Phenological Response of Flood Plain Forest Ecosystem Species to Climate Change during 1961–2021
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phenological Data
2.2. Meteorological Data
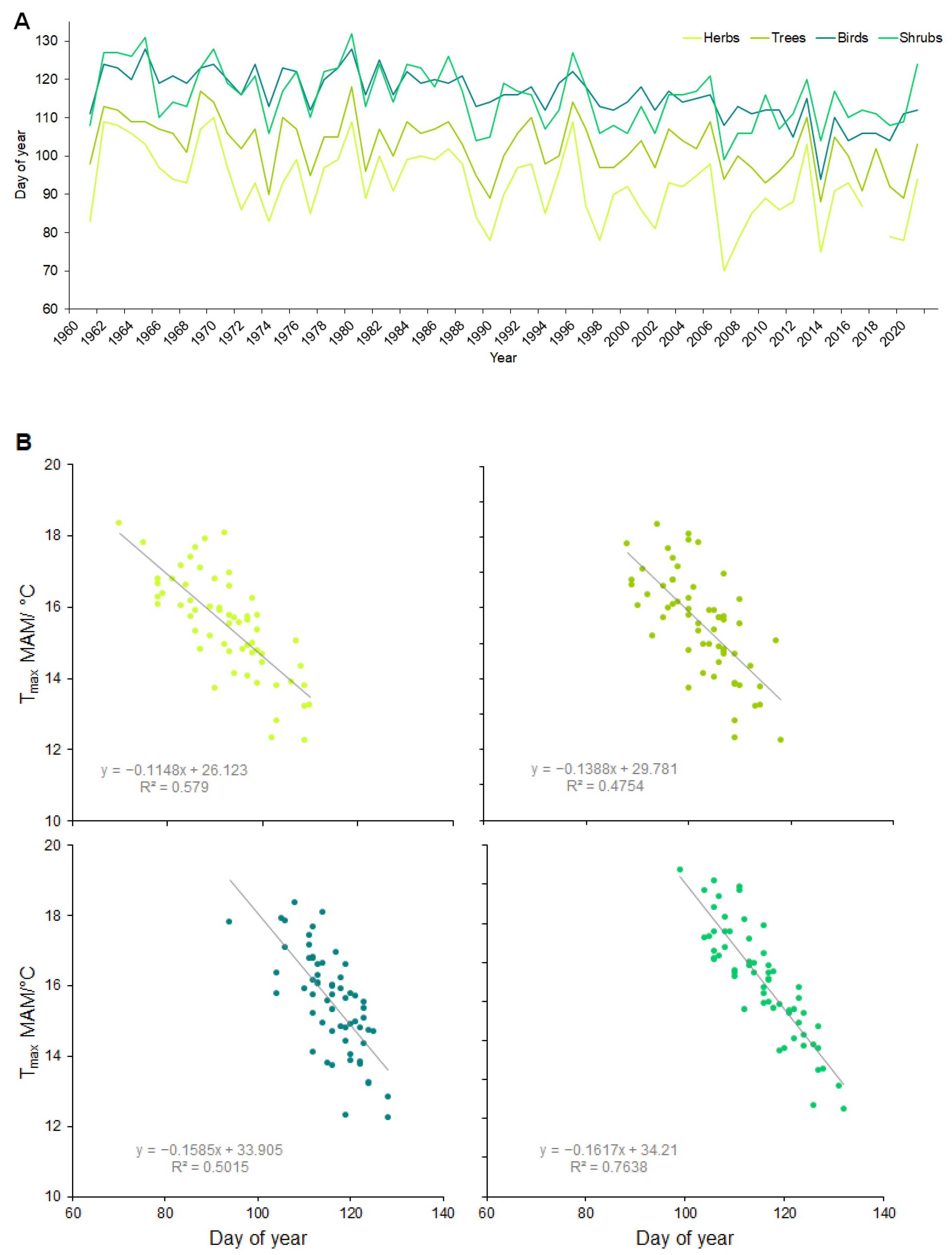
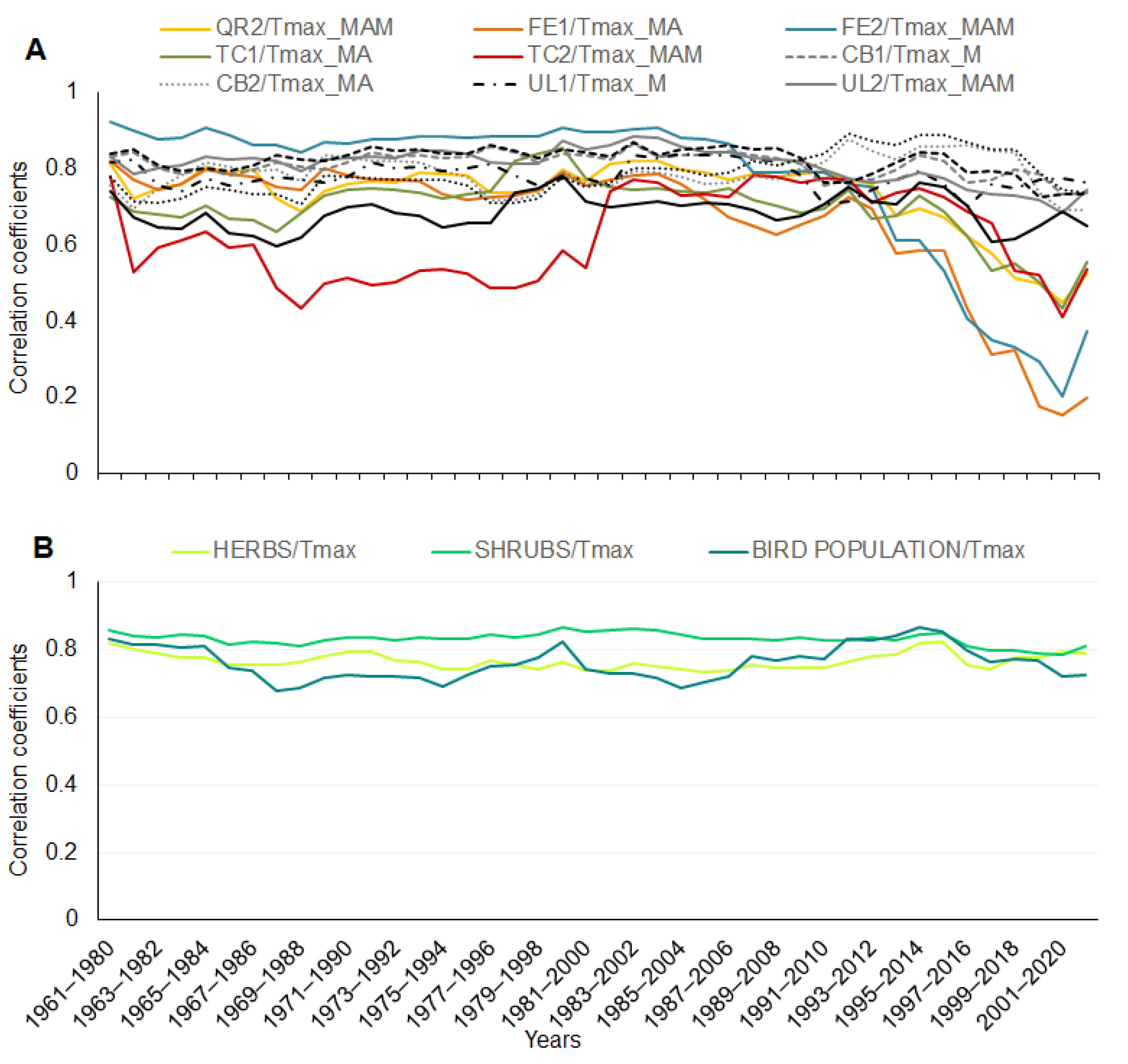
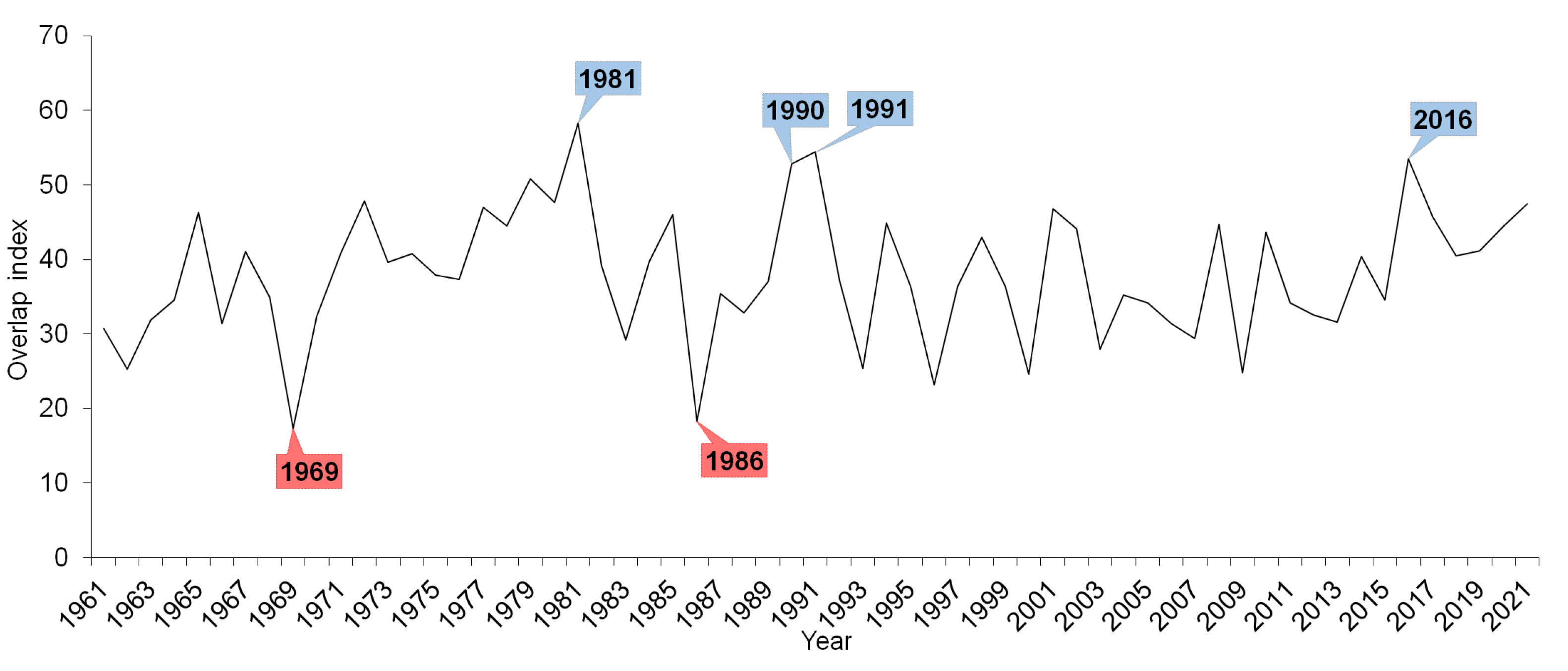
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Beard, K.H.; Kelsey, K.C.; Leffler, A.J.; Welker, J.M. The Missing Angle: Ecosystem Consequences of Phenological Mismatch. Trends Ecol. Evol. 2019, 34, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Büntgen, U.; Piermattei, A.; Krusic, P.J.; Esper, J.; Sparks, T.; Crivellaro, A. Plants in the UK flower a month earlier under recent warming. Proc. R. Soc. B 2022, 289, 20212456. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Yuan, Y.; Matiu, M.; Sparks, T.; Scheifinger, H.; Gehrig, R.; Estrella, N. Climate change fingerprints in recent European plant phenology. Glob Chang. Biol. 2020, 26, 2599–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Chang. Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cook, B.I.; Allen, J.M.; Crimmins, T.M.; Betancourt, J.L.; Travers, S.E.; Pau, S.; Regetz, J.; Davies, T.J.; Kraft, N.J.B.; et al. Warming experiments underpredict plant phenological responses to climate change. Nature 2012, 485, 494–497. [Google Scholar] [CrossRef]
