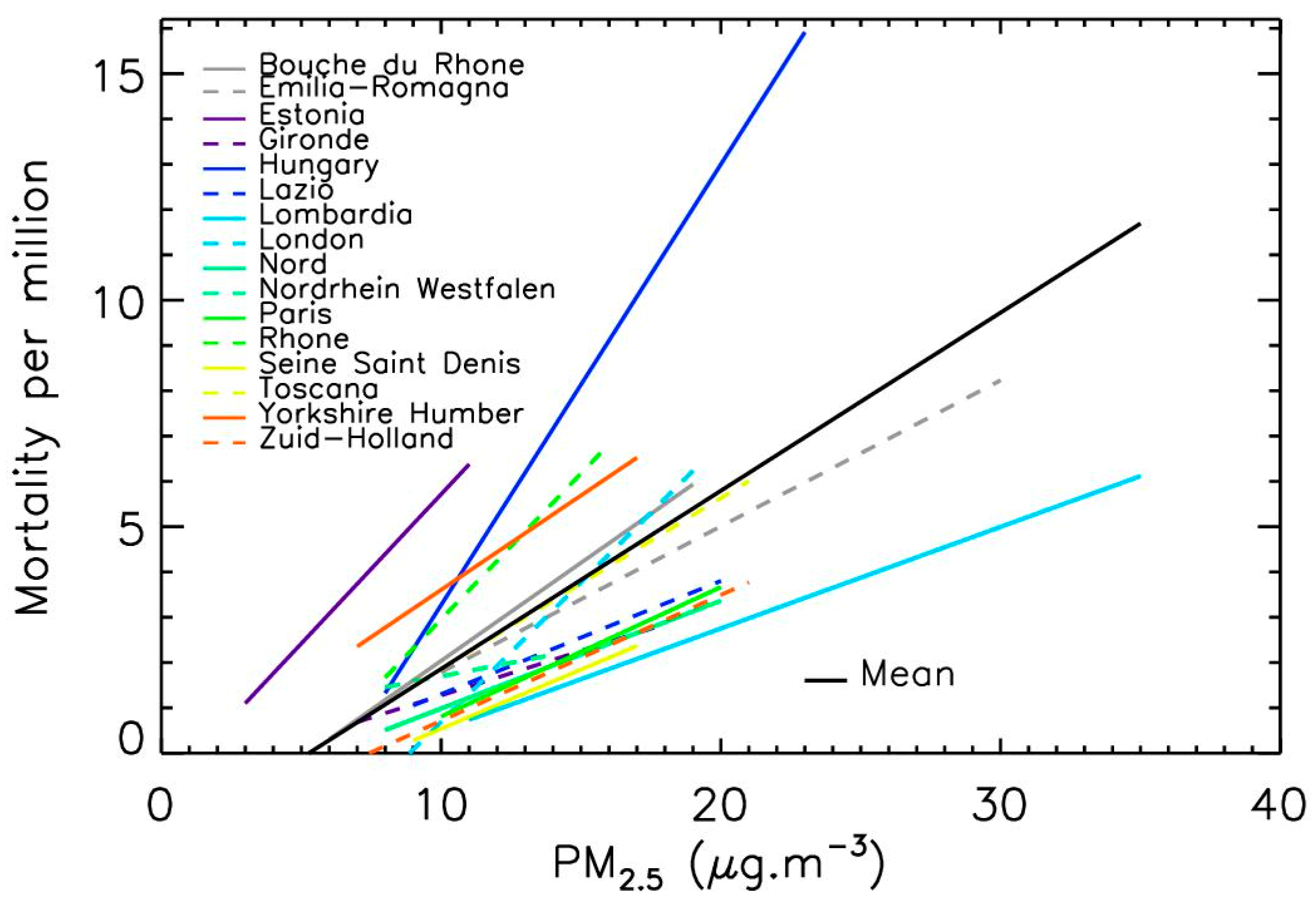
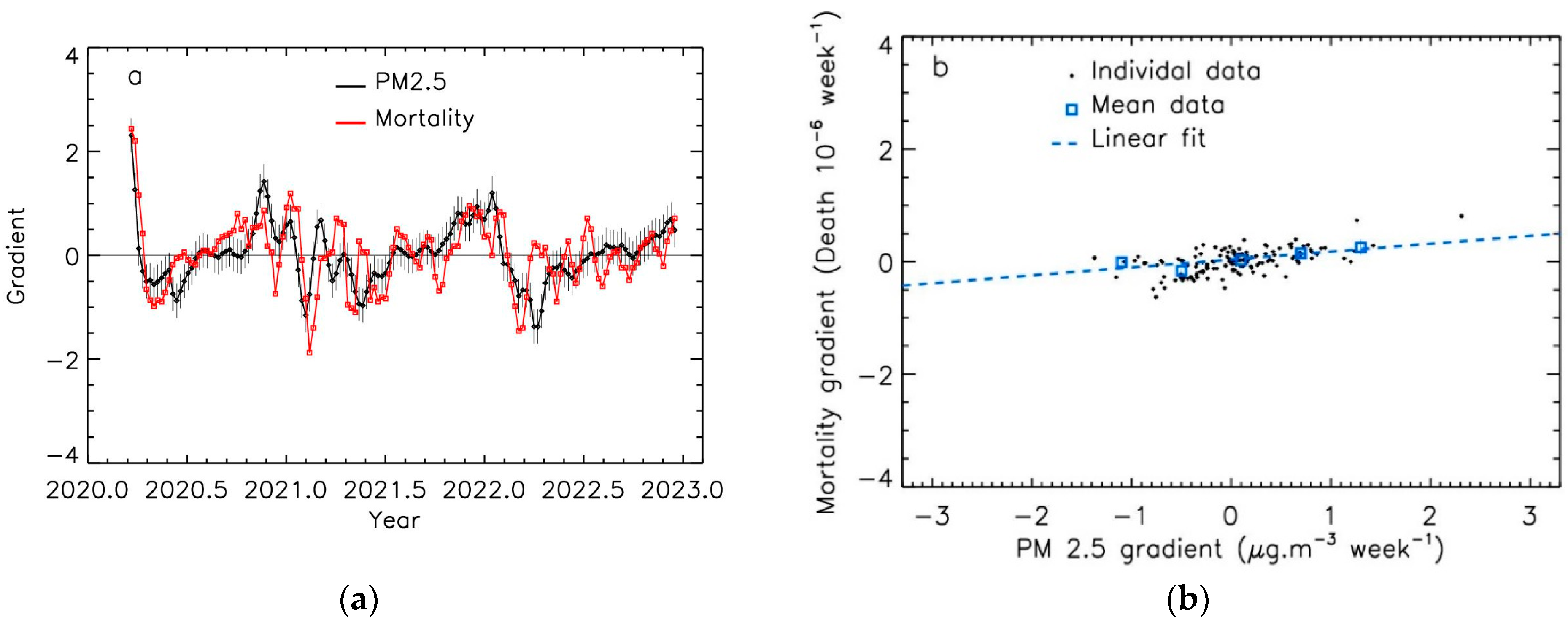
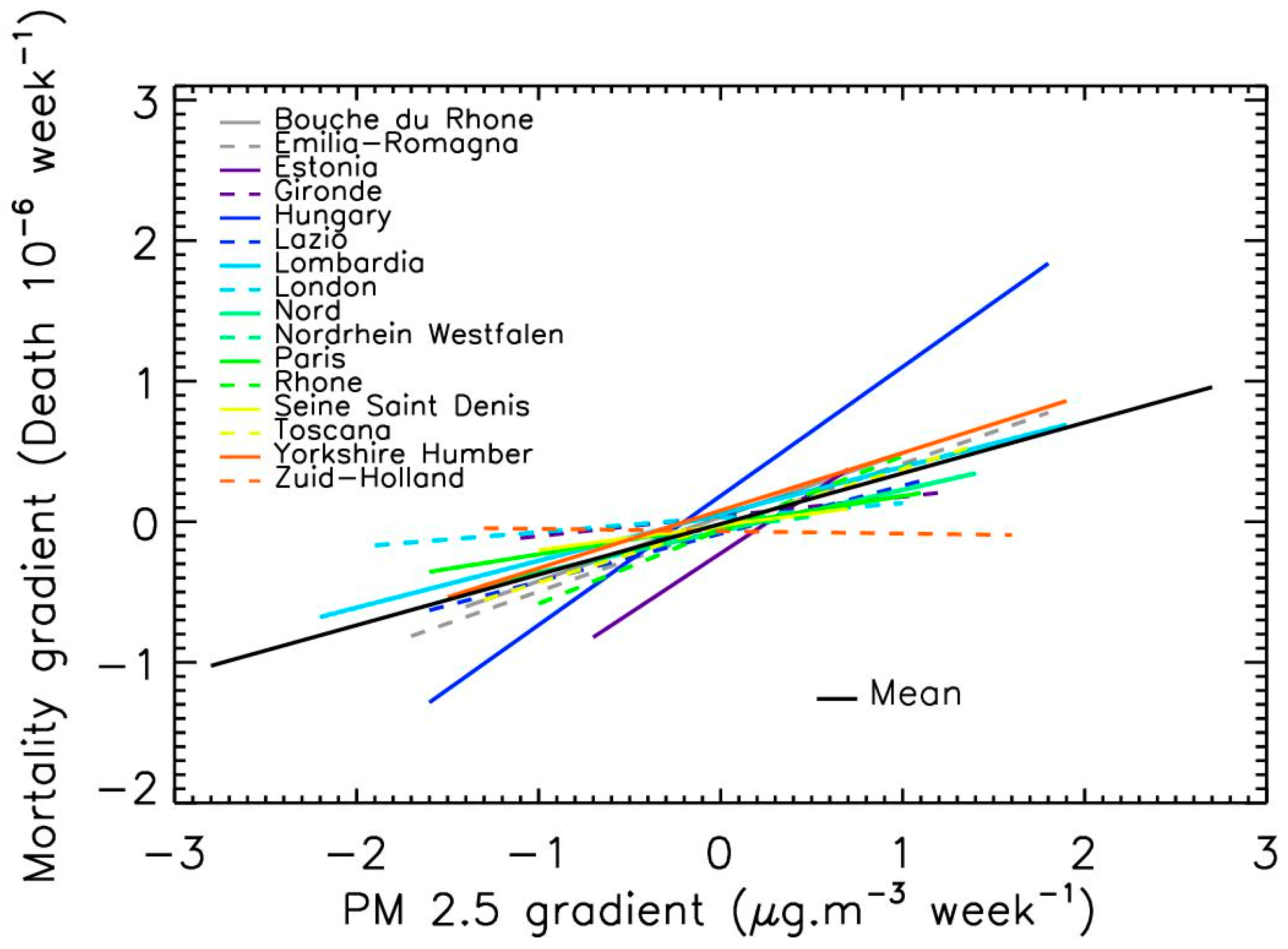
3.2. Linear Relation between PM2.5 Levels and COVID-19 Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seaton, A.; Godden, D.; MacNee, W.; Donaldson, K. Particulate air pollution and acute health effects. Lancet 1995, 345, 176–178. [Google Scholar]
- Beelen, R.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.; Nieuwenhuijsen, M.; et al. Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 2014, 383, 785–795. [Google Scholar] [PubMed]
- WHO (World Health Organization). Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; Report 2016; WHO Library Cataloguing-in-Publication Data; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.-H.; Mortimer, K.; Perez-Padilla, R.; Rice, R.; Riojas-Rodriguez, M.D.; Sood, H.; et al. Air Pollution and Noncommunicable Diseases A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. CHEST 2019, 155, 409–416. [Google Scholar]
- Thurston, G.D.; Kipen, H.; Annesi-Maesano, I.; Balmes, J.; Brook, R.D.; Cromar, K.; De Matteis, S.; Forastiere, F.; Forsberg, B.; Frampton, M.W.; et al. A joint ERS/ATS policy statement: What constitutes an adverse health effect of air pollution? An analytical framework. Eur. Respir. J. 2017, 49, 1600419. [Google Scholar] [PubMed]
- Horne, B.D.; Joy, E.A.; Hofmann, M.G.; Gesteland, P.H.; Cannon, J.B.; Lefler, J.S.; Blagev, D.P.; Korgenski, E.K.; Torosyan, N.; Hansen, G.I.; et al. Short-Term Elevation of Fine Particulate Matter Air Pollution and Acute Lower Respiratory Infection. Amer. J. Respir. Crit. Care Med. 2018, 198, 759–766. [Google Scholar]
- Robertson, S.; Miller, M.R. Ambient air pollution and thrombosis. Part. Fib. Toxicol. 2018, 15, 1. [Google Scholar]
- Miller, M.R. Oxidative stress and the cardiovascular effects of air pollution. Free Rad. Bio. Med. 2020, 151, 69–87. [Google Scholar]
- Glencross, D.A.; Ho, T.-R.; Camiña, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air pollution and its effects on the immune system. Free Rad. Biol. Med. 2020, 151, 56–68. [Google Scholar]
