High Resolution Chemical Stratigraphies of Atmospheric Depositions from a 4 m Depth Snow Pit at Dome C (East Antarctica)
Abstract
:1. Introduction
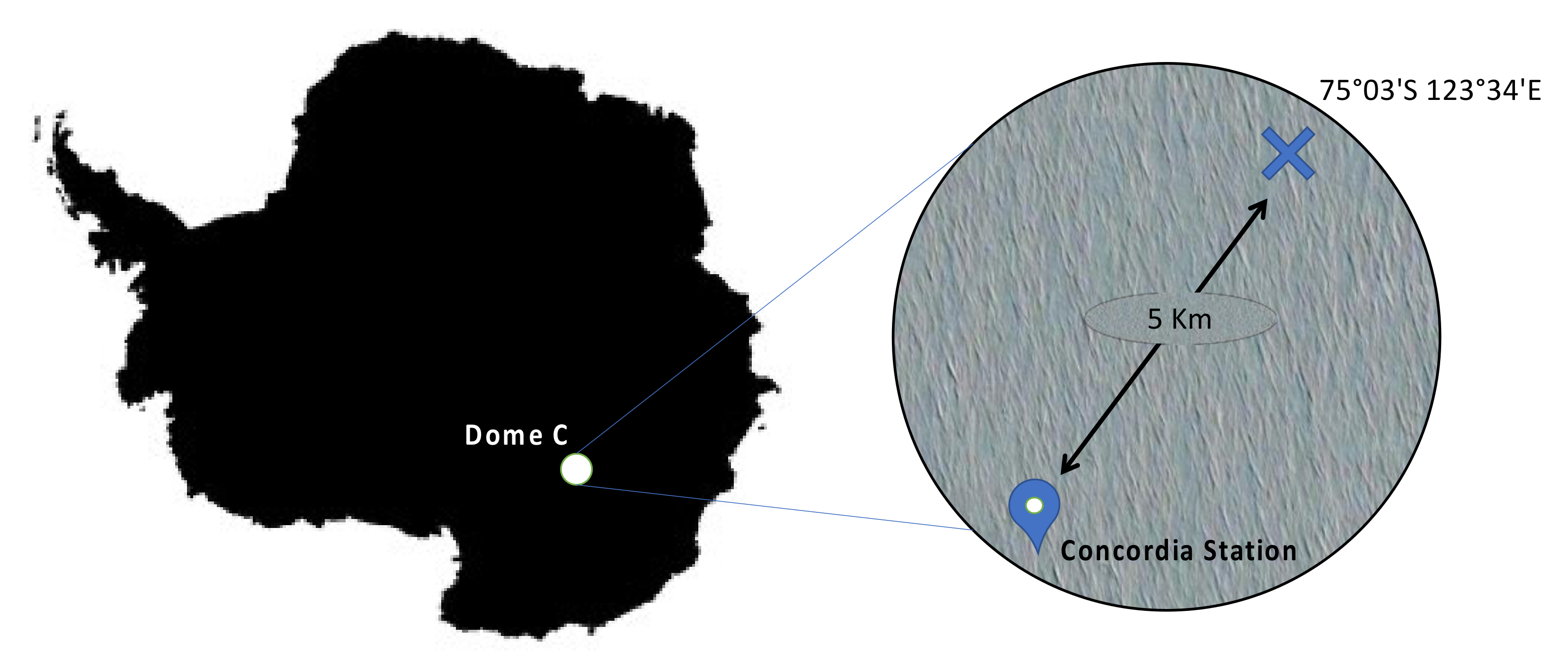
2. Sampling
3. Chemical Measurements
4. Results and Discussion
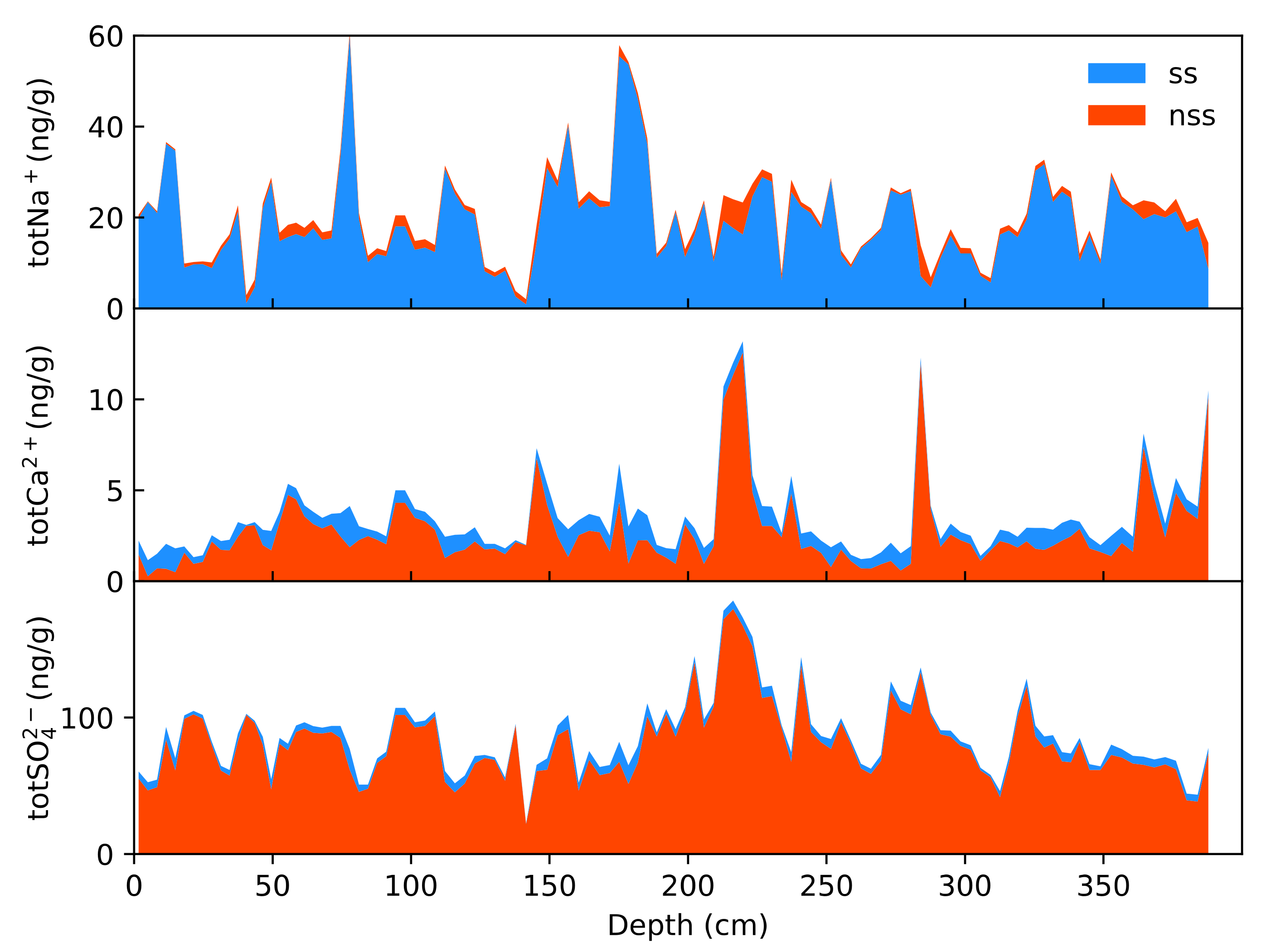
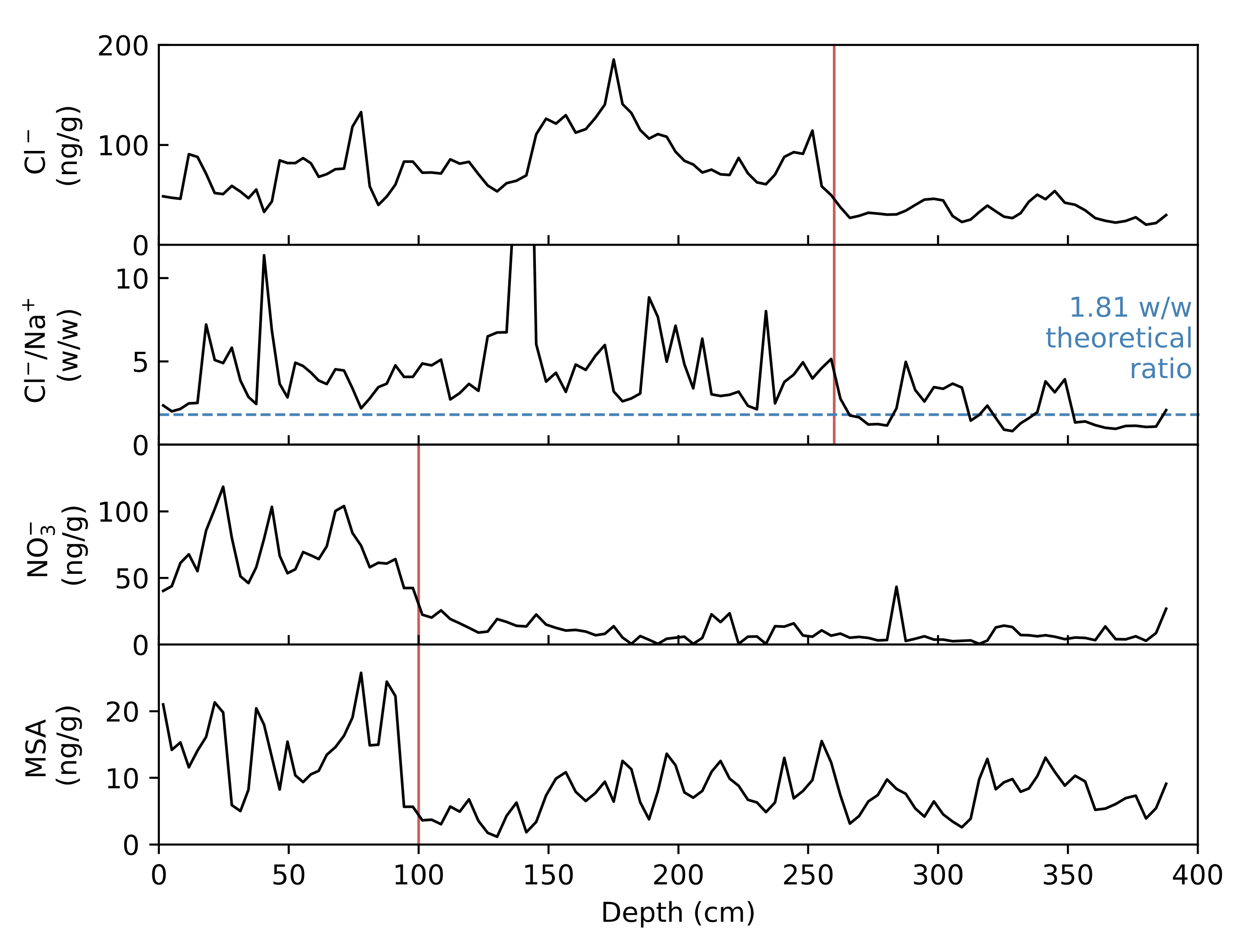
4.1. Sea Salt-Non-Sea Salt Contributions
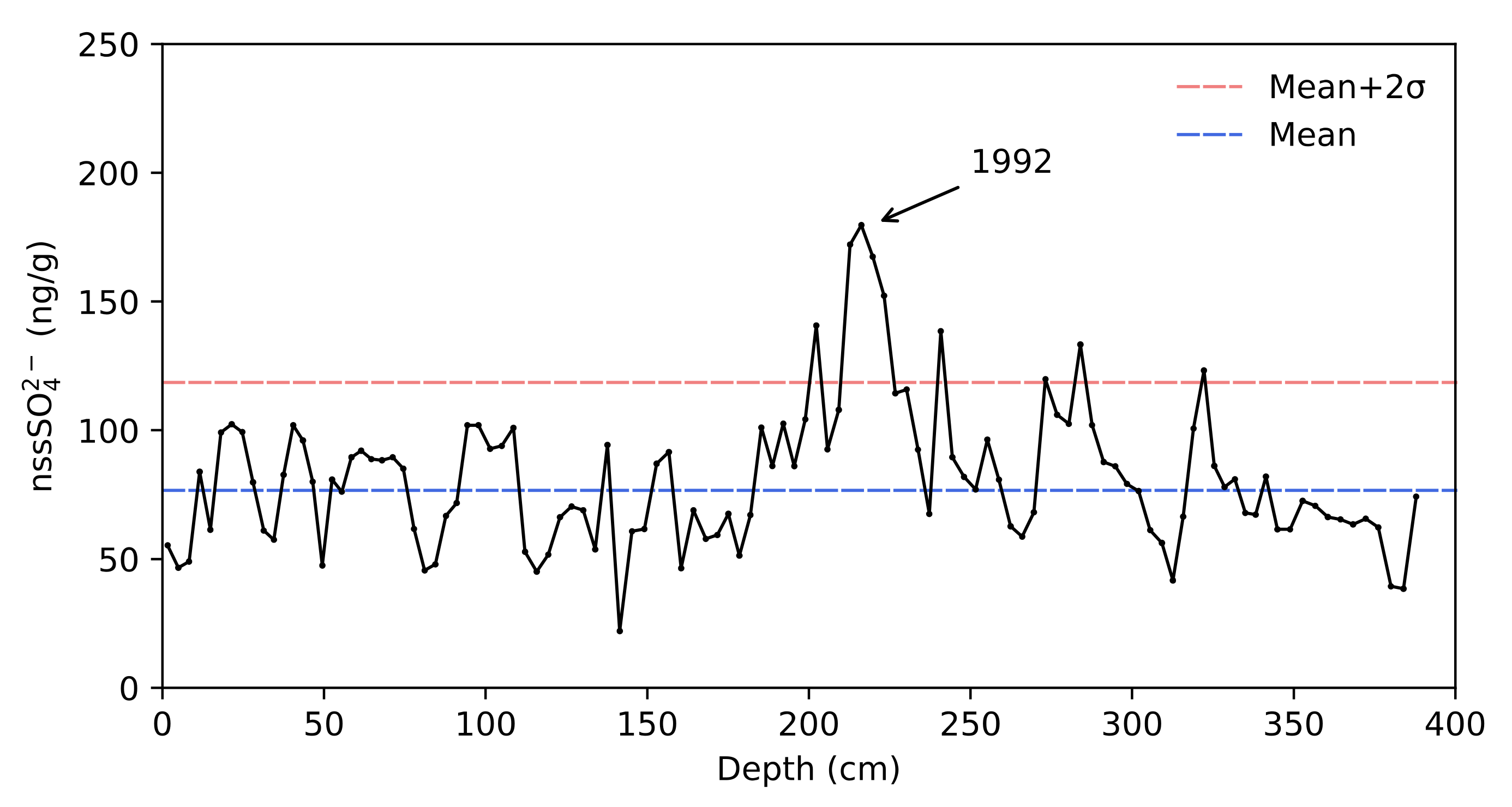
4.2. Dating
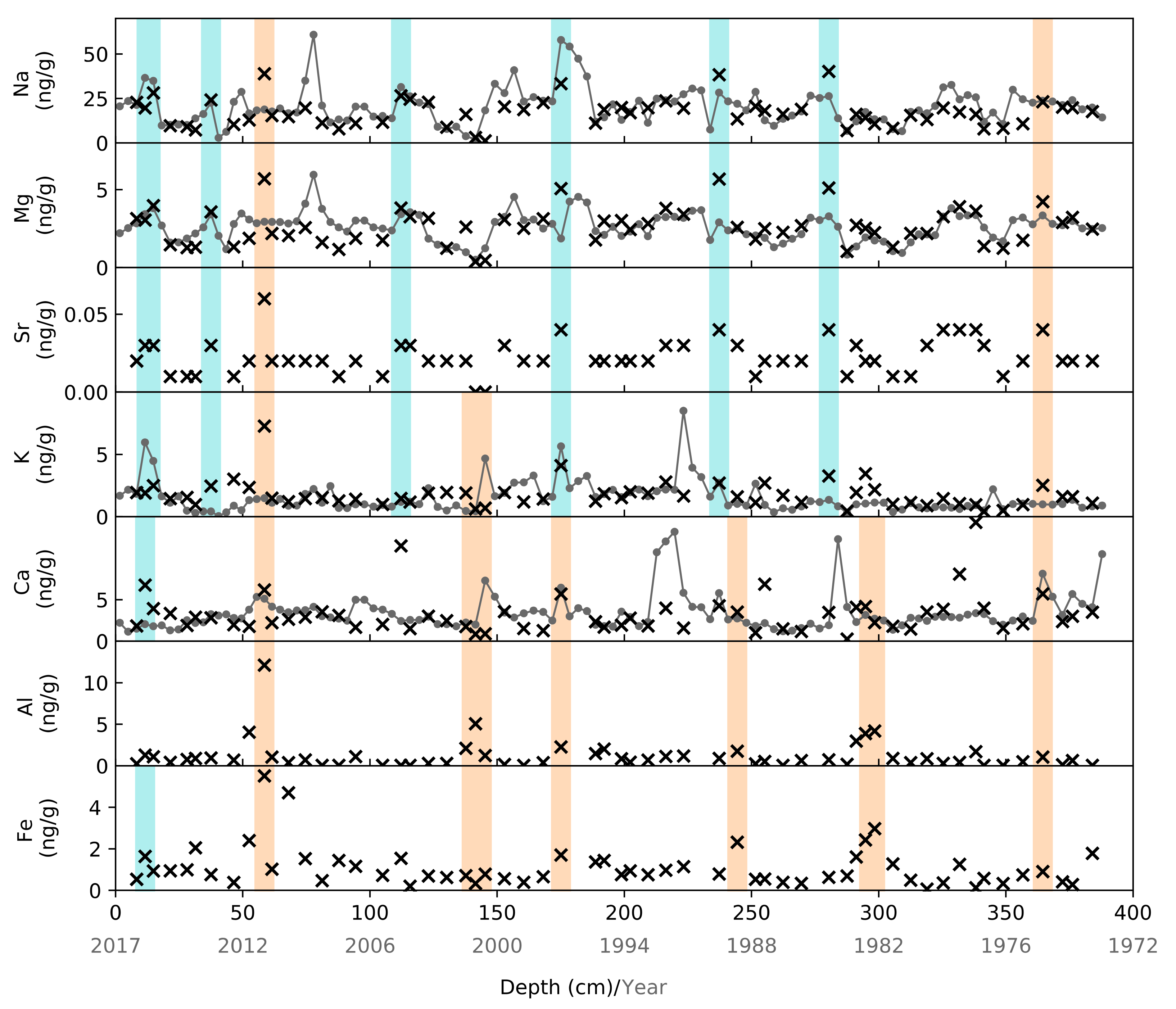
4.3. Primary Aerosol
4.4. Post-Depositional Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPICA Community Members. Eight glacial cycles from an Antarctic ice core. Nature 2004, 429, 623–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EPICA Community Members; Barbante, C.; Barnola, J.-M.; Becagli, S.; Beer, J.; Bigler, M.; Boutron, C.; Blunier, T.; Castellano, E.; Cattani, O.; et al. One-to-one coupling of glacial climate variability in Greenland and Antarctica. Nat. Cell Biol. 2006, 444, 195–198. [Google Scholar] [CrossRef]
- Wolff, E.; Barbante, C.; Becagli, S.; Bigler, M.; Boutron, C.; Castellano, E.; de Angelis, M.; Federer, U.; Fischer, H.; Fundel, F.; et al. Changes in environment over the last 800,000 years from chemical analysis of the EPICA Dome C ice core. Quat. Sci. Rev. 2010, 29, 285–295. [Google Scholar] [CrossRef]
- Abram, N.; Wolff, E.; Curran, M.A. A review of sea ice proxy information from polar ice cores. Quat. Sci. Rev. 2013, 79, 168–183. [Google Scholar] [CrossRef]
