Abstract
Biomonitoring studies have enormous benefits providing a fruitful and cost-efficient means of measuring environmental exposure to toxic chemicals. This study collected ambient air and pine tree components, including needles and 1-year-old and 2-year-old branches, for one year. Concentrations, potential sources and temporal variations of atmospheric polycyclic aromatic hydrocarbons (PAHs) were investigated. In general, lower concentration levels were observed in the warmer months. Ambient PAHs pose a serious public health threat and impose a need for calculating cancer risks. It was also intended to define the best tree component reflecting the ambient air PAHs. The consideration of the representative tree component minimizes the unnecessary laboratory processes and expenses in biomonitoring studies. The coefficient of divergence (COD), diagnostic ratio (DR) and principal component analysis (PCA) were employed to specify the PAH sources. As a result of the DR and PCA evaluations, the effect of the industrial area has emerged, besides the dominance of the pollutants originating from traffic and combustion. The results have shown that pine needles and branches were mainly affected by similar sources, which also influenced air concentrations. Inhalation cancer risk values were also calculated and they varied between 1.64 × 10−6 and 3.02 × 10−5. A potential risk increases in the colder season depending on the ambient air PAH concentrations.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are atmospheric pollutants that contain two or more benzene rings, which can have adverse effects on the ecosystem and human health due to their mutagenic and carcinogenic properties [1,2]. PAHs are organic pollutants that can be detected in air, vegetation, soil and water [3,4,5,6].
Evaluation of the presence and distribution of these organic pollutants in the ambient air requires analytical methodologies that allow them to be determined quickly and reliably. Air quality monitoring programs for this purpose are conducted through active or passive samplers. Vegetation [7,8,9] and semipermeable membrane devices (SPMDs) [10] are two common types of passive samplers used for atmospheric PAH sampling. Vegetation sampling for PAHs is a more economical alternative because special sampling equipment is not needed. Plants cover most of the soil surface [11]. The plants can have 6–14 times more surface zones than the corresponding soil surface [8]. Therefore, vegetation has an essential effect on the atmospheric cycle. Biomonitoring studies to assess PAH concentrations in various environments have become the standard tool since the late 1980s [12]. Many plants, lichen and tree species (deciduous and evergreen) have been used as bioindicators to determine urban air quality and pollutant sources [7,9,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Indeed, in 2004, the European Parliament proposed the use of bioindicators to assess the impact of PAH on ecosystems by Directive 2004/10/EC. Considering the bioaccumulation of PAHs, especially in tree leaves and branches, it is possible to consider vegetation as a prominent hydrocarbon collector.
Tree components are widely used in biomonitoring as reliable and cost-effective indicators of environmental pollution because they accumulate a wide range of airborne pollutants and thus, reflect the effects of existing air pollution [2,27,28]. The dynamic exchange of organic chemicals on plant surfaces occurs under the influence of both environmental conditions and the biological properties of the plant. Plant intake of organic substances is of great importance as it affects the environmental transport and fate of PAHs. The uptake of organic chemicals from plant surfaces was extensively searched in terms of diffusion and penetration. It was stated that plant lipids were the critical chemical component for the assimilation of organic pollutants [29,30]. The lipid-rich cuticle of plant materials is likely to accumulate such persistent pollutants mainly on the leaf surface with an atmospheric deposition [16].
Evergreen plants are preferred as they offer yearly sampling opportunities [21,23,31]. Pine tree components are frequently used in the estimation of PAH components and their distribution in the atmosphere [11,32,33,34]. The high-lipid wax structure of pine needles is one of the most remarkable plants in the monitoring studies carried out in recent years, as it allows the pollutants to be retained for a long time. However, PAH accumulation in tree components occurs in different amounts due to various environmental conditions such as distance to pollutant source, climatic conditions, terrain conditions, etc. [34]. Therefore, it is important to examine the distribution of PAH in pine tree components in different environments to understand the distribution of PAH in different regions. A quantitative correlation is tried to be established between PAH levels in the air and plants with recent approaches and modeling studies [35].
It is important to determine the main sources of atmospheric PAHs and present the air-tree component relationships for a better understanding of the fate and transport of PAHs in the environment. Identifying the sources of PAHs is also necessary to assess ecological risk and appropriate mitigation measures [36]. For this purpose, there are some generally accepted approaches. The diagnosis rates (DRs) and specific determinants of each selected PAH type, multivariate statistical methods and various emission inventory model approaches (Principal component analysis (PCA), positive matrix fraction (PMF)) are employed to define the sources of PAHs, make their source distributions and reveal their contribution [6,37].
Some PAHs are known as carcinogens and pose a serious threat to human health [38]. The International Cancer Research Agency [39] classifies some PAHs as carcinogens (Group 1, 2A or 2B). Biological monitoring of the exposure to PAHs has been an important topic of interest due to its widespread distribution and toxicological importance. PAHs can be exposed in many ways, such as inhalation of indoor–outdoor air and ingestion, including meals containing PAHs [40]. Exposure to PAHs also causes reproductive disorders and some diseases, including bladder and gastrointestinal cancers, as well as skin and lung cancers [41].
The main objectives of this study can be summarized as (i) to determine the possible sources of PAHs by the DR and PCA methods, (ii) to investigate which tree component better reflects the ambient air and (iii) to reveal the potential health risks of PAHs
2. Material Method
2.1. Sampling
The sampling was performed in Bursa, a densely populated city with developed industry. The samples of branches and needles were collected on a monthly basis for one year from a pine tree in a suburban–industrial area of Bursa. The location of the pine tree sampled is shown in Figure 1. The sampled pine tree was approximately 1.5 km from the Istanbul–Izmir highway. The Bursa Organized Industrial Zone (BOIZ) was located approximately 2.8 km from the tree. There were settlements in the northwest and northeast of the tree.
Figure 1.
The location of the sampled pine tree.
The samples of ambient air and pine tree components (branch and needle) were wrapped in aluminum foil and brought to the laboratory in airtight bags. Passive air samplers (PASs) containing polyurethane foam (PUF) discs [42,43] were used to determine ambient air concentrations of PAHs. In passive air sampling, increased pollutant accumulation in the PUF-disc medium (PDM) is explained by the deposition resistance and mass transfer in the PDM-air-section [43]. Mass loads per sampler were converted to concentrations in the air using sample and compound-specific effective air volumes. For passive samplers using PUF disk as an adsorbent, the sampling rate (R) values and site-specific values were estimated from the model and online tool [44,45]. Collected PUF discs were extracted in a Soxhlet extractor with a mixture of acetone/hexane (ACE/HEX) (v:v, 1/1) for 24 h, with each cycle for approximately 50 min. Prior to extraction, a recovery surrogate standard was added to each sample to determine the recovery level [20]. Then, fractionation and cleaning procedures were applied. Their details were presented elsewhere [5,20].
Branch and needle samples were collected from 1.5–2 m heights of the tree. It was paid attention that branch samples were collected as one-year-old and two-year-old.
2.2. Sample Preparation and Experimental Process
The pine needles were wiped with a napkin before extraction to obtain the particulate phase on their surfaces. Needle and branch (1-year-old and 2-year-old) samples were placed separately into dark-colored bottles. The branch and needle samples to be extracted were weighed approximately 5 g each. Fifty mL solvent consisting of dichloromethane and petroleum ether (DCM/PE, 1/1) and 1 mL surrogate standard were added to the bottles and kept in a shaker for approximately 16 h [20]. Then, ultrasonic extraction was applied to these samples for 15 min. Then, another solvent mixture (40 mL) consisting of acetone and hexane (ACE/HEX, 1:1) was added to the remaining samples and ultrasonic extraction was applied for 30 min to them [46]. Subsequently, the sample volume (90 mL) was reduced to 5 mL in a rotary evaporator (Laborota 4001 model, Heidolph, Germany) operating at 30 rpm and 22 °C. Afterward, 15 mL of HEX was added to the sample and its volume was reduced to 2 mL. Samples with decreased volumes were subjected to cleaning and fractionation in a column consisting of 3 g silicic acid, 2 g alumina and 2 g sodium sulfate [5,7,47]. The fractionation column was cleaned using 20 mL DCM and then 20 mL PE for possible contamination. Then, a 2 mL sample was added to the column and then 20 mL of DCM was poured into the column to collect the PAH compounds [5,43,48]. Then, the solvent volume was reduced to 5 mL in a rotary evaporator and the final volume was reduced to 2 mL after 15 mL of HEX was added. Thus, the process of converting the solvent to HEX was achieved. PAH compounds with a volume of 2 mL were reduced to a volume of 1 mL under pure N2 gas and taken into vials. The samples were stored in a deep freezer at −20 °C until the instrumental analysis stage [5]. The samples were analyzed for 14 individual PAH, including Acenaphthene (Ace), Fluorene (Fluo), Phenanthrene (Phe), Anthracene (Anth), Fluoranthene (Flt), Pyrene (Pyr), benz(a)anthracene (BaA), chrysene (Chr), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), indeno(1,2,3-cd)pyrene (Indeno), Dibenzo(ah)anthracene (DahA), benzo(g,h,i)perylene (BghiP).
2.3. GC Analysis
An analysis of PAHs was performed by an Agilent 7890A model gas chromatograph (GC) and mass spectrophotometer (MS) with an associated Agilent 5975C inert XL triaxial mass detector. The injection volume of each sample is 1 µL and injections were made in a splitless mode. A mass spectrophotometer was run in selected ion monitoring mode (SIM) for high sensitivity. A capillary column (HP 5-MS, 30 m × 250 µm × 0.25 μm) was used in the GC-MS. A modified PAH separation method was applied in the GC reading as follows: wait 1 min at 50 °C, 25 °C/min to 200 °C, 8 °C/min to 300 °C (wait 5.5 min), 5 °C/min to 310 °C, wait 3 min before the end [20].
2.4. Quality and Reliability Measures
One mL of the surrogate standard was added to each sample and blank samples to determine the analysis recovery level. The surrogate standard, including naphthalene-d8, acenaphthene-d10, phenantherened10, chrysene-d12 and perylene-d12, was added to the samples at a concentration of 4 ng/mL. The data with recoveries of 50–120% were included in the calculations.
Blank samples of a minimum of 10% of the total sample numbers were collected for each component to determine possible contamination during transport, sampling and analysis steps. The blank correction was applied by subtracting the mean of the blank from the sample values [5]. Three times the blank standard deviations and mean values were summed to obtain the limit of detection (LOD) values for each PAH compound. LOD has been calculated and the measurement results below this value were not evaluated [49,50]. LOD values ranged from 1.35 ng (DahA) to 37.85 ng (Nap) for PUF samples, from 9,72 ng (DahA) to 75.59 ng (Phe) for leaf samples and from 10.30 (BkF) to 58.20 (Phe) for branch samples.