- Coccia, M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total. Environ. 2020, 729, 138474. [Google Scholar]
- Conticini, E.; Frediani, B.; Caro, D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pol. 2020, 261, 114465. [Google Scholar]
- Frontera, A.; Cianfanelli, L.; Vlachos, K.; Landoni, G.; Cremona, G. Severe air pollution links to higher mortality in COVID-19 patients: The “double-hit” hypothesis. J. Infect. 2020, 81, 255–259. [Google Scholar]
- Fronza, R.; Lusic, M.; Schmidt, M.; Lucic, B. Spatial–Temporal Variations in Atmospheric Factors Contribute to SARS-CoV-2 Outbreak. Viruses 2020, 12, 588. [Google Scholar]
- Accarino, G.; Lorenzetti, S.; Aloisio, G. Assessing correlations between short-term exposure to atmospheric pollutants and Covid-19 spread in all Italian territorial areas. Environ. Pollut. 2021, 268, 115714. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, M.; Flahault, A.; Stoffel, M. Peaks of fine particulate matter may modulate the spreading and virulence of COVID-19. Earth Syst. Environ. 2020, 4, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bherwani, H.; Gautam, S.; Anjum, S.; Musugu, K.; Kumar, N.; Anshul, A.; Kumar, R. Air pollution aggravating COVID-19 lethality? Exploration in Asian cities using statistical models. Environ. Develop. Sustain. 2021, 23, 6408–6417. [Google Scholar] [CrossRef]
- Sidell, M.A.; Chen, Z.; Huang, B.Z.; Chow, T.; Eckel, S.P.; Martinez, M.P.; Lurmann, F.; Thomas, D.C.; Gilliland, F.D.; Xiang, A.H. Ambient air pollution and COVID-19 incidence during four 2020–2021 case surges. Environ. Res. 2022, 208, 112758. [Google Scholar]
- Bourdrel, T.; Annesi-Maesano, I.; Alahmad, B.; Maesano, C.N.; Bind, M.-D. The impact of outdoor air pollution on COVID-19: A review of evidence from in vitro, animal, and human studies. Eur. Resp. Rev. 2021, 30, 200242. [Google Scholar]
- Mehmood, K.; Saifullah, I.M.; Abzar, M.M. Can exposure to PM2.5 particles increase the incidence of coronavirus disease 2019? Sc. Total Environ. 2020, 741, 140441. [Google Scholar] [CrossRef] [PubMed]