- Cohen, J.M.; Lajeunesse, M.J.; Rohr, J.R. A global synthesis of animal phenological responses to climate change. Nat. Clim. Chang. 2018, 8, 224–228. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Henrys, P.A.; Hemming, D.; Bell, J.R.; Botham, M.S.; Burthe, S.; Helaouët, P.; Johns, D.G.; Jones, I.D.; Leech, D.I.; et al. Phenological sensitivity to climate across taxa and trophic levels. Nature 2016, 535, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Both, C.; Van Asch, M.; Bijlsma, R.G.; Van Den Burg, A.B.; Visser, M.E. Climate change and unequal phenological changes across four trophic levels: Constraints or adaptations? J. Anim. Ecol. 2009, 78, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Reed, T.E.; Jenouvrier, S.; Visser, M.E. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J. Anim. Ecol. 2012, 82, 131–144. [Google Scholar] [CrossRef]
- Wu, C.; Houb, X.; Penga, D.; Gonsamoc, A.; Xua, S. Land surface phenology of China’s temperate ecosystems over 1999–2013: Spatial–temporal patterns, interaction effects, covariation with climate and implications for productivity. Agric. For. Meteorol. 2016, 216, 177–187. [Google Scholar] [CrossRef]
- Meng, F.; Cui, S.; Wang, S.; Duan, J.; Jiang, L.; Zhang, Z.; Luo, C.; Wang, Q.; Zhou, Y.; Li, X.; et al. Changes in phenological sequences of alpine communities across a natural elevation gradient. Agric. For. Meteorol. 2016, 224, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.E.; Both, C.; Lambrechts, M.M. Global climate change leads to mistimed avian reproduction. Adv. Ecol. Res. 2004, 35, 89–110. [Google