- Delmonte, B.; Petit, J.R.; Maggi, V. Glacial to Holocene implications of the new 27000-year dust record from the EPICA Dome C (East Antarctica) ice core. Clim. Dyn. 2002, 18, 647–660. [Google Scholar]
- Traversi, R.; Usoskin, I.G.; Solanki, S.; Becagli, S.; Frezzotti, M.; Severi, M.; Stenni, B.; Udisti, R. Nitrate in Polar Ice: A New Tracer of Solar Variability. Sol. Phys. 2012, 280, 237–254. [Google Scholar] [CrossRef]
- Genthon, C.; Six, D.; Scarchilli, C.; Ciardini, V.; Frezzotti, M. Meteorological and snow accumulation gradients across Dome C, East Antarctic plateau. Int. J. Clim. 2015, 36, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Petit, J.R.; Jouzel, J.; Pourchet, M.; Merlivat, L. A detailed study of snow accumulation and stable isotope content in Dome C (Antarctica). J. Geophys. Res. Space Phys. 1982, 87, 4301–4308. [Google Scholar] [CrossRef]
- Fischer, H.; Fundel, F.; Ruth, U.; Twarloh, B.; Wegner, A.; Udisti, R.; Becagli, S.; Castellano, E.; Morganti, A.; Severi, M.; et al. Reconstruction of millennial changes in dust emission, transport and regional sea ice coverage using the deep EPICA ice cores from the Atlantic and Indian Ocean sector of Antarctica. Earth Planet. Sci. Lett. 2007, 260, 340–354. [Google Scholar] [CrossRef] [Green Version]
- Parrenin, F.; Cavitte, M.G.P.; Blankenship, D.D.; Chappellaz, J.; Fischer, H.; Gagliardini, O.; Masson-Delmotte, V.; Passalacqua, O.; Ritz, C.; Roberts, J.; et al. Is there 1.5-million-year-old ice near Dome C, Antarctica? Cryosphere 2017, 11, 2427–2437. [Google Scholar] [CrossRef] [Green Version]
