Methane, Nitrous Oxide, and Ammonia Emissions on Dairy Farms in Spain with or without Bio-Activator Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site and Description
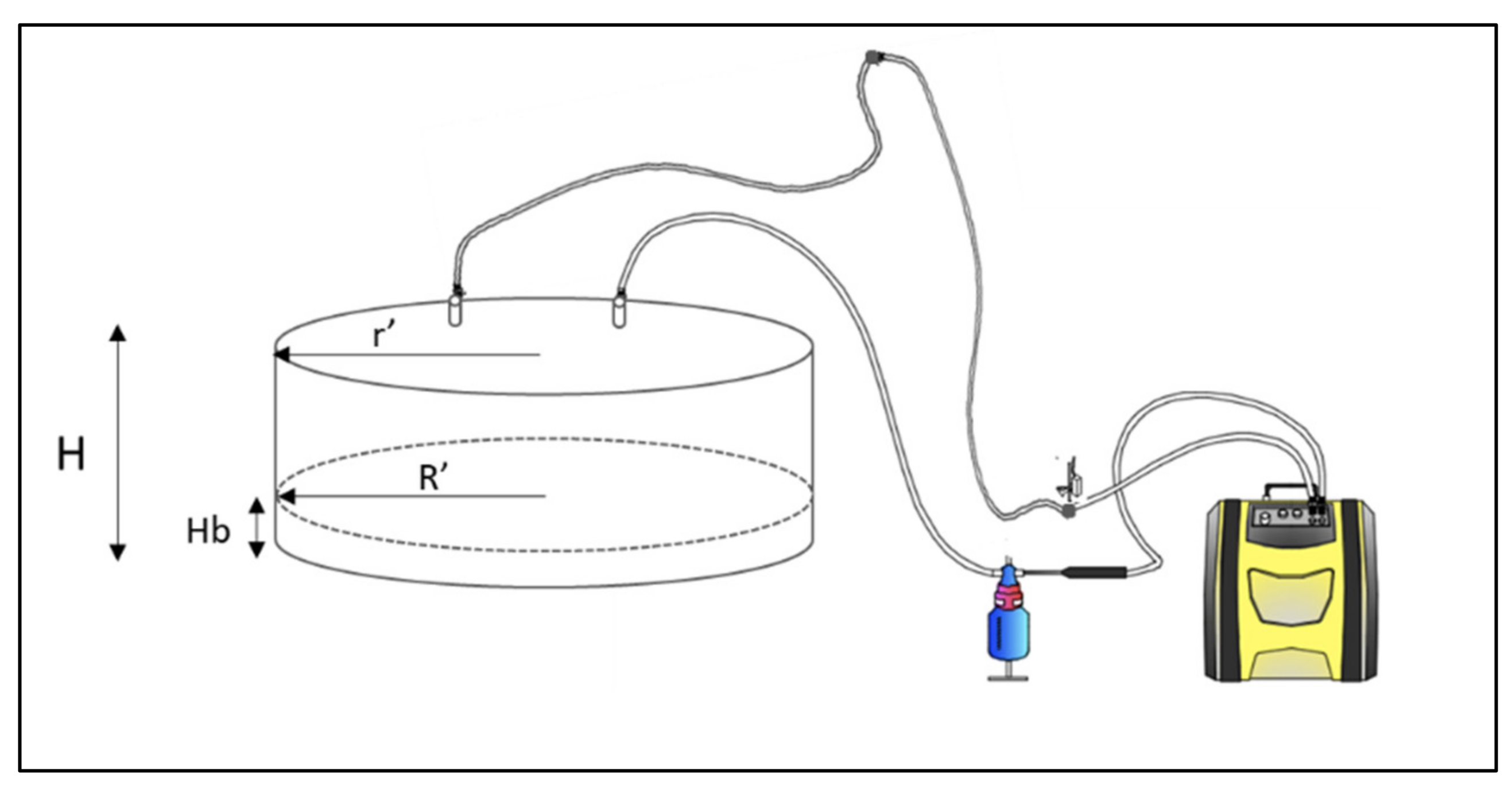
2.2. Gas Measurements and Sampling Procedure
2.3. Flux and Emission Rates
2.4. Bio-Activator
2.5. Microbiology in Slurry Samples
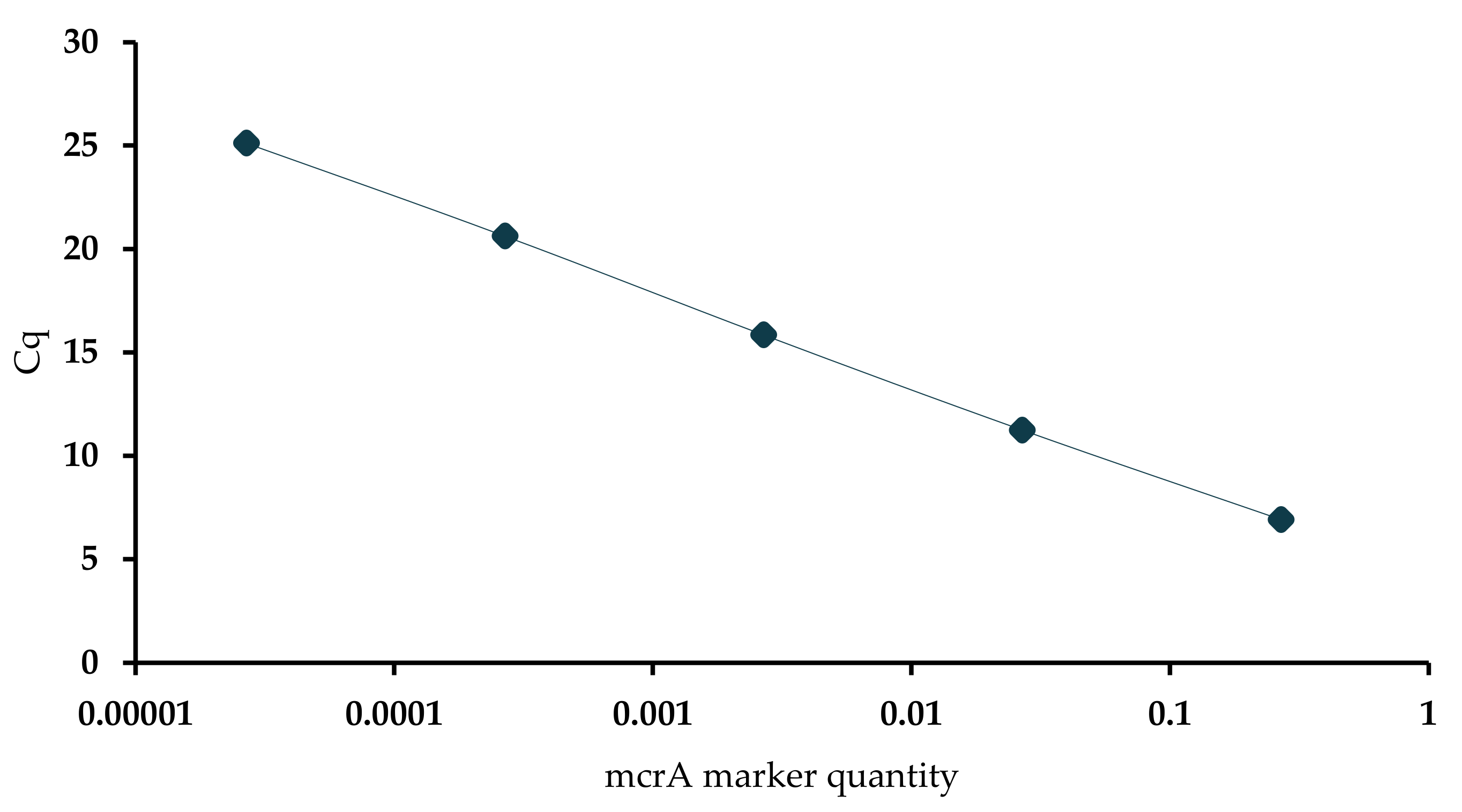
2.6. Quantitative PCR Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Slurry Emission Fluxes
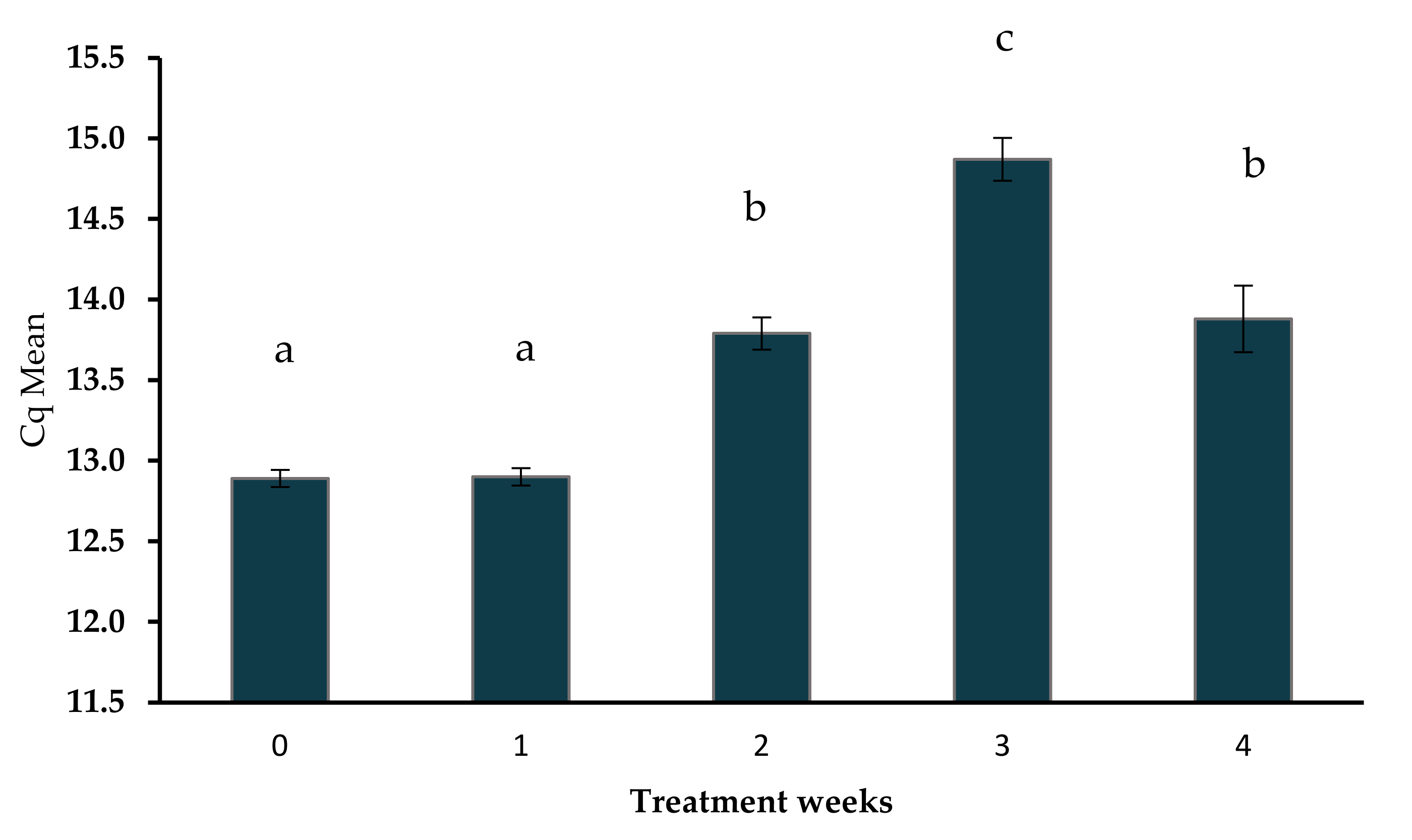
3.2. Bio-Activator and Population of Methanogens
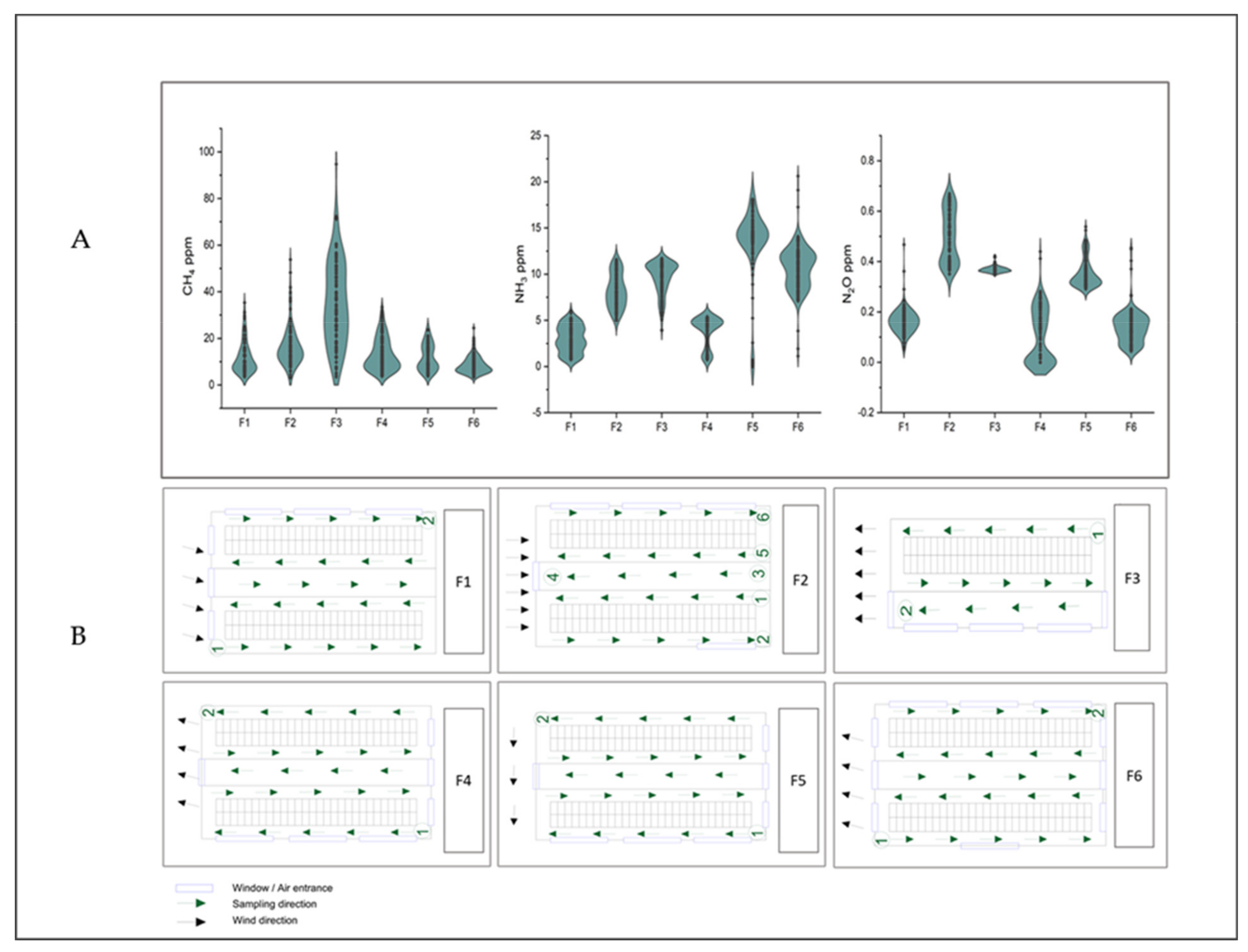
3.3. Emission Concentrations at the Barns
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gac, A.; Béline, F.; Bioteau, T.; Maguet, K. A French inventory of gaseous emissions (CH4, N2O, NH3) from livestock manure management using a mass-flow approach. Livest. Sci. 2007, 112, 252–260. [Google Scholar] [CrossRef]
- Jarvis, S.C.; Pain, B.F. Greenhouse Gas Emissions from Intensive Livestock Systems: Their Estimation and Technologies for Reduction. In Climate Change: Significance for Agriculture and Forestry; White, D.H., Howden, S.M., Eds.; Springer: Dordrecht, The Netherlands, 1994. [Google Scholar] [CrossRef]
- Webb, J.; Sommer, S.G.; Kupper, T.; Groenestein, K.; Hutchings, N.J.; Eurich-Menden, B.; Rodhe, L.; Misselbrook, T.H.; Amon, B. Emissions of Ammonia, Nitrous Oxide and Methane During the Management of Solid Manures. In Agroecology and Strategies for Climate Change; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews; Springer: Dordrecht, The Netherlands, 2012; Volume 8. [Google Scholar] [CrossRef]
- Sommer, S.G.; Hjorth, M.; Leahy, J.J.; Zhu, K.; Christel, W.; Sørensen, C.G. Pig slurry characteristics, nutrient balance and biogas production as affected by separation and acidification. J. Agric. Sci. 2015, 153, 177–191. [Google Scholar] [CrossRef]
- Mahmud, K.; Panday, D.; Mergoum, A.; Missaoui, A. Nitrogen Losses and Potential Mitigation Strategies for a Sustainable Agroecosystem. Sustainability 2021, 13, 2400. [Google Scholar] [CrossRef]