- Zoran, M.A.; Savastru, R.S.; Savastru, D.M.; Tautan, M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on Covid-19 in Milan, Italy. Sci. Total Environ. 2020, 738, 139825. [Google Scholar] [CrossRef]
- Annesi-Maesano, I.; Maesano, C.N.; Dessimond, B.; Prud’homme, J.; Colette, A.; Banerjee, S. Has the Spring 2020 lockdown modified the relationship between air pollution and Covid-19 mortality in Europe? Allergy 2020, 77, 1620–1622. [Google Scholar] [CrossRef]
- Coccia, M. How (un)sustainable Environments are Related to the Diffusion of COVID-19: The Relation between Coronavirus Disease 2019, Air Pollution, Wind Resource and Energy. Sustainability 2020, 12, 9709. [Google Scholar]
- Srivastava, A. COVID-19 and air pollution and meteorology-an intricate relationship: A review. Chemosphere 2021, 263, 128297. [Google Scholar]
- Yao, Y.; Pan, J.; Liu, Z.; Meng, X.; Wang, W.; Kan, H.; Wang, W. No association of Covid-19 transmission with temperature or UV radiation in Chinese cities. Eur. Respir. J. 2020, 55, 2000517. [Google Scholar] [CrossRef]
- McMullen, N.; Annesi-Maesano, I.; Renard, J.-B. Impact of rain precipitation on urban atmospheric particle matter measured at three locations in France between 2013 and 2019. Atmosphere 2021, 12, 769. [Google Scholar]
- Renard, J.-B.; Surcin, J.; Annesi-Maesano, I.; Delaunay, G.; Poincelet, E.; Dixsaut, G. Relation between PM2.5 pollution and COVID-19 mortality in Western Europe for the 2020–2022 period. Sci. Total Environ. 2022, 848, 157579. [Google Scholar]
- Wu, X.; Nethery, R.C.; Sabath, B.M.; Braun, D.; Dominici, F. Exposure to Air Pollution and COVID-19 Mortality in the United States. Sci. Adv. 2020, 6, eabd4049. [Google Scholar] [CrossRef] [PubMed]
- Travaglio, M.; Yu, Y.; Popovic, R.; Selley, L.; Leal, N.S.; Martins, L.M. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021, 268, 115859. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Ozgen, C.; Strobl, E. Air pollution exposure and COVID-19 in Dutch municipalities. Environ. Res. Econo. 2020, 76, 581–610. [Google Scholar] [CrossRef]
