Impact of Residual Water Vapor on the Simultaneous Measurements of Trace CH4 and N2O in Air with Cavity Ring-Down Spectroscopy
Abstract
1. Introduction
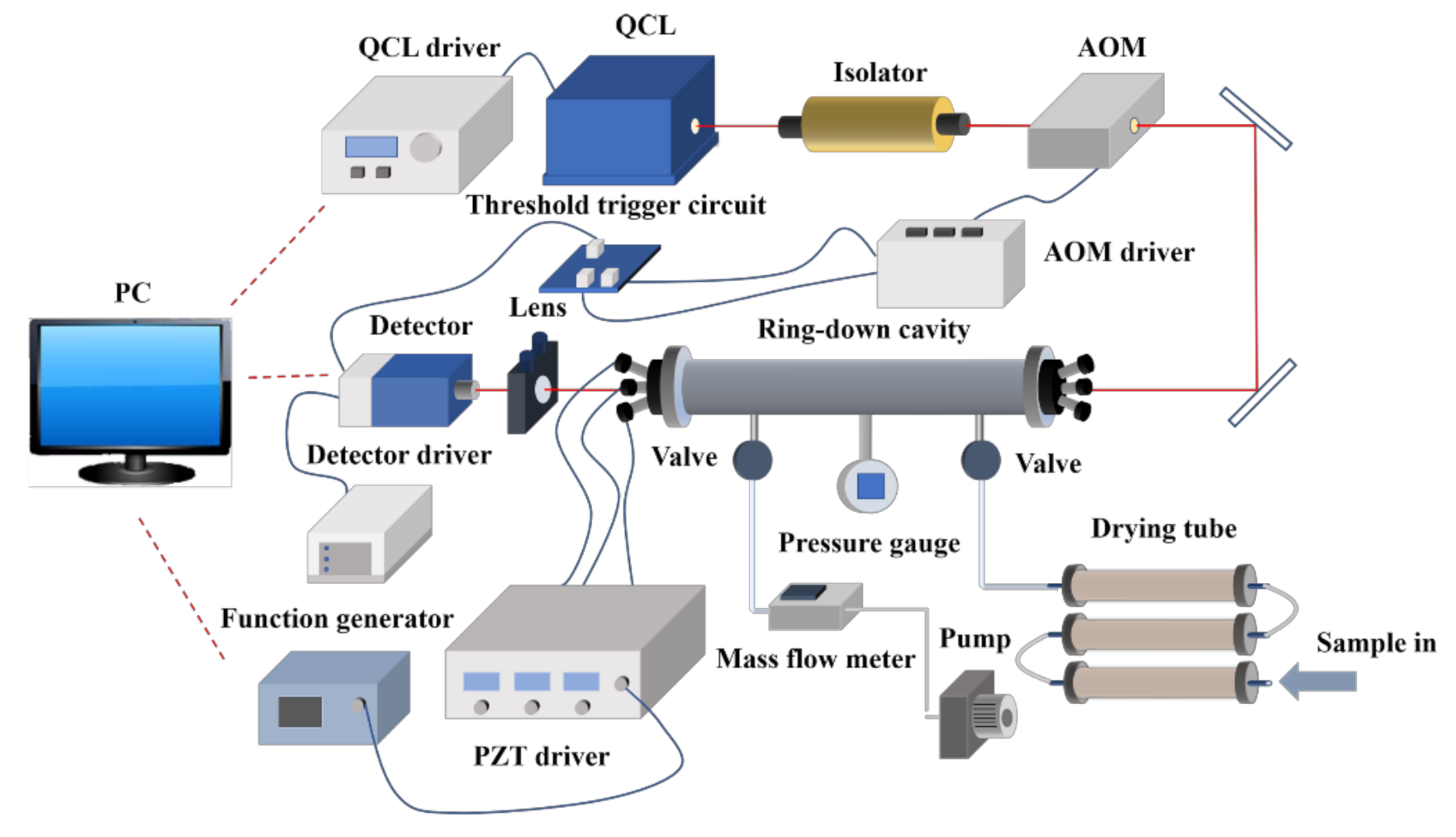
2. Experiments
3. Results and Discussion
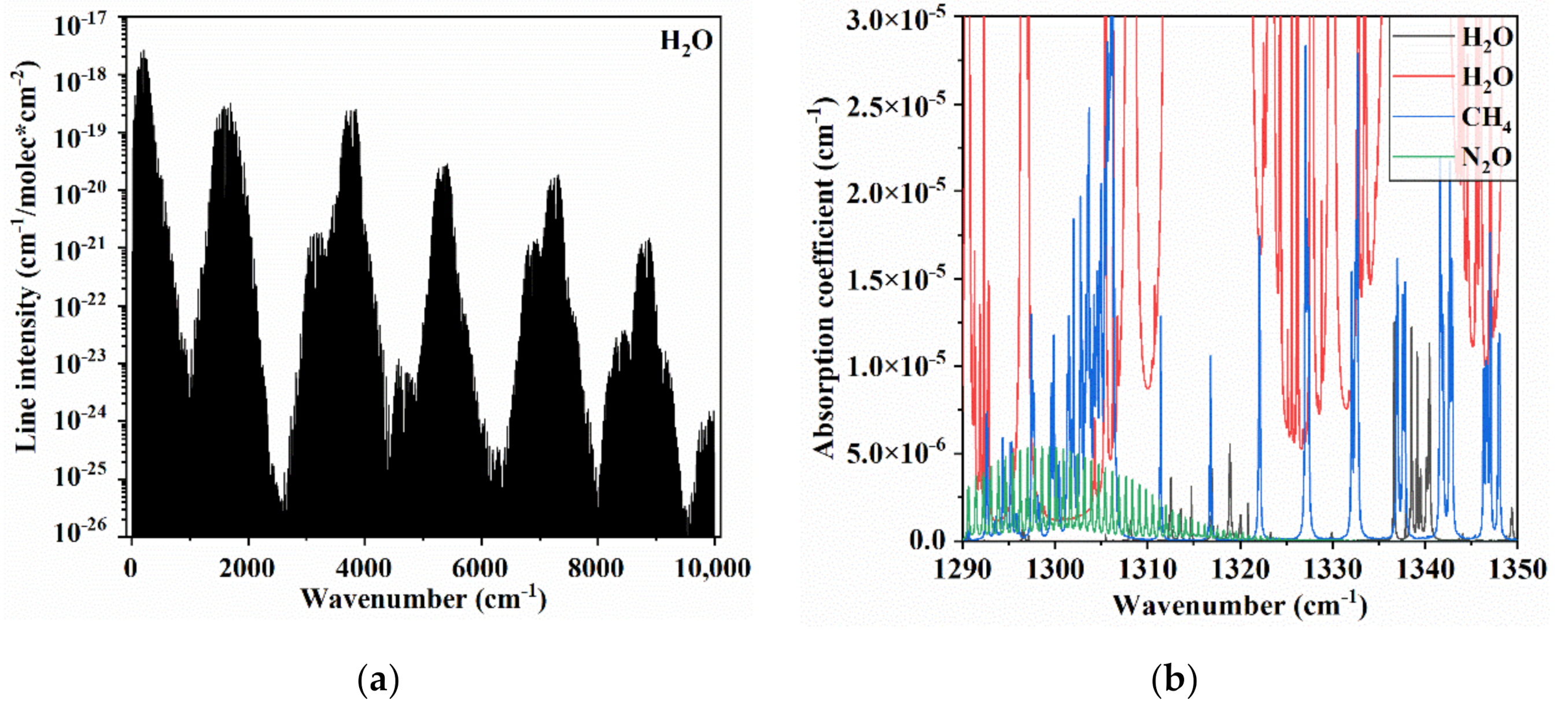
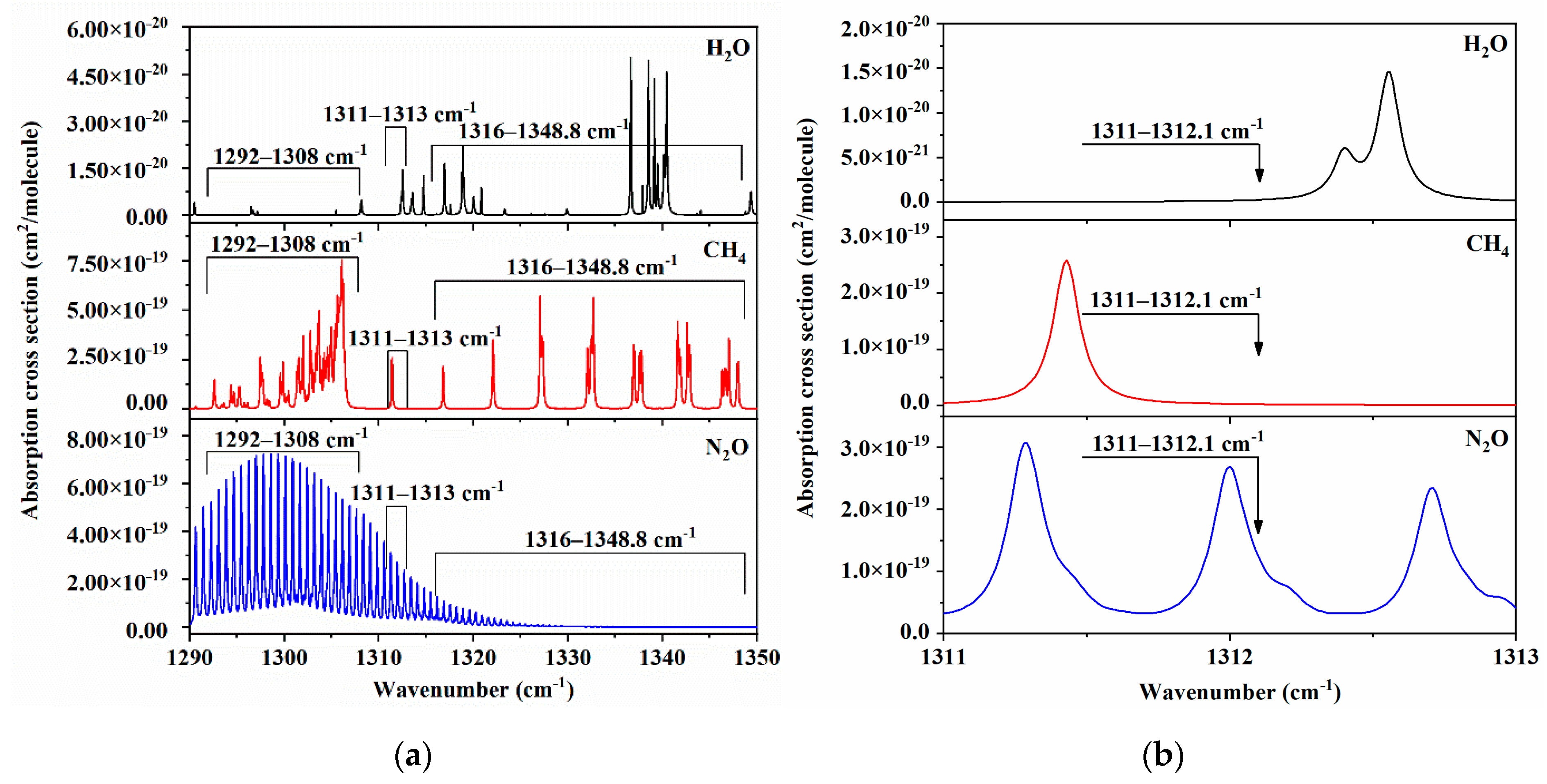
3.1. Spectral Range Selection
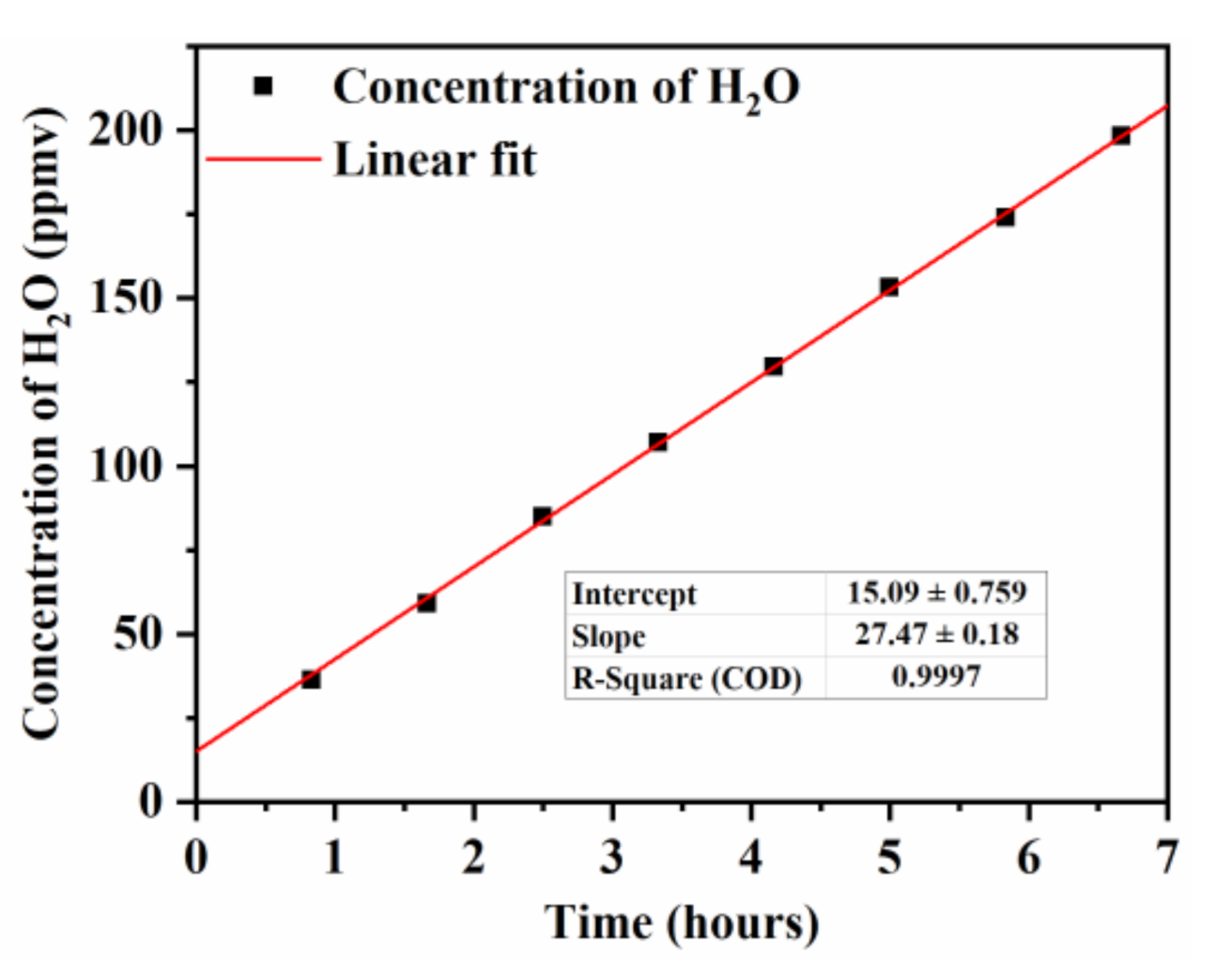
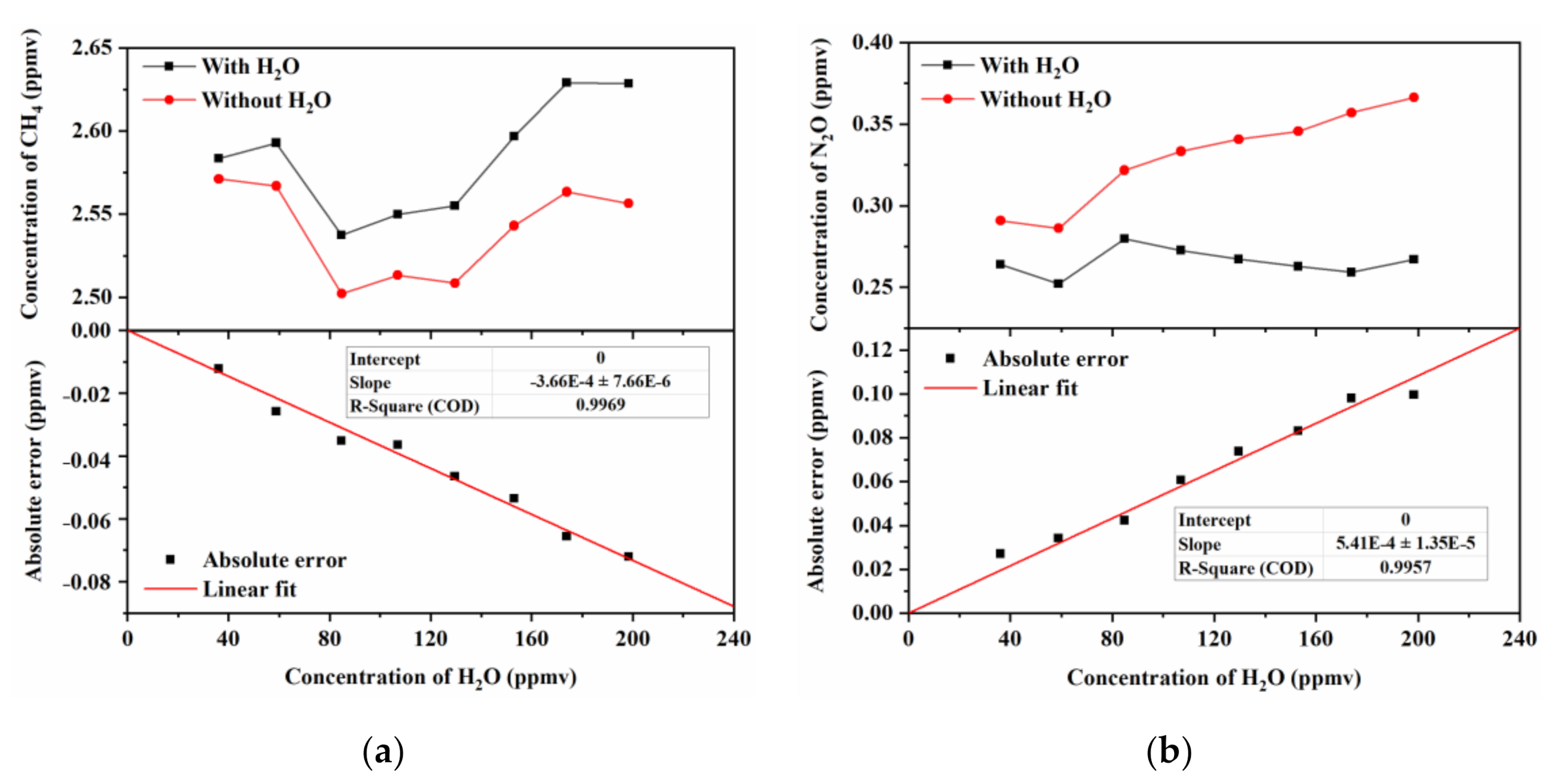
3.2. Impact of Residual Water Vapor on CH4 and N2O Measurements
3.3. CH4 and N2O Measurements of Flowing Air
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillett, N.P.; Stone, D.A.; Stott, P.A.; Nozawa, T.; Karpechko, A.Y.; Hegerl, G.C.; Wehner, M.F.; Jones, P.D. Attribution of polar warming to human influence. Nat. Geosci. 2008, 1, 750–754. [Google Scholar] [CrossRef]
- Boucher, O.; Friedlingstein, P.; Collins, B.; Shine, K.P. The indirect global warming potential and global temperature change potential due to methane oxidation. Environ. Res. Lett. 2009, 4, 4. [Google Scholar] [CrossRef]