- Traversi, R.; Becagli, S.; Castellano, E.; Cerri, O.; Morganti, A.; Severi, M.; Udisti, R. Study of Dome C site (East Antartica) variability by comparing chemical stratigraphies. Microchem. J. 2009, 92, 7–14. [Google Scholar] [CrossRef]
- Legrand, M.; Mayewski, P. Glaciochemistry of polar ice cores: A review. Rev. Geophys. 1997, 35, 219–243. [Google Scholar] [CrossRef] [Green Version]
- Legrand, M.; Wolff, E.; Wagenbach, D. Antarctic aerosol and snowfall chemistry: Implications for deep Antarctic ice-core chemistry. Ann. Glaciol. 1999, 29, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Kekonen, T.; Perämäki, P.; Moore, J. Comparison of analytical results for chloride, sulfate and nitrate obtained from adjacent ice core samples by two ion chromatographic methods. J. Environ. Monit. 2004, 6, 147–152. [Google Scholar] [CrossRef]
- Morganti, A.; Becagli, S.; Castellano, E.; Severi, M.; Traversi, R.; Udisti, R. An improved flow analysis–ion chromatography method for determination of cationic and anionic species at trace levels in Antarctic ice cores. Anal. Chim. Acta 2007, 603, 190–198. [Google Scholar] [CrossRef]
- Liu, K.; Hou, S.; Wu, S.; Zhang, W.; Zou, X.; Yu, J.; Song, J.; Sun, X.; Huang, R.; Pang, H.; et al. Assessment of heavy metal contamination in the atmospheric deposition during 1950–2016 A.D. from a snow pit at Dome A, East Antarctica. Environ. Pollut. 2021, 268, 115848. [Google Scholar] [CrossRef]
- Schwanck, F.; Simões, J.C.; Handley, M.; Mayewski, P.A.; Auger, J.D.; Bernardo, R.T.; Aquino, F.E. A 125-year record of climate and chemistry variability at the Pine Island Glacier ice divide, Antarctica. Cryosphere 2017, 11, 1537–1552. [Google Scholar] [CrossRef] [Green Version]
