The Air and Viruses We Breathe: Assessing the Effect the PM2.5 Air Pollutant Has on the Burden of COVID-19
Abstract
1. Introduction
2. Materials & Methods
2.1. Scenario Design
2.2. Model Framework
2.3. Model Parameterisation and Initialisation
2.4. Variants
2.5. Seasonality
2.6. Non-Pharmaceutical Interventions
2.7. Vaccinations
2.8. Treatment
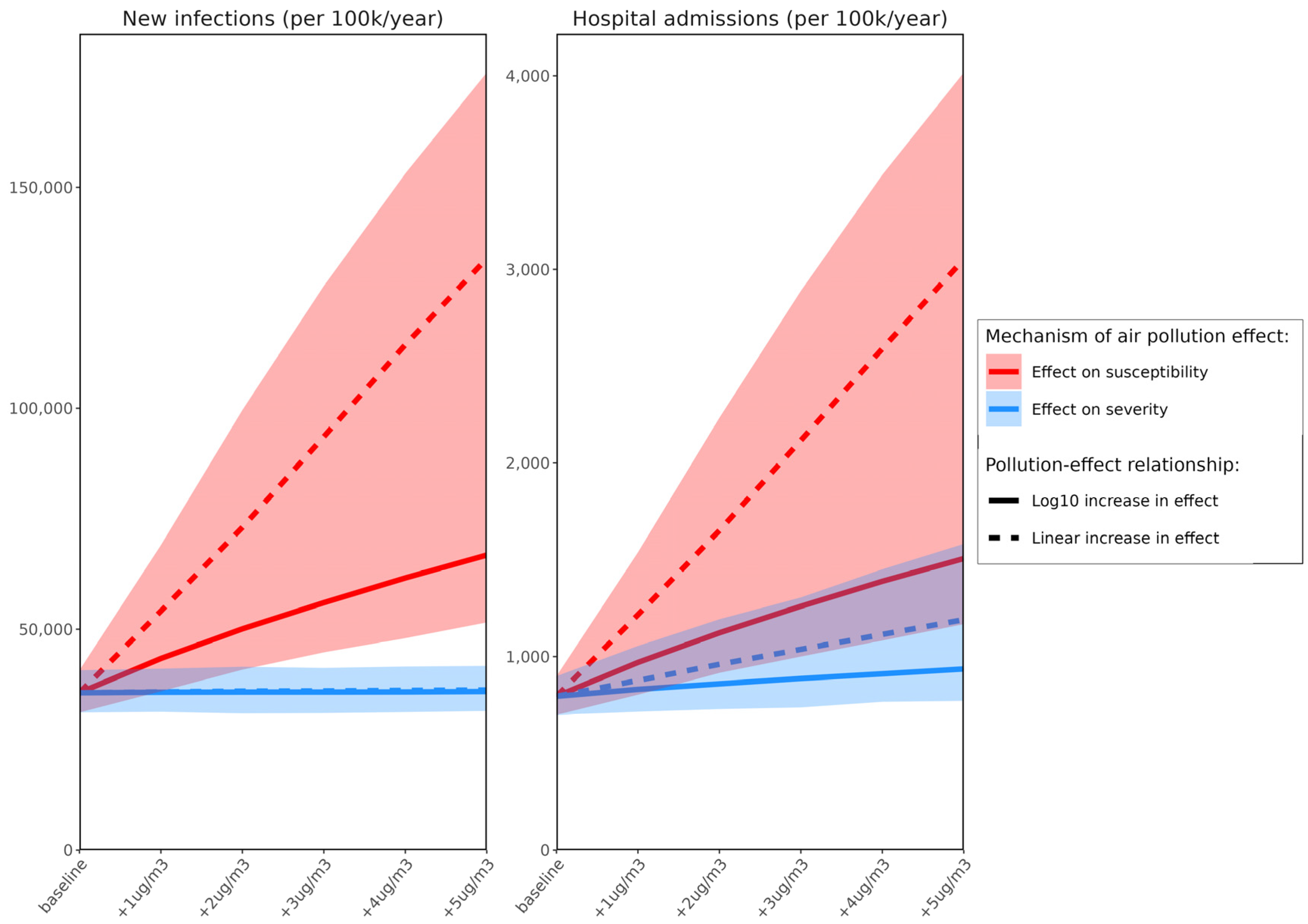