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; van Grinsven, H.; Winiwarter, W. Too much of a good thing. Nature 2011, 472, 159–161. [Google Scholar] [CrossRef]
- Cortés, A.; Feijoo, G.; Fernández, M.; Moreira, M.T. Pursuing the route to eco-efficiency in dairy production: The case of Galician area. J. Clean. Prod. 2021, 285, 124861. [Google Scholar] [CrossRef]
- Fernández, G. Economía Rural Y Agraria en Galicia; FG Estudios Sociales y Económicos: Madrid, Spain, 2001. [Google Scholar]
- Nemecek, T.; Olivier, H.E.; Dubois, D.; Gaillard, G.; Schaller, B.; Chervet, A. Life cycle assessment of Swiss farming systems: II. Extensive and intensive production. Agric. Syst. 2011, 104, 233–245. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Odor control in lagoons. J. Environ. Manag. 2013, 124, 62–71. [Google Scholar] [CrossRef]
- Chenu, C.; Cosentino, D. Microbial regulation of soil structural dynamics. In The Architecture and Biology of Soils: Life in Inner Space; CABI: Wallingford, UK, 2011; pp. 37–70. [Google Scholar] [CrossRef]
- Yang, T.; Siddique, K.H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health—A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. Available online: https://www.frontiersin.org/article/10.3389/fpls.2017.01617 (accessed on 1 March 2022). [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Saccá, M.L.; Caracciolo, A.B.; di Lenola, M.; Grenn, P. Ecosystem Services Provided by Soil Microorganisms. In Soil Biological Communities and Ecosystem Resilience; Springer: Dordrecht, The Netherlands, 2017; pp. 9–24. [Google Scholar] [CrossRef]
- Nkongolo, K.K.; Narendrula, K.R. Advances in monitoring soil microbial community dynamic and function. J. Appl. Genet. 2020, 61, 249–263. [Google Scholar] [CrossRef]
- Coonan, E.C.; Kirkby, C.A.; Kirkegaard, J.A.; Amidy, M.R.; Strong, C.L.; Richardson, A.E. Microorganisms and nutrient stoichiometry as mediators of soil organic matter dynamics. Nutr. Cycl. Agroecosyst. 2020, 117, 273–298. [Google Scholar] [CrossRef]
- Carney, K.M.; Matson, A. Plant Communities, Soil Microorganisms, and Soil Carbon Cycling: Does Altering the World Belowground Matter to Ecosystem Functioning? Ecosystems 2005, 8, 928–940. [Google Scholar] [CrossRef]