- Rapson, T.D.; Dacres, H. Analytical techniques for measuring nitrous oxide. TrAC Trends Anal. Chem. 2014, 54, 65–74. [Google Scholar] [CrossRef]
- Prather, M.J.; Hsu, J. Coupling of nitrous oxide and methane by global atmospheric chemistry. Science 2010, 330, 952–954. [Google Scholar] [CrossRef]
- Ren, W.; Jiang, W.; Tittel, F.K. Single-QCL-based absorption sensor for simultaneous trace-gas detection of CH4 and N2O. Appl. Phys. A 2014, 117, 245–251. [Google Scholar] [CrossRef]
- Hamilton, D.J.; Orr-Ewing, A.J. A Quantum cascade laser-based optical feedback cavity-enhanced absorption spectrometer for the simultaneous measurement of CH4 and N2O in air. Appl. Phys. A 2010, 102, 879–890. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2012, 24, 24. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef]
- Mahbub, P.; Noori, A.; Parry, J.S.; Davis, J.; Lucieer, A.; Macka, M. Continuous and real-time indoor and outdoor methane sensing with portable optical sensor using rapidly pulsed IR LEDs. Talanta 2020, 218. [Google Scholar] [CrossRef]
- Shao, L.; Fang, B.; Zheng, F.; Qiu, X.; He, Q.; Wei, J.; Li, C.; Zhao, W. Simultaneous detection of atmospheric CO and CH4 based on TDLAS using a single 2.3 μm DFB laser. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222. [Google Scholar] [CrossRef]
- Crosson, E. A cavity ring-down analyzer for measuring atmospheric levels of methane, carbon dioxide, and water vapor. Appl. Phys. A 2008, 92, 403–408. [Google Scholar] [CrossRef]
- Wheeler, M.D.; Newman, S.M.; Orr-Ewing, A.J.; Ashfold, M. Cavity ring-down spectroscopy. J. Chem. Soc. Faraday Trans. 1998, 94, 337–351. [Google Scholar] [CrossRef]
- Romanini, D.; Kachanov, A.; Sadeghi, N.; Stoeckel, F. CW cavity ring down spectroscopy. Chem. Phys. Lett. 1997, 264, 316–322. [Google Scholar] [CrossRef]
- Berden, G.; Peeters, R.; Meijer, G. Cavity ring-down spectroscopy: Experimental schemes and applications. Int. Rev. Phys. Chem. 2000, 19, 565–607. [Google Scholar] [CrossRef]
- De, A.; Banik, G.D.; Maity, A.; Pal, M.; Pradhan, M. Continuous wave external-cavity Quantum cascade laser-based high-resolution cavity ring-down spectrometer for ultrasensitive trace gas detection. Opt. Lett. 2016, 41, 1949–1952. [Google Scholar] [CrossRef]