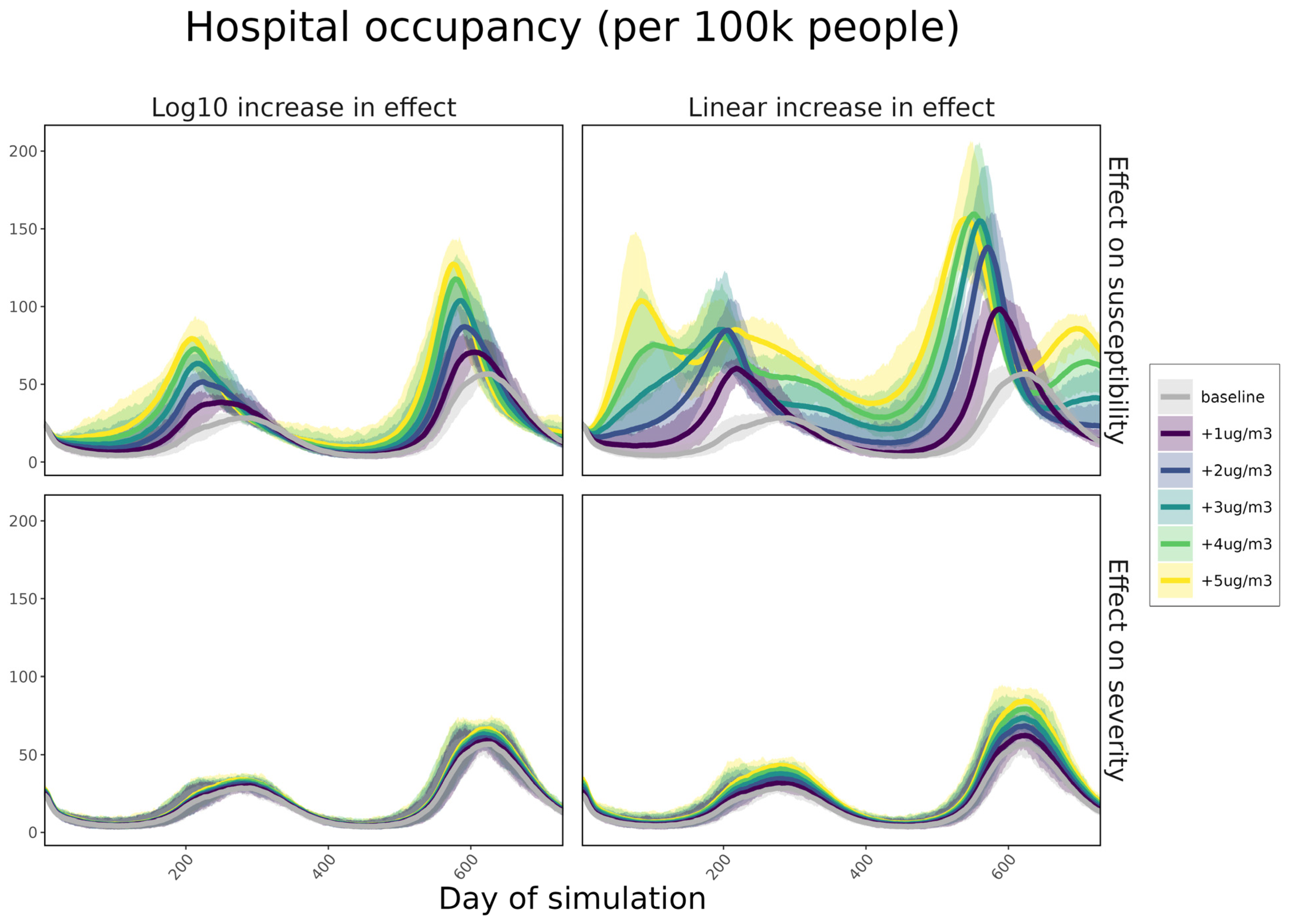
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Bank. What You Need to Know About Climate Change and Air Pollution; World Bank: Washington, DC, USA, 2022; Available online: https://www.worldbank.org/en/news/feature/2022/09/01/what-you-need-to-know-about-climate-change-and-air-pollution (accessed on 15 March 2023).
