Abstract
Exposure to airborne particulate matter (PM) has been associated with the transcriptional up-regulation of pro-inflammatory mediators. However, the effect of PM on post-transcriptional regulation of pro-inflammatory mediators has not been fully explored. In this study, we examined the acute effect of organic extracts from urban PM, rural PM and diesel exhaust particles (DEP) on the post-transcriptional control of interleukin-6 (IL-6) and interleukin-8 (CXCL8) using a human bronchial epithelial cell line. Both PM and DEP extracts induced the release of IL-6 and CXCL8 after 24 h of exposure. Time-course experiments were conducted to examine changes in mRNA steady-state levels and half-lives. The steady-state levels of CXCL8 mRNA increase at 15 min on cells exposed to both PM and DEP extracts. Meanwhile only the urban extract induced significant increases of IL-6 mRNA levels at 15 min. Indirect measurements of IL-6 mRNA half-life showed a dramatic increase in cells exposed to the organic extracts. CXCL8 mRNA half-life increases in cells exposed to PM extracts and not DEP extract. Nuclear run-ons demonstrated that the urban PM and DEP extracts promoted an up-regulation in the transcription rate of CXCL8 at 15 min but not for IL-6. Urban and rural PM influences the post-transcriptional control of CXCL8.
1. Introduction
Epidemiological, clinical, in vivo and in vitro studies have shown consistent associations between airborne particulate matter (PM) exposure and adverse respiratory effects [1]. In vitro studies have demonstrated that PM2.5 exposure can induce the production of pro-inflammatory cytokines and chemokines, such as interleukin-6 (IL-6) and interleukin-8 (CXCL8) [2,3,4,5,6,7,8,9,10,11,12]. Despite the extensive evidence relating PM exposure to the induction of pro-inflammatory mediators, the associated molecular mechanisms induced by such exposures are still not fully characterized [13].
Inflammatory responses are tightly regulated through transcriptional, translational and post-transcriptional mechanisms that modulate the rate of gene expression [14,15,16]. Post-transcriptional mechanisms can regulate the fate of mRNAs in association with RNA-binding proteins allowing cells to respond rapidly to extracellular stimulus by inducing different signaling pathways that lengthens or shortens target mRNA half-lives and thus the abundance of functional proteins [14,15,17,18].
One of the most important post-transcriptional control mechanisms involved in the regulation of mRNA half-lives of pro-inflammatory mediators is the AU-rich elements-mediated decay pathway. This pathway depends on cis-acting sequence elements rich in adenosine and uridine nucleotides, known as AU-rich elements (AREs), found in the 3’ untranslated region (3’UTR) of short-lived mRNAs [19]. These element sequences serve as binding sites to RNA-binding proteins collectively known as ARE-binding proteins (AREBPs) [19,20,21]. The general function of these proteins is to modulate the decay of target mRNAs by either stabilizing or destabilizing its mRNA target through the recruitment of different proteins involved in the degradation machinery [22,23]. Several studies have associated the heterogeneous nuclear ribonucleoproteins D (AUF-1) and the zinc-finger protein tristetraprolin (TTP) with the destabilization of cytokine mRNAs, such as IL-6 and CXCL8 [24]. The well-known HuR (ELAV-L protein 1) has been associated with the stability of these cytokine mRNAs [19].
A recent study provides evidence of the post-transcriptional regulation of CXCL8 in human lung cells exposed to aqueous cigarette smoke extracts by increasing the mRNA stability of its transcript in a p38-MAPK dependent manner [25]. The study suggests that the AREs-mediated decay pathway is responsible for the CXCL8 mRNA stabilization. However, to the best of our knowledge the effects of PM2.5 organic extracts in the post-transcriptional regulation of pro-inflammatory mediators have never been reported. Therefore, the objective of this study was to determine if exposure to PM2.5 had an effect on the post-transcriptional regulation of IL-6 and CXCL8 in the human bronchial epithelial cell line BEAS-2B. Here we report for the first time that exposure to PM organic extracts considerably increases the half-lives of IL-6 and CXCL8 mRNAs with a possible role for HuR.