2.5. Statistical Analysis of Data
Statistical calculations and evaluations were performed using the PASW Statistics 18—SPSS program. In this context, Spearman’s or Pearson’s correlation test, paired t-test and other relevant tests were applied to the data.
3. Results and Discussion
Monthly samples of pine needles, branches (1- and 2-year-old branches) and ambient air were collected during the one year to determine the distributions and concentrations of PAHs at the sampling point, which could be characterized as a suburban industrial area. Annual average Σ14PAH concentrations for pine needles, 1- and 2-year-old branches and ambient air were 756 ± 232 ng/g DW, 685 ± 350 ng/g DW and 587 ± 361 ng/g DW, respectively. When comparing with the literature data, it is crucial to have a region with similar characteristics, the number of PAH compounds studied and the type of tree species. The data obtained from this study agree with similar literature studies (Table 1). In this context, the values found in this study are similar to the values (Σ16PAH = 626 ± 306 ng/g DW for pine needles) found in a study by Cindoruk et al. [20] in a suburban region in Turkey. In another study conducted in the industrial area [4], the value found for pine needles was Σ16PAH = 414 ± 265 ng/g DW and Σ16PAH = 995 ± 643 ng/g DW for the branch. In another study conducted in another industrial region [33] (Σ16PAH =2157 ± 2098 for 2-year-old pine needles 1016 ± 684 for 1-year-old pine needles), much higher results were obtained. The distance from the industrial zone and the type of industrial activities significantly affect these concentrations. In this study, lower values were determined than the values determined by Odabasi et al. [33] due to the distance to the industrial zone. Obtained data from similar studies conducted in Italy [14] in an urban area containing industrial activities (Σ9PAH = 817.4 ng/g DW) and in Portugal [51] (Σ16PAH = 64–813 ng/g DW) prove that the data obtained in this study are reasonable values.

Table 1.
Some PAH biomonitoring data from pine needles and branches.
3.1. Identification of PAH Sources
Ambient air, pine needle and 1-year-old and 2-year-old pine branches samples were taken to analyze the 14 PAH compounds for a one-year period (December 2015–November 2016). In the literature, pollutants in ambient air have been monitored by using different plants and plant components. The coefficient of divergence (COD), diagnostic ratio (DR) and principal component analysis (PCA) were applied for the source apportionment justification.
3.1.1. Coefficient of Divergence (COD)
The coefficient of divergence (COD) is used to identify similarities or differences between pollutant composition profiles [54,55,56]. If the COD values are close to zero, the similarity can be mentioned between the two samples, and if it is close to one (>0.2), the difference and absolute heterogeneity can be mentioned [54,55]. There is also another method used to determine the relationship between two samples and it is known as Spearman’s correlation coefficient (Rs) [56]. Higher Rs (>0.7) and a lower COD (<0.2) indicate that the two samples have similar influences from the sources [46,55].
It was investigated which tree component was more representative of the ambient air. In this way, it may be appropriate to collect only this component from the tree to represent the atmospheric concentration, and the collection of other components will be prevented. A difference between PAH concentrations determined using the passive air sampler and tree components is expected. This is mainly because the PUFs (polyurethane foams) in the passive air sampler were exposed to atmospheric concentrations for one month in this study. Theoretically, PUFs accumulate all PAHs on them during this one-month sampling period and do not let PAHs leave back into the atmosphere. However, there is an active exchange between air and tree components, although there is an accumulation in the tree components during the one-month sampling period. As a result, a difference in PAH levels between tree components and PUF discs is likely to be observed. When all data are evaluated in the light of this basic expectation (annual Σ14PAH), the Rs and p values between air and needle, air and 1-year-old branch, and air and 2-year-old branch samples were calculated to be 0.672 and <0.001, 0.652 and <0.001, 0.769 and <0.001, respectively. According to the ‘p’ results, there is an acceptable relationship between all tree components and ambient air concentrations. Thus, tree components can be used to explain ambient air PAH concentrations. However, Spearman’s correlation values (Rs) indicated that the best correlation value was found between air and the 2-year-old branch samples.
The monthly COD and Rs values obtained between ambient air and tree components (air vs. needle, air vs. 1-year-old branch and air vs. 2-year-old branch) are given in Figure 2. The COD values between air-needle, air-1-year-old branch and air-1-year-old branch samples were determined as >0.815, >0.798 and >0.780, respectively. When the COD and Rs values were examined, it was determined that there were lower COD and higher Rs values between the air and 2-year-old branch samples. Generally, a decrease in Rs values was observed in all component pairs in the autumn and winter months (Figure 2). This decrease was observed more clearly in air-needle and air-1-year-old branch component pairs. It was clearly seen that the correlation between air and the 2-year-old branch was better than other components in almost all months. This finding probably indicates that 2-year-old branch samples are more successful in explaining air concentrations.
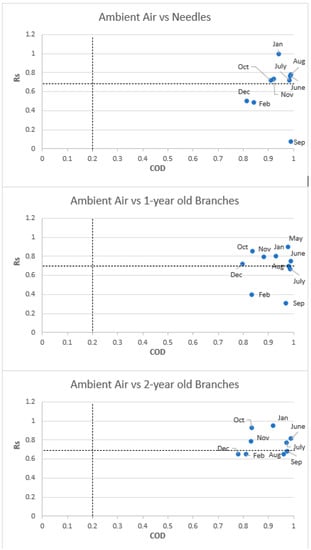
Figure 2.
Correlation coefficient (Rs) and coefficient of divergence (COD) values of PAHs for the ambient air vs. tree components.
The similarity of tree components (needle vs. 1-year-old branch, needle vs. 2-year-old branch, 1-year-old vs. 2-year-old branch) is evaluated. COD values were less than 0.437 among all tree component pairs. It can be concluded that the tree components were partially affected by the sources in a similar way. In particular, the lowest COD value (0.345) and the highest Rs value (Rs = 0.876; p < 0.001) among the component pairs belonged to the 1-year-old and 2-year-old branches. These two components are affected similarly by PAH sources and may be more successful in explaining each other.
In this study, the seasonal COD and Rs results obtained for needles and branches (needle-1-year-old branch, needle-2-year-old branch and 1- and 2-year-old branches) are given in Figure 3. Higher COD values were observed in the winter and autumn, yet the lowest COD values were detected in the summer. This finding is reasonable because tree components were exposed to more PAH in terms of sources and concentrations in winter and autumn. The result indicated that PAH sources that existed at the site affected tree components to different extents. On the other hand, in the summer months, lower COD values revealed more consistency between each tree component pair. Probably, this was mainly due to lower concentrations and fewer sources of PAHs. In general, there was good consistency between 1- and 2-year-old branch samples with Rs > 0.86 in all seasons (Table 2).
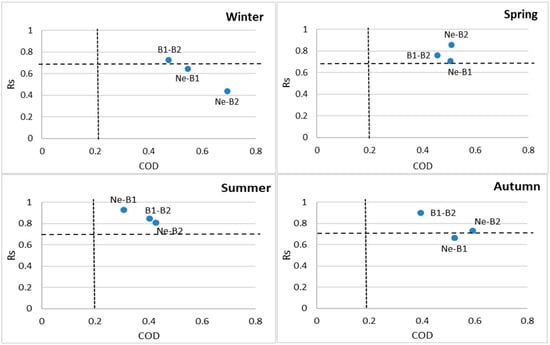
Figure 3.
Correlation coefficient (Rs) and coefficient of divergence (COD) values of PAHs for tree components in all seasons. Note: Ne, B1 and B2 refer to the needle, 1-year-old branch and 2-year-old branch, respectively.

Table 2.
The correlation coefficient (Rs) values.
The relationships between air and tree components were also investigated by considering the molecular weights of the PAH components. It was found that tree components data were more successful in describing air concentrations in medium molecular weight PAHs (Table 3). The correlation values (Rs and p) between air vs. needle (n = 28), air vs. 1-year-old branch (n = 27) and air vs. 2-year-old branch (n = 28) samples for medium molecular weight (MMW) PAHs were determined as 0.436 and 0.020, 0.524 and 0.005, 0.790 and <0.001, respectively. The correlation values (Rs and p) between air vs. needle, air vs. 1-year-old branch and air vs. 2-year-old branch samples for light molecular weight (LMW) PAHs were determined as 0.228 and 0.363, 0.274 and 0.243, 0.370 and 0.119, respectively. These values for heavy molecular weight PAHs were 0.067 and 0.736, −0.186 and 0.354, −0.381 and 0.089 for air vs. needle (n = 28), air vs. 1-year-old branch (n = 27) and air vs. 2-year-old branch (n = 28), respectively. According to the ‘p’ results, there is an acceptable relationship between all tree components and ambient air concentrations. In the evaluation of medium molecular weight PAHs, the best correlation was found between the air vs. 2-year-old branch values (Rs = 0.790; p < 0.001).

Table 3.
Rs values between air and tree components according to molecular weights of PAHs.
Considering the MMW PAH data, the tree components had lower COD values among themselves. For example, the COD values for the needle vs. 1-year-old branch, needle vs. 2-year-old branch and 1-year-old branch vs. 2-year-old branch samples were 0.374, 0.368 and 0.279, respectively. This result indicated that tree components were affected by similar sources and they absorbed the PAHs similarly. Moreover, the COD values between air vs. tree components were also calculated and they were high. These values, for MMW PAH, were 0.950, 0.936 and 0.928 for air vs. needle, air vs. 1-year-old branch and air vs. 2-year-old branch samples, respectively. In terms of correlation values between tree components and air, the highest Rs values were calculated between 1-year-old vs. 2-year-old branch (0.775; <0.001) and air vs. 2-year-old branch (0.790; <0.001) (Table 4).

Table 4.
Rs values among each tree component pair.
3.1.2. Diagnostic Ratios
Diagnosis ratios among some PAHs are considered to be the “fingerprint” of an emission source, as it presents specific characteristics in the form of molecular patterns versus their mechanism of formation [57,58]. Different PAH ratios are examined to evaluate some of the PAH sources or source groups. The sampling point was exposed to many sources, such as industrial zone, residential areas and highways. Table 5 summarizes the diagnosis rates calculated according to the ambient air values.
