Seasonal Variation Characteristics of Bacteria and Fungi in PM2.5 in Typical Basin Cities of Xi’an and Linfen, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site and PM2.5 Collection
2.2. DNA Extraction, PCR Amplification, and Illumina Sequencing
2.3. Sequence Analyses
2.4. Statistical Analysis
3. Results and Discussion
3.1. Microbial Richness and Diversity
3.2. Community Compositions of Microorganisms
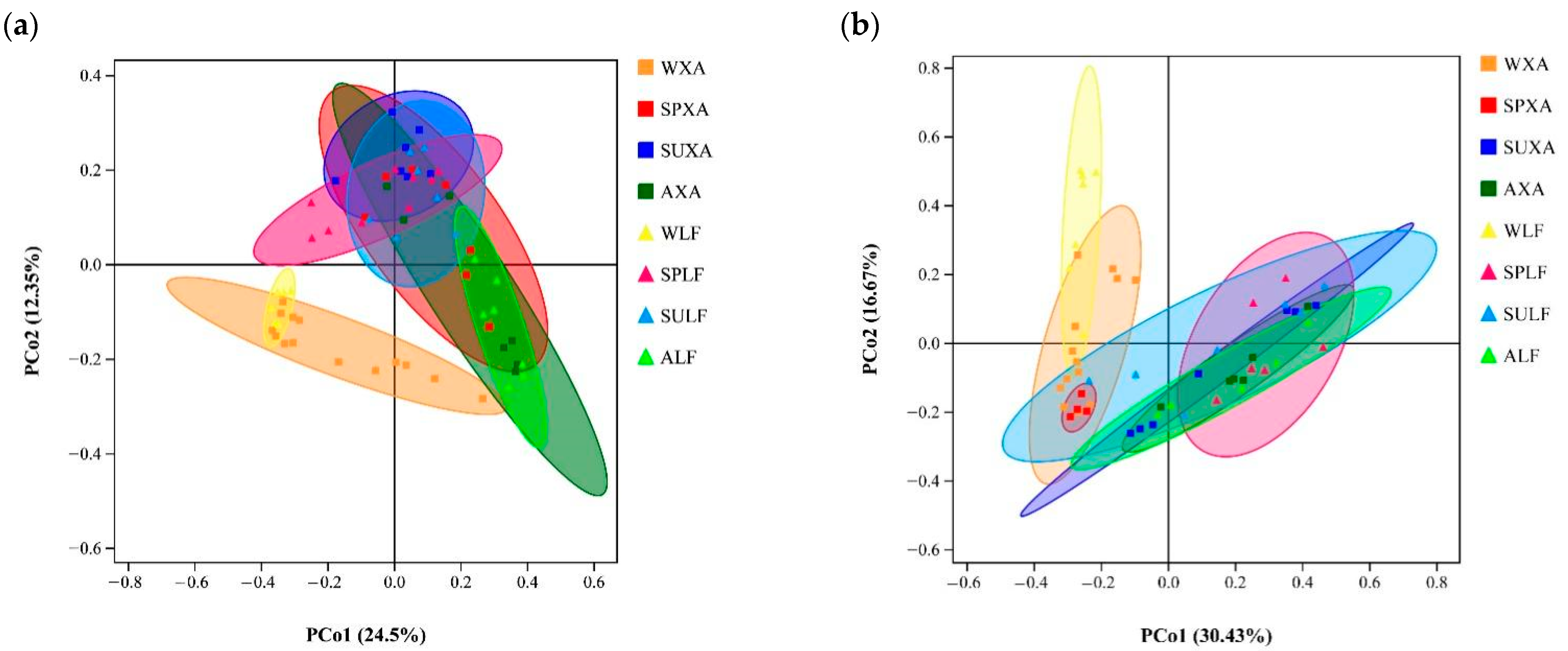
3.3. Comparative Analysis of Bacterial and Fungal Community Structures
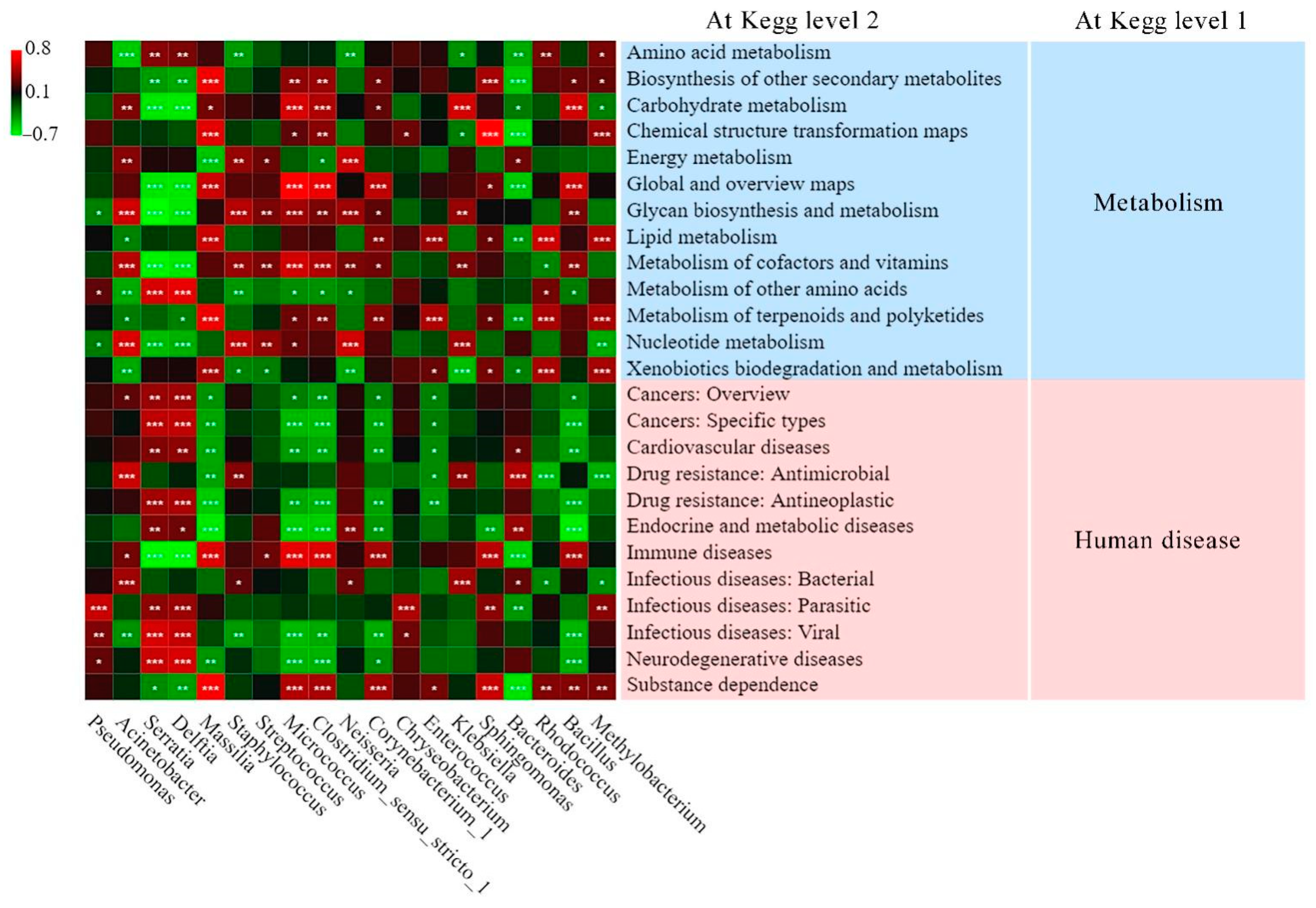
3.4. Environmental Influences on Microbial Communities
3.5. Ecological Function Analysis of Microorganisms
3.5.1. Predicted Functional Analysis of Bacterial Community through Tax4Fun2
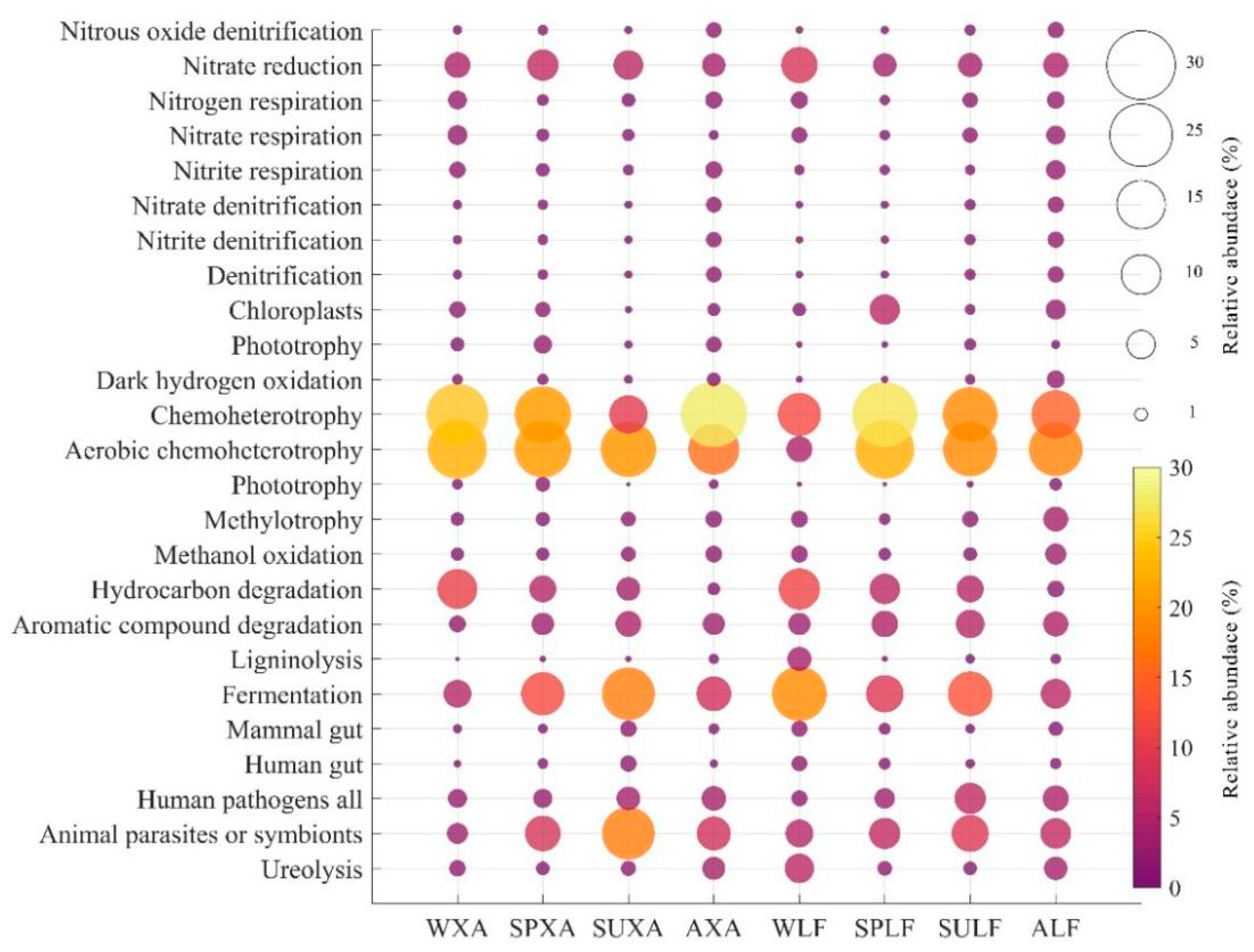
3.5.2. Predicted Functional Analysis of Bacterial Communities Using FAPROTAX
3.5.3. Analysis of Pathogenic and Functional Fungi
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, S.; Hu, M.; Zamora, M.L.; Peng, J.; Shang, D.; Zheng, J.; Du, Z.; Wu, Z.; Shao, M.; Zeng, L. Elucidating severe urban haze formation in China. Proc. Natl. Acad. Sci. USA 2014, 111, 17373–17378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnett, R.T.; Pope, C.A., III; Ezzati, M.; Olives, C.; Lim, S.S.; Mehta, S.; Shin, H.H.; Singh, G.; Hubbell, B.; Brauer, M.; et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ. Health Perspect. 2014, 122, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, K.K.; Ethan, C.J.; Yu, Y.; Shale, K.; Fan, Y.; Liu, F.; Rong, J. The effect of ambient air pollution on circulatory mortality: A short-term exposure assessment in Xi’an, China. Environ. Sci. Pollut. Res. 2019, 26, 22512–22521. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Ho, K.F.; Hu, T.; Sun, J.; Duan, J.; Huang, Y.; Lui, K.H.; Cao, J. Characterization of chemical components and cytotoxicity effects of indoor and outdoor fine particulate matter (PM2.5) in Xi’an, China. Environ. Sci. Pollut. Res. 2019, 26, 31913–31923. [Google Scholar] [CrossRef] [PubMed]
- Degobbi, C.; Lopes, F.D.; Carvalho-Oliveira, R.; Muñoz, J.E.; Saldiva, P.H. Correlation of fungi and endotoxin with PM2.5 and meteorological parameters in atmosphere of Sao Paulo, Brazil. Atmos. Environ. 2011, 45, 2277–2283. [Google Scholar] [CrossRef]
- Grinn-Gofroń, A.; Strzelczak, A.; Wolski, T. The relationships between air pollutants, meteorological parameters and concentration of airborne fungal spores. Environ. Pollut. 2011, 159, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W. Bioaerosols in the earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, A.; Hu, Z.; Sun, H. Study on the potential relationships between indoor culturable fungi, particle load and children respiratory health in Xi’an, China. Build. Environ. 2014, 80, 105–114. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, H.; Zhang, H.; Zhang, X.; Zhou, M.; Lou, L.; Zheng, P.; Xi, C.; Hu, B. Temporal discrepancy of airborne total bacteria and pathogenic bacteria between day and night. Environ. Res. 2020, 186, 109540. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, Z.; Zhao, Z.; Zhang, C.; Fu, Y.; Li, J.; Zhang, C.; Lu, B.; Qian, J. Biological and chemical compositions of atmospheric particulate matter during hazardous haze days in Beijing. Environ. Sci. Pollut. Res. 2018, 25, 34540–34549. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Zou, Z.; Zheng, Y.; Li, J.; Shen, F.; Wu, C.Y.; Wu, Y.; Hu, M.; Yao, M. Ambient bioaerosol particle dynamics observed during haze and sunny days in Beijing. Sci. Total Environ. 2016, 550, 751–759. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, C.; Lu, R.; Liu, P.; Wang, B.; Du, S.; Jin, C.; Deng, S.; Li, Y. Characteristics of ambient bioaerosols during haze episodes in China: A review. Environ. Pollut. 2018, 243, 1930–1942. [Google Scholar] [CrossRef]
- Ouyang, W.; Gao, B.; Cheng, H.; Zhang, L.; Wang, Y.; Lin, C.; Chen, J. Airborne bacterial communities and antibiotic resistance gene dynamics in PM2.5 during rainfall. Environ. Int. 2020, 134, 105318. [Google Scholar] [CrossRef]
- Du, P.; Du, R.; Lu, Z.; Ren, W.; Fu, P. Variation of bacterial and fungal community structures in PM2.5 collected during the 2014 APEC summit periods. Aerosol Air Qual. Res. 2018, 18, 444–455. [Google Scholar] [CrossRef]
- Park, J.; Li, P.F.; Ichijo, T.; Nasu, M.; Yamaguchi, N. Effects of Asian dust events on atmospheric bacterial communities at different distances downwind of the source region. J. Environ. Sci. 2018, 72, 133–139. [Google Scholar] [CrossRef]
- Liu, T.; Chen, L.W.A.; Zhang, M.; Watson, J.G.; Chow, J.C.; Cao, J.; Chen, H.; Wang, W.; Zhang, J.; Zeng, C. Bioaerosol concentrations and size distributions during the autumn and winter seasons in an industrial city of central China. Aerosol Air Qual. Res. 2019, 19, 1095–1104. [Google Scholar] [CrossRef]
- Wei, M.; Li, M.; Xu, C.; Xu, P.; Liu, H. Pollution characteristics of bioaerosols in PM2.5 during the winter heating season in a coastal city of northern China. Environ. Sci. Pollut. Res. 2020, 27, 27750–27761. [Google Scholar] [CrossRef]
- Bowers, R.M.; Clements, N.; Emerson, J.B.; Wiedinmyer, C.; Hannigan, M.P.; Fierer, N. Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environ. Sci. Technol. 2013, 47, 12097–12106. [Google Scholar] [CrossRef]
- Zhang, S.; Du, R.; Chen, H.; Ren, W.; Du, P. Seasonal variation of microbial activity and pathogenic bacteria under non-serious pollution levels in Beijing. Aerosol Air Qual. Res. 2019, 19, 1798–1807. [Google Scholar] [CrossRef]
- Qi, Y.; Li, Y.; Xie, W.; Lu, R.; Mu, F.; Bai, W.; Du, S. Temporal-spatial variations of fungal composition in PM2.5 and source tracking of airborne fungi in mountainous and urban regions. Sci. Total Environ. 2020, 708, 135027. [Google Scholar] [CrossRef]
- Chakrawarti, M.K.; Singh, M.; Yadav, V.P.; Mukhopadhyay, K. Temporal dynamics of air bacterial communities in a university health centre using Illumina MiSeq sequencing. Aerosol Air Qual. Res. 2020, 20, 966–980. [Google Scholar] [CrossRef] [Green Version]