- Rütting, T.; Schleusner, P.; Hink, L.; Prosser, J.I. The contribution of ammonia-oxidizing archaea and bacteria to gross nitrification under different substrate availability. Soil Biol. Biochem. 2021, 160, 108353. [Google Scholar] [CrossRef]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Soong, L.J.; Fuchslueger, L.; Sara, M.J.; Torn, S.M.; Janssen, A.I.; Penuelas, J.; Richter, A. Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling. Glob. Chang. Biol. 2020, 26, 1953–1961. [Google Scholar] [CrossRef]
- Li, K.; Cao, R.; Mo, S.; Yao, R.; Ren, Z.; Wu, J. Swine Manure Composting with Compound Microbial Inoculants: Removal of Antibiotic Resistance Genes and Their Associations with Microbial Community. Front. Microbiol. 2020, 11, 592592. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2020.592592 (accessed on 15 March 2022). [CrossRef]
- De Souza, R.S.C.; Armanhi, J.S.L.; Arru, P. From Microbiome to Traits: Designing Synthetic Microbial Communities for Improved Crop Resiliency. Front. Plant Sci. 2020, 11, 1179. Available online: https://www.frontiersin.org/article/10.3389/fpls.2020.01179 (accessed on 3 March 2022). [CrossRef]
- Mageed, T.A.A.E.; Rady, M.M.; Taha, R.S.; el Azeam, S.A.; Simpson, C.R.; Semida, W.M. Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci. Hortic. 2020, 261, 108930. [Google Scholar] [CrossRef]
- Hidalgo, D.; Corona, F.; Marroquín, J.M.M. Manure biostabilization by effective microorganisms as a way to improve its agronomic value. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Qu, G.; Cai, Y.; Lv, P.; Ma, X.; Xie, R.; Xu, Y.; Ning, P. Effect of EM microbial agent on aerobic composting for dairy cattle manure. Int. J. Environ. Sci. Technol. 2019, 16, 6945–6958. [Google Scholar] [CrossRef]
- Sarfraz, R.; Hussain, A.; Sabir, A.; Fekih, I.B.; Ditta, A.; Xing, S. Role of biochar and plant growth promoting rhizobacteria to enhance soil carbon sequestration—A review. Environ. Monit. Assess. 2019, 191, 51. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, M.; Acosta, M.; Kiese, R.; Altimir, N.; Brümmer, C.; Crill, P.; Darenova, E.; Fuß, R.; Gielen, B.; Graf, A. Standardisation of chamber technique for CO2, N2O and CH4 fluxes measurements from terrestrial ecosystems. Int. Agrophysics 2018, 32, 569–587. [Google Scholar] [CrossRef]