The Indeno/(Indeno+BghiP) ratio can be used to distinguish between diesel and gasoline vehicle emissions [6,59,60,61], as well as to evaluate grass, wood and coal combustion emissions [62]. According to the calculated Indeno/(Indeno+BghiP) ratios, changing between 0.08 and 0.61, the contribution of petrogenic and pyrolytic emissions was observed throughout the year (Table 5). Industrial, domestic and traffic-related sources around the sampling point were detected as important factors based on these findings. When the data for the spring (0.43 ± 0.15) and summer (0.43 ± 0.05) months were evaluated, the gasoline-related sources became dominant as the heating-related sources decreased [62].

Table 5.
Diagnostic ratios of atmospheric PAHs.
Table 5.
Diagnostic ratios of atmospheric PAHs.
| Diagnostic Ratio | ||||
|---|---|---|---|---|
| Value | Potential Source | Reference | This Study | |
| Indeno/(Indeno+BghiP) | <0.2 >0.5 0.2–0.5 0.4 0.3–0.7 0.56 0.48 0.19 0.32 0.32 0.36 0.35 0.42 | Petrogenic Grass, wood and coal combustion Petroleum combustion Gasoline Diesel engine Coal Coal combustion Diesel vehicles Gasoline vehicles Natural gas combustion Oil combustion Vegetation combustion Wood combustion | [63] [6] [6] [64] [60] [65] [62] [62] [62] [62] [62] [62] [62] | 0.08–0.61 |
| BaA/(BaA+Chr) | 0.2–0.35 >0.35 <0.2 >0.35 0.53 0.73 0.79 0.46 0.65 0.50 0.39 0.50 0.59 | Coal combustion Vehicle emission Petrogenic Combustion Vehicle emission Diesel engine Wood burning Coal combustion Diesel vehicles Gasoline vehicles Natural gas combustion Oil combustion Wood combustion | [66] [67] [63] [63] [57] [68] [57] [62] [62] [62] [62] [62] [62] | 0.55–1.00 |
| Phe/Ant | <10 >15 | Pyrogenic Petrogenic | [69] [69] | 1.00–48.19 |
| BaP/BghiP | <0.6 >0.6 | Non-traffic emissions Traffic emissions | [66] [66] | 0.38–1.36 |
| Fluo/(Fluo+Py) | <0.5 >0.5 | Petrol emissions Diesel emissions | [66] [70] | 0.61–0.66 |
| BaA/Chr | <0.2 >0.35 0.20–0.35 <0.35 >0.35 >0.4 <0.4 | Petrogenic, Combustion Petroleum or combustion Coal combustion Vehicle emission Fresh particles, Ageing (photolysis) | [63] [63] [63] [67] [67] [71] [71] | 1.21–2.97 |
| LMW/HMW | <1 >1 | Pyrolytic Petrogenic | [72] [72] | 0.74–2.61 |
| ΣCOMB/ΣPAHs | >0.49 0.34 0.7–0.73 0.19–0.39 >0.7 0.41 0.51 0.30 0.8–0.9 | Industrial site Remote site Combusiton source: Non-catalyst-equipped vehicles Petrogenic Pyrogenic Non-catalyst-equipped Catalyst-equipped Heavy duty diesel trucks Coal burning | [73] [73] [74] [60] [68] [68] [68] [68] [75] | 0.28–0.57 |
Similarly, BaA/(BaA+Chr) ratios also revealed the contribution of vehicle emissions and combustion in our study [59,67]. Phe/Ant ratio is one of the most frequently used combinations that distinguish pollutant sources from pyrogenic (combustion source) and petrogenic (unburned petroleum product) [76]. In this study, when the seasonal Phe/Ant ratio was evaluated, pyrogenic sources were dominant in winter (5.36 ± 7.55). The diagnostic ratio of BaP/BghiP is used to determine the significance of non-traffic emissions (<0.6) [77]. The values calculated in our study showed that traffic emissions were significant in both summer and autumn (0.71 ± 0.28 and 0.98 ± 0.54), respectively. Fluo/(Fluo+Py) values greater than 0.50 are reported for diesel emissions, while values less than 0.50 are indicative of gasoline emissions [70]. In the sampling region, petroleum emissions (0.20 ± 0.35 in summer, 0.43 ± 0.37 in autumn) dominate according to Fluo/(Fluo+Py) ratios.
Diagnostic ratios of PAH can also provide information about the aging of air masses [6,71,78]. If BaA/Chr ratios are higher than 0.4, it indicates the presence of fresh emissions and relatively little photolysis of the air mass, ratios below 0.4 indicate that the primary sources of PAHs are not local, the air masses are old, that is, convection from other regions [71]. In evaluating this ratio, it has been shown that even transporting PAHs more than a few kilometers could be sufficient to cause a change in source identification interpretation [79]. Differences in travel distances between pairs of PAHs suggest that the use of molecular detection rates for source identification requires careful consideration. BaA/(BaA+Chr) ratios were found to be the most robust diagnostic ratio for air concentrations among molecular diagnostic rates [79]. The results of the present study showed that fresh emissions (2.29 ± 0.70 in winter, 1.23 ± 0.02 in autumn) were released, as expected, from local sources. When the BaA/(BaA+Chr) ratio was evaluated to determine the source of the emissions, it also indicated combustion and traffic emissions (0.69±0.70 in winter, 0.55 ± 0.004 in autumn).
According to the literature, LMW (light molecular weight)/HMW(heavy molecular weight) <1 is an indicator of pyrogenic sources such as fossil fuel or incomplete combustion of wood, while LMW/HMW> 1 becomes an indicator of a petrogenic source, including oil (split oil) or petroleum products [80,81]. In this study, LMW/HMW ratios in the ambient air samples showed a significant dominance of petrogenic sources in winter and autumn with values of 1.92 ± 0.62 and 1.92 ± 0.53, respectively, while a value close to 1 in summer and spring (1.05 ± 0.27 and 1.06 ± 0.44, respectively) revealed the effect of both petrogenic and pyrogenic sources.
Sojinu et al. (2010) [82] conducted studies on 11 plants from the Delta State of Nigeria between April and July 2008 for biomonitoring studies of PAHs. They used Phe/Ant, Flt/Pyr to identify PAH sources and BaA/(BaA+Chr) and Indeno/(Indeno+BghiP) ratios for a more detailed distinction. The most commonly used combination to distinguish between pyrogenic and petrogenic sources is the Phe/Ant ratio to the Flt/Pyr ratio [52,76]. Phe/Ant ratios below 10 and Flt/Pyr ratios above 1 indicate pyrogenic sources [69]. In our study, Phe/Ant ratios varied between 2.23 and 33.23 in pine needles, while this ratio changed from 7.09 to 16.81 and from 4.13 to 25.36 in 1- and 2-year-old pine branches, respectively. High Phe/Ant ratios in pine needles can be attributed to the degradation of Ant in pine needles or the presence of more traveled sources [52,74]. While Flt/Pyr ratios were between 0–9.23 in pine needles and varied between 0.22 and 2.41, and 0.35 and 3.07 in 1- and 2-year-old pine branches, respectively. According to these ratios, pyrolytic and mixed sources were dominant in pine branches and needles as PAH sources while petrogenic and indeterminate sources could be evaluated in some months (Figure 4). When the air samples in the months with uncertain sources were examined, it was revealed that the sources could be defined as pyrolytic and mixed sources in these months.
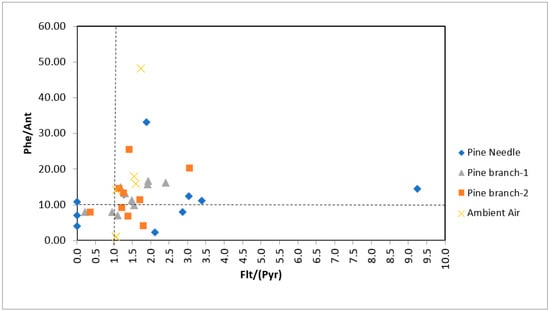
Figure 4.
Phe/Ant ratios against Flt/Pyr ratios for the tree components and ambient air. Note: Pine branch-1 and Pine branch-2 refer to 1-year-old and 2-year-old branches.
Indeno/(Indeno+BghiP) ratios (Table 6) in pine needles ranged from 0 to 1.00. This ratio was between 0.31–1.00 and 0–0.56 in 1- and 2-year-old pine branches, respectively. BaA/(BaA+Chr) ratios vary between 0–1.00, 0–0.93 and 0–0.70 in pine needles, 1- and 2-year-old branches, respectively. BaA/(BaA+Chr) and Indeno/(Indeno+BghiP) results indicated that most of the sources can be considered as combustion and mixed while the rest can be considered petroleum-related (Figure 5).
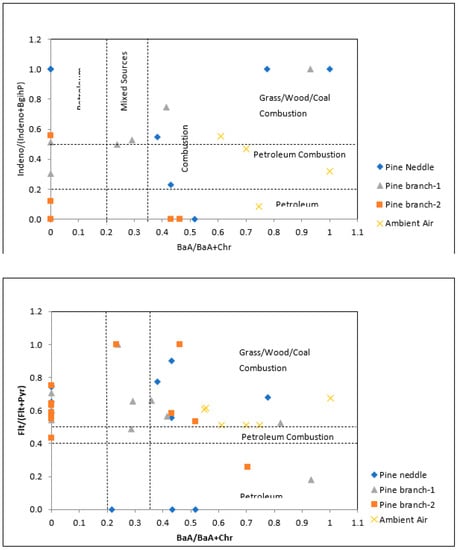
Figure 5.
PAH cross-plots for the ratios of Indeno/(Indeno+BghiP) and Flt/(Flt+Py) against BaA/(BaA+Chr) ratios in the studied tree components and ambient air. Note: Pine branch-1 and Pine branch-2 refer to 1-year-old and 2-year-old branches.
When the Flt/(Flt+Py) and BaA/(BaA+Chr) ratios are examined (Figure 5), it is clear that the PAH sources in pine needles and pine branches for this study are mainly coming from combustion. In addition to this, it is clear that there is also a petroleum-derived PAH distribution. When examined in terms of ambient air data, it is seen that all data are caused by combustion. A parallel result emerges when the Indeno/(Indeno+BghiP) and BaA/(BaA+Chr) ratios are examined.