- Núñez, A.; García, A.M.; Moreno, D.A.; Guantes, R. Seasonal changes dominate long-term variability of the urban air microbiome across space and time. Environ. Int. 2021, 150, 106423. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Y.; Gao, J.F.; Pan, K.L.; Li, D.C.; Dai, H.H.; Li, X. More obvious air pollution impacts on variations in bacteria than fungi and their co-occurrences with ammonia-oxidizing microorganisms in PM2.5. Environ. Pollut. 2019, 251, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Q.; Deng, Y.; Wang, Y.; Wang, X.; Zhang, H.; Sun, X.; Ouyang, Z. Meteorological factors had more impact on airborne bacterial communities than air pollutants. Sci. Total Environ. 2017, 601, 703–712. [Google Scholar] [CrossRef]
- Zhou, Y.; Lai, Y.; Tong, X.; Leung, M.H.; Tong, J.C.; Ridley, I.A.; Lee, P.K. Airborne bacteria in outdoor air and air of mechanically ventilated buildings at city scale in Hong Kong across seasons. Environ. Sci. Technol. 2020, 54, 11732–11743. [Google Scholar] [CrossRef]
- Chen, H.; Du, R.; Zhang, Y.; Zhang, S.; Ren, W.; Du, P. Survey of background microbial index in inhalable particles in Beijing. Sci. Total Environ. 2021, 757, 143743. [Google Scholar] [CrossRef]
- Park, E.H.; Heo, J.; Kim, H.; Yi, S.-M. The major chemical constituents of PM2.5 and airborne bacterial community phyla in Beijing, Seoul, and Nagasaki. Chemosphere 2020, 254, 126870. [Google Scholar] [CrossRef]
- Du, P.; Du, R.; Ren, W.; Lu, Z.; Fu, P. Seasonal variation characteristic of inhalable microbial communities in PM2.5 in Beijing city, China. Sci. Total Environ. 2018, 610, 308–315. [Google Scholar] [CrossRef]
- Ren, Y.X.; Yang, L.; Liang, X. The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Bioresour. Technol. 2014, 171, 1–9. [Google Scholar] [CrossRef]
- Pan, Y.; Pan, X.; Xiao, H.; Xiao, H. Structural characteristics and functional implications of PM2.5 bacterial communities during fall in Beijing and Shanghai, China. Front. Microbiol. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Wei, M.; Xu, C.; Xu, X.; Zhu, C.; Li, J.; Lv, G. Characteristics of atmospheric bacterial and fungal communities in PM2.5 following biomass burning disturbance in a rural area of North China Plain. Sci. Total Environ. 2019, 651, 2727–2739. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, Q.; Fu, X.; Zheng, L.; Dong, J.; Wang, J.; Guo, S. Feedback of airborne bacterial consortia to haze pollution with different PM2.5 levels in typical mountainous terrain of Jinan, China. Sci. Total Environ. 2019, 695, 133912. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 265–270. [Google Scholar]
- Chao, A.; Bunge, J. Estimating the number of species in a stochastic abundance model. Biometrics 2002, 58, 531–539. [Google Scholar] [CrossRef]
- Gou, H.; Lu, J.; Li, S.; Tong, Y.; Xie, C.; Zheng, X. Assessment of microbial communities in PM1 and PM10 of Urumqi during winter. Environ. Pollut. 2016, 214, 202–210. [Google Scholar] [CrossRef]
- Li, Y.; Lu, R.; Li, W.; Xie, Z.; Song, Y. Concentrations and size distributions of viable bioaerosols under various weather conditions in a typical semi-arid city of Northwest China. J. Aerosol. Sci. 2017, 106, 83–92. [Google Scholar] [CrossRef]
- Kong, L.; Tan, Q.; Feng, M.; Qu, Y.; An, J.; Liu, X.; Cheng, N.; Deng, Y.; Zhai, R.; Wang, Z. Investigating the characteristics and source analyses of PM2.5 seasonal variations in Chengdu, Southwest China. Chemosphere 2020, 243, 125267. [Google Scholar] [CrossRef] [PubMed]