- Enström, A.; Haatainen, T.; Suharto, A.; Giebels, M.; Lee, K.Y. Introducing a new GHG emission calculation approach for alternative methane reduction measures in the wastewater treatment of a palm oil mill. Environ. Dev. Sustain. 2019, 21, 3065–3076. [Google Scholar] [CrossRef]
- Experts in Genomics—AllGenetics. Available online: https://www.allgenetics.eu/ (accessed on 16 March 2022).
- Angel, R.; Claus, P.; Conrad, R. Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. ISME J. 2012, 6, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, L.M.; Regan, J.M. mcrA-targeted real-time quantitative PCR method to examine methanogen communities. Appl. Environ. Microbiol. 2009, 75, 4435–4442. [Google Scholar] [CrossRef]
- Kupper, T.; Häni, C.; Neftel, A.; Kincaid, C.; Bühler, M.; Amon, B.; Andrew, V. Ammonia and greenhouse gas emissions from slurry storage—A review. Agric. Ecosyst. Environ. 2020, 300, 106963. [Google Scholar] [CrossRef]
- Baral, K.; Jégo, G.; Amon, B.; Bol, R.; Chantigny, M.; Olesen, J.; Petersen, S. Greenhouse gas emissions during storage of manure and digestates: Key role of methane for prediction and mitigation. Agric. Syst. 2018, 166, 26–35. [Google Scholar] [CrossRef]
- Weslien, P.; Klemedtsson, L.; Svensson, L.; Galle, B.; Kasimir, A.A.; Gustafsson, A. Nitrogen losses following application of pig slurry to arable land. Soil Use Manag. 1998, 114, 200–208. [Google Scholar] [CrossRef]
- Ni, K.; Köster, J.R.; Seidel, A.; Pacholski, A. Field measurement of ammonia emissions after nitrogen fertilization—A comparison between micrometeorological and chamber methods. Eur. J. Agron. 2015, 71, 115–122. [Google Scholar] [CrossRef]
- Amon, B.; Kryvoruchko, V.; Moitzi, G.; Amon, T.; Zechmeister, B.S. Methane, nitrous oxide and ammonia emissions during storage and after application of dairy cattle and pig slurry and influence of slurry treatment. Agric. Ecosyst. Environ. 2006, 112, 153–162. [Google Scholar] [CrossRef]