- Bertinetti, S.; Ardini, F.; Caiazzo, L.; Grotti, M. Determination of major elements in Antarctic snow by inductively coupled plasma optical emission spectrometry using a total-consumption sample introduction system. Spectrochim. Acta Part B Spectrosc. 2021, 181, 106231. [Google Scholar] [CrossRef]
- Urbini, S.; Frezzotti, M.; Gandolfi, S.; Vincent, C.; Scarchilli, C.; Vittuari, L.; Fily, M. Historical behaviour of Dome C and Talos Dome (East Antarctica) as investigated by snow accumulation and ice velocity measurements. Glob. Planet. Chang. 2008, 60, 576–588. [Google Scholar] [CrossRef]
- Bertinetti, S.; Ardini, F.; Vecchio, M.A.; Caiazzo, L.; Grotti, M. Isotopic analysis of snow from Dome C indicates changes in the source of atmospheric lead over the last fifty years in East Antarctica. Chemosphere 2020, 255, 126858. [Google Scholar] [CrossRef]
- Caiazzo, L.; Becagli, S.; Frosini, D.; Giardi, F.; Severi, M.; Traversi, R.; Udisti, R. Spatial and temporal variability of snow chemical composition and accumulation rate at Talos Dome site (East Antarctica). Sci. Total. Environ. 2016, 550, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Grotti, M.; Soggia, F.; Todolì, J.L. Ultratrace analysis of Antarctic snow samples by reaction cell inductively coupled plasma mass spectrometry using a total-consumption micro-sample-introduction system. Analyst 2008, 133, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Paredes, E.; Grotti, M.; Mermet, J.M.; Todolí, J.L. Heated-spray chamber-based low sample consumption system for inductively coupled plasma spectrometry. J. Anal. Spectrom. 