Impacts of Permafrost Degradation on Carbon Stocks and Emissions under a Warming Climate: A Review
Abstract
:1. Introduction
2. Carbon Stocks in Permafrost Regions
2.1. Soil Organic Carbon (SOC) Stocks in Permafrost Regions
2.2. Subsea Permafrost Carbon Storage
2.3. Methane Hydrate Storages in Permafrost Regions
3. Biodegradability of Permafrost Organic Carbon (POC)
4. Carbon Emissions in Regions of Degrading Permafrost
4.1. Atmospheric CH4 and CO2 Emission Induced by Permafrost Degradation
4.2. Lateral Carbon Flux in Regions of Degrading Permafrost
5. Modeling and Projecting Permafrost Carbon Feedback to Climate Warming
6. Summary, Inadequacies, and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hugelius, G.; Strauss, J.; Zubrzycki, S.; Harden, J.D.; Schuur, E.A.G.; Ping, C.L.; Schirrmeister, L.; Grosse, G.; Michaelson, G.J.; Koven, C.D. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 2014, 11, 6573–6593. [Google Scholar] [CrossRef] [Green Version]
- Schuur, E.A.G.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef]
- Zimov, S.A.; Davydov, S.P.; Zimova, G.M.; Davydova, A.I.; Schuur, E.A.G.; Dutta, K.; Chapin, F.S., III. Permafrost carbon: Stock and decomposability of a globally significant carbon pool. Geophys. Res. Lett. 2006, 33, L20502. [Google Scholar] [CrossRef] [Green Version]
- Zimov, S.A.; Schuur, E.A.G.; Chapin, F.S., III. Permafrost and the global carbon budget. Science 2006, 312, 1612–1613. [Google Scholar] [CrossRef] [PubMed]
- Köchy, M.; Hiederer, R.; Freibauer, A. Global distribution of soil organic carbon–Part 1: Masses and frequency distributions of SOC stocks for the tropics, permafrost regions, wetlands, and the world. Soil 2015, 1, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Intergovernmental Panel of Climate Change (IPCC). Climate Change 2021: The Physical Science Basis, Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel of Climate Change (IPCC): Cambridge, UK; New York, NY, USA, 2021.
- Peng, X.; Frauenfeld, O.W.; Jin, H.; Du, R.; Qiao, L.; Zhao, Y.; Mu, C.; Zhang, T. Assessment of temperature changes on the Tibetan Plateau during 1980–2018. Earth Space Sci. 2021, 8, e2020EA001609. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Bockheim, J.; Canadell, J.G.; Euskirchen, E.; Field, C.B.; Goryachkin, S.V.; Hagemann, S.; Kuhry, P.; Lafleur, P.M.; Lee, H.; et al. Vulnerability of permafrost carbon to climate change: Implications for the global carbon cycle. Bioscience 2008, 58, 701–714. [Google Scholar] [CrossRef]
- Van Huissteden, J.; Dolman, A.J. Soil carbon in the Arctic and the permafrost carbon feedback. Curr. Opin. Environ. Sust. 2012, 4, 545–551. [Google Scholar] [CrossRef]
- Hope, D.; Billett, M.F.; Cresser, M.S. A review of the export of carbon in river water: Fluxes and processes. Environ. Pollut. 1994, 84, 301–324. [Google Scholar] [CrossRef]
- Fabre, C.; Sauvage, S.; Tananaev, N. Assessment of sediment and organic carbon exports into the Arctic ocean: The case of the Yenisei River basin. Water Resour. 2019, 158, 118–135. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Jin, H.; Yu, C.; Bense, V. Dissolved organic carbon in permafrost regions: A review. Sci. China Earth Sci. 2019, 62, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Meybeck, M. River transport of organic carbon to the ocean. In Flux of Organic Carbon by Rivers to the Oceans Report of a Workshop Held at the NAS Study Center, Woods Hole, MA, USA, 21–25 September 1980; CONF-8009140; United States Department of Energy: Washinton, DC, USA, 1981; pp. 219–269. [Google Scholar]
- Tanski, G.; Wagner, D.; Knoblauch, C.; Fritz, M.; Lantuit, H. Rapid CO2 release from eroding permafrost in seawater. Geophys. Res. Lett. 2019, 46, 11244–11252. [Google Scholar] [CrossRef] [Green Version]
- Rosentreter, J.A.; Borges, A.V.; Deemer, B.R.; Holgerson, M.A.; Liu, S.; Song, C.; Melack, J.; Raymond, P.A.; Duarte, C.M.; Allen, G.H.; et al. Half of global methane emissions come form highly variable aquatic ecosystem sources. Nat. Geosci. 2021, 14, 225–239. [Google Scholar] [CrossRef]
- Jorgenson, M.T.; Shur, Y.L.; Pullman, E.R. Abrupt increase in permafrost degradation in Arctic Alaska. Geophys. Res. Lett. 2006, 33, L02503. [Google Scholar] [CrossRef]
- Natali, S.M.; Watts, J.D.; Rogers, B.M.; Potter, S.; Ludwig, S.M.; Selbmann, A.K.; Sullivan, P.F.; Abbott, B.W.; Arndt, K.A.; Birch, L.; et al. Large loss of CO2 in winter observed across the northern permafrost region. Nat. Clim. Chang. 2019, 9, 852–857. [Google Scholar] [CrossRef]