2. Experiments
2.1. PM2.5 Extract Preparation
Airborne PM2.5 samples were collected between January and September 2001 at two locations in Puerto Rico (PR), an urban industrialized area (Guaynabo) in the north, and a rural coastal reference site at the east, Fajardo (a natural reserve) [26]. A description of sampling sites is provided in the supplementary material. A total of 49 PTFE and quartz filters from the urban area and 63 filters from the rural costal site were extracted. The filters were extracted overnight using a Soxhlet apparatus in a 150 mL solvent mixture of acetone:hexane of 1:1 (v/v) and dried under a gentle nitrogen stream as previously described [5]. Dried organic extracts were weighed and resuspended in dimethyl sulfoxide (DMSO), the carrier, (99.9% Certified A.C.S., Fisher Scientific, Pittsburgh, PA, USA) at a final concentration of 100 mg/mL. The total amount of organic extracts recovered were 37.9 mg for the urban site and 18.6 mg for the rural site. A similar extraction was performed on 50 mg of diesel exhaust particles (DEP) (Standard Reference Material 1650a) obtained from the National Institute of Standards & Technology (Gaithersburg, MD, USA). The dried organic extract of DEP was resuspended in DMSO at a final concentration of 100 mg/mL.
2.2. Cell Culture and Maintenance
BEAS-2B cell line (ATCC®cat. No. CRL-9609, Manassas, VA, USA) was cultured in Keratinocyte Basal Medium-2 (KGM-2) without calcium (Ca++) and supplemented with gentamicin sulfate, amphotericin-B, insulin, human epidermal growth factor, epinephrine, transferrin, bovine pituitary extract and hydrocortisone (KGM®-2 BulletKit®, Lonza Bioscience, Walkersville, MD, USA). The cells were maintained in 75-cm2 cell culture-treated vented cap flasks (Corning, Inc. Lowell, MA, USA) in a humidified 5% CO2 atmosphere at 37 °C. Cells between passages 52–65 were used for the experiments. Gentamicin sulfate was not added to the media during experiments.
2.3. Dose-Response Experiments
Cells were seeded onto 96-well plates at a density of 1.0 × 105 cells/well and exposed for 24 h at different concentrations (25, 50, 75 and 100 µg/mL) of the organic extracts in 0.1% DMSO diluted in media (carrier). Positive controls were exposed with 10 µg/mL of Escherichia coli lipopolysaccharides 0111: B4 (LPS) (Sigma-Aldrich, Saint Louis, MO, USA) diluted in media, and 10 µg/mL of DEP extract in 0.1% of DMSO. Cells kept in media and 0.1% DMSO were used as negative control. Cell supernatants were collected at the end of the exposure and analyzed for IL-6 and CXCL8 with a human multiplex-bead assay (Millipore Corporation, Billerica, MA) using the dual laser flow analyzer Luminex100 (Luminex Corp., Austin, TX, USA). Cell viability was assessed using the Neutral Red Assay as described elsewhere [27].
2.4. Time-Course Experiments
Cells were seeded (1.0 × 105 cells/well) onto a 96-well plate and exposed to 25 µg/mL of the organic extracts and 10 µg/mL of DEP organic extract for the following time points: 0 min, 15 min, 30 min, 60 min and 120 min. Positive controls were exposed to 10 µg/mL of LPS and negative controls to media, and 0.1% DMSO in media. Total RNA was isolated immediately after each exposure using the RNAqueous-96 RNA isolation kit (Ambion, Carlsbad, CA, USA). The mRNA half-lives were calculated by linear regression of the time-course data between the time points of 15 min and 120 min.