2009, 24, 903–910. [Google Scholar] [CrossRef]
- Grotti, M.; Ardini, F.; Todolì, J.L. Total introduction of microsamples in inductively coupled plasma mass spectrometry by high-temperature evaporation chamber with a sheathing gas stream. Anal. Chim. Acta 2013, 767, 14–20. [Google Scholar] [CrossRef]
- Grotti, M.; Soggia, F.; Ardini, F.; Magi, E.; Becagli, S.; Traversi, R.; Udisti, R. Year-round record of dissolved and particulate metals in surface snow at Dome Concordia (East Antarctica). Chemosphere 2015, 138, 916–923. [Google Scholar] [CrossRef]
- Legrand, M.; Delmas, R.J. Soluble impurities in four Antarctic ice cores over the last 30,000 years. Ann. Glaciol. 1988, 10, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Röthlisberger, R.; Mulvaney, R.; Wolff, E.; Hutterli, M.A.; Bigler, M.; Sommer, S.; Jouzel, J. Dust and sea salt variability in central East Antarctica (Dome C) over the last 45 kyrs and its implications for southern high-latitude climate. Geophys. Res. Lett. 2002, 29, 24-1–24-4. [Google Scholar] [CrossRef]
- Bowen, H.J.M. Environmental Chemistry of the Elements; Academic Press: London, UK, 1979. [Google Scholar]
- Nozaki, Y. A Fresh Look at Element Distribution in the North Pacific. EOS, American Geophysical Union. 1997. Available online: http://www.agu.org/eos_elec/97025e.html (accessed on 22 June 2021).