- Murton, J.B.; Edwards, M.E.; Lozhkin, A.V.; Anderson, P.M.; Savvinov, G.N.; Bakulina, N.; Bondarenko, O.V.; Cherepanova, M.V.; Danilov, P.P.; Boeskorov, V. Preliminary paleoenvironmental analysis of permafrost deposits at Batagaika megaslump, Yana Uplands, northeast Siberia. Quat. Res. 2017, 87, 314–330. [Google Scholar] [CrossRef] [Green Version]
- Fritz, M.; Opel, T.; Tanski, G.; Herzschuh, U.; Meyer, H.; Eulenburg, A.; Lantuit, H. Dissolved organic carbon (DOC) in Arctic ground ice. Cryosphere 2015, 9, 737–752. [Google Scholar] [CrossRef] [Green Version]
- Elberling, B. Annual soil CO2 effluxes in the High Arctic: The role of snow thickness and vegetation type. Soil Biol. Biochem. 2007, 39, 646–654. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Abbott, B.W.; Bowden, W.B.; Brovkin, V.; Camill, P.; Canadell, J.G.; Chanton, J.P.; Chapin, F.S., III; Christensen, T.R.; Ciais, P.; et al. Expert assessment of vulnerability of permafrost carbon to climate change. Clim. Chang. 2013, 119, 359–374. [Google Scholar] [CrossRef] [Green Version]
- Plaza, C.; Pegoraro, E.; Bracho, R.; Celis, G.; Crummer, K.G.; Hutchings, J.A.; Hicks Pries, C.E.; Mauritz, M.; Natali, S.; Salmon, V.G.; et al. Direct observation of permafrost degradation and rapid soil carbon loss in tundra. Nat. Geosci. 2019, 12, 627–631. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, F.; Kang, L.; Zhang, D.; Kou, D.; Mao, C.; Qin, S.; Zhang, Q.; Yang, Y. Large-scale evidence for microbial response and associated carbon release after permafrost thaw. Glob. Chang. Biol. 2020, 27, 3218–3229. [Google Scholar] [CrossRef]
- Monique, B. The buried carbon bomb. Nature 2021, 591, 360–362. [Google Scholar]
- Ping, C.-L.; Michaelson, G.J.; Jorgenson, M.T.; Kimble, J.M.; Epstein, H.; Romanovsky, V.E.; Walker, D.A. High stocks of soil organic carbon in the North American Arctic region. Nat. Geosci. 2008, 1, 615–619. [Google Scholar] [CrossRef]
- Burnham, J.H.; Sletten, R.S. Spatial distribution of soil organic carbon in northwest Greenland and underestimates of high Arctic carbon stores. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Strauss, J.; Schirrmeister, L.; Grosse, G.; Wetterich, S.; Ulrich, M.; Herzschuh, U.; Hubberten, H.W. The deep permafrost carbon pool of the Yedoma region in Siberia and Alaska. Geophys. Res. Lett. 2013, 40, 6165–6170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; Peng, S.; Ciais, P.; Zech, R.; Krinner, S.; Zimov, G. Simulating soil organic carbon in yedoma deposits during the Last Glacial Maximum in a land surface model. Geophys. Res. Lett. 2016, 43, 5133–5142. [Google Scholar] [CrossRef] [Green Version]
- Tarnocai, C. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycles 2009, 23, GB2023. [Google Scholar] [CrossRef]
- Bockheim, J.G.; Haus, N.W. Distribution of organic carbon in the soils of Antarctica. In Soil Carbon; Hartemink, A.E., McSweeney, K., Eds.; Springer: Cham, Switzerland, 2014; pp. 373–380. [Google Scholar]
- Lindgren, A.; Hugelius, G.; Kuhry, P. Extensive loss of past permafrost carbon but a net accumulation into present-day soils. Nature 2018, 560, 219–222. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, C.J.A.; Warkentin, I.G. Global estimates of boreal forest carbon stocks and flux. Glob. Planet. Chang. 2015, 128, 24–30. [Google Scholar] [CrossRef]
- Turunen, J.; Tomppo, E.; Tolonen, K.; Reinikainen, A. Estimating carbon accumulation rates of undrained mires in Finland–application to boreal and subarctic regions. Holocene 2002, 12, 79–90. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, T.; Wu, Q.; Peng, X.; Cao, B.; Zhang, X.; Cao, B.; Cheng, G. Editorial: Organic carbon pools in permafrost regions on the Qinghai–Xizang (Tibetan) Plateau. Cryosphere 2015, 9, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Shmelev, D.; Veremeeva, A.; Kraev, G.; Kholodov, A.; Spencer, R.; Walker, W.; Rivkinaet, E. Estimation and sensitivity of carbon storage in permafrost of North-Eastern Yakutia. Permafr. Periglac. Process. 2017, 28, 379–390. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, X.; Wang, Z.; Sheng, Y.; Fang, H.; Zhao, Y.; Hu, G.; Li, W.; Pang, Q.; Shi, J.; et al. Soil organic carbon and total nitrogen pools in permafrost zones of the Qinghai-Tibentan Plateau. Sci. Rep. 2018, 8, 3656. [Google Scholar] [CrossRef]