- Bragoszewska, E.; Mainka, A.; Pastuszka, J.S. Concentration and size distribution of culturable bacteria in ambient air during spring and winter in Gliwice: A typical urban area. Atmosphere 2017, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Hwang, G.B.; Jung, J.H.; Jeong, T.G.; Lee, B.U. Effect of hybrid UV-thermal energy stimuli on inactivation of S. epidermidis and B. subtilis bacterial bioaerosols. Sci. Total Environ. 2010, 408, 5903–5909. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, H.; Wang, W.; Liu, J.; Meng, Q.; Wang, W. Characteristics of bacterial and fungal aerosols during the autumn haze days in Xi’an, China. Atmos. Environ. 2015, 122, 439–447. [Google Scholar] [CrossRef]
- Tang, J.W. The effect of environmental parameters on the survival of airborne infectious agents. J. R. Soc. Interface 2009, 6, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Daş, E.; Gürakan, G.C.; Bayındırlı, A. Effect of controlled atmosphere storage, modified atmosphere packaging and gaseous ozone treatment on the survival of Salmonella Enteritidis on cherry tomatoes. Food Microbiol. 2006, 23, 430–438. [Google Scholar] [CrossRef]
- Gao, M.; Yan, X.; Qiu, T.; Han, M.; Wang, X. Variation of correlations between factors and culturable airborne bacteria and fungi. Atmos. Environ. 2016, 128, 10–19. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, H.; Yao, X.; Zhou, M.; Wang, J.; He, Z.; Zhang, H.; Lou, L.; Mao, W. Effect of air pollution on the total bacteria and pathogenic bacteria in different sizes of particulate matter. Environ. Pollut. 2018, 233, 483–493. [Google Scholar] [CrossRef]
- Du, P.; Du, R.; Ren, W.; Lu, Z.; Zhang, Y.; Fu, P. Variations of bacteria and fungi in PM2.5 in Beijing, China. Atmos. Environ. 2018, 172, 55–64. [Google Scholar] [CrossRef]
- Cabral, J.P. Can we use indoor fungi as bioindicators of indoor air quality? Historical perspectives and open questions. Sci. Total Environ. 2010, 408, 4285–4295. [Google Scholar] [CrossRef]
- Abd Aziz, A.; Lee, K.; Park, B.; Park, H.; Park, K.; Choi, I.G.; Chang, I.S. Comparative study of the airborne microbial communities and their functional composition in fine particulate matter (PM2.5) under non-extreme and extreme PM2.5 conditions. Atmos. Environ. 2018, 194, 82–92. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, W.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Inhalable microorganisms in Beijing’s PM2.5 and PM10 pollutants during a severe smog event. Environ. Sci. Technol. 2014, 48, 1499–1507. [Google Scholar] [CrossRef]
- Després, V.; Nowoisky, J.; Klose, M.; Conrad, R.; Andreae, M.; Pöschl, U. Characterization of primary biogenic aerosol particles in urban, rural, and high-alpine air by DNA sequence and restriction fragment analysis of ribosomal RNA genes. Biogeosciences 2007, 4, 1127–1141. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Li, Y.; Li, W.; Xie, Z.; Fan, C.; Liu, P.; Deng, S. Bacterial community structure in atmospheric particulate matters of different sizes during the haze days in Xi’an, China. Sci. Total Environ. 2018, 637, 244–252. [Google Scholar] [CrossRef]