Scholar]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Bauer, Z.; Bartosova, L.; Bauerova, J.; Trnka, M.; Stepanek, P.; Mozny, M.; Dubrovsky, M.; Nyklova, E.; Zalud, Z. Observed Phenological Response of Ecosystems to the Climate: Part I. Flood-Plain Forest; Global Change Research Centre, Academy of Sciences of the Czech Republic: Staré Město, Czech Republic, 2014; ISBN 978-80-87902-00-4. [Google Scholar]
- Durant, J.M.; Hjermann, D.; Ottersen, G.; Stenseth, N.C. Climate and the match or mismatch between predator requirements and resource availability. Clim. Res. 2007, 33, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.; Both, C. Shifts in phenology due to global climate change: The need for a yardstick. Proc. R. Soc. B 2005, 272, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, S.J.; Sparks, T.H.; Frederiksen, M.; Burthe, S.; Bacon, P.J.; Bell, J.R.; Botham, M.S.; Brereton, T.M.; Bright, P.W.; Carvalho, L.; et al. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob. Chang. Biol. 2010, 16, 3304–3313. [Google Scholar] [CrossRef] [Green Version]
- Stenseth, N.C.; Mysterud, A. Climate, changing phenology, and other life history traits: Nonlinearity and match-mismatch to the environment. Proc. Natl. Acad. Sci. USA 2002, 99, 13379–13381. [Google Scholar] [CrossRef] [Green Version]
- Walther, R.G. Community and ecosystem responses to recent climate change. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2019–2024. [Google Scholar] [CrossRef]