- Coker, E.S.; Cavalli, L.; Fabrizi, E.; Guastella, G.; Lippo, E.; Parisi, M.L.; Pontarollo, N.; Rizzati, M.; Varacca, A.; Vergalli, S. The Effects of Air Pollution on COVID-19 Related Mortality in Northern Italy. Environ. Resour. Econ. 2020, 76, 611–634. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, R.M.; Cicerelli, R.E.; de Almeida, T.; Requia, W.J. Air pollution and COVID-19 mortality in Brazil. Atmosphere 2022, 14, 5. [Google Scholar] [CrossRef]
- Zang, S.T.; Luan, J.; Li, L.; Yu, H.X.; Wu, Q.J.; Chang, Q.; Zhao, Y.H. Ambient air pollution and COVID-19 risk: Evidence from 35 observational studies. Environ. Res. 2022, 204 Pt B, 112065. [Google Scholar]
- Bossak, B.H.; Andritsch, S. COVID-19 and Air Pollution: A spatial analysis of particulate matter concentration and pandemic-associated mortality in the US. Int. J. Environ. Res. Public Health 2022, 19, 729. [Google Scholar]
- Semczuk-Kaczmarek, K.; Rys-Czaporowska, A.; Sierdzinski, J.; Kaczmarek, L.D.; Szymanski, F.M.; Platek, A.E. Association between air pollution and COVID-19 mortality and morbidity. Intern. Emerg. Med. 2022, 17, 467–473. [Google Scholar] [PubMed]
- Shao, L.; Cao, Y.; Jones, T.; Santosh, M.; Silva, L.F.O.; Ge, S.; da Boit, K.; Feng, X.; Zhang, M.; BéruBé, K. COVID-19 mortality and exposure to airborne PM2.5: A lag time correlation, Science of The Total Environment. Sci. Total Environ. 2022, 806, 151286. [Google Scholar]
- Renard, J.-B.; Marchand, C. High Resolution Mapping of PM2.5 Concentrations in Paris (France) Using Mobile Pollutrack Sensors Network in 2020. Atmosphere 2021, 12, 529. [Google Scholar] [CrossRef]
- Johns Hopkins University & Medicine, Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/ (accessed on 22 January 2023).
- Comunian, S.; Dongo, D.; Milani, C.; Palestini, P. Air Pollution and Covid-19: The Role of particulate matter in the spread and increase of COVID-19’s morbidity and mortality. Int. J. Environ. Res. Pub. Health 2020, 17, 4487. [Google Scholar]
- Renard, J.-B.; Dulac, F.; Durand, P.; Bourgeois, Q.; Denjean, C.; Vignelles, D.; Couté, B.; Jeannot, M.; Verdier, N.; Mallet, M. In situ measurements of desert dust particles above the western Mediterranean Sea with the balloon-borne Light Optical Aerosol Counter/sizer (LOAC) during the ChArMEx campaign of summer 2013. Atmos. Chem. Phys. 2018, 18, 3677–3699. [Google Scholar]