- Dong, S.R.; Han, Y.J.; Jing, W.; Zeng, C.L.; Zhu, K.H.; Chen, X.J.; Liu, Y.M.; Zou, X.Q.; Zheng, S.L.; Wen, Z.H. Distribution of Microbiota in Fine Particulate Matter Particles in Guangzhou, China. Biomed. Environ. Sci. 2020, 33, 306–314. [Google Scholar]
- Jia, J.; Gomes-Silva, G.; Plath, M.; Pereira, B.B.; UeiraVieira, C.; Wang, Z. Shifts in bacterial communities and antibiotic resistance genes in surface water and gut microbiota of guppies (Poecilia reticulata) in the upper Rio Uberabinha, Brazil. Ecotox. Environ. Saf. 2021, 211, 111955. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, X.; Zhang, M.; Yang, W.; Di, H.J.; Ma, L.; Liu, W.; Li, B. The application of Bacillus Megaterium alters soil microbial community composition, bioavailability of soil phosphorus and potassium, and cucumber growth in the plastic shed system of North China. Agric. Ecosyst. Environ. 2021, 307, 107236. [Google Scholar] [CrossRef]
- Wu, T.; Li, X.B.; Xu, J.; Liu, L.X.; Ren, L.L.; Dong, B.; Li, W.; Xie, W.W.; Yao, Z.G.; Chen, Q.F. Diversity and functional characteristics of endophytic bacteria from two grass species growing on an oil-contaminated site in the Yellow River Delta, China. Sci. Total Environ. 2021, 767, 144340. [Google Scholar] [CrossRef]
- White, D.C.; Sutton, S.D.; Ringelberg, D.B. The genus Sphingomonas: Physiology and ecology. Curr. Opin. Biotechnol. 1996, 7, 301–306. [Google Scholar] [CrossRef]
- McLeod, M.P.; Warren, R.L.; Hsiao, W.W.; Araki, N.; Myhre, M.; Fernandes, C.; Miyazawa, D.; Wong, W.; Lillquist, A.L.; Wang, D. The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse. Proc. Natl. Acad. Sci. USA 2006, 103, 15582–15587. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Zhang, T.; Su, J.; Zhao, L.L.; Wang, H.; Fang, X.M.; Zhang, Y.Q.; Liu, H.Y.; Yu, L.Y. Diversity and composition of airborne fungal community associated with particulate matters in Beijing during haze and non-haze days. Front. Microbiol. 2016, 7, 487. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Li, D.; Li, B.; Sun, S.; Geng, J.; Ma, L.; Qi, H. Exploring the effects of haze pollution on airborne fungal composition in a cold megacity in Northeast China. J. Clean Prod. 2021, 280, 124205. [Google Scholar] [CrossRef]
- Griffin, D.W. Terrestrial microorganisms at an altitude of 20,000 m in Earth’s atmosphere. Aerobiologia 2004, 20, 135–140. [Google Scholar] [CrossRef]
- Fröhlich-Nowoisky, J.; Burrows, S.; Xie, Z.; Engling, G.; Solomon, P.; Fraser, M.; Mayol-Bracero, O.; Artaxo, P.; Begerow, D.; Conrad, R. Biogeography in the air: Fungal diversity over land and oceans. Biogeosciences 2012, 9, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.M.; Li, C.S. Seasonal fungus prevalence inside and outside of domestic environments in the subtropical climate. Atmos. Environ. 1994, 28, 3125–3130. [Google Scholar] [CrossRef]