- Valter, J.; Reitschläger, J.D. Operation Guideline for Phenological Stations, Methodic Instruction No. 10, Wood Plants, 2nd ed.; Czech Hydrometeorological Institute: Prague, Czech Republic, 2009. [Google Scholar]
- Štěpánek, P.; Zahradníček, P.; Brázdil, R.; Tolasz, R. Metodologie Kontroly a Homogenizace Časových řad v Klimatologii (Methodology of Data Quality Kontrol and Homogenization of Time Series in Climatology); ČHMÚ: Prague, Czech Republic, 2011; p. 118. ISBN 978-80-86690-97-1. [Google Scholar]
- Štěpánek, P.; Zahradníček, P.; Huth, R. Interpolation Techniques Used for Data Quality Control and Calculation of Technical Series: An Example of Central European Daily Time Series. Időjárás 2011, 115, 87–98. [Google Scholar]
- Štěpánek, P.; Zahradníček, P.; Farda, A. Experiences with data quality control and homogenization of daily records of various meteorological elements in the Czech Republic in the period 1961–2010. Időjárás 2013, 117, 123–141. [Google Scholar]
- Squintu, A.A.; Schrier, G.; Štěpánek, P.; Zahradníček, P.; Klein Tank, A. Comparison of homogenization methods for daily temperature series against an observation-based benchmark dataset. Arch. Meteorol. Geophys. Bioclimatol. Ser. B 2020, 140, 285–301. [Google Scholar] [CrossRef] [Green Version]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 1 April 2022).
- Stepanek, P. An Clim—Software for Time Series Analysis. Department of Geography, Faculty of Natural Sciences, MU, Brno, 1.47 MB. 2008. Available online: http://www.climahom.eu/AnClim.html (accessed on 1 April 2022).