The ratio of ΣCOMB (specific main combustion compounds including Flt, Pyr, BaA, Chry, BbF, BkF, BaP, Indeno and BghiP) to the total PAHs (ΣCOMB/ΣPAHs) in pine needles varies between 0.49 and 0.65 in industrial areas, while in remote areas this ratio drops to 0.34 [73]. The high ΣCOMB/ΣPAH ratios in our site (Figure 6) represent PAHs released from combustion [83]. The ΣCOMB/ΣPAHs is also an important ratio used to distinguish petrogenic additives and combustion sources [68,70,74,84,85,86], and the value of 0.41 represents a non-catalyst equipped vehicle, 0.51 catalyst-equipped vehicle and 0.3 heavy-duty diesel trucks. On the other hand, Cecinato et al. (1999) [75] stated that this value being 0.8–0.9 indicates coal burning. In our study, while the ΣCOMB/ΣPAHs values (Table 6) in pine needles were in the range of 0.09–0.80 with an average of 0.40 ± 0.19, a similar ratio, calculated for the 1- and 2-year-old branches and ambient air (0.47 ± 0.19, 0.41 ± 0.15 and 0.41 ± 0.10, respectively), reveals the contribution of the mixed PAH source distribution, where the industrial region, petrogenic sources and coal burning are also influential (Figure 6).
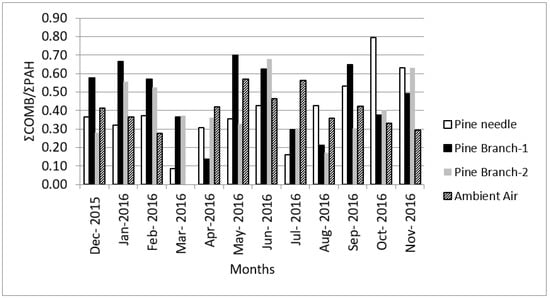
Figure 6.
ΣCOMB/ΣPAHs ratios for the tree components and ambient air. Note: Pine branch-1 and Pine branch-2 refer to 1-year-old and 2-year-old branches.
BFs (BbF, BkF and BjF)/BghiP ratios are utilized to distinguish combustion sources [87]. BFs/BghiP value of 7.11–11.2 shows the effect of industrial furnaces [88]. The values obtained in this study were 9.59 ± 12.67 for needles, 2.57 ± 1.33 and 1.83 ± 2.26 for 1- and 2-year-old pine branches, respectively. These values support industrial activities and traffic contributions. While the Flt/(Flt+Pyr) ratio ranged from 0 to 0.90 and in pine needles, values between 0.18–1.00 and 0.26–1.00 were calculated for 1- and 2-year-old pine branches, respectively. Flt/(Flt+Pyr) ratios above 0.50 are characteristic of grass, wood or coal-burning [23,63,73]. Considering the location of our sampling point, the determined results are reasonable.
3.1.3. Principle Component Analysis (PCA)
In addition to diagnostic ratios, principal component analysis (PCA) is employed to estimate the relative distribution of each source and identify the primary sources of PAH emissions [66]. The standardized distribution of various factors to different concentrations of PAHs is given in Tables S1 and S2. Each principal component is obtained with different factor loadings and determined and evaluated as possible pollution source indicators. In association with PAH, those with component weights ≥0.25 were evaluated as criteria [27]. In this assessment, those with component weights of 1–0.75 were strong; 0.75–0.50 were moderate and 0.5–0.3 indicated weak effects [89,90].
The PCA results for ambient air, pine needles and branches are given in Tables S1 and S2. PCA determined two components for ambient air data. Two factors explained the majority of the variance (91.84%). PC1 was responsible for 63.81% of the variance and mainly had a substantial load for PAH compounds such as Ace, Fluo, Phe, BaA, Chr, BbF, BkF, BaP, Indeno and BghiP, while compounds Flt, Pyr and DahA had a moderate load. A load of Ace, Fluo and Phe compounds characterizes wood, coal and biomass combustion [91]. The predominance of 5- and 6-ring PAHs is an indicator of gasoline traffic load and incomplete combustion [27]. In previous studies, BaA, BaP and BghiP have been reported to be markers of motor vehicle emissions and oil combustion [66,92,93,94]. According to the 2016 Bursa Province environmental status report [95], it has been stated that there was illegal construction near the sampling point (Yolçatı district). Illegal structures are not allowed to use natural gas because they have no permission to be connected to the natural gas distribution network. In the same report, it was stated that SO2 values were high, especially as a result of burning coal for heating purposes. While this contributes to light PAH compounds, the highway was also thought to contribute significantly to heavy PAH compounds. Then, this source could be defined as a mixed source (traffic + heating) indicator of road traffic and combustion for heating purposes.

Table 6.
Diagnostic ratios of PAHs in pine needles/branches.
Table 6.
Diagnostic ratios of PAHs in pine needles/branches.
| Diagnostic Ratio | ||||
|---|---|---|---|---|
| Value | Potential Source | Reference | This Study | |
| Indeno/(Indeno+BghiP) | >0.50 <0.20 | Solid fuel (Grass, wood or coal) combustion Asphalt, tire particles, motor oils and uncombusted fuels, petrogenic | [96,97] [63,98,99] | 0–1 (Needle) 0.31–1 (1-year-old branches) 0–0.56 (2-year-old branches) |
| BaA/(BaA+Chr) | 0.2–0.35 >0.35 | Petroleum and fuel oil combustion, Solid fuel (Grass, wood or coal) combustion | [6] [6] | 0–1(Needle) 0–0.93 (1-year-old branches) 0–0.70 (2-year-old branches) |
| Flt/(Flt+Py) | <0.40 >0.50 0.40–0.50 | Petroleum Solid fuel (Grass, wood or coal) combustion Fossil fuel combustion petroleum and fuel oil combustion | [63] [23,96,97,100] [6] | 0–0.90 (Needle) 0.18-1 (1-year-old branches) 0.26–1 (2-year-old branches) |
| ΣCOMB/ΣPHs | >0.49 0.34 0.7–0.73 0.19–0.39 >0.7 0.41 0.51 0.30 0.8–0.9 | Industrial site Remote site Combustion source: non-catalyst-equipped vehicles Petrogenic Pyrogenic non-catalyst-equipped catalyst-equipped heavy duty diesel trucks Coal burning | [101] [101] [74] [60] [68] [68] [68] [68] [75] | 0.09–0.80 (Needle) 0.14–0.70 (1-year-old branches) 0.17–0.68 (2-year-old branches) |
The second component explained with 28.03%, PAH compounds such as BaP and DahA have a moderate load, while Ace, Fluo, Indeno compounds have a light load. BaP, DahA and Indeno compounds originate from vehicle (gasoline and diesel) exhaust emissions [102,103]. Therefore, this component explains the traffic emissions.
As a result of the PCA analysis, the characteristic sources of variances overlap with the sources represented in the diagnosis rates, explained in the above section. It has been revealed that both pyrogenic and petrogenic sources were dominant in diagnosis rates, the dominance of PAHs due to heating increases in cold weather; besides, the contribution of traffic emissions was quite large and there was a variance representing the industrial zone located near the sampling point.
Five components (90.76%) representing the majority of the variance were determined by PCA analysis in pine needles. The high number of components in needles may be due to fluctuations in PAH concentrations in the needle being more affected by biological processes [104] than concentrations in air. The first group component PC1 is responsible for 30.5% of the variance and the main component is BkF (0.805), BaP (0.941), Indeno (0.866) and DahA (0.797) compounds. Syed et al. (2017) [105] stated that a source with a high concentration of BghiP, Indeno and BaP might originate from combustion due to the predominance of heavy PAHs. Many researchers have stated that BaP, Indeno and DhaA are released from vehicle emissions [106,107,108] and these compounds can be defined as emissions released from gasoline vehicles [109,110,111] in particular. Therefore, the PC1 component is considered to represent vehicle (gasoline and diesel) emissions.
The second component with a high Chr compound and moderate Ant, Pyr and BbF loadings, explaining the total variance at the rate of 24.96%. BaA, Chr and BbF are used as specific indicators of coal combustion in industries [112,113,114,115]. Chr has also been stated as the natural gas emission characteristic in previous studies [60,116,117] and the steel industry emission together with BaA [115]. While diesel emissions are rich in Flt, Chr and Pyr, Ant, Phen, BaP, Flt and Pyr are evaluated as markers of wood-burning [115]. In another study, Fla, Pyr, Phe, Ant and BaA were evaluated as coal combustion profiles [102]. BbF compound originates from vehicle (gasoline and diesel) exhaust emissions [103]. This component represents a mixed source as an indicator of industrial and domestic heating (coal, natural gas) combustion and traffic emissions.
PC3 variance (17.31%), with moderate loading of Ace, Fluo, Phe and BaA compounds, characterizes wood and coal combustion [58], as well as being the main compounds emitted from coke ovens (Fluo, Ace, Nap, Phe, Ant ve Flt)[118,119]. This occurrence reveals the industrial zone’s influence.
The fourth source with moderate Anth and weak Pyr loading explains 9.95% of the data variance. Ant refers to a petrogenic source (unburned) [107,120] and it is treated more as vehicle emissions in the source allocation [120]. Anth has been evaluated as an indicator of wood burning in some sources [121]. Pyr is an indicator of gasoline vehicle emissions [119]. Therefore, this component is thought to represent vehicle emissions as in PC1. In the PCA analysis evaluation, the fifth source (8.05%) with moderately positive BghiP loading and weak Fluo, Phe, Anth and Pyr (coal, wood and diesel combustion emissions) [119] loadings can be evaluated as combustion emissions.
On the other hand, four components represent a significant part of the variance (84.56%) in 1-year branches with PCA analysis performed on pine branches. The PC1 component explained 28.43% of the total variance. In the first group of components (PC1), Phe, Ant, Pyr, Chr and Flt compounds have a high load. Phe, Ant, Pyr, Chr and Flt compounds have a high load on PC1. Studies by Ciaparra et al. (2009) [122] and Jang et al. (2013) [123] indicated that a similar profile formed by Ant, BaA and Pyr derivatives partially reflects emissions from the steel industry. BaA, Chr and BbF are used as specific indicators of coal combustion in industries [112,113,114]. Phe, Ant and Flt compounds are the main components emitted from coke ovens [119]. These compounds are thought to originate from industrial emissions from the industrial area near the sampling point.
The second component has high Ace and Indeno and moderate Fluo, BbF, DahA and BghiP and weak Phe loading, explaining 24.17% of the total variance. Indeno originates from vehicle (gasoline and diesel) exhaust emissions [102]. BbF, BghiP and Indeno come from vehicle emissions [119], Indeno, BghiP and DahA are typical traffic emissions [91,124], Ace, Fluo and Phe compounds load wood, coal and characterize biomass combustion [58,91]. Therefore, this component is a mixed source representing pyrogenic and petrogenic sources.
The third component (21.1%) has a weak load of Flt and BghiP. This composition is typically the source of vehicle emissions (gasoline and diesel) [103]. However, some researchers [125] stated that Flt is an indicator of coal combustion. This variance indicates the vehicle and the source of combustion. The last component (PC4), which explains 10.87% of the variance, has strong BaP (0.828) and weak Anth and Indeno loadings. While BaP is an important indicator of petroleum and diesel emissions [80], Indeno and DahA come from gasoline vehicle emissions [108,109,110,111], Anth indicates a petrogenic source [107,120]. Therefore, the PC4 component is considered to represent vehicle emissions.