- Udisti, R.; Dayan, U.; Becagli, S.; Busetto, M.; Frosini, D.; Legrand, M.; Lucarelli, F.; Preunkert, S.; Severi, M.; Traversi, R.; et al. Sea spray aerosol in central Antarctica. Present atmospheric behaviour and implications for paleoclimatic reconstructions. Atmos. Environ. 2012, 52, 109–120. [Google Scholar] [CrossRef]
- Piccardi, G.; Becagli, S.; Traversi, R.; Udisti, R. Fractionating phenomena. altitude induced, on snow composition in Northern Victoria Land (Antarctica). Ital. Res. Antarct. Atmos. 1996, 51, 229–245. [Google Scholar]
- Wolff, E.W.; Rankin, A.M.; Röthlisberger, R. An ice core indicator of Antarctic sea ice production? Geophys. Res. Lett. 2003, 30, 2158. [Google Scholar] [CrossRef]
- Becagli, S.; Proposito, M.; Benassai, S.; Gragnani, R.; Magand, O.; Traversi, R.; Udisti, R. Spatial distribution of biogenic sulphur compounds (MSA, nssSO4 2–) in the northern Victoria Land–Dome C–Wilkes Land area, East Antarctica. Ann. Glaciol. 2005, 41, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Bertler, N.; Mayewski, P.A.; Aristarain, A.; Barrett, P.; Becagli, S.; Bernardo, R.; Bo, S.; Xiao, C.; Curran, M.; Qin, D.; et al. Snow chemistry across Antarctica. Ann. Glaciol. 2005, 41, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Hammer, C.U.; Clausen, H.B.; Dansgaard, W.; Gundestrup, N.; Johnsen, S.J.; Reeh, N. Dating of Greenland ice cores by flow models, isotopes, volcanic debris, and continental dust. J. Glaciol. 1978, 20, 82. [Google Scholar] [CrossRef] [Green Version]
- Delmas, R.J.; Legrand, M.; Aristarain, A.J.; Zanolini, F. Volcanic deposits in Antarctic snow and ice. J. Geophys. Res. Space Phys. 1985, 90, 12901. [Google Scholar] [CrossRef]
- Cole-Dai, J.; Mosley-Thompson, E. The Pinatubo eruption in South Pole snow and its potential value to ice-core paleovolcanic records. Ann. Glaciol. 1999, 29, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Castellano, E.; Becagli, S.; Hansson, M.; Hutterli, M.; Petit, J.R.; Rampino, M.R.; Severi, M.; Steffensen, J.P.; Traversi, R.; Udisti, R. Holocene volcanic history as recorded in the sulfate stratigraphy of the European Project for Ice Coring in Antarctica Dome C (EDC96) ice core. J. Geophys. Res. Space Phys. 2005, 110, 110. [Google Scholar] [CrossRef]
- Nardin, R.; Amore, A.; Becagli, S.; Caiazzo, L.; Frezzotti, M.; Severi, M.; Stenni, B.; Traversi, R. Volcanic Fluxes Over the Last Millennium as Recorded in the Gv7 Ice Core (Northern Victoria Land, Antarctica). Geosciences 2020, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Benassai, S.; Becagli, S.; Gragnani, R.; Magand, O.; Proposito, M.; Fattori, I.; Traversi, R.; Udisti, R. Sea-spray deposition in Antarctic coastal and plateau areas from ITASE traverses. Ann. Glaciol. 2005, 41, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Traversi, R.; Becagli, S.; Castellano, E.; Largiuni, O.; Migliori, A.; Severi, M.; Frezzotti, M.; Udisti, R. Spatial and temporal distribution of environmental markers from Coastal to Plateau areas in Antarctica by firn core chemical analysis. Int. J. Environ. Anal. Chem. 2004, 84, 457–470. [Google Scholar] [CrossRef]
- Tuohy, A.; Bertler, N.; Neff, P.; Edwards, R.; Emanuelsson, D.; Beers, T.; Mayewski, P. Transport and deposition of heavy metals in the Ross Sea Region, Antarctica. J. Geophys. Res. Atmos. 2015, 120, 120. [Google Scholar] [CrossRef]
- Grotti, M.; Soggia, F.; Ardini, F.; Magi, E.; Grotti, M.; Soggia, F.; Ardini, F.; Magi, E. Major and trace element partitioning between dissolved and particulate phases in Antarctic surface snow. J. Environ. Monit. 2011, 13, 2511–2520. [Google Scholar] [CrossRef]
- Gabrielli, P.; Planchon, F.; Hong, S.; Lee, K.H.; Hur, S.D.; Barbante, C.; Ferrari, C.P.; Petit, J.R.; Lipenkov, V.; Cescon, P. Trace elements in Vostok Antarctic ice during the last four climatic cycles. Earth Planet. Sci. Lett. 2005, 234, 249–259. [Google Scholar] [CrossRef]