- Ding, J.; Wang, T.; Piao, S. The paleoclimate footprint in the soil carbon stock of the Tibetan permafrost region. Nat. Commun. 2019, 10, 4195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Wu, T.; Wu, X.; Wei, X.; Mu, C.; Li, R.; Hu, G.; Zou, D.; Zhu, X.; Chen, J.; et al. Soil organic carbon distribution for 0-3 m soils at 1 km2 scale of the frozen ground in the Third Pole Regions. Earth Syst. Sci. Data 2021. [Google Scholar] [CrossRef]
- Egli, M.; Mirabella, A.; Sartori, G.; Zanelli, R.; Bischof, S. Effect of north and south exposure on weathering rates and clay mineral formation in alpine soils. Cetena 2006, 67, 155–174. [Google Scholar] [CrossRef]
- Dymov, A.A.; Zhangurov, E.V.; Startsev, V.V. Soil of the northern part of the Subpolar Urals: Morphology, physicochemical properties, and carbon and nitrogen pools. Euras. Soil Sci. 2013, 46, 459–467. [Google Scholar] [CrossRef]
- Miller, D.C.; Birkeland, P.W. Soil catena variation along an alpine climatic transact, northern Peruvian Andes. Geoderma 1992, 55, 211–223. [Google Scholar] [CrossRef]
- Pascual, D.; Kuhry, P.; Raudina, T. Soil organic carbon storage in a mountain permafrost area of Central Asia (High Altai, Russia). Ambio 2021, 50, 2022–2037. [Google Scholar] [CrossRef] [PubMed]
- Bockheim, J.G.; Munroe, J.S. Organic carbon pools and genesis of alpine soils with permafrost: A review. Arct. Antarct. Alp. Res. 2014, 46, 987–1006. [Google Scholar] [CrossRef] [Green Version]
- Ran, Y.; Li, X.; Cheng, G.; Zhang, T.; Wu, Q.B.; Jin, H.; Jin, R. Distribution of permafrost in China: An overview of existing permafrost maps. Permafr. Periglac. Process. 2012, 23, 322–333. [Google Scholar] [CrossRef]
- Ran, Y.; Li, X.; Cheng, G. Climate warming over the past half century has led to thermal degradation of permafrost on the Qinghai–Tibet Plateau. Cryosphere 2018, 12, 595–608. [Google Scholar] [CrossRef] [Green Version]
- Ran, Y.; Li, X.; Cheng, G.; Nan, Z.; Che, J.; Sheng, Y.; Wu, Q.; Jin, H.; Luo, D.; Tang, Z.; et al. Mapping the permafrost stability on the Tibetan Plateau for 2005–2015. Sci. China Earth Sci. 2021, 64, 62–79. [Google Scholar] [CrossRef]
- Ran, Y.; Li, X.; Cheng, G.; Che, J.; Aalto, J.; Karjalainen, O.; Hjort, J.; Luoto, M.; Jin, H.; Obu, J.; et al. New high-resolution estimates of the permafrost thermal state and hydrothermal conditions over the Northern Hemisphere. Earth Syst. Sci. Data Discuss. 2021. [Google Scholar] [CrossRef]
- Lambeck, K.; Rouby, H.; Purcell, A.; Sun, Y.; Sambridge, M. Sea level and global ice volumes from the Last Glacial Maximum to the Holocene. Proc. Natl. Acad. Sci. USA 2014, 111, 15296–15303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayedi, S.S.; Abbott, B.W.; Thornton, B.F.; Frederick, J.M.; Vonk, J.E.; Overduin, P.; Schädel, C.; Schuur, E.A.G.; Bourbonnais, A.; Demidov, N.; et al. Subsea permafrost carbon stocks and climate change sensitivity estimated by expert assessment. Environ. Res. Lett. 2020, 15, 124075. [Google Scholar] [CrossRef]
- Vonk, J.E.; Sánchez-García, L.; Van Dongen, B.E.; Alling, V.; Kosmach, D.; Charkin, A.; Semiletov, I.P.; Dudarev, O.V.; Shakhova, N.; Roos, P.; et al. Activation of old carbon by erosion of coastal and subsea permafrost in Arctic Siberia. Nature 2012, 489, 137–140. [Google Scholar] [CrossRef]
- Thornton, B.; Wik, M.; Crill, P. Double-counting challenges the accuracy of high-latitude methane inventories. Geophy. Res. Lett. 2016, 12, 569–577. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Leifer, I.; Sergienko, V.; Salyuk, D.; Kosmach, D.; Chernykh, C.; Stubbs, D.; Nicolsky, V. Tumskoy Ebullition and storm-induced methane release from the East Siberian Arctic Shelf. Nat. Geosci. 2014, 7, 64–70. [Google Scholar] [CrossRef]
- Maslin, M.; Owen, M.; Betts, R.; Day, S.; Jones, T.D.; Ridgwell, A. Gas hydrates: Past and future geohazard? Philos. Trans. Soc. A 2010, 368, 2369–2393. [Google Scholar] [CrossRef]
- MacDonald, G.J. Role of methane clathrates in past and future climates. Clim. Chang. 1990, 16, 247–281. [Google Scholar] [CrossRef]
- Ruppel, C. Permafrost-associated gas hydrate: Is it really approximately 1% of the global system? J. Chem. Eng. Data 2014, 60, 429–436. [Google Scholar] [CrossRef]
- Wang, X.; Pan, L.; Lau, H.; Zang, M.; Li, L.; Zhou, Q. Reservoir volume of gas hydrate stability zones in permafrost regions of China. Appl. Energy 2018, 225, 486–500. [Google Scholar] [CrossRef]
- Jin, H.; Cheng, G. Methane emissions in permafrost regions. Adv. Earth Sci. 1997, 12, 226–283. (In Chinese) [Google Scholar]
- Ruppel, C.D.; Kessler, J.D. The interaction of climate change and methane hydrates. Rev. Geophys. 2017, 55, 126–168. [Google Scholar] [CrossRef]