- Renard, J.-B.; Michoud, V.; Giacomoni, J. Vertical Profiles of Pollution Particle Concentrations in the Boundary Layer above Paris (France) from the Optical Aerosol Counter LOAC Onboard a Touristic Balloon. Sensors 2020, 20, 1111. [Google Scholar] [PubMed]
- Stolzenburg, M.R.; McMurry, P.H. Method to assess performance of scanning mobility particle sizer (SMPS) instruments and software. Aeros. Sci. Technol. 2018, 52, 609–613. [Google Scholar] [CrossRef]
- Coccia, M. Sources, diffusion and prediction in COVID-19 pandemic: Lessons learned to face next health emergency. AIMS Public Health 2023, 10, 145–168. [Google Scholar] [CrossRef]


| Location Name | Population (106 Inhabitants) | Source of PM2.5 Data | Mean PM2.5 Level | Starting Date |
|---|---|---|---|---|
| Bouche du Rhone (France) | 2.0 | Pollutrack | Medium | Mid-December 2020 |
| Emilia-Romagna (Italia) | 4.5 | Air quality network | High | Mid-April 2020 |
| Estonia | 1.3 | Air quality network | Low | Mid-December 2020 |
| Gironde (France) | 1.6 | Air quality network | Low | Mid-March 2020 |
| Hungary | 9.7 | Air quality network | Medium | Mid-April 2020 |
| Lazio (Italia) | 5.9 | Air quality network | Medium | Mid-December 2020 |
| Lombardy (Italia) | 10.1 | Air quality network | High | Mid-December 2020 |
| London (Great Britain) | 9.0 | Pollutrack | Medium | End May 2020 |
| Nord (France) | 2.6 | Pollutrack | Medium | Early January 2021 |
| Nordrhein-Westfalen (Germany) | 17.9 | Air quality network | Low | Mid-April 2020 |
| Paris (France) | 2.2 | Pollutrack | High | Mid-December 2020 |
| Rhone (France) | 1.9 | Air quality network | Medium | Mid-December 2020 |
| Seine Saint-Denis (France) | 1.7 | Air quality network | Medium | Mid-December 2020 |
| Toscana (Italia) | 3.7 | Air quality network | Medium | Mid-December 2020 |
| Yorkshire and the Humber (Great Britain) | 5.5 | Air quality network | Low | Early July 2020 |