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Ladányi, M.; Persely, S.; Szabó, T.; Soltész, M.; Nyéki, J.; Szabó, Z. The application of A HEAT SUM MODEL for the budburst of sour cherry varieties grown at Újfehértó. Int. J. Hortic. Sci. 2009, 15, 105–112. [Google Scholar] [CrossRef]
- Renner, S.S.; Zohner, C.M. Climate Change and Phenological Mismatch in Trophic Interactions among Plants, Insects, and Vertebrates. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 165–182. [Google Scholar] [CrossRef]
- Kowalska, N.; Šigut, L.; Stojanović, M.; Fischer, M.; Kyselova, I.; Pavelka, M. Analysis of floodplain forest sensitivity to drought. Phil. Trans. R. Soc. B 2020, 375, 20190518. [Google Scholar] [CrossRef]
- Cook, B.I.; Wolkovich, E.M.; Davies, T.J.; Ault, T.R.; Betancourt, J.L.; Allen, J.; Bolmgren, K.; Cleland, E.E.; Crimmins, T.M.; Kraft, N.J.B.; et al. Sensitivity of spring phenology to warming across temporal and spatial climate gradients in two independent databases. Ecosystems 2012, 15, 1283–1294. [Google Scholar] [CrossRef]
- Tang, J.; Körner, C.; Muraoka, H.; Piao, S.; Shen, M.; Thackeray, S.J.; Yang, X.I. Emerging opportunities and challenges in phenology: A review. Ecosphere 2016, 7, e01436. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Huang, J.G.; Ma, Q.; Hänninen, H.; Tremblay, F.; Bergeron, Y. Long-term changes in the impacts of global warming on leaf phenology of four temperate tree species. Glob. Chang. Biol. 2019, 25, 997–1004. [Google Scholar] [CrossRef]
- Fu, Y.; Piao, S.; Vitasse, Y.; Zhao, H.; De Boeck, H.J.; Liu, Q.; Yang, H.; Weber, U.; Hänninen, H.; Janssens, I. Increased heat requirement for leaf flushing in temperate woody species over 1980–2012: Effects of chilling, precipitation and insolation. Glob. Chang. Biol. 2015, 21, 2687–2697. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, H.; Piao, S.; Peaucelle, M.; Peng, S.; Zhou, G.; Ciais, P.; Huang, M.; Menzel, A.; Penuelas, J.; et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature 2015, 526, 104–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.T.; Inouye, D.W.; McKinney, A.M.; Colautti, R.I.; Mitchell-Olds, T. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc. R. Soc. B 2012, 279, 3843–3852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalupa, J. Geobiocenologické Podklady Pro Návrh Péče O Přírodní Rezervaci Plačkův Les a říčKa ŠAtava. Bachelor’s Thesis, Mendel University in Brno, Brno, Czech Republic, 2007; 46p. [Google Scholar]