- Kretschmer, K.; Biastoch, A.; Rüpke, L.; Burwicz, E. Modeling the fate of methane hydrates under global warming. Glob. Biogeochem. Cycle 2015, 29, 610–625. [Google Scholar] [CrossRef]
- Liesowska, A. More Than 400 Sealed ‘Craters’ Are Ticking Time Bombs from a Total 7000+ Arctic Permafrost Mounds. Available online: https://siberiantimes.com/other/others/news/more-than-300-sealed-craters-are-ticking-time-bombs-from-a-total-7000-plus-arctic-permafrost-mounds (accessed on 30 June 2021).
- Schädel, C.; Schuur, E.A.G.; Bracho, R.; Elberling, B.; Knoblauch, C.; Lee, H.; Luo, Y.; Shaver, G.R.; Turetsky, M.R. Circumpolar assessment of permafrost C quality and its vulerability over time using long-term incubation data. Glob. Chang. Biol. 2014, 20, 641–652. [Google Scholar] [CrossRef]
- Treat, C.C.; Jones, M.C.; Alder, J.; Sannel, A.B.K.; Camill, P.; Frolking, S. Predicted vulnerability of carbon in permafrost peatlands with future climate change and permafrost thaw in Western Canada. Biogeosciences 2021, 126, e2020JG005872. [Google Scholar] [CrossRef]
- Elberling, B.; Michelsen, A.; Schädel, C. Long-term CO2 production following permafrost thaw. Nat. Clim. Chang. 2013, 3, 890–894. [Google Scholar] [CrossRef]
- Chen, L.; Liang, J.; Qin, S. Determinants of carbon release from the active layer and permafrost deposits on the Tibetan Plateau. Nat. Commun. 2016, 7, 13046. [Google Scholar] [CrossRef] [Green Version]
- Kuhry, P.; Básra, J.; Blok, D.; Elberling, B.; Faucherre, S.; Hugelius, G.; Jørgensen, C.; Richter, A.; Šantrůčková, H.; Weiss, N. Lability classification of soil organic matter in the norhern permafrost region. Biogeosciences 2020, 17, 361–379. [Google Scholar] [CrossRef] [Green Version]
- Schuur, E.A.G.; Vogel, J.G.; Crummer, K.G.; Lee, H.; Sickman, J.O.; Osterkamp, T.E. The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature 2009, 495, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Mackelprang, R.; Waldrop, M.P.; DeAngelis, K.M.; David, M.M.; Chavarria, K.L.; Blazewicz, S.J.; Rubin, E.M.; Jansson, J.K. Metagenomic analysis of a permafrost microbial community reveals a rapid response to thaw. Nature 2011, 480, 368–371. [Google Scholar] [CrossRef] [Green Version]
- Xue, K.; Yuan, M.; Shi, Z.J.; Qin, Y.; Deng, Y.; Cheng, L.; Wu, L.; He, Z.; Van Nostrand, J.D.; Bracho, R.; et al. Tundra soil carbon is vulnerable to rapid microbial decomposition under climate warming. Nat. Clim. Chang. 2016, 6, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Kwon, M.J.; Jung, J.Y.; Tripathi, B.M.; Göckede, M.; Lee, Y.K.; Kim, M. Dynamics of microbial communities and CO2 and CH4 fluxes in the tundra ecosystems of the changing Arctic. J. Microbiol. 2019, 57, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, L.; Hu, G.; Liu, G.; Li, W.; Ding, Y. Permafrost and land cover as controlling factors for light fraction organic matter on the southern Qinghai-Tibetan Plateau. Sci. Total Environ. 2018, 613–614, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Peng, Y.; Chen, L.; Yang, G.; Abbott, B.W.; Zhang, D.; Fang, K.; Wang, G.; Wang, J.; Yu, J.; et al. Warming alters surface soil organic matter composition despite unchanged carbon stocks in a Tibetan permafrost ecosystem. Funct. Ecol. 2019, 34, 911–922. [Google Scholar] [CrossRef]
- Natali, S.M.; Schuur, E.A.G.; Mauritz, M.; Schade, J.D.; Celis, G.; Crummer, K.G.; Johnston, C.; Krapek, J.; Pegoraro, E.; Salmon, V.G. Permafrost thaw soil moisture driving CO2 and CH4 release from upland tundra. J. Geophys. Res. Biogeosci. 2015, 120, 525–537. [Google Scholar] [CrossRef]
- Treat, C.C.; Natali, S.M.; Ernakovich, J.; Iversen, C.M.; Lupascu, M.; Mcguire, A.D.; Norby, R.J.; Chowdhury, T.R.; Richter, A.; Šantrůčková, H.; et al. A pan-Arctic synthesis of CH4 and CO2 production from anoxic soil incubations. Glob. Chang. Biol. 2015, 21, 2787–2803. [Google Scholar] [CrossRef]
- Roulet, N.T.; Ash, R.; Moore, T.R. Low boreal wetlands as a source of atmospheric methane. J. Geophys. Res. Atmos. 1992, 97, 3739–3749. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Wu, J.; Cheng, G.; Nakano, T.; Sun, G.Y. Methane emissions from wetlands on the Qinghai-Tibet Plateau. Chin. Sci. Bull. 1999, 44, 2282–2286. [Google Scholar] [CrossRef]
- Dutta, K.; Schuur, E.A.G.; Neff, J.C.; Zimov, S.A. Potential carbon release from permafrost soils of northeastern Siberia. Glob. Chang. Biol. 2006, 12, 2336–2351. [Google Scholar] [CrossRef]