In 2-year-old pine branches, 88.19% of the variance is represented by five components. When the compound loads were evaluated in the PCA analysis, sources similar to the ones detected with 1-year-old pine branches emerged. PC1 (31.07%) component, which has a high load for Phe (0.895) and Flt (0.91) compounds and moderately loaded for Ace, Anth, Pyr and Chr compounds, is a component of the steel industry and pyrolytic sources [126] can be considered as a mixed source representing together.
The second component, which explains 23.61% of the total variance, has high BkF (0.824) and BbF (0.772) compounds and moderate BaA, Chr and BaP loads. While high loadings of BkF have been associated with coal burning in previous studies [91] and BbF with diesel vehicle emissions [119], BaP and BaA are important indicators for petroleum and diesel emissions [80]. In another study, Anth, BaA, Chr and BaP were evaluated as biomass and coal combustion indicators [124,127]. This component can be considered a mixed source.
The other two components explaining the variance at 15.75% (PC3) and 10.54% (PC4) rates were also associated with moderate Fluo (0.637), BaA (0.535), DahA (0.746) and moderately Indeno (0.63), BghiP (0.543) for PC3 and PC4, respectively. Fluo refers to combustion (coal, wood and diesel) and vehicle emissions [119], DahA [109] and Indeno [11,128] are defined by gasoline emissions. Therefore, these two components are considered to represent vehicle emissions.
The fifth component, explaining 7.23% of the total variance, was associated with moderate Pyr and weakly Ace, Anth, Pyr, BaA, Chr and Indeno compounds. Ace and Anth comes from burning source [91], and the profile of Anth, Pyr and BaA derivatives reflects emissions from the steel industry [122,123]. In this case, this component can indicate the effect of the industrial zone.
Generally, it was determined that ambient air, pine needles and branches reflect the relative distribution of all possible pollutants in the sampling area. Pine needles and branches have been a good tool to reveal the industrial emission source and the traffic effect.
3.2. Carcinogenic Potential
Sixteen PAHs have been listed among priority pollutants by the United States Environmental Protection Agency (US-EPA). Adverse health effects (carcinogens, mutagens and teratogens) from PAH exposure are a major concern [38]. The International Agency for Research on Cancer (IARC) has classified some of the PAH strains in terms of carcinogenic effects. The most important health effect that can be expected if PAHs are inhaled is the risk of lung cancer [41]. The concentrations of PAH species were calculated as BaP equivalent concentrations (BaPeq) using TEF values [129] of each PAH type in order to reveal the carcinogenic potential that may occur as a result of inhalation of atmospheric PAHs. In this study, BaP equivalent concentrations (BaPeq) Σ14BaPeq values range from 0.006 to 0.71 ng/m3 throughout the year (Table S3). These values are below both the international standard value (10 ng/m3) and the WHO standard value (1 ng/m3) [130]. Average values of Σ14BaPeq were calculated as 0.33 ± 0.18, 0.02 ± 0.02, 0.05 ± 0.03 and 0.35 ± 0.33 ng/m3 in winter, spring, summer and autumn samples, respectively. The highest Σ14BaPeq data were reached in winter and autumn.
Lifetime lung cancer risk was calculated using the following formula [131]:
where UR is the respiratory unit risk of exposure to BaP (the theoretical upper limit probability of contracting cancer when exposed to BaP at a concentration of one nanogram per cubic meter over a 70-year lifetime) [132,133]. In this study, UR is equal to 8.7 × 10−5 per ng/m3 was taken based on an epidemiology study according to the World Health Organization (WHO) [134].
The calculated lung cancer risk values are 2.85 × 10−5, 1.64 × 10−6, 4.40 × 10−6 and 3.02 × 10−5 in winter, spring, summer and autumn samples, respectively. These risk values calculated for the study area indicate that US EPA poses cancer risks because it exceeds the guideline safety value of 10−6 (a cancer case in a population of one million people for life) [131,135,136,137]. These values indicate that the risk of epidemiological lung cancer as a result of lifetime exposure to total ambient PAHs is significant and should be considered in maintaining health in the future. In winter and autumn, lung cancer risk values increased by 6.49 and 6.88 times, respectively, compared to the summer season. Risk values increase in parallel with the increase in PAH concentrations caused by heating in the autumn and winter months. Cold seasons pose a higher potential risk to human health.
4. Conclusions
Ambient air, pine needles and branch samples were collected for a one-year period. Diagnosis ratios and PCA analyses applied to all data sets revealed that needles and branches could effectively detect and monitor all contaminant sources in the sampling area. As a result of the PCA analysis, the characteristic sources of variances coincide with the sources represented in the diagnosis ratios. It has been demonstrated that both pyrogenic and petrogenic sources are predominant in diagnosis ratios, PAHs caused by warming in a cold weather increase; besides, the contribution of traffic emission is quite large and there is a variance representing the industrial zone located near the sampling point. The calculated cancer risk values rise with the increase in pollutant concentrations depending on the heating in the autumn and winter months. In winter and autumn, lung cancer risk values increased by 6.49 and 6.88 times, respectively, compared to the summer season. Calculated risk values revealed that epidemiological lung cancer risk is important and should be considered in future health protection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atmos13111938/s1, Table S1. PCA analyses results for PAHs in the ambient air and pine needles; Table S2. PCA analyses results for PAHs in the one-year-old and two-year-old branches; Table S3. Total BaP equivalent concentrations (Σ14BaPeq) values in this study.
Author Contributions
Y.T. Conceptualization, methodology, processing raw data, review and editing, funding acquisition, supervision, project administration. S.C.E. processing raw data, writing original draft preparation, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scientific and Technological Research Council of Turkey (TUBITAK Project No. 114Y577); the Bursa Uludag University Scientific Research Projects (Project No. DDP (MH)-2020/11).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to A Egemen Sakın, Burak Çalışkan and Berfu Bükler for their help in the experimental studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdallah, M.A.E.; Atia, N.N. Atmospheric concentrations, gaseous-particulate distribution, and carcinogenic potential of polycyclic aromatic hydrocarbons in Assiut, Egypt. Environ. Sci. Pollut. Res. 2014, 21, 8059–8069. [Google Scholar] [CrossRef] [PubMed]
- Fasani, D.; Fermo, P.; Barroso, P.J.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Analytical method for biomonitoring of PAH using leaves of bitter orange trees (Citrus Aurantium): A case study in South Spain. Water Air Soil. Pollut. 2016, 227, 360. [Google Scholar] [CrossRef]
- Salihoglu, N.K.; Salihoglu, G.; Tasdemir, Y.; Cindoruk, S.S.; Yolsal, D.; Ruken, O.; Karaca, G. Comparison of polycyclic aromatic hydrocarbons levels in sludges from municipal and industrial wastewater treatment plants. Arch. Environ. Contam. Toxicol. 2010, 58, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.F.; Esen, F.; Tasdemir, Y. Biomonitoring and source identification of Polycyclic Aromatic Hydrocarbons (PAHs) using pine tree components from three different sites in Bursa, Turkey. Arch. Environ. Contam. Toxicol. 2020, 78, 646–657. [Google Scholar] [CrossRef]
- Tasdemir, Y.; Esen, F. Urban air PAHs: Concentrations, temporal changes and gas/particle partitioning at a traffic site in Turkey. Atmos. Res. 2007, 84, 1–12. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Sanli, G.E.; Tasdemir, Y. Accumulations and temporal trends of Polychlorinated Biphenyls (PCBs) in olive tree components. Environ. Geochem. Health 2022, 44, 2577–2594. [Google Scholar] [CrossRef] [PubMed]
- Simonich, S.L.; Hites, R.A. Importance of vegetation in removing polycyclic aromatic hydrocarbons from the atmosphere. Nature 1994, 370, 49–51. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef]
- Huckins, J.N.; Tubergen, M.W.; Manuweera, G.K. Semipermeable membrane devices containing model lipid: A new approach to monitoring the Bioavaiiability of Lipophilic contaminants and estimating their bioconcentration potential. Chemosphere 1990, 20, 533–552. [Google Scholar] [CrossRef]
- Odabasi, M.; Dumanoglu, Y.; Ozgunerge Falay, E.; Tuna, G.; Altiok, H.; Kara, M.; Bayram, A.; Tolunay, D.; Elbir, T. Investigation of spatial distributions and sources of Persistent Organic Pollutants (POPs) in a heavily polluted industrial region using tree components. Chemosphere 2016, 160, 114–125. [Google Scholar] [CrossRef]
- Ratola, N.; Amigo, J.M.; Oliveira, M.S.N.; Araújo, R.; Silva, J.A.; Alves, A. Differences between Pinus Pinea and Pinus Pinaster as bioindicators of polycyclic aromatic hydrocarbons. Environ. Exp. Bot. 2011, 72, 339–347. [Google Scholar] [CrossRef]
- Oishi, Y. Comparison of pine needles and mosses as bio-indicators for polycyclic aromatic hydrocarbons. J. Environ. Prot. 2013, 4, 106–113. [Google Scholar] [CrossRef]