- Horák, J. Jihomoravské Lužní Lesy (Flood Plain Forests of South MORAVIA); Typological Study; Mendel University in Brno: Brno, Czech Republic, 1961; 247p. [Google Scholar]
- Brázdil, R.; Zahradnícek, P.; Dobrovolný, P.; Štěpánek, P.; Trnka, M. Observed changes in precipitation during recent warming: The Czech Republic, 1961–2019. Int. J. Climatol. 2021, 41, 3881–3902. [Google Scholar] [CrossRef]
- Brázdil, R.; Dobrovolný, P.; Mikšovský, J.; Pišoft, P.; Trnka, M.; Možný, M.; Balek, J. Documentary-based climate reconstructions in the Czech Lands 1501–2020 CE and their European context. Clim. Past 2022, 18, 935–959. [Google Scholar] [CrossRef]
- Rims, R.A.; Henden, J.A.; Killengreen, S.T. Collapsing population cycles. Trends Ecol. Evol. 2008, 23, 79–86. [Google Scholar]
- Peňuelas, J.; Rico, L.; Ogaya, R.; Jump, A.S.; Terradas, J. Summer season and long-term drought increase the richness of bacteria and fungi in the foliar phyllosphere of Quercus ilex in a mixed Mediterranean forest. Plant Biol. 2012, 14, 565–575. [Google Scholar] [CrossRef]
- Reneerkens, J.; Schmidt, N.M.; Gilg, O.; Hansen, J.; Hansen, L.H.; Moreau, J.; Piersma, T. Effects of food abundance and early clutch predation on reproductive timing in a high Arctic shorebird exposed to advancements in arthropod abundance. Ecol. Evol. 2016, 6, 7375–7386. [Google Scholar] [CrossRef]
- Mayor, S.J.; Guralnick, R.P.; Tingley, M.W.; Otegui, J.; Withey, J.C.; Elmendorf, S.; Andrew, M.; Leyk, S.; Pearse, I.S.; Schneider, D.C. Increasing phenological asynchrony between spring green-up and arrival of migratory birds. Sci. Rep. 2017, 7, 1902. [Google Scholar] [CrossRef] [Green Version]
- Ross, M.V.; Alisauskas, E.T.; Douglas, D.C.; Kellett, D.K. Decadal declines in avian herbivore reproduction: Density-dependent nutrition and phenological mismatch in the Arctic. Ecology 2017, 98, 1869–1883. [Google Scholar] [CrossRef]
- Illán, J.G.; Gutierrez, D.; Diez, S.B.; Wilson, R.J. Elevational trends in butterfly phenology: Implications for species responses to climate change. Ecol. Entomol. 2012, 37, 134–144. [Google Scholar] [CrossRef]
- Hansen, B.B.; Grøtan, V.; Aanes, R.; Sæther, B.E.; Stien, A.; Fuglei, E.; Ims, R.A.; Yoccoz, N.G.; Pedersen, Å.Ø. Climate events synchronize the dynamics of a resident vertebrate community in the high Arctic. Science 2013, 339, 313–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
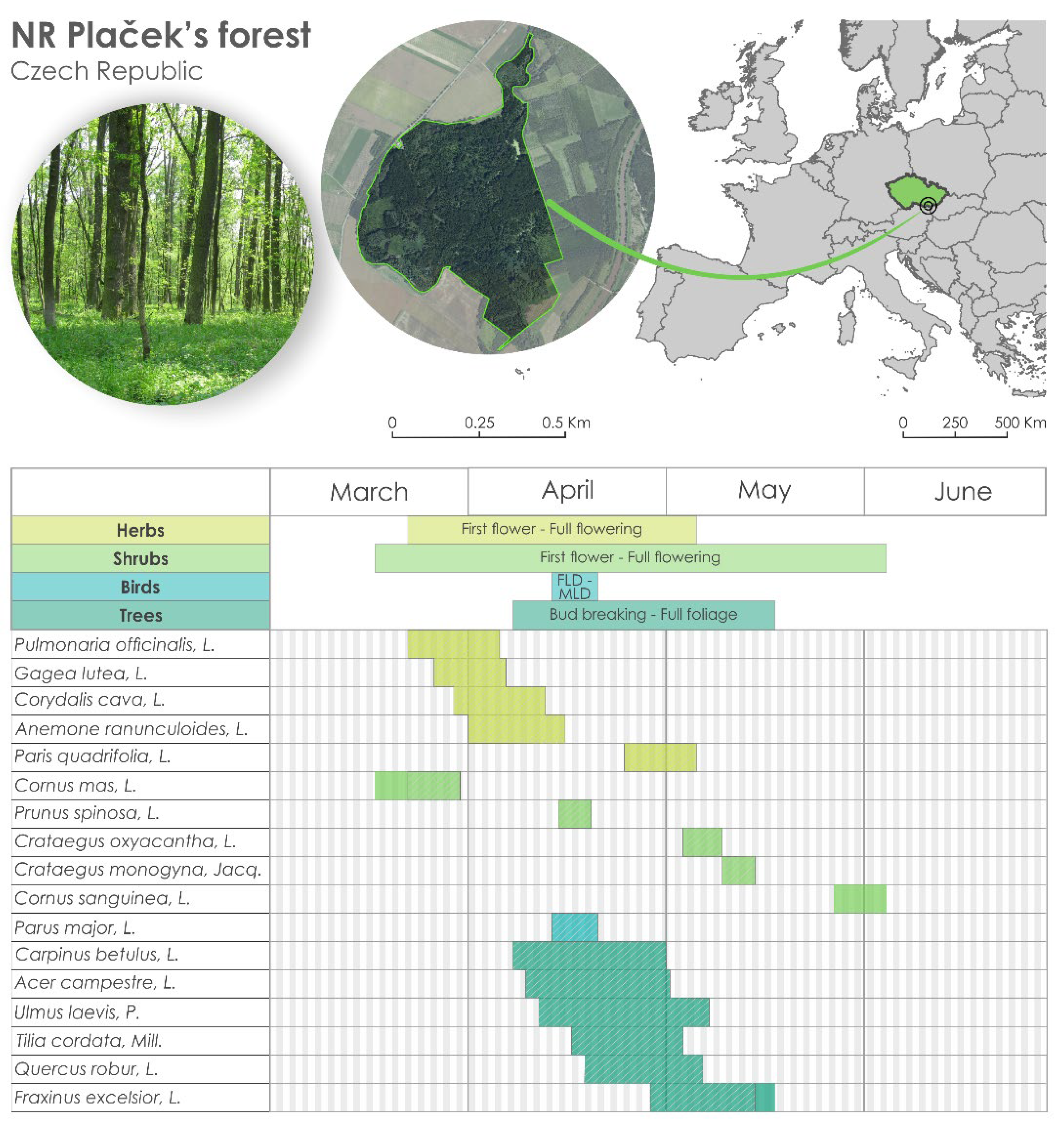

| Nature Reserve | Altitude/m | Latitude | Longitude | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vranovice | Plačkek’ forest | 170 | 48°56′54″ | 16°35′50″ | |||||||