- Hollesen, J.; Elberling, B.; Jansson, P.E. Future active layer dynamics and carbon dioxide production from thawing permafrost layers in Northeast Greenland. Glob. Chang. Biol. 2011, 17, 911–926. [Google Scholar] [CrossRef]
- Van Huissteden, J. Thawing Permafrost: Permafrost Carbon a Warm Arctic; Springer International Publishing: Cham, Swizerland, 2020. [Google Scholar]
- Wei, D.; Tarchen, T.; Dai, D.; Wang, Y.; Wang, Y. Revisiting the role of CH4 emissions from alpine wetlands on the Tibetan Plateau: Evidence from two in situ measurements at 4758 and 4320 m above sea level. J. Geophys. Res. Biogeosci. 2015, 120, 1741–1750. [Google Scholar] [CrossRef]
- Tao, Z.; Shen, C.; Gao, Q.; Sun, Y.; Yi, W.; Li, Y. Soil organic carbon storage and soil CO2 flux in the alpine meadow ecosystem. Sci. China Earth Sci. 2007, 50, 1103–1114. [Google Scholar] [CrossRef]
- Virkkala, A.M.; Aalto, J.; Rogers, B.M.; Tagesson, T.; Treat, C.C.; Natali, S.M.; Watts, J.D.; Potter, S.; Lehtonen, A.; Mauritz, M. Statistical upscaling of ecosystem CO2 fluxes across the terrestrial tundra and boreal domain: Regional patterns and uncertainties. Glob. Chang. Biol. 2021, 27, 4040–4059. [Google Scholar] [CrossRef]
- Jørgensen, C.; Johansen, K.; Westergaard-Nielsen, A.; Elberling, B. Net regional methane sink in High Arctic soils of northeast Greenland. Nat. Geosci. 2015, 8, 20–23. [Google Scholar] [CrossRef]
- Lupascu, M.; Welker, J.M.; Seibt, U.; Maseyk, K.; Xu, X.; Czimczik, C.I. High Arctic reduces permafrost carbon feedbacks to climate warming. Nat. Clim. Chang. 2014, 4, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Voigt, C.; Marushchak, M.E.; Mastepanov, M.; Lamprecht, R.E.; Christensen, T.R.; Dorodnikov, M.; Jackowicz-Korczyński, M.; Lindgren, A.; Lohila, A.; Nykänen, H.; et al. Ecosystem carbon response of an Arctic peatland to simulated permafrost thaw. Glob. Chang. Biol. 2019, 25, 1746–1764. [Google Scholar] [CrossRef] [Green Version]
- Takakai, F.; Desyatkin, A.; Larry, C.; Fedorov, A.; Desyatkin, R.; Hatano, R. CH4 and N2O emissions from a forest-alas ecosystem in the permafrost taiga forest region, eastern Siberia, Russia. J. Geophys. Res. Biogeosci. 2008, 113, G02002. [Google Scholar] [CrossRef]
- Desyatkin, A.; Takakai, F.; Fedorov, R.; Nikolaeva, M.; Desyatkin, R.; Hatano, R. CH4 emission from different stages of thermokarst formation in Central Yakutia, East Siberia. Soil Sci. Plant Nut. 2009, 55, 558–570. [Google Scholar] [CrossRef] [Green Version]
- Walter, K.M.; Smith, L.C.; Chapin, F.S. Methane bubbling from northern lakes: Present and future contributions to the global methane budget. Philos. Trans. A Math. Phys. Eng. Sci. 2007, 365, 1657–1676. [Google Scholar] [CrossRef]
- Lindgren, P.R.; Grosse, G.; Walter, A.K.M.; Meyer, F.J. Detection and spatiotemporal analysis of methane ebullition on thermokarst lake ice using high-resolution optical aerial imagery. Biogeosciences 2016, 13, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Lara, H.; Bouchard, F.; Laurion, I.; Séjourné, A.; Marlin, C.; Hatté, C.; Costard, F.; Fedorov, A.; Desyatkin, A. Seasonal patterns in greenhouse gas emissions from thermokarst lakes in Central Yakutia (Eastern Siberia). Limnol. Oceanogr. 2020, 66, S98–S116. [Google Scholar]
- Park, H.; Fedorov, A.; Konstantinov, P.; Hiyama, T. Numeriacal assessments fo excess ice impacts on permafrost aned greenhouse gases in a Siberian tundra site under a warming climate. Front. Earth Sci. 2021, 9, 704447. [Google Scholar] [CrossRef]
- Olefeldt, D.; Goswami, S.; Grosse, G.; Hayes, D.; Hugelius, G.; Kuhry, P.; McGuire, A.D.; Romanovsky, V.E.; Sannel, A.B.; Schuur, E.A.; et al. Circumpolar distribution and carbon storage of thermokarst landscapes. Nat. Commun. 2016, 7, 13043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turetsky, M.R.; Abbott, B.W.; Jones, M.C.; Anthony, K.W.; Olefeldt, D.; Schuur, E.A.G.; Grosse, G.; Kuhry, P.; Hugelius, G.; Koven, C.; et al. Carbon release through abrupt permafrost thaw. Nat. Geosci. 2020, 13, 138–143. [Google Scholar] [CrossRef]
- Hu, F.; Higuera, P.; Walsh, J.; Chapman, W.; Duffy, P.; Brubaker, L.; Chipman, M. Tundra burning in Alaska: Linkages to climatic change and sea ice retreat. J. Geophys. Res. Biogeosci. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Natali, S.M.; Holdren, J.P.; Rogers, B.M.; Treharne, R.; Duffy, P.B.; Pomerance, R.; MacDonald, E.N. Permafrost carbon feedbacks threaten global climate goals. Proc. Natl. Acad. Sci. USA 2021, 118, e2100163118. [Google Scholar] [CrossRef]