2.5. Quantitative Real Time PCR
First strand cDNA was synthesized using the VersoTM SYBR® Green 2-Step QRT-PCR Fluorescein Kit (Thermo Scientific Inc. Foster City, CA, USA). For each qPCR reaction, 100 ng of cDNA was combined with 10 µL of KappaTM SYBR® Fast qPCR master mix (KAPA BIOSYSTEMS, Wilmington, MA, USA), and 10 µM of both forward (FWD) and reverse (REV) specific primers for IL-6 (FWD primer: GAACTCCTTCTCCACAAGCG and REV primer: TTTTCTGCCAGTGCCTCTTT) and CXCL8 (FWD primer: CTGCGCCAACACAGAAATTA and REV primer: ATTGCATCTGGCAACCCTAC). The final reaction volume was set to 20 µl. All qPCR reactions were run in a Realplex qPCR (Eppendorf, Hauppauge, NY, USA) with the following conditions: initial hot start step at 95 °C for 2 min, 40 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 20 s, extension at 72 °C for 30 s, and a melting curve as the final step. Beta-actin was used to normalize the results (FWD primer: GGACTTCGAGCAAGAGATGG and REV primer: AGCACTGTGTTGGCGTACAG).
2.6. Nuclear Run-On Assays
Nuclear run-on assays were performed to examine changes in the transcription rate of IL-6 and CXCL8 at times when peak induction of mRNA steady-state levels was observed. We used nuclear run on assays instead of actinomycin D since it has been reported that actinomycin D by itself may promote the stabilization of mRNAs [28,29]. This method allowed us to determine possible transcriptional contributions to mRNA steady-state levels. In order to perform these assays, BEAS-2B were seeded onto 24-well plates at a cell density of 5.0 × 105 cells/well and exposed to 10 µg/mL of DEP extract, and 25 µg/mL of rural and urban organic extracts for 15 min and 60 min. After exposure, non-radioactive nuclear run-on assays were performed as previously described by Patrone et al. [30], with modifications. Briefly, cells were trypsinized, centrifuged for 10 min at 10,000 rpm at 4 °C, and washed once with PBS and once with Hypotonic Buffer N (0.5 M HEPES pH 7.5, 1 M MgCl2, 3 M KCl supplemented with complete protease inhibitor cocktail (ROCHE Diagnostics, Indianapolis, IN, USA, 67 µM of PMSF and 100mM DTT). Cells were then lysed by incubation for 1 h at 4 °C with 250 µL of Hypotonic Buffer N/0.5% NP-40 followed by the addition of 32 µL of 1.67 M sucrose. Nuclear pellets were collected after centrifugation for 10 min at 10,000 rpm at 4 °C and washed with 250 µL of ice-cold Freezing Buffer N (10 mM HEPES, 2 mM MgCl2, 25 mMKCl, 250 mM sucrose) supplemented with protease inhibitors. Purified nuclei were stored in 200 µL of Freezing Buffer N at −80 °C until further analysis. In vitro transcription reactions were carried out with 500,000 nuclei and 88 µL of 2× Transcription Buffer (200 mM KCL, 20 mM Tris-HCl pH 8.0, 5 mM MgCl2, 4mM DTT, 4 mM each ATP, CTP and GTP, 200 mM sucrose and 20% glycerol). In order to initiate the reactions, 4 µL of 10 mM biotin-16-UTP (ROCHE, Indianapolis, IN, USA) was added and incubated at 30 °C. After 2 h, the reaction was stopped by adding 250 mM CaCl2 and 60 U of RNAse-free DNAse I (Ambion, Austin, TX, USA), and then incubating for 30 min at 37 °C. Nuclear RNA was extracted with 200 µL of TRIzol (Sigma-Aldrich, St. Louis, MO, USA), and biotin-labeled mRNAs isolated using streptavidin-magnetic beads (Dynabeads, Invitrogen, Carlsbad, CA, USA) and resuspended in 30 µL of DEPC water. The cDNA synthesis and qPCR reactions were performed as described above.