- Curl, R.F.; Capasso, F.; Gmachl, C.; Kosterev, A.A.; McManus, B.; Lewicki, R.; Pusharsky, M.; Wysocki, G.; Tittel, F.K. Quantum cascade lasers in chemical physics. Chem. Phys. Lett. 2010, 487, 1–18. [Google Scholar] [CrossRef]
- Wysocki, G.; Lewicki, R.; Curl, R.; Tittel, F.; Diehl, L.; Capasso, F.; Troccoli, M.; Höfler, G.; Bour, D.; Corzine, S.; et al. Widely tunable mode-hop free external cavity Quantum cascade lasers for high resolution spectroscopy and chemical sensing. Appl. Phys. A 2008, 92, 305–311. [Google Scholar] [CrossRef]
- Banik, G.D.; Som, S.; Maity, A.; Pal, M.; Maithani, S.; Mandal, S.; Pradhan, M. An EC-QCL based N2O sensor at 5.2 μm using cavity ring-down spectroscopy for environmental applications. Anal. Methods 2017, 9, 2315–2320. [Google Scholar] [CrossRef]
- Maity, A.; Pal, M.; Banik, G.D.; Maithani, S.; Pradhan, M. Cavity ring-down spectroscopy using an EC-QCL operating at 7.5 µm for direct monitoring of methane isotopes in air. Laser Phys. Lett. 2017, 14. [Google Scholar] [CrossRef]
- Tang, J.; Li, B.; Wang, J. High-precision measurements of nitrous oxide and methane in air with cavity ring-down spectroscopy at 7.6 µm. Atmos. Meas. Tech. 2019, 12, 2851–2861. [Google Scholar] [CrossRef]