- MacLean, R.; Oswood, M.W.; Irons, J.G., III; McDowell, W.H. The effect of permafrost on stream biogeochemistry: A case study of two streams in the Alaskan (U.S.A.) taiga. Biogeochemistry 1999, 47, 239–267. [Google Scholar] [CrossRef]
- Guo, L.; Ping, C.-L.; Macdonald, R.W. Mobilization pathways of organic carbon from permafrost to arctic rivers in a changing climate. Geophys. Res. Lett. 2007, 34, l13603. [Google Scholar] [CrossRef]
- Pawson, R.R.; Evans, M.G.; Allott, T.E.H.A. Fluvial carbon flux from headwater peatland streams: Significance of particulate carbon flux. Earth Surf. Proc. Land. 2012, 37, 1203–1212. [Google Scholar] [CrossRef]
- Juutinen, S.; Väliranta, M.; Kuutti, V.; Laine, A.M.; Virtanen, T.; Seppä, H.; Weckström, J.; Tuittila, E.S. Short-term and long-term carbon dynamics in a northern peatland-stream-lake continuum: A catchment approach. J. Geophys. Res. Biogeosci. 2013, 118, 171–183. [Google Scholar] [CrossRef]
- Walvoord, M.A.; Striegl, R.G. Increased groundwater to stream discharge from permafrost thawing in the Yukon River basin: Potential impacts on lateral export of carbon and nitrogen. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef] [Green Version]
- Giesler, R.; Lyon, S.W.; Mörth, C.M.; Karlsson, J.; Karlsson, E.M.; Jantze, E.J.; Destouni, G.; Humborg, C. Catchment-scale dissolved carbon concentrations and export estimates across six subarctic streams in northern Sweden. Biogeosciences 2014, 11, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Song, C.; Wan, Z.; Lu, Y.; Wang, L. Dynamics of dissolved organic carbon release from a permafrost wetland catchment in northeast China. J. Hydrol. 2015, 531, 919–928. [Google Scholar] [CrossRef]
- Tank, S.E.; Striegl, R.G.; McClelland, J.W.; Kokelj, S.V. Multi-decadal increases in dissolved organic carbon and alkalinity flux from the Mackenzie drainage basin to the Arctic Ocean. Environ. Res. Lett. 2016, 11, 054015. [Google Scholar] [CrossRef]
- McClelland, J.W.; Holmes, M.R.; Peterson, J.B.; Raymond, A.P.; Striegl, G.R. Particulate organic carbon and nitrogen export from major Arctic rivers. Glob. Biogeochem. Cycle 2016, 30, 629–643. [Google Scholar] [CrossRef]
- Lamoureux, S.F.; Lafrenière, M.J. Seasonal fluxes and age of particulate organic carbon exported from Arctic catchments impacted by localized permafrost slope disturbances. Environ. Res. Lett. 2014, 9, 045002. [Google Scholar] [CrossRef] [Green Version]
- Striegl, R.G.; Dornblaser, M.M.; Aiken, G.R.; Wickland, K.P.; Raymond, R.A. Carbon export and cycling by the Yukon, Tanana, and Porcupine rivers, Alaska. Water Resour. Res. 2007, 43, W02411. [Google Scholar] [CrossRef] [Green Version]
- Worrall, F.; Burt, T. Time sreies analysis of long-term river dissolved organic carbon records. Hydrol. Process. 2004, 18, 893–912. [Google Scholar] [CrossRef]
- Findlay, S.E.G. Increased carbon trnasport in the Hudson River: Unexpected consequance of nitrogen deposition? Front. Ecol. Environ. 2005, 3, 133–137. [Google Scholar] [CrossRef]
- Frey, K.E.; Smith, L.C. Amplied carbon release form vast West Siberian peatlands by 2100. Geophys. Res. Lett. 2005, 32, L09401. [Google Scholar] [CrossRef] [Green Version]
- Striegl, R.G.; Aiken, G.R.; Dornblaser, M.M.; Raymond, P.A.; Wickland, K.P. A decreased in discharge-normailized DOC export by the Yukon River during summer through autumn. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Ma, Q. Research on In-Stream Dissolved Organic Carbon Dynamics and Mechanisms in the Source Area of the Yellow River on the Northeastern Qinghai-Tibet Plateau, Southwest, China. Ph.D. Thesis, Chinese Academy of Sciences, Lanzhou, China, 2020. (In Chinese with English abstract). [Google Scholar]
- Mu, C.; Zhang, F.; Chen, X.; Ge, S.; Mu, M.; Jia, L.; Wu, Q.; Zhang, T. Carbon and mercury export from the Arctic rivers and response to permafrost degradation. Water Res. 2019, 161, 54–60. [Google Scholar] [CrossRef]
- Beel, C.; Lamoureux, S.; Orwin, J.; Pope, M.; Scott, N. Differential impact of thermal and physical permafrost disturbances on High Arctic dissolved and particulate fluvial fluxes. Sci. Rep. 2020, 10, 11836. [Google Scholar] [CrossRef]
- Serikova, S.S.; Pokrovsky, O.S.; Ala-Aho, P.; Kazantsev, V.; Kirpotin, S.N.; Kopysov, S.G.; Krickov, I.V.; Laudon, H.; Manasypov, R.M.; Shirokova, L.S.; et al. High riverine CO2 emissions at the permafrost boundary of Western Siberia. Nat. Geosci. 2018, 11, 825–829. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xia, X.; Liu, S.; Zhang, S.; Li, S.; Wang, J.; Wang, G.; Gao, H.; Zhang, Z.; Wang, Q.; et al. Significant methane ebullition from alpine permafrost rivers on the East Qinghai–Tibet Plateau. Nat. Geosci. 2020, 13, 349–354. [Google Scholar] [CrossRef]