- Piccardo, M.T.; Pala, M.; Bonaccurso, B.; Stella, A.; Redaelli, A.; Paola, G.; Valerio, F. Pinus Nigra and Pinus Pinaster needles as passive samplers of polycyclic aromatic hydrocarbons. Environ. Pollut. 2005, 133, 293–301. [Google Scholar] [CrossRef]
- Ratola, N.; Lacorte, S.; Alves, A.; Barceló, D. Analysis of polycyclic aromatic hydrocarbons in pine needles by gas chromatography-mass spectrometry: Comparison of different extraction and clean-up procedures. J. Chromatogr. A 2006, 1114, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Simonich, S.L.; Hites, R.A. Organic pollutant accumulation in vegetation. Environ. Sci. Technol. 1995, 29, 2905–2914. [Google Scholar] [CrossRef]
- Tomashuk, T.A.; Truong, T.M.; Mantha, M.; McGowin, A.E. Atmospheric polycyclic aromatic hydrocarbon profiles and sources in pine needles and particulate matter in Dayton, Ohio, USA. Atmos. Environ. 2012, 51, 196–202. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Tian, F.; Yang, M.; Liu, C.; Li, Y.-F. Application of positive matrix factorization to identify potential sources of PAHs in Soil of Dalian, China. Environ. Pollut. 2009, 157, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Zítková, J.; Hegrová, J.; Keken, Z.; Ličbinský, R. Impact of road salting on scots pine (Pinus Sylvestris) and Norway Spruce (Picea Abies). Ecol. Eng. 2021, 159, 106129. [Google Scholar] [CrossRef]
- Cindoruk, S.S.; Sakin, A.E.; Tasdemir, Y. Levels of persistent organic pollutants in pine tree components and ambient air. Environ. Pollut. 2020, 256, 113418. [Google Scholar] [CrossRef]
- De Nicola, F.; Maisto, G.; Prati, M.V.; Alfani, A. Temporal variations in PAH concentrations in Quercus Ilex L. (Holm Oak) leaves in an urban area. Chemosphere 2005, 61, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Hubai, K.; Kováts, N.; Sainnokhoi, T.-A.; Teke, G. Accumulation pattern of polycyclic aromatic hydrocarbons using Plantago Lanceolata L. as passive biomonitor. Environ. Sci. Pollut. Res. 2022, 29, 7300–7311. [Google Scholar] [CrossRef] [PubMed]
- Lehndorff, E.; Schwark, L. Biomonitoring of air quality in the cologne conurbation using pine needles as a passive sampler—Part II: Polycyclic Aromatic Hydrocarbons (PAH). Atmos. Environ. 2004, 38, 3793–3808. [Google Scholar] [CrossRef]
- Librando, V.; Perrini, G.; Tomasello, M. Biomonitoring of atmospheric PAHs by evergreen plants: Correlations and applicability. Polycycl. Aromat. Compd. 2002, 22, 549–559. [Google Scholar] [CrossRef]
- Marsili, M.; Stracquadanio, M.; Trombini, C.; Vassura, I. The epicuticular wax of laurus nobilis leaves as a passive sampler of polycyclic aromatic hydrocarbons in ambient air. Fresenius Environ. Bull. 2001, 10, 26–30. [Google Scholar]
- Meharg, A.A.; Wright, J.; Dyke, H.; Osborn, D. Polycyclic Aromatic Hydrocarbon (PAH) dispersion and deposition to vegetation and soil following a large scale chemical fire. Environ. Pollut. 1998, 99, 29–36. [Google Scholar] [CrossRef]
- Kargar, N.; Matin, G.; Matin, A.A.; Buyukisik, H.B. Biomonitoring, status and source risk assessment of Polycyclic Aromatic Hydrocarbons (PAHs) using honeybees, pine tree leaves, and propolis. Chemosphere 2017, 186, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Tarricone, K.; Wagner, G.; Klein, R. Toward standardization of sample collection and preservation for the quality of results in biomonitoring with trees—A critical review. Ecol. Indic. 2015, 57, 341–359. [Google Scholar] [CrossRef]
- Chefetz, B.; Xing, B. Relative role of aliphatic and aromatic moieties as sorption domains for organic compounds: A review. Environ. Sci. Technol. 2009, 43, 1680–1688. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, L. Sorption of polycyclic aromatic hydrocarbons to carbohydrates and lipids of ryegrass root and implications for a sorption prediction model. Environ. Sci. Technol. 2009, 43, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- van Drooge, B.L.; Garriga, G.; Grimalt, J.O. Polycyclic aromatic hydrocarbons in pine needles (Pinus Halepensis) along a spatial gradient between a traffic intensive urban area (Barcelona) and a nearby natural park. Atmos. Pollut. Res. 2014, 5, 398–403. [Google Scholar] [CrossRef]
- Chrabąszcz, M.; Mróz, L. Tree bark, a valuable source of information on air quality. Pol. J. Environ. Stud. 2017, 26, 453–466. [Google Scholar] [CrossRef]
- Odabasi, M.; Ozgunerge Falay, E.; Tuna, G.; Altiok, H.; Kara, M.; Dumanoglu, Y.; Bayram, A.; Tolunay, D.; Elbir, T. Biomonitoring the spatial and historical variations of Persistent Organic Pollutants (POPs) in an industrial region. Environ. Sci. Technol. 2015, 49, 2105–2114. [Google Scholar] [CrossRef]
- Oishi, Y. Comparison of moss and pine needles as bioindicators of transboundary polycyclic aromatic hydrocarbon pollution in Central Japan. Environ. Pollut. 2018, 234, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fan, Z.T.; Wu, X.; Meng, Q.; Wang, S.W.; Tang, X.; Ohman-Strickland, P.; Georgopoulos, P.; Zhang, J.; Bonanno, L.; et al. Spatial variation of volatile organic compounds in a “hot spot” for air pollution. Atmos. Environ. 2008, 42, 7329–7338. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Ali-Musstjab-Akber-Shah Eqani, S.; Malik, R.N.; Katsoyiannis, A.; Zhang, G.; Zhang, Y.; Li, J.; Xiang, L.; Jones, K.C.; Shinwari, Z.K. Occurrence, finger printing and ecological risk assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in the Chenab River, Pakistan. J. Environ. Monit. 2011, 13, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Dvorská, A.; Lammel, G.; Klánová, J. Use of diagnostic ratios for studying source apportionment and reactivity of ambient polycyclic aromatic hydrocarbons over Central Europe. Atmos. Environ. 2011, 45, 420–427. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- IARC Working Group. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 92, 1–853. [Google Scholar]
- ACGIH (American Conference of Governmental Industrial Hygienists). Polycyclic Aromatic Hydrocarbons (PAHs) Biologic Exposure Indices (BEI) Cincinnati; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2005. [Google Scholar]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne Polycyclic Aromatic Hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Esen, F.; Evci, Y.M.; Tasdemir, Y. Evaluation and application of a passive air sampler for Polycylic Aromatic Hydrocarbons (PAHs). J. Environ. Sci. Heal. Part A 2017, 52, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Evci, Y.M.; Esen, F.; Taşdemir, Y. Monitoring of long-term outdoor concentrations of PAHs with passive air samplers and comparison with meteorological data. Arch. Environ. Contam. Toxicol. 2016, 71, 246–256. [Google Scholar] [CrossRef] [PubMed]
- The University of Iowa. PUF-PAS Sampling Rate Model Interface. Available online: http://s-iihr41.iihr.uiowa.edu/pufpas_model/ (accessed on 28 April 2021).
- Schuster, J.K.; Harner, T.; Eng, A.; Rauert, C.; Su, K.; Hornbuckle, K.C.; Johnson, C.W. Tracking POPs in global air from the first 10 years of the GAPS network (2005 to 2014). Environ. Sci. Technol. 2021, 55, 9479–9488. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.F.; Esen, F.; Tasdemir, Y. Characterization, source apportionment, air/plant partitioning and cancer risk assessment of atmospheric PAHs measured with tree components and passive air sampler. Environ. Res. 2021, 194, 110508. [Google Scholar] [CrossRef]
- Esen, F.; Cindoruk, S.S.; Taşdemir, Y. Ambient concentrations and gas/particle partitioning of polycyclic aromatic hydrocarbons in an urban site in Turkey. Environ. Forensics 2006, 7, 303–312. [Google Scholar] [CrossRef]
- Vardar, N.; Tasdemir, Y.; Odabasi, M.; Noll, K.E. Characterization of atmospheric concentrations and partitioning of PAHs in the Chicago atmosphere. Sci. Total Environ. 2004, 327, 163–174. [Google Scholar] [CrossRef]
- Birgul, A.; Tasdemir, Y. Concentrations, gas-particle partitioning, and seasonal variations of polycyclic aromatic hydrocarbons at four sites in Turkey. Arch. Environ. Contam. Toxicol. 2015, 68, 46–63. [Google Scholar] [CrossRef]
- Eker, G. Bursa’nin Zeytinlik Arazilerindeki Topraklarda Poliaromatik Hidrokarbon (Pah) Konsantrasyonlarinin Bölgesel Deǧişimi. J. Fac. Eng. Archit. Gazi Univ. 2017, 32, 607–616. [Google Scholar] [CrossRef][Green Version]
- Fernández-Varela, R.; Ratola, N.; Alves, A.; Amigo, J.M. Relationship between levels of polycyclic aromatic hydrocarbons in pine needles and socio-geographic parameters. J. Environ. Manage 2015, 156, 52–61. [Google Scholar] [CrossRef]
- Ratola, N.; Amigo, J.M.; Alves, A. Levels and sources of PAHs in selected sites from portugal: Biomonitoring with Pinus Pinea and Pinus Pinaster needles. Arch. Environ. Contam. Toxicol. 2010, 58, 631–647. [Google Scholar] [CrossRef][Green Version]