- Sang, J.; Zhou, S.; Zhang, L.; He, T.; Li, J. Impact of H2O on atmospheric CH4 measurement in near-infrared absorption spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 237. [Google Scholar] [CrossRef]
- Deng, H.; Sun, J.; Liu, N.; Wang, H.; Yu, B.; Li, J. Impact of H2O broadening effect on atmospheric CO and N2O detection near 4.57 μm. J. Mol. Spectrosc. 2017, 331, 34–43. [Google Scholar] [CrossRef]
- Owen, K.; Es-Sebbar, E.-T.; Farooq, A. Measurements of NH3 linestrengths and collisional broadening coefficients in N2, O2, CO2, and H2O near 1103.46cm−1. J. Quant. Spectrosc. Radiat. Transf. 2013, 121, 56–68. [Google Scholar] [CrossRef]
- Chen, H.; Winderlich, J.; Gerbig, C.; Hoefer, A.; Rella, C.W.; Crosson, E.R.; Van Pelt, A.D.; Steinbach, J.H.; Kolle, O.; Beck, V.; et al. High-accuracy continuous airborne measurements of greenhouse gases (CO2 and CH4) using the cavity ring-down spectroscopy (CRDS) technique. Atmos. Meas. Tech. 2010, 3, 375–386. [Google Scholar] [CrossRef]