2.7. HuR Abundance
HuR was measured in the cytoplasm because is well known that changes in the post-transcriptional control of IL-6 and CXCL8 have been associated with the presence of AREs in the 3’UTR region of their mRNAs. These changes result from the activation, and thus cytoplasmic translocation of different AREBPs. HuR is one of the most studied AREBP, and is known that once activated, this protein shuttles to the cytoplasm and stabilized ARE-mRNAs [14]. In order to explore if the activation of HuR is associated with the stabilization of IL-6 and CXCL8 induced by the urban and rural extracts dot blots were performed to determine if there are a cytoplasmic abundance of HuR in exposed cells. BEAS-2B were seeded onto 24-well plates (5.0 × 105 cells/well) and exposed to 10 µg/mL of DEP extract, and 25 µg/mL of the urban and rural PM organic extracts for 15 min and 60 min. Cytoplasmic proteins were extracted using the method of Lahiri et al., [31] with the following modifications. Briefly, BEAS-2B cells were washed with 300 µL of TBS and then incubated for 15 min with 400 µL ice-cold buffer (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, and 2 µL Complete Protease Inhibitor cocktail). Cells were scraped from the plate and transferred to a new tube followed by the addition of 25 µL of Triton X-100. Cell homogenates were centrifuged for 5 min at 1500× g at 4 °C and supernatants containing the cytoplasmic fraction were collected. Protein concentrations were determined by the BioRad Quick Start Bradford Protein (BioRad, Hercules, CA, USA) assay following the manufacturer’s protocol. A total of 45 µg of protein was blotted onto a nitrocellulose membrane using a dot blot apparatus. Membranes were blocked in TBST (20 mM Tris-HCl, 150 mM NaCl and 0.1% Tween 20) with 5% non-fat milk for 1 h at RT and incubated overnight at 4 °C with 1:200 dilution of the appropriate antibody, HuR or actin (sc-5261and sc-1616; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation, the membranes were washed four times with TBST and then incubated overnight at 4 °C with a 1:1000 dilution of the appropriate secondary HRP-conjugated antibody (sc-2020 and sc-2005; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After four washes with TBST, the blots were developed with ECL reagents (Santa Cruz Biotechnology, Santa Cruz, CA, USA) following the manufacturer’s protocol. The chemiluminescence signal was captured using the Eagle Eye image analyzer (Stratagene, San Diego, CA, USA) and quantitated using the ImageJ software (NIH, Bethesda, MD, USA).
2.8. Statistical Analysis
Data analyses were performed using a one-way analysis of variance (ANOVA) followed by the appropriate post hoc comparisons tests (Dunnet, Bonferroni–Scheffé, Tukey–Kramer) and Pairwise T-test when appropriate. The criterion for statistical significance was set at p ≤ 0.05. Statistical analyses were performed with the software packages InStat 3, and SPSS.
3. Results
3.1. Validation of the Experimental Conditions
In order to validate the experimental conditions, positive control cells were exposed to 10 µg/mL of LPS and negative control cells were exposed to media alone. In addition, DEP organic extract was used as a reference material for PM exposures. After 24 h of exposure, IL-6 and CXCL8 levels were measured in the cells supernatants. Results showed that IL-6 levels were three times higher in positive controls when compared to negative controls (Figure 1A), while CXCL8 were 27 times higher in positive controls (Figure 1B). Similar results were observed in cells exposed to DEP extract when compared to cells exposed to the carrier alone; with IL-6 levels 2.7 times higher (Figure 1A) and CXCL8 levels seven times higher in DEP extract exposed cells (Figure 1B).
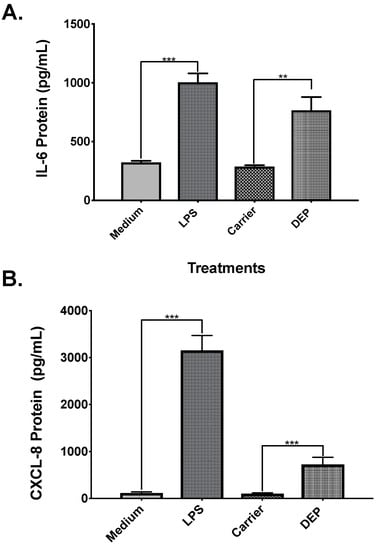
Figure 1.