- Çalişkan Eleren, S.; Tasdemir, Y. Levels, distributions, and seasonal variations of Polycyclic Aromatic Hydrocarbons (PAHs) in ambient air and pine components. Environ. Monit. Assess 2021, 193, 253. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Pervez, S.; Chow, J.C.; Matawle, J.L.; Watson, J.G.; Sahu, R.K.; Srivastava, A.; Tiwari, S.; Pervez, Y.F.; Deb, M.K. Coarse Particle (PM10–2.5) source profiles for emissions from domestic cooking and industrial process in Central India. Sci. Total Environ. 2018, 627, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, C.; Ding, X.; Wang, X.; Fu, Q.; Zhao, Q.; Zhang, Y.; Duan, Y.; Qiu, X.; Zheng, M. Sources and spatial distribution of particulate polycyclic aromatic hydrocarbons in Shanghai, China. Sci. Total Environ. 2017, 584–585, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Guo, S.; Li, X.; Wang, J. Temporal and spatial variations of Polycyclic Aromatic Hydrocarbons (PAHs) in soils from a typical organic sewage irrigation area. Sci. Total Environ. 2018, 613–614, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Dickhut, R.M.; Canuel, E.A.; Gustafson, K.E.; Liu, K.; Arzayus, K.M.; Walker, S.E.; Edgecombe, G.; Gaylor, M.O.; MacDonald, E.H. Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay Region. Environ. Sci. Technol. 2000, 34, 4635–4640. [Google Scholar] [CrossRef]
- Khalili, N.R.; Scheff, P.A.; Holsen, T.M. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 533–542. [Google Scholar] [CrossRef]
- Kaur, S.; Senthilkumar, K.; Verma, V.K.; Kumar, B.; Kumar, S.; Katnoria, J.K.; Sharma, C.S. Preliminary analysis of polycyclic aromatic hydrocarbons in air particles (PM10) in Amritsar, India: Sources, apportionment, and possible risk implications to humans. Arch. Environ. Contam. Toxicol. 2013, 65, 382–395. [Google Scholar] [CrossRef]
- Kavouras, I.G.; Koutrakis, P.; Tsapakis, M.; Lagoudaki, E.; Stephanou, E.G.; Von Baer, D.; Oyola, P. Source apportionment of urban particulate aliphatic and Polynuclear Aromatic Hydrocarbons (PAHs) using multivariate methods. Environ. Sci. Technol. 2001, 35, 2288–2294. [Google Scholar] [CrossRef]
- Ravindra, K.; Wauters, E.; Tyagi, S.K.; Mor, S.; Van Grieken, R. Assessment of air quality after the implementation of Compressed Natural Gas (CNG) as fuel in public transport in Delhi, India. Environ. Monit. Assess 2006, 115, 405–417. [Google Scholar] [CrossRef][Green Version]
- Galarneau, E. Source specificity and atmospheric processing of airborne PAHs: Implications for source apportionment. Atmos. Environ. 2008, 42, 8139–8149. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River Basin: A Critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Caricchia, A.M.; Chiavarini, S.; Pezza, M. Polycyclic aromatic hydrocarbons in the urban atmospheric particulate matter in the city of Naples (Italy). Atmos. Environ. 1999, 33, 3731–3738. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Shahsavani, S.; Hoseini, M.; Dehghani, M.; Fararouei, M. Characterisation and potential source identification of polycyclic aromatic hydrocarbons in atmospheric particles (PM10) from urban and suburban residential areas in Shiraz, Iran. Chemosphere 2017, 183, 557–564. [Google Scholar] [CrossRef]
- Akyüz, M.; Çabuk, H. Gas–particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey. Sci. Total Environ. 2010, 408, 5550–5558. [Google Scholar] [CrossRef]
- Rogge, W.F.; Hildemann, L.M.; Mazurek, M.A.; Cass, G.R.; Simoneit, B.R.T. Sources of Fine Organic Aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks. Environ. Sci. Technol. 1993, 27, 636–651. [Google Scholar] [CrossRef]
- Baumard, P.; Budzinski, H.; Michon, Q.; Garrigues, P.; Burgeot, T.; Bellocq, J. Origin and bioavailability of PAHs in the mediterranean sea from mussel and sediment records. Estuar. Coast Shelf Sci. 1998, 47, 77–90. [Google Scholar] [CrossRef]
- Hoseini, M.; Yunesian, M.; Nabizadeh, R.; Yaghmaeian, K.; Ahmadkhaniha, R.; Rastkari, N.; Parmy, S.; Faridi, S.; Rafiee, A.; Naddafi, K. Characterization and risk assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in urban atmospheric particulate of Tehran, Iran. Environ. Sci. Pollut. Res. 2016, 23, 1820–1832. [Google Scholar] [CrossRef]
- Lohmann, R.; Northcott, G.L.; Jones, K.C. Assessing the contribution of diffuse domestic burning as a source of PCDD/Fs, PCBs, and PAHs to the U.K. atmosphere. Environ. Sci. Technol. 2000, 34, 2892–2899. [Google Scholar] [CrossRef]
- Wilcke, W. Global patterns of Polycyclic Aromatic Hydrocarbons (PAHs) in soil. Geoderma 2007, 141, 157–166. [Google Scholar] [CrossRef]
- Sun, F.; Wen, D.; Kuang, Y.; Li, J.; Li, J.; Zuo, W. Concentrations of heavy metals and polycyclic aromatic hydrocarbons in needles of Masson Pine (Pinus Massoniana L.) growing nearby different industrial sources. J. Environ. Sci. 2010, 22, 1006–1013. [Google Scholar] [CrossRef]
- Hwang, H.M.; Wade, T.L.; Sericano, J.L. Concentrations and source characterization of polycyclic aromatic hydrocarbons in pine needles from Korea, Mexico, and United States. Atmos. Environ. 2003, 37, 2259–2267. [Google Scholar] [CrossRef]
- Cecinato, A.; Marino, F.; Di Filippo, P.; Lepore, L.; Possanzini, M. Distribution of N-Alkanes, polynuclear aromatic hydrocarbons and nitrated polynuclear aromatic hydrocarbons between the fine and coarse fractions of inhalable atmospheric particulates. J. Chromatogr. A 1999, 846, 255–264. [Google Scholar] [CrossRef]
- Orecchio, S.; Gianguzza, A.; Culotta, L. Absorption of polycyclic aromatic hydrocarbons by Pinus bark: Analytical method and use for environmental pollution monitoring in the Palermo Area (Sicily, Italy). Environ. Res. 2008, 107, 371–379. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Terzi, E.; Cai, Q.Y. On the use of PAH molecular diagnostic ratios in sewage sludge for the understanding of the PAH sources. Is this use appropriate? Chemosphere 2007, 69, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Shabbaj, I.I.; Alghamdi, M.A.; Khoder, M.I. Street dust—bound polycyclic aromatic hydrocarbons in a Saudi Coastal City: Status, profile, sources, and human health risk assessment. Int. J. Environ. Res. Public Health 2018, 15, 2397. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, A.; Breivik, K. Model-based evaluation of the use of polycyclic aromatic hydrocarbons molecular diagnostic ratios as a source identification tool. Environ. Pollut. 2014, 184, 488–494. [Google Scholar] [CrossRef]
- Mishra, N.; Ayoko, G.A.; Morawska, L. Atmospheric polycyclic aromatic hydrocarbons in the urban environment: Occurrence, toxicity and source apportionment. Environ. Pollut. 2016, 208, 110–117. [Google Scholar] [CrossRef]
- Soclo, H.H.; Garrigues, P.; Ewald, M. Origin of Polycyclic Aromatic Hydrocarbons (PAHs) in coastal marine sediments: Case Studies in Cotonou (Benin) and Aquitaine (France) areas. Mar. Pollut. Bull. 2000, 40, 387–396. [Google Scholar] [CrossRef]
- Sojinu, O.S.; Sonibare, O.O.; Ekundayo, O.; Zeng, E.Y. Biomonitoring potentials of Polycyclic Aromatic Hydrocarbons (PAHs) by higher plants from an oil exploration site, Nigeria. J. Hazard Mater. 2010, 184, 759–764. [Google Scholar] [CrossRef]
- Yang, H.H.; Lai, S.O.; Hsieh, L.T.; Hsueh, H.J.; Chi, T.W. Profiles of PAH emission from steel and iron industries. Chemosphere 2002, 48, 1061–1074. [Google Scholar] [CrossRef]
- Khedidji, S.; Balducci, C.; Ladji, R.; Cecinato, A.; Perilli, M.; Yassaa, N. Chemical composition of particulate organic matter at industrial, university and forest areas located in Bouira Province, Algeria. Atmos. Pollut. Res. 2017, 8, 474–482. [Google Scholar] [CrossRef]
- Ladji, R.; Yassaa, N.; Balducci, C.; Cecinato, A. Particle size distribution of N-alkanes and Polycyclic Aromatic Hydrocarbons (PAHS) in urban and industrial aerosol of Algiers, Algeria. Environ. Sci. Pollut. Res. 2014, 21, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Sienra, M.D.R.; Rosazza, N.G.; Préndez, M. Polycyclic aromatic hydrocarbons and their molecular diagnostic ratios in urban atmospheric respirable particulate matter. Atmos. Res. 2005, 75, 267–281. [Google Scholar] [CrossRef]
- Mantis, J.; Chaloulakou, A.; Samara, C. PM10-bound Polycyclic Aromatic Hydrocarbons (PAHs) in the greater area of Athens, Greece. Chemosphere 2005, 59, 593–604. [Google Scholar] [CrossRef]
- Yang, H.H.; Lee, W.J.; Chen, S.J.; Lai, S.O. PAH Emission from various industrial stacks. J. Hazard Mater. 1998, 60, 159–174. [Google Scholar] [CrossRef]
- Azid, A.; Juahir, H.; Ezani, E.; Toriman, M.E.; Endut, A.; Rahman, M.N.A.; Yunus, K.; Kamarudin, M.K.A.; Hasnam, C.N.C.; Saudi, A.S.M.; et al. Identification source of variation on regional impact of air quality pattern using chemometric. Aerosol Air Qual. Res. 2015, 15, 1545–1558. [Google Scholar] [CrossRef]
- Iqbal, K.; Ahmad, S.; Dutta, V. Pollution mapping in the urban segment of a tropical river: Is Water Quality Index (WQI) enough for a nutrient-polluted river? Appl. Water Sci. 2019, 9, 197. [Google Scholar] [CrossRef]
- Dong, T.T.T.; Lee, B.K. Characteristics, toxicity, and source apportionment of Polycylic Aromatic Hydrocarbons (PAHs) in road dust of Ulsan, Korea. Chemosphere 2009, 74, 1245–1253. [Google Scholar] [CrossRef]
- Ho, K.F.; Lee, S.C.; Chiu, G.M.Y. Characterization of selected volatile organic compounds, polycyclic aromatic hydrocarbons and carbonyl compounds at a roadside monitoring station. Atmos. Environ. 2002, 36, 57–65. [Google Scholar] [CrossRef]
- Li, A.; Jang, J.K.; Scheff, P.A. Application of EPA CMB8.2 model for source apportionment of sediment PAHS in Lake Calumet, Chicago. Environ. Sci. Technol. 2003, 37, 2958–2965. [Google Scholar] [CrossRef]
- Ma, W.L.; Li, Y.F.; Qi, H.; Sun, D.Z.; Liu, L.Y.; Wang, D.G. Seasonal variations of sources of Polycyclic Aromatic Hydrocarbons (PAHs) to a northeastern urban city, China. Chemosphere 2010, 79, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Çed ve Çevre İzinleri Şube Müdürlüğü, Ç.V.Ş.İ. Bursa İli 2016 Yılı Çevre Durum Raporu. 2017. Available online: https://webdosya.csb.gov.tr/db/ced/editordosya/Bursa_icdr2016.pdf (accessed on 28 April 2021).