- WHO. Air Pollution; WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/health-topics/air-pollution#tab=tab_2 (accessed on 15 March 2023).
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A.; et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Chang. 2022, 12, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Conticini, E.; Frediani, B.; Caro, D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Env. Pollut. 2020, 261, 114465. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Marsland, A.L.; Kinnee, E.J.; Tunno, B.J.; Manuck, S.B.; Gianaros, P.J.; Clougherty, J.E. Long-Term Ambient Air Pollution Exposures and Circulating and Stimulated Inflammatory Mediators in a Cohort of Midlife Adults. Env. Health Perspect. 2021, 129, 57007. [Google Scholar] [CrossRef] [PubMed]
- Woodby, B.; Arnold, M.M.; Valacchi, G. SARS-CoV-2 infection, COVID-19 pathogenesis, and exposure to air pollution: What is the connection? Ann. N. Y. Acad. Sci. 2021, 1486, 15–38. [Google Scholar] [CrossRef]
- Pozzer, A.; Dominici, F.; Haines, A.; Witt, C.; Münzel, T.; Lelieveld, J. Regional and global contributions of air pollution to risk of death from COVID-19. Cardiovasc. Res. 2020, 116, 2247–2253. [Google Scholar] [CrossRef]
- WHO. Coronavirus Dashboard; WHO: Geneva, Switzerland, 2023; Available online: https://covid19.who.int/?mapFilter=deaths (accessed on 15 March 2023).
- Abel, D.W.; Holloway, T.; Harkey, M.; Meier, P.; Ahl, D.; Limaye, V.S.; Patz, J.A. Air-quality-related health impacts from climate change and from adaptation of cooling demand for buildings in the eastern United States: An interdisciplinary modeling study. PLoS Med. 2018, 15, e1002599. [Google Scholar] [CrossRef]
- Veronesi, G.; De Matteis, S.; Calori, G.; Pepe, N.; Ferrario, M.M. Long-term exposure to air pollution and COVID-19 incidence: A prospective study of residents in the city of Varese, Northern Italy. Occup. Env. Med. 2022, 79, 192–199. [Google Scholar] [CrossRef]
- De Angelis, E.; Renzetti, S.; Volta, M.; Donato, F.; Calza, S.; Placidi, D.; Lucchini, R.G.; Rota, M. COVID-19 incidence and mortality in Lombardy, Italy: An ecological study on the role of air pollution, meteorological factors, demographic and socioeconomic variables. Env. Res. 2021, 195, 110777. [Google Scholar] [CrossRef]
- Travaglio, M.; Yu, Y.; Popovic, R.; Selley, L.; Leal, N.S.; Martins, L.M. Links between air pollution and COVID-19 in England. Env. Pollut. 2021, 268, 115859. [Google Scholar] [CrossRef]
- Kogevinas, M.; Castaño-Vinyals, G.; Karachaliou, M.; Espinosa, A.; de Cid, R.; Garcia-Aymerich, J.; Carreras, A.; Cortés, B.; Pleguezuelos, V.; Jiménez, A.; et al. Ambient Air Pollution in Relation to SARS-CoV-2 Infection, Antibody Response, and COVID-19 Disease: A Cohort Study in Catalonia, Spain (COVICAT Study). Env. Health Perspect. 2021, 129, 117003. [Google Scholar] [CrossRef]
- Alsaber, A.R.; Setiya, P.; Al-Sultan, A.T.; Pan, J. Exploring the impact of air pollution on COVID-19 admitted cases. Jpn. J. Stat. Data Sci. 2022, 5, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Nethery, R.C.; Sabath, M.B.; Braun, D.; Dominici, F. Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci. Adv. 2020, 6, eabd4049. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Cao, Y.; Jones, T.; Santosh, M.; Silva, L.F.; Ge, S.; da Boit, K.; Feng, X.; Zhang, M.; Bérubé, K. COVID-19 mortality and exposure to airborne PM2.5: A lag time correlation. Sci. Total. Environ. 2022, 806, 151286. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, Z.-F.; Froines, J.; Zhao, J.; Wang, H.; Yu, S.-Z.; Detels, R. Air pollution and case fatality of SARS in the People’s Republic of China: An ecologic study. Env. Health 2003, 2, 15. [Google Scholar] [CrossRef]