- Wallace, C.J.; Jeon, C.; Anderson, C.N.; Havey, D.K. H2O Broadening of a CO2 line and its nearest neighbors near 6360 cm−1. J. Phys. Chem. A 2011, 115, 13804–13810. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- Rella, C.W.; Chen, H.; Andrews, A.E.; Filges, A.; Gerbig, C.; Hatakka, J.; Karion, A.; Miles, N.; Richardson, S.J.; Steinbacher, M.; et al. High accuracy measurements of dry mole fractions of carbon dioxide and methane in humid air. Atmos. Meas. Tech. 2013, 6, 837–860. [Google Scholar] [CrossRef]
- Chen, H.; Karion, A.; Rella, C.W.; Winderlich, J.; Gerbig, C.; Filges, A.; Newberger, T.; Sweeney, C.; Tans, P.P. Accurate measurements of carbon monoxide in humid air using the cavity ring-down spectroscopy (CRDS) technique. Atmos. Meas. Tech. 2013, 6, 1031–1040. [Google Scholar] [CrossRef]
- Zellweger, C.; Steinbacher, M.; Buchmann, B. Evaluation of new laser spectrometer techniques for in-situ carbon monoxide measurements. Atmos. Meas. Tech. 2012, 5, 2555–2567. [Google Scholar] [CrossRef]