| Average values | |||||||||||
| February | March | April | May | FM | MA | AM | FMA | MAM | FMAM | Year | |
| Mean daily air temperature (°C) | 0.8 | 5 | 10.2 | 14.8 | 2.9 | 7.6 | 12.5 | 5.3 | 10 | 7.7 | 9.8 |
| Maximum daily air temperature (°C) | 4.4 | 9.8 | 16 | 20.8 | 7.1 | 12.9 | 18.4 | 10.1 | 15.5 | 12.8 | 14.7 |
| Minimum daily air temperature (°C) | −2.9 | 0.3 | 4.3 | 8.9 | −1.3 | 2.3 | 6.6 | 0.6 | 4.5 | 2.7 | 5.0 |
| Precipitation (mm) | 23 | 26 | 30 | 55 | 49 | 56 | 85 | 78 | 111 | 134 | 473 |
| Linear trends in 1961–2021 | |||||||||||
| February | March | April | May | FM | MA | AM | FMA | MAM | FMAM | Year | |
| Mean daily air temperature (°C) | 1.69 | 1.5 | 1.05 | 1.05 | 1.59 | 1.27 * | 1.05 * | 1.42 * | 1.2 ** | 1.32 ** | 1.45 *** |
| Maximum daily air temperature (°C) | 2.5 * | 2.74 * | 2.35 ** | 1.24 | 2.62 * | 2.55 *** | 1.8 ** | 2.53 *** | 2.11 *** | 2.21 *** | 1.89 *** |
| Minimum daily air temperature (°C) | 0.89 | 0.25 | −0.24 | 0.86 | 0.57 | 0.05 | 0.31 | 0.3 | 0.29 | 0.44 | 1.0 ** |
| Total precipitation (mm) | −3.13 | 4.18 | −10.73 | −6.23 | 1.05 | −6.55 | −16.96 | −9.68 | −12.78 | −15.91 | 6.32 |
| Species | Phenophases | Abbreviation | Days/1961–2021 (Days/Decade) | Temperature Correlation | r | |
|---|---|---|---|---|---|---|
| Herbs | Common Lungwort (Pulmonaria officinalis, L.) | P1 | PO1 | 12.5 * (2.1) | Tmax/FM | 0.81 |
| P2 | PO2 | 7.8 * (1.3) | Tmax/M | 0.79 | ||
| Yellow Starof-Bethlehem (Gagea lutea, L.) | P1 | GL1 | 20.7 *** (3.5) | Tmax/FM | 0.77 | |
| P2 | GL2 | 19.6 *** (3.3) | Tmax/M | 0.75 | ||
| Bulbous Corydalis (Corydalis cava, L.) | P1 | CC1 | 15.1 ** (2.6) | Tmax/M | 0.79 | |
| P2 | CC2 | 14.0 *** (2.4) | Tmax/MA | 0.74 | ||
| Yellow Wood Anemone (Anemone ranunculoides, L) | P1 | AR1 | 13.7 ** (2.3) | Tmax/FM | 0.82 | |
| P2 | AR2 | 11.1 ** (1.9) | Tmax/MA | 0.78 | ||
| Herb Paris (Paris quadrifolia, L.) | P1 | PQ1 | 10.4 ** (1.8) | Tmax/MA | 0.81 | |
| P2 | PQ2 | 10.8 *** (1.8) | Tmax/MA | 0.81 | ||
| Shrubs | European Cornel (Cornus mas, L.) | P1 | CM1 | 11.5 (1.9) | Tmax/FM | 0.91 |
| P2 | CM2 | 9 (1.5) | Tmax/FM | 0.85 | ||
| Blackthorn (Prunus spinosa, L.) | P1 | PS1 | 10.07 ** (1.7) | Tmax/FMA | 0.85 | |
| P2 | PS2 | 10.1 ** (1.7) | Tmax/FMA | 0.83 | ||
| English Hawthorn (Crataegus oxyacantha, L.) | P1 | CO1 | 15.4 *** (2.6) | Tmax/MAM | 0.86 | |
| P2 | CO2 | 15.4 *** (2.6) | Tmax/MAM | 0.89 | ||