- Shattock, A.J.; Le Rutte, E.A.; Dünner, R.P.; Sen, S.; Kelly, S.L.; Chitnis, N.; Penny, M.A. Impact of vaccination and non-pharmaceutical interventions on SARS-CoV-2 dynamics in Switzerland. Epidemics 2022, 38, 100535. [Google Scholar] [CrossRef]
- Le Rutte, E.A.; Shattock, A.J.; Chitnis, N.; Kelly, S.L.; Penny, M.A. Modelling the impact of Omicron and emerging variants on SARS-CoV-2 transmission and public health burden. Commun. Med. 2022, 2, 93. [Google Scholar] [CrossRef]
- Kelly, S.L.; Le Rutte, E.A.; Richter, M.; Penny, M.A.; Shattock, A.J. COVID-19 Vaccine Booster Strategies in Light of Emerging Viral Variants: Frequency, Timing, and Target Groups. Infect. Dis. Ther. 2022, 11, 2045–2061. [Google Scholar] [CrossRef]
- World Population Prospects. Population; UN DESA Population Division: Geneva, Switzerland, 2022; Available online: https://population.un.org/wpp/Download/Standard/Population/ (accessed on 15 March 2023).
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P.A. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Env. Res. 2022, 209, 112911. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Assessment of the Further Emergence and Potential Impact of the SARS-Cov-2 Omicron Variant of Concern in the Context of The Ongoing Delta VOC Transmission in the EU/EEA, 18th Update; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-assessment-further-emergence-omicron-18th-risk-assessment (accessed on 15 March 2023).
- Hemmer, C.J.; Löbermann, M.; Reisinger, E.C. COVID-19: Epidemiology and mutations: An update. Radiologe. 2021, 61, 880–887. [Google Scholar] [CrossRef]
- van Elsland, S.L. People with Omicron Variant Less Likely Hospitalised Compared to Delta Variant; Imperial College London: London, UK, 2022; Available online: https://www.imperial.ac.uk/news/234736/people-with-omicron-variant-less-likely/ (accessed on 15 March 2023).
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Fisman, D.N.; Tuite, A.R. Evaluation of the relative virulence of novel SARS-CoV-2 variants: A retrospective cohort study in Ontario, Canada. CMAJ 2021, 193, E1619–E1625. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Steain, M.; Triccas, J.A.; Sigal, A.; Davenport, M.P.; Cromer, D. Analysis: A meta-analysis of Early Results to predict Vaccine efficacy against Omicron. Medrxiv 2021. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Del Cura-Bilbao, A.; López-Mendoza, H.; Chaure-Pardos, A.; Vergara-Ugarriza, A.; Guimbao-Bescós, J. Effectiveness of 3 COVID-19 Vaccines in Preventing SARS-CoV-2 Infections, January–May 2021, Aragon, Spain. Emerg. Infect. Dis. 2022, 28, 591–598. [Google Scholar] [CrossRef]
- Chivese, T.; Matizanadzo, J.T.; Musa, O.A.H.; Hindy, G.; Furuya-Kanamori, L.; Islam, N.; Al-Shebly, R.; Shalaby, R.; Habibullah, M.; Al-Marwani, T.A.; et al. The prevalence of adaptive immunity to COVID-19 and reinfection after recovery–a comprehensive systematic review and meta-analysis. Pathog. Glob. Health 2022, 116, 269–281. [Google Scholar] [CrossRef]
- CDC. Science Brief: SARS-CoV-2 Infection-Induced and Vaccine-Induced Immunity; CDC: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html (accessed on 15 March 2023).