- Chan, J.F.; Lau, S.K.; Yuen, K.Y.; Woo, P.C. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg. Microbes Infect. 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Fang, Z.; Ouyang, Z.; Zheng, H.; Wang, X. Concentration and size distribution of culturable airborne microorganisms in outdoor environments in Beijing, China. Aerosol Sci. Technol. 2008, 42, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Smets, W.; Moretti, S.; Denys, S.; Lebeer, S. Airborne bacteria in the atmosphere: Presence, purpose, and potential. Atmos. Environ. 2016, 139, 214–221. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Qu, Y.; Wang, J.; An, J.; Zhang, Y.; Zhang, F. Formation mechanism of continuous extreme haze episodes in the megacity Beijing, China, in January 2013. Atmos. Res. 2015, 155, 192–203. [Google Scholar] [CrossRef]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Cheng, N. VOC characteristics, sources and contributions to SOA formation during haze events in Wuhan, Central China. Sci. Total Environ. 2019, 650, 2624–2639. [Google Scholar] [CrossRef]
- Xu, C.; Chen, J.; Wang, Z.; Chen, H.; Feng, H.; Wang, L.; Xie, Y.; Wang, Z.; Ye, X.; Kan, H. Diverse bacterial populations of PM2.5 in urban and suburb Shanghai, China. Front. Environ. Sci. Eng. 2021, 15, 1–10. [Google Scholar] [CrossRef]
- Ho, H.M.; Rao, C.Y.; Hsu, H.H.; Chiu, Y.H.; Liu, C.M.; Chao, H.J. Characteristics and determinants of ambient fungal spores in Hualien, Taiwan. Atmos. Environ. 2005, 39, 5839–5850. [Google Scholar] [CrossRef]
- Tan, J.; Duan, J.; He, K.; Ma, Y.; Duan, F.; Chen, Y.; Fu, J. Chemical characteristics of PM2.5 during a typical haze episode in Guangzhou. J. Environ. Sci. 2009, 21, 774–781. [Google Scholar] [CrossRef]
- Mouli, P.; Mohan, S.; Reddy, S. Assessment of microbial (bacteria) Concentrations of ambient air at semi-arid urban region: Influence of meteorological factors. Appl. Ecol. Environ. Res. 2005, 3, 139–149. [Google Scholar] [CrossRef]
- Wei, M.; Liu, H.; Chen, J.; Xu, C.; Li, J.; Xu, P.; Sun, Z. Effects of aerosol pollution on PM2.5-associated bacteria in typical inland and coastal cities of northern China during the winter heating season. Environ. Pollut. 2020, 262, 114188. [Google Scholar] [CrossRef]
- Xu, C.; Wei, M.; Chen, J.; Sui, X.; Zhu, C.; Li, J.; Zheng, L.; Sui, G.; Li, W.; Wang, W. Investigation of diverse bacteria in cloud water at Mt. Tai, China. Sci. Total Environ. 2017, 580, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Hameed, A.A.; Khoder, M.; Ibrahim, Y.; Saeed, Y.; Osman, M.; Ghanem, S. Study on some factors affecting survivability of airborne fungi. Sci. Total Environ. 2012, 414, 696–700. [Google Scholar] [CrossRef]
- Pan, Y.; Luo, L.; Xiao, H.; Zhu, R.; Xiao, H. Spatial variability of inhalable fungal communities in airborne PM2.5 across Nanchang, China. Sci. Total Environ. 2020, 746, 141171. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, X.; Ju, F.; Zhan, H.; Jiang, B.; Kang, H.; Xie, Z. Airborne bacterial community diversity, source and function along the Antarctic Coast. Sci. Total Environ. 2021, 765, 142700. [Google Scholar] [CrossRef]
- Wei, M.; Xu, C.; Chen, J.; Zhu, C.; Li, J.; Lv, G. Characteristics of bacterial community in cloud water at Mt Tai: Similarity and disparity under polluted and non-polluted cloud episodes. Atmos. Chem. Phys. 2017, 17, 5253–5270. [Google Scholar] [CrossRef] [Green Version]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef]