- Becagli, S.; Proposito, M.; Benassai, S.; Flora, O.; Genoni, L.; Gragnani, R.; Largiuni, O.; Pili, S.L.; Severi, M.; Stenni, B.; et al. Chemical and isotopic snow variability in East Antarctica along the 2001/02 ITASE traverse. Ann. Glaciol. 2004, 39, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Lambert, F.R.; Delmonte, B.; Petit, J.-R.; Bigler, M.; Kaufmann, P.R.; Hutterli, M.A.; Stocker, T.F.; Ruth, U.; Steffensen, J.P.; Maggi, V. Dust-climate couplings over the past 800,000 years from the EPICA Dome C ice core. Nature 2008, 452, 616–619. [Google Scholar] [CrossRef] [Green Version]
- Marino, F.; Castellano, E.; Nava, S.; Chiari, M.; Ruth, U.; Wegner, A.; Lucarelli, F.; Udisti, R.; Delmonte, B.; Maggi, V. Coherent composition of glacial dust on opposite sides of the East Antarctic Plateau inferred from the deep EPICA ice cores. Geophys. Res. Lett. 2009, 36, 23703. [Google Scholar] [CrossRef]
- Planchon, F.A.; Boutron, C.F.; Barbante, C.; Cozzi, G.; Gaspari, V.; Wolff, E.; Ferrari, C.P.; Cescon, P. Changes in heavy metals in Antarctic snow from Coats Land since the mid-19th to the late-20th century. Earth Planet. Sci. Lett. 2002, 200, 207–222. [Google Scholar] [CrossRef]
- De Angelis, M.; Legrand, M.; Petit, J.R.; Barkov, N.I.; Korotkevitch, Y.S.; Kotlyakov, V.M. Soluble and insoluble impurities along the 950 m deep Vostok ice core (Antarctica)—Climatic implications. J. Atmos. Chem. 1983, 1, 215–239. [Google Scholar] [CrossRef]
- Traversi, R.; Barbante, C.; Gaspari, V.; Fattori, I.; Largiuni, O.; Magaldi, L.; Udisti, R. Aluminium and iron record for the last 28 kyr derived from the Antarctic EDC96 ice core using new CFA methods. Ann. Glaciol. 2004, 39, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Jickells, T.D.; Anderson, K.; Andersen, K.K.; Baker, A.R.; Bergametti, G.; Brooks, N.; Cao, J.J.; Boyd, P.; Duce, R.A.; Hunter, K.A.; et al. Global Iron Connections Between Desert Dust, Ocean Biogeochemistry, and Climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Conway, T.; Wolff, E.; Röthlisberger, R.; Mulvaney, R.; Elderfield, H. Constraints on soluble aerosol iron flux to the Southern Ocean at the Last Glacial Maximum. Nat. Commun. 2015, 6, 7850. [Google Scholar] [CrossRef] [Green Version]
- Burgay, F.; Barbaro, E.; Cappelletti, D.; Turetta, C.; Gallet, J.-C.; Isaksson, E.; Stenni, B.; Dreossi, G.; Scoto, F.; Barbante, C.; et al. First discrete iron(II) records from Dome C (Antarctica) and the Holtedahlfonna glacier (Svalbard). Chemosphere 2021, 267, 129335. [Google Scholar] [CrossRef] [PubMed]
- Koffman, B.G.; Dowd, E.G.; Osterberg, E.C.; Ferris, D.; Hartman, L.H.; Wheatley, S.D.; Kurbatov, A.V.; Wong, G.J.; Markle, B.; Dunbar, N.W.; et al. Rapid transport of ash and sulfate from the 2011 Puyehue-Cordón Caulle (Chile) eruption to West Antarctica. J. Geophys. Res. Atmos. 2017, 122, 8908–8920. [Google Scholar] [CrossRef]
- Boening, C.; Willis, J.K.; Landerer, F.W.; Nerem, R.S.; Fasullo, J. The 2011 La Niña: So strong, the oceans fell. Geophys. Res. Lett. 2012, 39, 19602. [Google Scholar] [CrossRef] [Green Version]
- Henderson, P.; Henderson, G.M. The Cambridge Handbook of Earth Science Data; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Wagnon, P.; Delmas, R.J.; Legrand, M.; Wagnon, P.; Delmas, R.J.; Legrand, M. Loss of volatile acid species from upper firn layers at Vostok, Antarctica. J. Geophys. Res. Space Phys. 1999, 104, 3423–3431. [Google Scholar] [CrossRef]
- Wagenbach, D.; Ducroz, F.; Mulvaney, R.; Keck, L.; Minikin, A.; Legrand, M.; Hall, J.S.; Wolff, E.; Wagenbach, D.; Ducroz, F.; et al. Sea-salt aerosol in coastal Antarctic regions. J. Geophys. Res. Space Phys. 1998, 103, 10961–10974. [Google Scholar] [CrossRef] [Green Version]
- Legrand, M.; Delmas, R. The ionic balance of Antarctic snow: A 10-year detailed record. Atmos. Environ. 1984, 18, 1867–1874. [Google Scholar] [CrossRef]
- Grannas, A.M.; Jones, A.E.; Dibb, J.; Ammann, M.; Anastasio, C.; Beine, H.J.; Bergin, M.; Bottenheim, J.; Boxe, C.S.; Carver, G.; et al. An overview of snow photochemistry: Evidence, mechanisms and impacts. Atmos. Chem. Phys. Discuss. 2007, 7, 4329–4373. [Google Scholar] [CrossRef] [Green Version]