- Flores, L.; Garfí, M.; Pena, R.; García, J. Promotion of full-scale constructed wetlands in the wine sector: Comparison of greenhouse gas emissions with activated sludge systems. Sci. Total Environ. 2021, 770, 145326. [Google Scholar] [CrossRef]
- Sommer, S.G.; Petersen, S.O.; Sørensen, P.; Poulsen, H.D.; Møller, H.B. Methane and carbon dioxide emissions and nitrogen turnover during liquid manure storage. Nutr. Cycl. Agroecosyst. 2007, 78, 27–36. [Google Scholar] [CrossRef]
- Barret, M.; Gagnon, N.; Topp, E.; Masse, L.; Massé, D.I.; Talbot, G. Physico-chemical characteristics and methanogen communities in swine and dairy manure storage tanks: Spatio-temporal variations and impact on methanogenic activity. Water Res. 2013, 47, 737–746. [Google Scholar] [CrossRef][Green Version]
- Duan, Y.F.; Soud, W.A.A.; Brejnrod, A.; Sørensen, S.J.; Elsgaard, L.; Petersen, S.O.; Boon, N. Methanotrophs, methanogens and microbial community structure in livestock slurry surface crusts. J. Appl. Microbiol. 2014, 117, 1066–1078. [Google Scholar] [CrossRef]
- Golec, A.F.C.; Pérez, G.; Lokare, C. Effective Microorganisms: Myth or reality? Microorganismos efi caces: Mito o realidad? Rev. Peru. Biol. 2007, 14, 315–319. Available online: http://sisbib.unmsm.edu.pe/BVRevistas/biologia/biologiaNEW.htmhttp://sisbib.unmsm.edu.pe/BVRevistas/biologia/biologiaNEW.htm (accessed on 27 February 2022).
- Cookson, J.T. Bioremediation Engineering: Design and Application; Cookson, J.T., Jr., Ed.; McGraw-Hill: New York, NY, USA, 1995. Available online: http://www.loc.gov/catdir/toc/mh022/94026856.html (accessed on 1 February 2022).
- Higa, T.; Parr, J.F. Beneficial and Effective Microorganisms for a Sustainable Agriculture and Environment; International Nature Farming Research Center: Atami, Japan, 1994. [Google Scholar]
- Redondo, L.B. Análisis y Caracterización de Purines para la Obtención de Estruvita y Biogas (Analysis and Characterization of Slurry to Obtain Struvite and Biogas); Valencia Polytechnic University: Valencia, Spain, 2015. [Google Scholar]
- Kim, S.Y.; Pramanik, P.; Bodelier, P.L.E.; Kim, J. Cattle Manure Enhances Methanogens Diversity and Methane Emissions Compared to Swine Manure under Rice Paddy. PLoS ONE 2014, 9, e113593. [Google Scholar] [CrossRef]