- Waelbroeck, C.; Monfray, P.; Oechel, W.C.; Hastings, S.; Vourlitis, G. The impact of permafrost thawing on the carbon dynamics of tundra. Geophys. Res. Lett. 1997, 24, 229–232. [Google Scholar] [CrossRef]
- Khvorostyanov, D.V.; Krinner, G.; Ciaia, P.; Heimann, M.; Zimov, S.A. Vulnerability of permafrost carbon to global warming. Part I: Model description and role of heat generated by organic matter decomposition. Tellus B 2008, 60B, 250–264. [Google Scholar] [CrossRef] [Green Version]
- Koven, C.D.; Ringeval, B.; Friedlingstein, P.; Ciais, P.; Cadule, P.; Khvorostyanov, D.; Krinner, G.; Tarnocai, C. Permafrost carbon-climate feedbacks accelerate global warming. Proc. Natl. Acad. Sci. USA 2011, 108, 14769–14774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDougall, A.H.; Avis, C.A.; Weaver, A.J. Significant contribution to climate warming from the permafrost carbon feedback. Nat. Geosci. 2012, 5, 719–721. [Google Scholar] [CrossRef]
- Schaphoff, S.; Heyder, U.; Ostberg, S.; Gerten, D.; Heinke, J.; Lucht, W. Contribution of permafrost soils to the global carbon budget. Environ. Res. Lett. 2013, 8, 014026. [Google Scholar] [CrossRef]
- Von Deimling, T.S.; Grosse, G.; Strauss, J.; Schirrmeister, L.; Morgenstern, A.; Schaphoff, S.; Meinshausen, M.; Boike, J. Observation-based modelling of permafrost carbon fluxes with accounting for deep carbon deposits and thermokarst activity. Biogeosciences 2015, 12, 3469–3488. [Google Scholar] [CrossRef] [Green Version]
- Burke, E.J.; Burke, E.J.; Ekici, A.; Huang, Y.; Chadburn, S.E.; Huntingford, C.; Ciais, P.; Friedlingstein, P.; Peng, S.; Krinner, G. Quantifiying uncertainties of permafrost carbon-cliamte feedbacks. Biogeosciences 2017, 14, 3051–3066. [Google Scholar] [CrossRef] [Green Version]
- Walter, A.K.; Von Deimling, T.S.; Nitze, I.; Frolking, S.; Emond, A.; Daanen, R.; Anthony, P.; Lindgren, P.; Jones, B.; Grosse, G. 21st-century modeled permafrost carbon emissions accelerated by abrupt thaw beneath lakes. Nat. Commun. 2018, 9, 3262. [Google Scholar] [CrossRef]
- Krinner, G.; Viovy, N.; De Noblet-Ducoudré, N.; Ogée, J.; Polcher, J.; Friedlingstein, P.; Ciais, P.; Sitch, S.; Prentice, C.I. A dynamic global vegetation model for studies of the coupled atmosphere-biosphere system. Glob. Biogeochem. Cycles 2005, 19, GB1015. [Google Scholar] [CrossRef]
- Holloway, J.E.; Lewkowicz, A.G.; Douglas, T.A.; Li, X.; Turetsky, M.R.; Baltzer, J.L.; Jin, H. Impact of wildfire on permafrost landscapes: A review of recent advances and future prospects. Permafr. Periglac. Process. 2020, 31, 371–382. [Google Scholar] [CrossRef]
- Li, X.Y.; Jin, H.J.; Wang, H.W.; Marchenko, S.S.; Shan, W.; Luo, D.L.; He, R.X.; Spektor, V.; Huang, Y.D.; Li, X.Y.; et al. Influences of wildfires on the permafrost environment: A review. Adv. Clim. Chang. Res. 2021, 12, 48–65. [Google Scholar] [CrossRef]
- Saito, K.; Machiya, H.; Iwahana, G.; Ohno, H.; Yokohata, T. Mapping simulated circum-Arctic organic carbon, ground ice, and vulnerability of ice-rich permafrost to degradation. Progr. Earth Planet Sci. 2020, 7, 31. [Google Scholar] [CrossRef]
- Yokohata, T.; Saito, K.; Ito, A.; Ohno, H.; Tanaka, K.; Hajima, T.; Iwahana, G. Future projection of greenhouse gas emissions due to permafrost degradation using a simple numerical scheme with a global land surface model. Prog. Earth Planet. Sci. 2020, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Machiya, H.; Iwahana, G.; Yokohata, T.; Ohno, H. Numerical model to simulate long-term soil organic carbon and ground ice budget with permafrost and ice sheets (SOC-ICE-v1.0). Geosci. Model Dev. 2021, 14, 521–542. [Google Scholar] [CrossRef]
- Mekonnen, Z.; Riley, W.; Berner, L.; Bouskill, N.; Torn, M.; Iwahana, G.; Breen, A.; Myers-Smith, I.; Criado, M.; Liu, Y.; et al. Arctic tundra shrubification: A review of mechanisms and impacts on ecosystem carbon balance. Environ. Res. Lett. 2021, 16, 053001. [Google Scholar] [CrossRef]

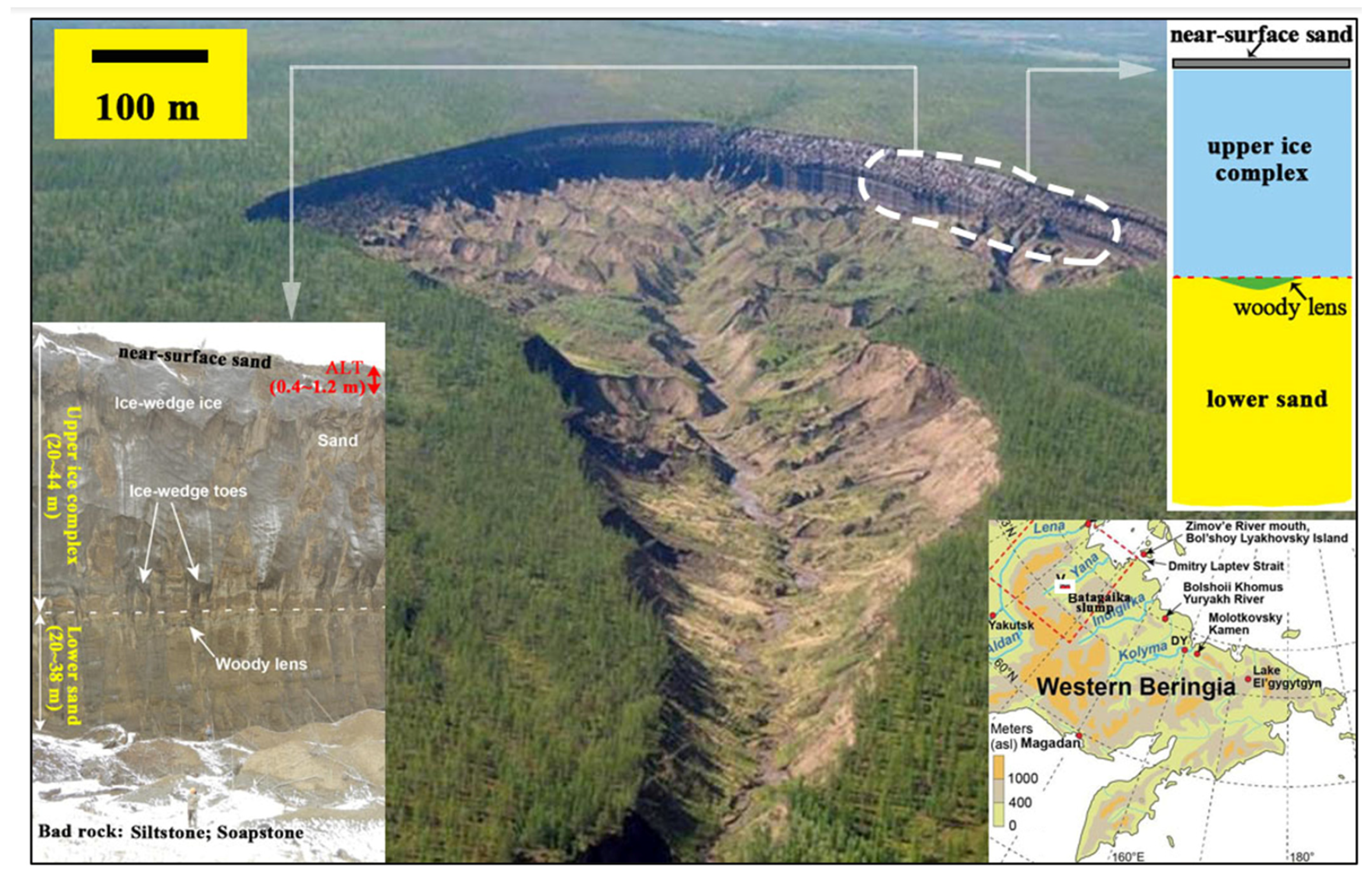
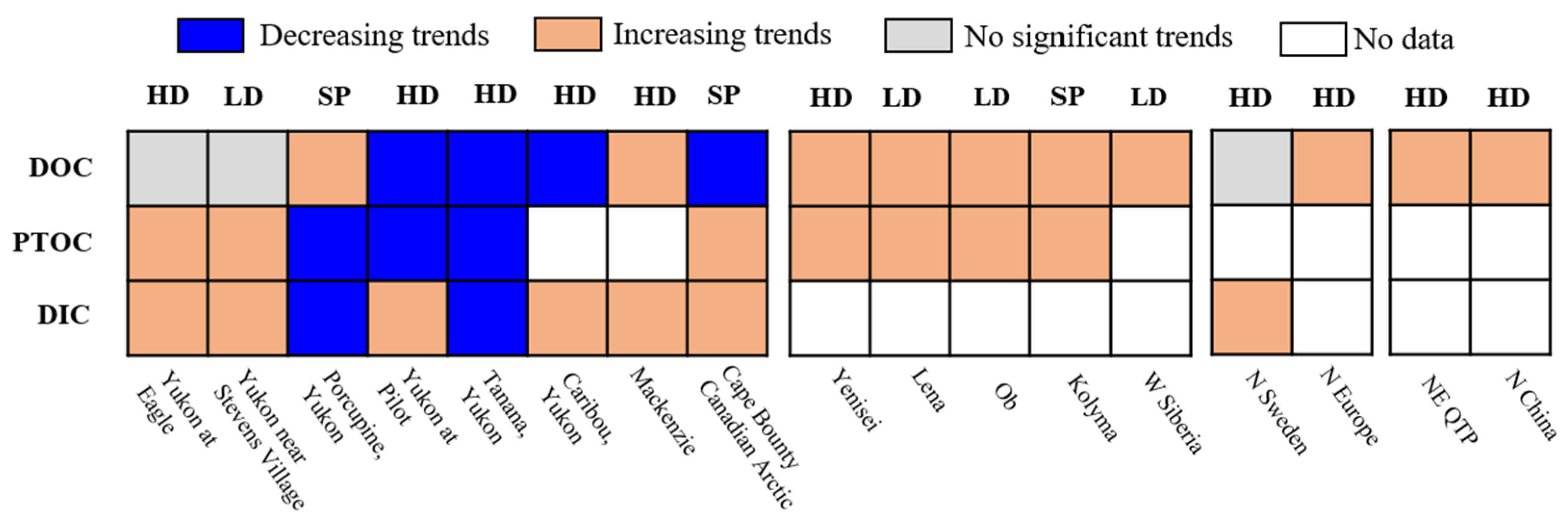
| Regions | Soil Horizons or Depths (m) | Time Period | Areal Extent (106 km2) | Soil Organic Carbon Density (kg·m−3) | Carbon Stock (Pg C) | References |
|---|---|---|---|---|---|---|
| Latitudinal permafrost regions (1460–1600 Pg C) | ||||||
| Arctic/Antarctic | ||||||
| Arctic | 0–0.25 | present | 5.6 | 18.81 | ~26.33 | [26] |