| Zuid-Holland (The Netherlands) | 3.7 | Pollutrack | Medium | End July 2020 |
| Location Name (Country) | Integration Width (Weeks) | Shift (Weeks) | Correlation without Applying the Adjustments | Correlation When Applying the Adjustments |
|---|---|---|---|---|
| Bouche du Rhone (FR) | 7 | 2 | 0.56 | 0.82 |
| Emilia-Romagna (IT) | 9 | 0 | 0.52 | 0.76 |
| Estonia (EE) | 14 | 0 | 0.28 | 0.60 |
| Gironde (FR) | 11 | 0 | 0.36 | 0.81 |
| Hungary (HU) | 7 | 1 | 0.56 | 0.83 |
| Lazio (IT) | 9 | 2 | 0.37 | 0.79 |
| Lombardia (IT) | 9 | 0 | 0.47 | 0.78 |
| London (GB) | 8 | 0 | 0.25 | 0.45 |
| Nord (FR) | 14 | 1 | 0.41 | 0.78 |
| Nordrhein-Westfalen (DE) | 7 | 0 | 0.17 | 0.42 |
| Paris (FR) | 8 | 1 | 0.33 | 0.62 |
| Rhone (FR) | 7 | 1 | 0.40 | 0.73 |
| Seine Saint-Denis (FR) | 10 | 0 | 0.41 | 0.64 |
| Toscana (IT) | 8 | 3 | 0.53 | 0.80 |
| Yorkshire (GB) | 5 | 0 | 0.25 | 0.41 |
| Zuid-Holland (NL) | 8 | 0 | 0.29 | 0.51 |
| Integration Time for PM2.5 Mean Level (Week) | Shift between COVID-19 Mortality and PM2.5 Peaks (Week) | Rate of Mortality Increase per µg·m−3 of PM2.5 Increase |
|---|---|---|
| 8.8 ± 2.5 | 0.7 ± 0.8 | 40 ± 20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renard, J.-B.; Surcin, J.; Annesi-Maesano, I.; Poincelet, E. Temporal Evolution of PM2.5 Levels and COVID-19 Mortality in Europe for the 2020–2022 Period. Atmosphere 2023, 14, 1222. https://doi.org/10.3390/atmos14081222
Renard J-B, Surcin J, Annesi-Maesano I, Poincelet E. Temporal Evolution of PM2.5 Levels and COVID-19 Mortality in Europe for the 2020–2022 Period. Atmosphere. 2023; 14(8):1222. https://doi.org/10.3390/atmos14081222
Chicago/Turabian StyleRenard, Jean-Baptiste, Jérémy Surcin, Isabella Annesi-Maesano, and Eric Poincelet. 2023. "Temporal Evolution of PM2.5 Levels and COVID-19 Mortality in Europe for the 2020–2022 Period" Atmosphere 14, no. 8: 1222. https://doi.org/10.3390/atmos14081222
APA StyleRenard, J.-B., Surcin, J., Annesi-Maesano, I., & Poincelet, E. (2023). Temporal Evolution of PM2.5 Levels and COVID-19 Mortality in Europe for the 2020–2022 Period. Atmosphere, 14(8), 1222. https://doi.org/10.3390/atmos14081222