- Freeman, D.J.; Cattell, F.C.R. Woodburning as a source of atmospheric polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 1990, 24, 1581–1585. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Jones, A.D.; Turn, S.Q.; Williams, R.B. Emission factors for polycyclic aromatic hydrocarbons from biomass burning. Environ. Sci. Technol. 1996, 30, 2462–2469. [Google Scholar] [CrossRef]
- Van Metre, P.C.; Mahler, B.J. Contribution of PAHs from coal-tar pavement sealcoat and other sources to 40 U.S. lakes. Sci. Total Environ. 2010, 409, 334–344. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, F.; Lancellotti, C.; Prati, M.V.; Maisto, G.; Alfani, A. Biomonitoring of PAHs by using Quercus Ilex leaves: Source diagnostic and toxicity assessment. Atmos. Environ. 2011, 45, 1428–1433. [Google Scholar] [CrossRef]
- Budzinski, H.; Jones, I.; Bellocq, J.; Piérard, C.; Garrigues, P. Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Mar. Chem. 1997, 58, 85–97. [Google Scholar] [CrossRef]
- Orecchio, S. PAHs Associated with the Leaves of Quercus Ilex L.: Extraction, GC-MS analysis, distribution and sources. Assessment of air quality in the Palermo (Italy) Area. Atmos. Environ. 2007, 41, 8669–8680. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, W.; Chen, Y.; Zhang, S.; Feng, Q.; Hou, H.; Chen, F. Spatial variability of PAHs and microbial community structure in surrounding surficial soil of coal-fired power plants in Xuzhou, China. Int. J. Environ. Res. Public Health 2016, 13, 878. [Google Scholar] [CrossRef]
- Yang, J.; Xu, W.; Cheng, H. Seasonal variations and sources of airborne Polycyclic Aromatic Hydrocarbons (PAHs) in Chengdu, China. Atmosphere 2018, 9, 63. [Google Scholar] [CrossRef]
- Klánová, J.; Čupr, P.; Baráková, D.; Šeda, Z.; Anděl, P.; Holoubek, I. Can pine needles indicate trends in the air pollution levels at remote sites? Environ. Pollut. 2009, 157, 3248–3254. [Google Scholar] [CrossRef] [PubMed]
- Syed, J.H.; Iqbal, M.; Zhong, G.; Katsoyiannis, A.; Yadav, I.C.; Li, J.; Zhang, G. Polycyclic Aromatic Hydrocarbons (PAHs) in Chinese forest soils: Profile composition, spatial variations and source apportionment. Sci. Rep. 2017, 7, 2692. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.C.; Chang, C.N.; Wu, Y.S.; Fu, P.P.C.; Yang, I.L.; Chen, M.H. Characterization, identification of ambient air and road dust polycyclic aromatic hydrocarbons in central Taiwan, Taichung. Sci. Total Environ. 2004, 327, 135–146. [Google Scholar] [CrossRef]
- Motelay-Massei, A.; Ollivon, D.; Garban, B.; Tiphagne-Larcher, K.; Zimmerlin, I.; Chevreuil, M. PAHs in the Bulk Atmospheric deposition of the Seine River Basin: Source identification and apportionment by ratios, multivariate statistical techniques and scanning electron microscopy. Chemosphere 2007, 67, 312–321. [Google Scholar] [CrossRef]
- Sulong, N.A.; Latif, M.T.; Sahani, M.; Khan, M.F.; Fadzil, M.F.; Tahir, N.M.; Mohamad, N.; Sakai, N.; Fujii, Y.; Othman, M.; et al. Distribution, sources and potential health risks of Polycyclic Aromatic Hydrocarbons (PAHs) in PM2.5 Collected during different monsoon seasons and haze episode in Kuala Lumpur. Chemosphere 2019, 219, 1–14. [Google Scholar] [CrossRef]
- Guo, H.; Lee, S.C.; Ho, K.F.; Wang, X.M.; Zou, S.C. Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmos. Environ. 2003, 37, 5307–5317. [Google Scholar] [CrossRef]
- Park, S.U.; Kim, J.G.; Jeong, M.J.; Song, B.J. Source identification of atmospheric polycyclic aromatic hydrocarbons in industrial complex using diagnostic ratios and multivariate factor analysis. Arch. Environ. Contam. Toxicol. 2011, 60, 576–589. [Google Scholar] [CrossRef]
- Jamhari, A.A.; Sahani, M.; Latif, M.T.; Chan, K.M.; Tan, H.S.; Khan, M.F.; Mohd Tahir, N. Concentration and source identification of Polycyclic Aromatic Hydrocarbons (PAHs) in PM10 of urban, industrial and semi-urban areas in Malaysia. Atmos. Environ. 2014, 86, 16–27. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, Y.; Tian, C.; Lin, T.; Hu, L.; Li, J.; Zhang, G. Application of PMF receptor model merging with PAHs signatures for source apportionment of black carbon in the continental shelf surface sediments of the Bohai and Yellow Seas, China. J. Geophys. Res. Ocean 2016, 121, 1346–1359. [Google Scholar] [CrossRef]
- Hussain, K.; Hoque, R.R. Seasonal attributes of urban soil PAHs of the Brahmaputra Valley. Chemosphere 2015, 119, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Rahman, M.; Prakash, A.; Hoque, R.R. Street dust bound PAHs, carbon and heavy metals in Guwahati City—Seasonality, toxicity and sources. Sustain. Cities Soc. 2015, 19, 17–25. [Google Scholar] [CrossRef]
- Lakhani, A. Source apportionment of particle bound polycyclic aromatic hydrocarbons at an industrial location in Agra, India. Sci. World J. 2012, 2012, 781291. [Google Scholar] [CrossRef]
- Callén, M.S.; Iturmendi, A.; López, J.M. Source apportionment of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons by a PMF receptor model. assessment of potential risk for human health. Environ. Pollut. 2014, 195, 167–177. [Google Scholar] [CrossRef]
- Lee, J.H.; Gigliotti, C.L.; Offenberg, J.H.; Eisenreich, S.J.; Turpin, B.J. Sources of polycyclic aromatic hydrocarbons to the Hudson River Airshed. Atmos. Environ. 2004, 38, 5971–5981. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Teng, Y.-G.; Wang, J.-S. Source apportionment of Polycyclic Aromatic Hydrocarbons (PAHs) in surface sediments of the Rizhao Coastal Area (China) using diagnostic ratios and factor analysis with nonnegative constraints. Sci. Total Environ. 2012, 414, 293–300. [Google Scholar] [CrossRef]
- Jiao, H.; Wang, Q.; Zhao, N.; Jin, B.; Zhuang, X.; Bai, Z. Distributions and sources of Polycyclic Aromatic Hydrocarbons (PAHs) in soils around a chemical plant in Shanxi, China. Int. J. Environ. Res. Public Health 2017, 14, 1198. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Wang, H.; Chen, G. Source apportionment of PAHs using two mathematical models for mangrove sediments in Shantou Coastal Zone, China. Estuaries Coasts 2011, 34, 950–960. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, C.; Chai, X.; He, J.; Cai, Y.; Ren, M.; Yan, B.; Peng, P.; Fu, J. The behaviors and fate of Polycyclic Aromatic Hydrocarbons (PAHs) in a coking wastewater treatment plant. Chemosphere 2012, 88, 174–182. [Google Scholar] [CrossRef]
- Ciaparra, D.; Aries, E.; Booth, M.J.; Anderson, D.R.; Almeida, S.M.; Harrad, S. Characterisation of volatile organic compounds and polycyclic aromatic hydrocarbons in the ambient air of steelworks. Atmos. Environ. 2009, 43, 2070–2079. [Google Scholar] [CrossRef]
- Jang, E.; Alam, M.S.; Harrison, R.M. Source apportionment of polycyclic aromatic hydrocarbons in urban air using positive matrix factorization and spatial distribution analysis. Atmos. Environ. 2013, 79, 271–285. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, Z.; Cao, J.; Xia, L. Source and ecological risk characteristics of PAHs in sediments from Qinhuai River and Xuanwu Lake, Nanjing, China. J. Chem. 2017, 2017, 3510796. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.; Liu, M.; Zheng, X.; Liu, Y.; Wang, Q.; Liu, W. PAHs in organic film on glass window surfaces from Central Shanghai, China: Distribution, sources and risk assessment. Environ. Geochem. Health 2014, 36, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Jeon, H.J.; Kim, Y.C.; Yang, S.H.; Choi, H.; Kim, T.O.; Lee, S.E. Monitoring polycyclic aromatic hydrocarbon concentrations and distributions in rice paddy soils from Gyeonggi-Do, Ulsan, and Pohang. Appl. Biol. Chem. 2019, 62, 18. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, G.; Bi, X.; Feng, Y.; Mai, B.; Fu, J. Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China. Environ. Sci. Technol. 2005, 39, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.Z.; Li, W.H.; Shi, G.L.; Feng, Y.C.; Wang, Y.Q. Relationships between PAHs and PCBs, and quantitative source apportionment of PAHs toxicity in sediments from Fenhe Reservoir and Watershed. J. Hazard Mater. 2013, 248–249, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, I.C.T.; LaGoy, P.K. Toxic Equivalency Factors (TEFs) for Polycyclic Aromatic Hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Hertel, R.F.; Rosner, G.; Kielhorn, J.; Artelt, S.; Boehncke, A.; Creutzenberg, O.; Mangelsdorf, I.; Menichini, E.; Grover, P.L.; Blok, J.; et al. Selected Non-Heterocyclic Polycyclic Aromatic Hydrocarbons; World Health Organization: Geneva, Switzerland, 1998; ISBN 9241572027.
- Callén, M.S.; López, J.M.; Iturmendi, A.; Mastral, A.M. Nature and sources of particle associated Polycyclic Aromatic Hydrocarbons (PAH) in the atmospheric environment of an urban area. Environ. Pollut. 2013, 183, 166–174. [Google Scholar] [CrossRef]
- Cal-EPA. Air Toxics Hot Spots Program Risk Assessment Guidelines Part II: Technical Support Document for Describing Available Cancer Potency Factors; Office of Environmental Health Hazards Assessment: Oakland, CA, USA, 2005.
- OEHHA. Benzo[a]Pyrene as a Toxic Air Contaminant—California Air Resources Board Office of Environmental Health Hazard-Assessment; Office of Environmental Health Hazards Assessment: Oakland, CA, USA, 1994.
- World Health Organization. Air quality guidelines for Europe. Environ. Sci. Pollut. Res. 1996, 3, 23. [Google Scholar] [CrossRef]
- Ramírez, N.; Cuadras, A.; Rovira, E.; Marcé, R.M.; Borrull, F. Risk assessment related to atmospheric polycyclic aromatic hydrocarbons in gas and particle phases near industrial sites. Environ. Health Perspect. 2011, 119, 1110–1116. [Google Scholar] [CrossRef]
- Taghvaee, S.; Sowlat, M.H.; Hassanvand, M.S.; Yunesian, M.; Naddafi, K.; Sioutas, C. Source-specific lung cancer risk assessment of ambient PM2.5-bound Polycyclic Aromatic Hydrocarbons (PAHs) in Central Tehran. Environ. Int. 2018, 120, 321–332. [Google Scholar] [CrossRef]
- U.S. EPA. Regional Screening Levels for Chemical Contaminants at Superfund Sites; U.S. Environmental Protection Agency: Washington, DC, USA, 2015.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).