- Butenhoff, C.; Khalil, M. Correction for water vapor in the measurement of atmospheric trace gases. Chemosphere 2002, 47, 823–836. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Li, B.; Wang, J.; Zhao, B.; Yang, P. Impact of Residual Water Vapor on the Simultaneous Measurements of Trace CH4 and N2O in Air with Cavity Ring-Down Spectroscopy. Atmosphere 2021, 12, 221. https://doi.org/10.3390/atmos12020221
Wei Q, Li B, Wang J, Zhao B, Yang P. Impact of Residual Water Vapor on the Simultaneous Measurements of Trace CH4 and N2O in Air with Cavity Ring-Down Spectroscopy. Atmosphere. 2021; 12(2):221. https://doi.org/10.3390/atmos12020221
Chicago/Turabian StyleWei, Qianhe, Bincheng Li, Jing Wang, Binxing Zhao, and Ping Yang. 2021. "Impact of Residual Water Vapor on the Simultaneous Measurements of Trace CH4 and N2O in Air with Cavity Ring-Down Spectroscopy" Atmosphere 12, no. 2: 221. https://doi.org/10.3390/atmos12020221
APA StyleWei, Q., Li, B., Wang, J., Zhao, B., & Yang, P. (2021). Impact of Residual Water Vapor on the Simultaneous Measurements of Trace CH4 and N2O in Air with Cavity Ring-Down Spectroscopy. Atmosphere, 12(2), 221. https://doi.org/10.3390/atmos12020221