- Usher, A.D. The global COVID-19 treatment divide. Lancet 2022, 399, 779–782. [Google Scholar] [CrossRef]
- Liu, J.; Pan, X.; Zhang, S.; Li, M.; Ma, K.; Fan, C.; Lv, Y.; Guan, X.; Yang, Y.; Ye, X.; et al. Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: A multicenter randomized controlled study. Lancet Reg. Health West. Pac. 2023, 33, 100694. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Feng, Z.; Song, Y. Influenza incidence and air pollution: Findings from a four-year surveillance study of prefecture-level cities in China. Public Health Front. 2022, 10, 1071229. [Google Scholar] [CrossRef]
- Domingo, J.L.; Marquès, M.; Rovira, J. Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. A review. Env. Res. 2020, 188, 109861. [Google Scholar] [CrossRef]
- Villeneuve, P.J.; Goldberg, M.S. Ecological studies of COVID-19 and air pollution: How useful are they? Env. Epidemiol. 2022, 6, e195. [Google Scholar] [CrossRef]
- Feng, B.; Wang, W.; Zhou, B.; Zhou, Y.; Wang, J.; Liao, F. Mapping the long-term associations between air pollutants and COVID-19 risks and the attributable burdens in the continental United States. Env. Pollut. 2023, 324, 121418. [Google Scholar] [CrossRef] [PubMed]
- Nottmeyer, L.; Armstrong, B.; Lowe, R.; Abbott, S.; Meakin, S.; O’Reilly, K.M.; von Borries, R.; Schneider, R.; Royé, D.; Hashizume, M.; et al. The association of COVID-19 incidence with temperature, humidity, and UV radiation–A global multi-city analysis. Sci. Total. Environ. 2022, 854, 158636. [Google Scholar] [CrossRef] [PubMed]
- Sera, F.; Armstrong, B.; Abbott, S.; Meakin, S.; O’Reilly, K.; von Borries, R.; Schneider, R.; Royé, D.; Hashizume, M.; Pascal, M.; et al. A cross-sectional analysis of meteorological factors and SARS-CoV-2 transmission in 409 cities across 26 countries. Nat. Commun. 2021, 12, 5968. [Google Scholar] [CrossRef] [PubMed]
- WHO. COP26 Special Report on Climate Change and Health: The Health Argument for Climate Action; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240036727 (accessed on 15 March 2023).
- Kirwa, K.; Eckert, C.M.; Vedal, S.; Hajat, A.; Kaufman, J.D. Ambient air pollution and risk of respiratory infection among adults: Evidence from the multiethnic study of atherosclerosis (MESA). BMJ Open Resp. Res. 2021, 8, e000866. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, S.L.; Shattock, A.J.; Ragettli, M.S.; Vienneau, D.; Vicedo-Cabrera, A.M.; de Hoogh, K. The Air and Viruses We Breathe: Assessing the Effect the PM2.5 Air Pollutant Has on the Burden of COVID-19. Atmosphere 2023, 14, 887. https://doi.org/10.3390/atmos14050887
Kelly SL, Shattock AJ, Ragettli MS, Vienneau D, Vicedo-Cabrera AM, de Hoogh K. The Air and Viruses We Breathe: Assessing the Effect the PM2.5 Air Pollutant Has on the Burden of COVID-19. Atmosphere. 2023; 14(5):887. https://doi.org/10.3390/atmos14050887
Chicago/Turabian StyleKelly, Sherrie L., Andrew J. Shattock, Martina S. Ragettli, Danielle Vienneau, Ana M. Vicedo-Cabrera, and Kees de Hoogh. 2023. "The Air and Viruses We Breathe: Assessing the Effect the PM2.5 Air Pollutant Has on the Burden of COVID-19" Atmosphere 14, no. 5: 887. https://doi.org/10.3390/atmos14050887
APA StyleKelly, S. L., Shattock, A. J., Ragettli, M. S., Vienneau, D., Vicedo-Cabrera, A. M., & de Hoogh, K. (2023). The Air and Viruses We Breathe: Assessing the Effect the PM2.5 Air Pollutant Has on the Burden of COVID-19. Atmosphere, 14(5), 887. https://doi.org/10.3390/atmos14050887