- Win, E.; Win, K.K.; Kimura, S.D.B.; Oo, A.Z. Influence of rice varieties, organic manure and water management on greenhouse gas emissions from paddy rice soils. PLoS ONE 2021, 16, e0253755. [Google Scholar] [CrossRef]
- Petersen, S.O.; Dorno, N.; Lindholst, S.; Feilberg, A.; Eriksen, J. Emissions of CH4, N2O, NH3 and odorants from pig slurry during winter and summer storage. Nutr. Cycl. Agroecosyst. 2013, 95, 103–113. [Google Scholar] [CrossRef]
- Baldé, H.; Andrew, C.V.; Burtt, S.D.; Wagner-Riddle, C.; Evans, L.; Gordon, R.; Desjardins, R.L.; MacDonald, J.D. Ammonia emissions from liquid manure storages are affected by anaerobic digestion and solid-liquid separation. Agric. For. Meteorol. 2018, 258, 80–88. [Google Scholar] [CrossRef]
- Zhu, J. A review of microbiology in swine manure odor control. Agric. Ecosyst. Environ. 2000, 78, 93–106. [Google Scholar] [CrossRef]
- Sommer, S.G.; Christensen, M.; Schmidt, T.; Jensen, L. Animal Manure Recycling: Treatment and Management; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Nielsen, D.A.; Nielsen, L.P.; Schramm, A.; Revsbech, N. Oxygen Distribution and Potential Ammonia Oxidation in Floating, Liquid Manure Crusts. J. Environ. Qual. 2010, 39, 1813–1820. [Google Scholar] [CrossRef]
- van der Stelt, B.; Temminghoff, E.J.M.; van Vliet, P.C.J.; van Riemsdijk, W.H. Volatilization of ammonia from manure as affected by manure additives, temperature and mixing. Bioresour. Technol. 2007, 98, 3449–3455. [Google Scholar] [CrossRef]
- Külling, D.R.; Dohme, F.; Menzi, H.; Sutter, F.; Lischer, P.; Kreuzer, M. Methane Emissions of Differently Fed Dairy Cows and Corresponding Methane and Nitrogen Emissions from their Manure during Storage. Env. Monit Assess 2000, 79, 150. [Google Scholar] [CrossRef]
- Jun, P.; Gibbs, M.; Gaffney, K. CH4 and N2O Emissions from Livestock Manure. Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories. 2002. Available online: https://www.ipcc-nggip.iges.or.jp/public/gp/bgp/4_2_CH4_and_N2O_Livestock_Manure.pdf (accessed on 15 February 2022).