| Common hawthorn (Crataegus monogyna, Jacq.) | P1 | CMo1 | 13.4 *** (2.3) | Tmax/MAM | 0.88 | |
| P2 | CMo2 | 13.0 *** (2.2) | Tmax/MAM | 0.91 | ||
| Common Dogwood (Cornus sanguinea, L.) | P1 | CS1 | 15.4 *** (2.6) | Tmax/MAM | 0.85 | |
| P2 | CS2 | 15.7 *** (2.7) | Tmax/AM | 0.86 | ||
| Trees | Common Hornbeam (Carpinus betulus, L.) | P1 | CB1 | 9.2 ** (1.6) | Tmax/M | 0.82 |
| P2 | CB2 | 7.1 * (1.2) | Tmax/MA | 0.81 | ||
| European White Elm (Ulmus laevis, P.) | P1 | UL1 | 8.8 * (1.5) | Tmax/M | 0.78 | |
| P2 | UL2 | 10.7 *** (1.8) | Tmax/MAM | 0.85 | ||
| English Oak (Quercus robur, L.) | P1 | QR1 | 11.9 *** (2.0) | Tmax/MA | 0.75 | |
| P2 | QR2 | 10.4 *** (1.8) | Tmax/MAM | 0.82 | ||
| European ash (Fraxinus excelsior, L.) | P1 | FE1 | 10.3 *** (1.7) | Tmax/MA | 0.79 | |
| P2 | FE2 | 8.6 ** (1.4) | Tmax/MAM | 0.88 | ||
| Field maple (Acer campestre, L.) | P1 | AC1 | 5.9 (1.0) | Tmax/M | 0.83 | |
| P2 | AC2 | 7.5 * (1.0) | Tmax/MA | 0.81 | ||
| Small-leaved lime (Tilia cordata, Mill.) | P1 | TC1 | 6.2 (1.0) | Tmax/MA | 0.76 | |
| P2 | TC2 | 6.4 * (1.1) | Tmax/MAM | 0.72 | ||
| Birds | Great Tit (Parus major, L.) | P1 | PM1 | 7.8 ** (1.3) | Tmax/MA | 0.75 |
| P2 | PM2 | 8.5 *** (1.4) | Tmax/MA | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartošová, L.; Dížkova, P.; Bauerová, J.; Hájková, L.; Fischer, M.; Balek, J.; Bláhová, M.; Možný, M.; Zahradníček, P.; Štěpánek, P.; et al. Phenological Response of Flood Plain Forest Ecosystem Species to Climate Change during 1961–2021. Atmosphere 2022, 13, 978. https://doi.org/10.3390/atmos13060978
Bartošová L, Dížkova P, Bauerová J, Hájková L, Fischer M, Balek J, Bláhová M, Možný M, Zahradníček P, Štěpánek P, et al. Phenological Response of Flood Plain Forest Ecosystem Species to Climate Change during 1961–2021. Atmosphere. 2022; 13(6):978. https://doi.org/10.3390/atmos13060978
Chicago/Turabian StyleBartošová, Lenka, Petra Dížkova, Jana Bauerová, Lenka Hájková, Milan Fischer, Jan Balek, Monika Bláhová, Martin Možný, Pavel Zahradníček, Petr Štěpánek, and et al. 2022. "Phenological Response of Flood Plain Forest Ecosystem Species to Climate Change during 1961–2021" Atmosphere 13, no. 6: 978. https://doi.org/10.3390/atmos13060978
APA StyleBartošová, L., Dížkova, P., Bauerová, J., Hájková, L., Fischer, M., Balek, J., Bláhová, M., Možný, M., Zahradníček, P., Štěpánek, P., Žalud, Z., & Trnka, M. (2022). Phenological Response of Flood Plain Forest Ecosystem Species to Climate Change during 1961–2021. Atmosphere, 13(6), 978. https://doi.org/10.3390/atmos13060978