- Lou, J.; Gu, H.; Wang, H.; An, Q.; Xu, J. Complete genome sequence of Massilia sp. WG5, an efficient phenanthrene-degrading bacterium from soil. J. Biotechnol. 2016, 218, 49–50. [Google Scholar] [CrossRef]
- Ventorino, V.; Sannino, F.; Piccolo, A.; Cafaro, V.; Carotenuto, R.; Pepe, O. Methylobacterium populi VP2: Plant growth-promoting bacterium isolated from a highly polluted environment for polycyclic aromatic hydrocarbon (PAH) biodegradation. Sci. World J. 2014, 2014, 931793. [Google Scholar] [CrossRef] [Green Version]
- Ye, D.; Siddiqi, M.A.; Maccubbin, A.E.; Kumar, S.; Sikka, H.C. Degradation of polynuclear aromatic hydrocarbons by Sphingomonas paucimobilis. Environ. Sci. Technol. 1995, 30, 136–142. [Google Scholar] [CrossRef]
- Robinson, C.V.; Elkins, M.R.; Bialkowski, K.M.; Thornton, D.J.; Kertesz, M.A. Desulfurization of mucin by Pseudomonas aeruginosa: Influence of sulfate in the lungs of cystic fibrosis patients. J. Med. Microbiol. 2012, 61, 1644–1653. [Google Scholar] [CrossRef]
- Noto, M.J.; Boyd, K.L.; Burns, W.J.; Varga, M.G.; Peek, R.M.; Skaar, E.P. Toll-like receptor 9 contributes to defense against Acinetobacter baumannii infection. Infect. Immun. 2015, 83, 4134–4141. [Google Scholar] [CrossRef] [Green Version]
- Hejazi, A.; Falkiner, F. Serratia marcescens. J. Med. Microbiol. 1997, 46, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Ray, M.; Lim, D.K. A rare polymicrobial keratitis involving Chryseobacterium meningosepticum and Delftia acidovorans in a cosmetic contact lens wearer. Eye Contact Lens Sci. Clin. Pract. 2013, 39, 192–193. [Google Scholar] [CrossRef]
- Lin, F.Y.C.; Weisman, L.E.; Troendle, J.; Adams, K. Prematurity is the major risk factor for late-onset group B streptococcus disease. J. Infect. Dis. 2003, 188, 267–271. [Google Scholar] [CrossRef]
- Aykac, K.; Ozsurekci, Y.; Tuncer, O.; Sancak, B.; Cengiz, A.B.; Kara, A.; Ceyhan, M. Six cases during 2012–2015 and literature review of Chryseobacterium indologenes infections in pediatric patients. Can. J. Microbiol. 2016, 62, 812–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trost, E.; Blom, J.; de Castro Soares, S.; Huang, I.H.; Al-Dilaimi, A.; Schröder, J.; Jaenicke, S.; Dorella, F.A.; Rocha, F.S.; Miyoshi, A. Pangenomic study of Corynebacterium diphtheriae that provides insights into the genomic diversity of pathogenic isolates from cases of classical diphtheria, endocarditis, and pneumonia. J. Bacteriol. 2012, 194, 3199–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.L.; Shao, M.F.; Wang, Q.; Wang, L.T.; Fang, W.Y.; Ouyang, F.; Li, J. Airborne microbial communities in the atmospheric environment of urban hospitals in China. J. Hazard. Mater. 2018, 349, 10–17. [Google Scholar] [CrossRef]