- Steinberg, L.M.; Regan, J.M. Phylogenetic Comparison of the Methanogenic Communities from an Acidic, Oligotrophic Fen and an Anaerobic Digester Treating Municipal Wastewater Sludge. Appl. Environ. Microbiol. 2008, 74, 6663–6671. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Olle, M.; Williams, I.H. Effective microorganisms and their influence on vegetable production—A review. J. Hortic. Sci. Biotechnol. 2013, 88, 380–386. [Google Scholar] [CrossRef]
- Magali, A.; Rezende, F.A.; Tomita, C.K.; Uesugi, C.H. Fungicidas cúpricos, cloretos de benzalcônio e composto dos ponteiros causada por em goiabeiras. Trop. Plant Pathol. 2008, 33, 288–294. [Google Scholar]
- Beevi, N.D.; Qadri, S.M.H. Biological control of mulberry root rot disease (Fusarium spp.) with antagonistic microorganisms. J. Biopestic. 2010, 3, 90. [Google Scholar]
- Aryantha, N.; Guest, D.I. Bokashi (EM) as a Bio-control Agent to Suppress the Growth of Phytophthora Cinnamomi Rands. School of Botany, The University of Melbourne, Parkville 3052, Victoria. Available online: https://www.researchgate.net/publication/267795255_Bokashi_EM_as_a_Bio-control_Agent_to_Suppress_the_Growth_of_Phytophthora_Cinnamomi_Rands (accessed on 22 February 2022).
- Smirnova, I.; Sadanov, A. Application of Agriculturally Important Microorganisms for Biocontrol of Root Rot Infection of Sugar Beet. Arch. Phytopathol. Plant Prot. 2019, 52, 698–713. [Google Scholar] [CrossRef]
- Mitter, B.; Brader, G.; Pfaffenbichler, N.; Sessitsch, A. Next generation microbiome applications for crop production—Limitations and the need of knowledge-based solutions. Curr. Opin. Microbiol. 2019, 49, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Zabalgogeazcoa, I.; de Aldana, B.R.V.; Martinez-Medina, A. Untapping the potential of plant mycobiomes for applications in agriculture. Curr. Opin. Plant Biol. 2021, 60, 102034. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qiu, Z.; Ye, J.; Verma, J.P.; Li, J.; Singh, B.K. Effective colonisation by a bacterial synthetic community promotes plant growth and alters soil microbial community. J. Sustain. Agric. Environ. 2021, 1, 30–42. [Google Scholar] [CrossRef]
- Gibby, A.; Lancaster, E. Use of effective microorganisms®(EM®) for sustainable pathogen control in food safety. In Proceedings of the 2018 4th International Conference on Universal Village (UV), Boston, MA, USA, 21–24 October 2018; pp. 1–5. [Google Scholar]
- Newbold, C.J.; Rode, L.M. Dietary additives to control methanogenesis in the rumen. Int. Congr. Ser. 2006, 1293, 138–147. [Google Scholar] [CrossRef]
- Chaucheyras, F.; Fonty, G.; Bertin, G.; Goue, P. In Vitro H2 utilization by a ruminal acetogenic bacterium cultivated alone or in association with an archaea methanogen is stimulated by a probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1995, 61, 3466–3467. [Google Scholar] [CrossRef]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2010, 4, 351–365. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.L.; Hwang, S.W.; Eom, J.H.; Kim, S.H.; Kang, S.W.; Cho, J.S.; Seo, D.C. Correction to: Characteristics of ammonia gas emissions from soybean cultivation soils treated with mixed microorganisms. Appl. Biol. Chem. 2021, 64, 16. [Google Scholar] [CrossRef]
- Schmithausen, A.J.; Trimborn, M.; Büscher, W. Sources of nitrous oxide and other climate relevant gases on surface area in a dairy free stall barn with solid floor and outside slurry storage. Atmos. Environ. 2018, 178, 41–48. [Google Scholar] [CrossRef]
- Broucek, J. Nitrous Oxide Production from Cattle and Swine Manure. J. Anim. Behav. Biometeorol. 2016, 5, 13–19. [Google Scholar] [CrossRef]
| Barn | |||||||
|---|---|---|---|---|---|---|---|