Validation of the experimental conditions. A total of 5.0 × 104 BEAS-2B cells were seeded on 96-well plates. Positive control cells were exposed to 10 µg/mL of lipopolysaccharides (LPS), negative controls cells were exposed to Keratinocyte Basal Medium-2 (KGM-2), carrier cells were exposed to 0.1% dimethyl sulfoxide (DMSO) diluted in KGM-2 and diesel exhaust particles (DEP) cells were exposed to 10 µg/mL of DEP organic extract. Interleukin-6 (IL-6) release (A) and interleukin-8 (CXCL8) release (B) were measured from cell supernatants after 24 h of exposure. Values are expressed as the mean ± SEM (n = 3). ** p ≤ 0.01 *** p ≤ 0.001 compared to negative control.
3.2. Dose-Response Experiments
To determine the effects of rural and urban PM organic extracts in cell viability and the induction of the pro-inflammatory mediators IL-6 and CXCL8, a dose-response experiment was performed. Cell viability assays showed that neither the carrier nor LPS (Figure 2A) induced cell cytotoxicity at the tested concentrations. In cells exposed to DEP extract concentrations higher than 10 µg/mL, cell viability was reduced when compared to the carrier cells (Figure 2B). When cells were exposed to rural PM organic extract concentrations higher than 50 µg/mL, cell viability was significantly reduced (Figure 2C). In contrast, the urban PM organic extract did not show any cytotoxicity at the tested concentrations compared to the extract carrier (Figure 2D).
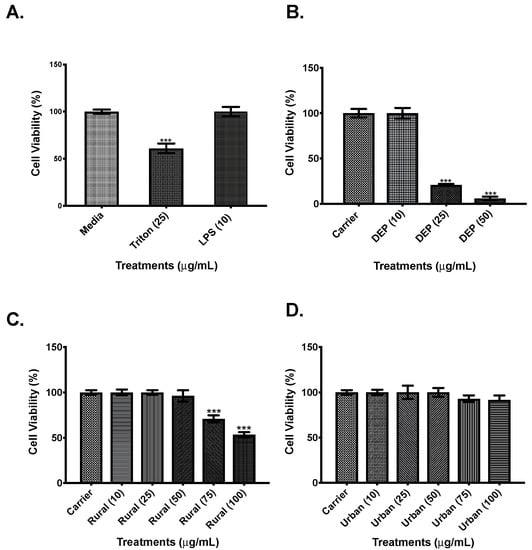
Figure 2.
Cell viability. A total of 1.0 × 105 BEAS-2B cells were exposed for 24 h to various treatments prior determination of their viability using the Neutral Red Assay. (A). Control cells were exposed to KGM-2 media alone (Medium), 25 µg/mL of TRITON X-100, and 10 µg/mL LPS. (B). Cells were exposed to 0.1% of DMSO diluted in media (carrier) and increasing concentrations of DEP organic extract. (C). Cells were exposed to different concentrations of the rural airborne particulate matter (PM) organic extract. (D). Cells were exposed to different concentrations of the urban PM organic extracts. Values are expressed as the mean ± SEM (n = 3), *** p ≤ 0.001 compared to media and to carrier (controls), when exposed to DEP organic extract, Rural PM organic extract, and Urban PM organic extract.
Results showed that cells exposed to rural PM had an IL-6 and CXCL8 expression significantly higher in comparison to cells exposed to carrier alone. As shown in Figure 3A, IL-6 protein levels of cells exposed to the lowest concentration of the rural organic extract were 1.3 times higher when compared to carrier and 1.4 times higher in cells exposed to the 25 μg/ml concentration. CXCL8 protein (Figure 3B) was 1.7 higher at the 25 μg/ml concentration of rural PM organic extract. Results for the urban PM extracts showed that secretion of IL-6 (Figure 4A) was significantly higher (1.25 fold) in cells exposed to 25 μg/ml when compared to carrier. No induction of CXCL8 protein levels (Figure 4B) was observed in the tested concentrations.
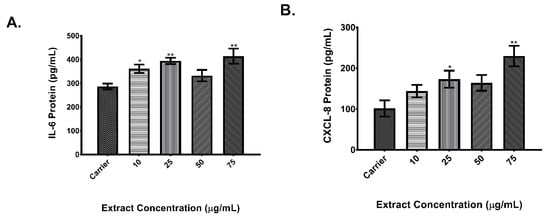
Figure 3.