- Röthlisberger, R.; Hutterli, M.A.; Wolff, E.; Mulvaney, R.; Fischer, H.; Bigler, M.; Goto-Azuma, K.; Hansson, M.E.; Ruth, U.; Siggaard-Andersen, M.-L.; et al. Nitrate in Greenland and Antarctic ice cores: A detailed description of post-depositional processes. Ann. Glaciol. 2002, 35, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Traversi, R.; Becagli, S.; Brogioni, M.; Caiazzo, L.; Ciardini, V.; Giardi, F.; Legrand, M.; Macelloni, G.; Petkov, B.; Preunkert, S.; et al. Multi-year record of atmospheric and snow surface nitrate in the central Antarctic plateau. Chemosphere 2017, 172, 341–354. [Google Scholar] [CrossRef]
- Oppenheimer, C.; Kyle, P.; Tsanev, V.; Mcgonigle, A.; Mather, T.; Sweeney, D. Mt. Erebus, the largest point source of NO2 in Antarctica. Atmos. Environ. 2005, 39, 6000–6006. [Google Scholar] [CrossRef]
- Mather, T.A.; Pyle, D.M.; Allen, A.G. Volcanic source for fixed nitrogen in the early Earth’s atmosphere. Geology 2004, 32, 905–908. [Google Scholar] [CrossRef]
- Legrand, M.; Feniet-Saigne, C. Methanesulfonic acid in South polar snow layers: A record of strong El Niño? Geophys. Res. Lett. 1991, 18, 187–190. [Google Scholar] [CrossRef]
- Becagli, S.; Castellano, E.; Cerri, O.; Curran, M.; Frezzotti, M.; Marino, F.; Morganti, A.; Proposito, M.; Severi, M.; Traversi, R.; et al. Methanesulphonic acid (MSA) stratigraphy from a Talos Dome ice core as a tool in depicting sea ice changes and southern atmospheric circulation over the previous 140 years. Atmos. Environ. 2009, 43, 1051–1058. [Google Scholar] [CrossRef]
- Curran, M.A.J.; van Ommen, T.D.; Morgan, V.I.; Phillips, K.L.; Palmer, A.S. Ice Core Evidence for Antarctic Sea Ice Decline Since the 1950s. Science 2003, 302, 1203–1206. [Google Scholar] [CrossRef]
- Weller, R.; Traufetter, F.; Fischer, H.; Oerter, H.; Piel, C.; Miller, H. Postdepositional losses of methane sulfonate, nitrate, and chloride at the European Project for Ice Coring in Antarctica deep-drilling site in Dronning Maud Land, Antarctica. J. Geophys. Res. Space Phys. 2004, 109. [Google Scholar] [CrossRef] [Green Version]
| Element | Analytical Method | Median (ng g−1) | Mean (ng g−1) | Std Dev |
|---|---|---|---|---|
| Al | ICP-OES | 0.68 | 1.16 | 1.82 |
| Ca | ICP-OES | 2.64 | 3.21 | 2.44 |
| Fe | ICP-OES | 0.76 | 1.09 | 0.98 |
| K | ICP-OES | 1.54 | 1.75 | 1.05 |
| Mg | ICP-OES | 2.53 | 2.61 | 1.17 |
| Na | ICP-OES | 17.00 | 17.21 | 8.08 |
| Sr | ICP-OES | 0.022 | 0.023 | 0.010 |
| Na+ | IC | 19.91 | 20.73 | 10.40 |
| K+ | IC | 1.09 | 1.50 | 1.25 |
| Mg2+ | IC | 2.61 | 2.61 | 0.89 |
| Ca2+ | IC | 2.86 | 3.43 | 2.22 |
| Cl− | IC | 60.61 | 66.24 | 33.37 |
| NO3− | IC | 12.58 | 25.70 | 29.55 |
| SO42− | IC | 83.28 | 86.38 | 27.79 |
| MSA | IC | 8.26 | 9.39 | 5.05 |
| Element | Na | K | Mg | Ca |
|---|---|---|---|---|
| IC/ICP (111/58) | 1.20 | 0.86 | 1.00 | 1.06 |
| IC/ICP (58/58) | 1.11 | 0.71 | 1.00 | 1.11 |
| Element | Na | K | Mg | Ca |
|---|---|---|---|---|
| Slope (±error) | 1.07 ± 0.05 | 0.72 ± 0.10 | 0.85 ± 0.04 | 0.68 ± 0.09 |
| Correlation coefficient | 0.93 | 0.68 | 0.93 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caiazzo, L.; Becagli, S.; Bertinetti, S.; Grotti, M.; Nava, S.; Severi, M.; Traversi, R. High Resolution Chemical Stratigraphies of Atmospheric Depositions from a 4 m Depth Snow Pit at Dome C (East Antarctica). Atmosphere 2021, 12, 909. https://doi.org/10.3390/atmos12070909
Caiazzo L, Becagli S, Bertinetti S, Grotti M, Nava S, Severi M, Traversi R. High Resolution Chemical Stratigraphies of Atmospheric Depositions from a 4 m Depth Snow Pit at Dome C (East Antarctica). Atmosphere. 2021; 12(7):909. https://doi.org/10.3390/atmos12070909
Chicago/Turabian StyleCaiazzo, Laura, Silvia Becagli, Stefano Bertinetti, Marco Grotti, Silvia Nava, Mirko Severi, and Rita Traversi. 2021. "High Resolution Chemical Stratigraphies of Atmospheric Depositions from a 4 m Depth Snow Pit at Dome C (East Antarctica)" Atmosphere 12, no. 7: 909. https://doi.org/10.3390/atmos12070909
APA StyleCaiazzo, L., Becagli, S., Bertinetti, S., Grotti, M., Nava, S., Severi, M., & Traversi, R. (2021). High Resolution Chemical Stratigraphies of Atmospheric Depositions from a 4 m Depth Snow Pit at Dome C (East Antarctica). Atmosphere, 12(7), 909. https://doi.org/10.3390/atmos12070909