| Farms | N Dairy Cattle | Animal Nutrition | Type of Farming | Storage Slurry Weeks | Bedding Material | ||
| F1 | 150 | 30–40% Corn Silage 20% Straw 11–13% Mixed Forage 15–20% Dry Organic Compounds | Intensive | 4 | Sand | ||
| F2 | 130 | 3 | |||||
| F3 | 120 | 4 | |||||
| F4 | 130 | 4 | |||||
| F5 | 100 | 3 | |||||
| F6 | 130 | 4 | |||||
| Slurry Tank | |||||||
| Farms | Temperature °C | pH | Density kg/m3 | Additional Inputs | Appearance | Type | Volume Slurry m3 |
| F1 | 16.8 | 6.9 | 1050 | Disinfectants, Drug Residues, Hormones, Formalin, Fungicide, Copper, Cow Milking | Dense | Semi-Open | 907.2 |
| F2 | 16.6 | 6.8 | 1050 | Liquid | 1500 | ||
| F3 | 14.8 | 6.7 | 1020 | Liquid | 750 | ||
| F4 | 16.7 | 6.9 | 1040 | Liquid | 665 | ||
| F5 | 14 | 6.9 | 1070 | Liquid | 1035 | ||
| F6 | 15.5 | 6.9 | 1020 | Semi-Dense | 1776 | ||
| Item | Farms (n = 3) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | SEM * | p-Value | |
| Emission Fluxes, g × m−2 × h−1 | ||||||||
| CH4 | 103.41 | 39.4 | 22.49 | 38.91 | 37.65 | 14.51 | 1.10 | 0.31 |
| N2O | 2.99 × 10−2 | 3.00 × 10−4 | 3.06 × 10−3 | 3.00 × 10−4 | 6.12 × 10−3 | 3.06 × 10−3 | 0.001 | 0.33 |
| NH3 | 0.13 | 0.25 | 0.29 | 0.32 | 0.43 | 0.15 | 0.01 | 0.16 |
| Emission Factor, g × kg−1 Slurry | ||||||||
| CH4 | 139.03 | 70.44 | 39.06 | 57.25 | 54.95 | 23.63 | 2.24 | 0.43 |
| N2O | 4.02 × 10−2 | 5.00 × 10−4 | 5.31 × 10−3 | 5.00 × 10−4 | 8.93 × 10−3 | 4.98 × 10−3 | 0.001 | 0.33 |
| NH3 | 0.17 | 0.44 | 0.51 | 0.46 | 0.62 | 0.24 | 0.02 | 0.16 |
| Analysis of Methanogen Communities Using qPCR in Slurry Samples | |||||
|---|---|---|---|---|---|
| Treatment Weeks | Cq Mean * | Cq SD ** | Quantity Mean ng in 50 µL | mcrA Copy Number in 50 µL *** | Cq Threshold |
| 0 | 12.89 | 0.05 | 3.12 × 10−2 | 7.11 × 107 | 2.66 |
| 1 | 12.90 | 0.05 | 3.11 × 10−2 | 7.09 × 107 | 2.66 |
| 2 | 13.79 | 0.10 | 1.98 × 10−2 | 4.51 × 107 | 2.66 |
| 3 | 14.87 | 0.13 | 1.15 × 10−2 | 2.63 × 107 | 2.66 |
| 4 | 13.88 | 0.21 | 1.90 × 10−2 | 4.33 × 107 | 2.66 |
| Treatment Weeks | t | p-Value |
|---|---|---|
| 0 vs. 1 | −0.15 | 0.89 |
| 0 vs. 2 | −13.80 | <0.001 |
| 0 vs. 3 | −23.90 | <0.001 |
| 0 vs. 4 | −8.05 | 0.001 |
| 1 vs. 2 | −13.64 | <0.001 |
| 2 vs. 3 | −11.16 | <0.001 |
| 2 vs. 4 | −0.64 | 0.55 |
| 3 vs. 4 | 6.965 | 0.002 |
| Treatment Weeks | mcrA Copy Number in 50 µL | Reduction in Copies with Treatment (%) |
|---|---|---|
| 0 | 7.11 × 107 | |
| 1 | 7.09 × 107 | 0.28 |
| 2 | 4.51 × 107 | 36.57 |
| 3 | 2.63 × 107 | 63.01 |
| 4 | 4.33 × 107 | 39.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San Martin Ruiz, M.; González Puelles, J.E.; Herra Bogantes, J.; Rivera-Méndez, W.; Reiser, M.; Kranert, M. Methane, Nitrous Oxide, and Ammonia Emissions on Dairy Farms in Spain with or without Bio-Activator Treatment. Atmosphere 2022, 13, 893. https://doi.org/10.3390/atmos13060893
San Martin Ruiz M, González Puelles JE, Herra Bogantes J, Rivera-Méndez W, Reiser M, Kranert M. Methane, Nitrous Oxide, and Ammonia Emissions on Dairy Farms in Spain with or without Bio-Activator Treatment. Atmosphere. 2022; 13(6):893. https://doi.org/10.3390/atmos13060893
Chicago/Turabian StyleSan Martin Ruiz, Macarena, Jesús Eugenio González Puelles, Juan Herra Bogantes, William Rivera-Méndez, Martin Reiser, and Martin Kranert. 2022. "Methane, Nitrous Oxide, and Ammonia Emissions on Dairy Farms in Spain with or without Bio-Activator Treatment" Atmosphere 13, no. 6: 893. https://doi.org/10.3390/atmos13060893
APA StyleSan Martin Ruiz, M., González Puelles, J. E., Herra Bogantes, J., Rivera-Méndez, W., Reiser, M., & Kranert, M. (2022). Methane, Nitrous Oxide, and Ammonia Emissions on Dairy Farms in Spain with or without Bio-Activator Treatment. Atmosphere, 13(6), 893. https://doi.org/10.3390/atmos13060893