- da Silva, L.; Bock, D.; Klafke, G.; Sanchotene, K.; Basso, R.; Benelli, J.; Poester, V.; da Silva, F.; Trilles, L.; Severo, C. Cryptococcosis in HIV-AIDS patients from Southern Brazil: Still a major problem. J. Mycol. Med. 2020, 30, 101044. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, A.; Kurnatowski, P.; Błaszkowska, J. Potentially pathogenic yeasts from soil of children’s recreational areas in the city of Łódź (Poland). Int. J. Occup. Med. Environ. Health 2013, 26, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Bigirimana, V.D.P.; Hua, G.K.H.; Nyamangyoku, O.I.; Hoefte, M. Rice Sheath Rot: An emerging ubiquitous destructive disease complex. Front. Plant Sci. 2015, 6, 1066:1–1066:16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, H.Y.; Dufault, N.S.; Walker, D.R.; Isard, S.A.; Schneider, R.W.; Giesler, L.J.; Wright, D.L.; Marois, J.J.; Hartman, G.L. From select agent to an established pathogen: The response to Phakopsora pachyrhizi (soybean rust) in North America. Phytopathology 2015, 105, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Zeigler, R.S. Recombination in Magnaporthe grisea. Annu. Rev. Phytopathol. 1998, 36, 249–275. [Google Scholar] [CrossRef]


| Sample | Sequence Numbers | OTUs | Chao 1 | ACE | Shannon | Simpson | Coverage | |
|---|---|---|---|---|---|---|---|---|
| WXA | 57706 | 676 | 759 | 732 | 4.26 | 0.937 | 99.88% | |
| SPXA | 37522 | 1162 | 1336 | 1303 | 5.48 | 0.982 | 99.46% | |
| SUXA | 38214 | 570 | 738 | 694 | 4.59 | 0.98 | 99.73% | |
| Bacteria | AXA | 35888 | 2684 | 3126 | 3079 | 6.47 | 0.996 | 98.62% |
| WLF | 49533 | 592 | 675 | 641 | 4.50 | 0.957 | 99.90% | |
| SPLF | 38228 | 701 | 824 | 802 | 4.58 | 0.961 | 99.68% | |
| SULF | 38070 | 862 | 966 | 942 | 5.08 | 0.983 | 99.62% | |
| ALF | 39175 | 2462 | 2789 | 2721 | 6.39 | 0.994 | 98.88% | |
| WXA | 78090 | 126 | 160 | 163 | 2.71 | 0.899 | 99.97% | |
| SPXA | 102636 | 141 | 161 | 162 | 2.60 | 0.858 | 99.98% | |
| SUXA | 55630 | 183 | 204 | 201 | 2.62 | 0.848 | 99.96% | |
| Fungi | AXA | 96725 | 126 | 143 | 142 | 3.17 | 0.934 | 99.98% |
| WLF | 77206 | 87 | 101 | 111 | 0.98 | 0.301 | 99.98% | |
| SPLF | 105503 | 130 | 144 | 144 | 2.55 | 0.849 | 99.98% | |
| SULF | 100109 | 115 | 141 | 162 | 2.92 | 0.887 | 99.98% | |
| ALF | 61915 | 110 | 132 | 127 | 2.84 | 0.869 | 99.97% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liu, W.; Li, J.; Sun, H.; Qian, Y.; Ding, L.; Ma, H.; Li, J. Seasonal Variation Characteristics of Bacteria and Fungi in PM2.5 in Typical Basin Cities of Xi’an and Linfen, China. Atmosphere 2021, 12, 809. https://doi.org/10.3390/atmos12070809
Wang S, Liu W, Li J, Sun H, Qian Y, Ding L, Ma H, Li J. Seasonal Variation Characteristics of Bacteria and Fungi in PM2.5 in Typical Basin Cities of Xi’an and Linfen, China. Atmosphere. 2021; 12(7):809. https://doi.org/10.3390/atmos12070809
Chicago/Turabian StyleWang, Sen, Wanyu Liu, Jun Li, Haotian Sun, Yali Qian, Liuyi Ding, Hao Ma, and Jiao Li. 2021. "Seasonal Variation Characteristics of Bacteria and Fungi in PM2.5 in Typical Basin Cities of Xi’an and Linfen, China" Atmosphere 12, no. 7: 809. https://doi.org/10.3390/atmos12070809
APA StyleWang, S., Liu, W., Li, J., Sun, H., Qian, Y., Ding, L., Ma, H., & Li, J. (2021). Seasonal Variation Characteristics of Bacteria and Fungi in PM2.5 in Typical Basin Cities of Xi’an and Linfen, China. Atmosphere, 12(7), 809. https://doi.org/10.3390/atmos12070809