| High Arctic | 0–0.3 | present | 1.068 | 31.21 | 10 ± 3 | [1] |
| High Arctic | 0–1 | present | 1.068 | 22.47 | 24 ± 8 | [1] |
| High Arctic | 1–2 | present | 1.068 | 6.55 | 7 ± 5 | [1] |
| High Arctic | 2–3 | present | 1.068 | 2.81 | 3 ± 3 | [1] |
| Yedoma 1 | >3 | present | 1.387 | 4–17 | 58–371 | [27] |
| Yedoma | >3 | LGM 2 | 1.32 | 16.32 | 390–446 | [28] |
| Siberian yedoma | >3 | present | 1 | 18 | 450 | [4] |
| Siberian yedoma | >3 | present | 1 | 18.5 | ~407 | [29] |
| Deltaic deposits | >3 | present | 0.08 | 8.3–56.2 | 91 ± 52 | [1] |
| Antarctic | <1 | present | 0.495 | 0.725 | [30] | |
| Antarctic Peninsula | <1 | present | 0.1 | 0.6 | [30] | |
| Circumpolar/boreal/Sub-Arctic regions | ||||||
| Circum-Arctic regions | 0–0.3 | present | 18.782 | 33.95 | 191.29 | [29] |
| Circum-Arctic regions | 0–0.3 | present | 17.8 | 40.64 | 217 ± 12 | [1] |
| Circum-Arctic regions | 0–1 | present | 18.782 | 26.40 | 495.8 | [29] |
| Circum-Arctic regions | 1–2 | present | 17.8 | 19.94 | 355 ± 81 | [1] |
| Circum-Arctic regions | 2–3 | present | 17.8 | 11.63 | 207 ± 42 | [1] |
| Circum-Arctic regions | 0–3 | present | 18.782 | 18.17 | 1024 | [29] |
| Circum-Arctic regions | 0–3 | present | 17.8 | 19.38 | 1035 ± 150 | [1] |
| Circum-Arctic regions | 0–3 | present | 17.8 | 1084 | [31] | |
| Circum-Arctic regions | 0–3 | LGM 1 | 29.3 | 790 | [31] | |
| Boreal forest | 0–3 | present | 12.0 | 4.17 | 150 | [32] |
| Boreal and subarctic peatland | 0–1.1 | present | 3.46 | 70.9–97.2 | 270–370 | [33] |
| Boreal and subarctic peatland | 0–2.3 | present | 3.345 | 59.14 | 455 | [32] |
| Boreal and subarctic peatland | 0–3 | present | 3.46 | 457–683 | [31] | |
| Boreal and subarctic peatland | 0–3 | LGM | 0.87 | 30 | [31] | |
| Elevational permafrost regions (21.7–42.7 Pg C) | ||||||
| Qinghai Tibet Plateau (QTP) | ||||||
| QTP | 0–1 | present | 1.35 | 12.81 | 17.3 ± 5.3 | [34] |
| QTP | 0–2 | present | 1.35 | 7.85 | 27.9 ± 8.0 | [34] |
| QTP | 0–3 | present | 1.35 | 3.78 | 33.3 ± 9.4 | [34] |
| QTP | >3 | present | 1.35 | 3.77 | 127.2 ± 37.3 | [34] |
| QTP | 0–2 | present | 1.48 | 6.20 | 18.34 ± 7.0 | [36] |
| QTP | 0–3 | present | 1.06 | 11.45 | 36.4 ± 2.5 | [37] |
| QTP | 0–3 | present | 1.72 | 4.20 | 21.69 | [38] |
| Alpine/mountain regions | ||||||
| Alps | 0–1 | present | 5 × 10−3 | 7–35 | 0.04–0.18 | [39] |
| Urals | 0–0.5 | present | 0.13 | 7.7–39.3 | 0.50–2.55 | [40] |
| Andes | 0–1 | present | 2.6 × 10−2 [1] | 5.2–88.3 | 0.1–2.3 | [41] |
| Altai (Russia) | 0–1 | present | 5.1 × 10−5 | 2.0–3.2 | [42] | |
| Coupled Models | Modeling Objectives | Spatial Resolution | Temporal Resolution | Reference |
|---|---|---|---|---|
| Hydrological and thermal model and biogeochemical model | net-CO2 flux | 1.9° × 1.2° | 1 day | [115] |
| Permafrost model and 1-D soil model | CO2 & CH4 fluxes by degrading permafrost | 0.5° × 0.5° | 5 days | [116] |
| ORCHIDEE (Organizing Carbon and Hydrology in Dynamics Ecosystems) model and CH4 module | net-CO2 and CH4 fluxes | 1.0° × 1.0° | 3 h | [117,123] |
| UVic ESCM (University of Victoria Earth System Climate Model) and permafrost model | CO2 flux by permafrost degradation | 3.6° × 1.8° | 5 days | [118] |
| LPJmL (Lund–Potsdam–Jena managed Land) model and permafrost module | Net ecosystem carbon exchange | 0.5° × 0.5° | 1 day | [119] |
| Two-dimension multi-pool model | CO2 and CH4 flux by permafrost degradation | 2.0° × 2.0° | 1 day | [120] |
| IMOGEN (Integrated Model Of Global Effects of climatic aNomalies) | net-CO2 flux | 2.5° × 3.75° | 30 min | [121] |
| CLM4.5BGC coupled with 3-D thermokarst lake model | CO2 and CH4 flux by degrading permafrost | 0.5° × 0.5° | 1 month | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Ma, Q. Impacts of Permafrost Degradation on Carbon Stocks and Emissions under a Warming Climate: A Review. Atmosphere 2021, 12, 1425. https://doi.org/10.3390/atmos12111425
Jin H, Ma Q. Impacts of Permafrost Degradation on Carbon Stocks and Emissions under a Warming Climate: A Review. Atmosphere. 2021; 12(11):1425. https://doi.org/10.3390/atmos12111425
Chicago/Turabian StyleJin, Huijun, and Qiang Ma. 2021. "Impacts of Permafrost Degradation on Carbon Stocks and Emissions under a Warming Climate: A Review" Atmosphere 12, no. 11: 1425. https://doi.org/10.3390/atmos12111425
APA StyleJin, H., & Ma, Q. (2021). Impacts of Permafrost Degradation on Carbon Stocks and Emissions under a Warming Climate: A Review. Atmosphere, 12(11), 1425. https://doi.org/10.3390/atmos12111425