Dose response of rural organic extract. A total of 5.0 × 104 BEAS-2B cells were seeded on 96-well plates and exposed to increasing concentrations of the rural PM organic extract. IL-6 (A) and CXCL8 (B) protein levels were measured from cell supernatants after 24 h of exposure. Values are expressed as the mean ± SEM (n = 3). * p ≤ 0.05, ** p ≤ 0.01 compared to carrier.
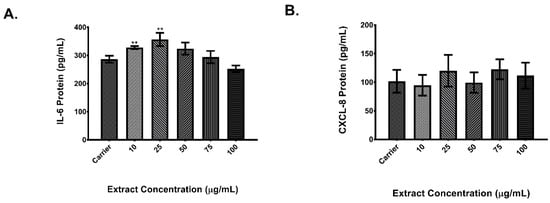
Figure 4.
Dose response of urban organic extract. A total of 5.0 × 104 BEAS-2B cells were seeded on 96-well plates and exposed to increasing concentrations of the urban PM organic extract. IL-6 (A) and CXCL8 (B) protein levels were measured from cell supernatants after 24 h of exposure. Values are expressed as the mean ± SEM (n = 3). ** p ≤ 0.01 compared to carrier.
3.3. Time Course Experiments
To evaluate the effects of rural and urban PM on the post-transcriptional control of IL-6 and CXCL8 a time-course was conducted to assess changes in their mRNA levels. Cells exposed to extracts from rural PM and DEP (Figure 5A,C, respectively) did not induce an increase in IL-6 mRNA levels. However, a significant peak increase in the mRNA levels of IL-6 was observed in cells exposed to urban PM extracts at 15 min (Figure 5B). To corroborate if this effect is the result of an up-regulation in the transcription rate of IL-6 mRNA a nuclear run-on assay was performed. As shown in Figure 5D there was no difference in the IL-6 transcription rate at 15 min of exposure but after 60 min of exposure there was a significant up-regulation of the transcription rate (~2 fold).
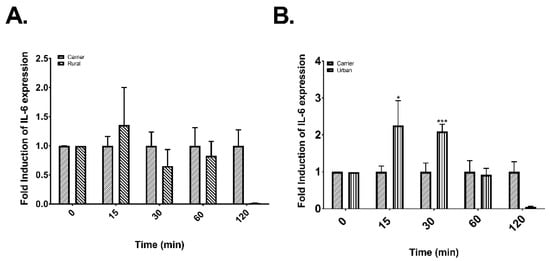
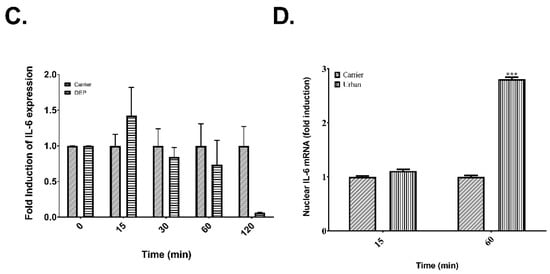
Figure 5.
Post-transcriptional control and nuclear expression of IL-6 in cells exposed to rural and urban organic extracts from PR and DEP organic extract. (A) Time course of cells exposed to 25 μg/mL of rural organic extract. (B) Time course of cells exposed to 25 μg/mL of urban organic extract. (C) Time course of cells exposed to 10 μg/mL of DEP organic extract. (D) Induction of the transcription rate of IL-6 transcript in response to urban organic extract exposure. Values are expressed as the mean ± SEM (n = 3). * p ≤ 0.05, *** p ≤ 0.001 compared to carrier.
Meanwhile for CXCL8, mRNA levels were significantly higher after 15 min of exposure to organic extracts from urban PM (Figure 6A), rural PM (Figure 6B) and DEP (Figure 6C). Also, there was another peak induction at 60 min in cells exposed to urban extracts (Figure 6A). In contrast to IL-6, the nuclear run-on data (Figure 6D) showed a significant difference in the transcription rate of CXCL8 at 15 min both for urban and DEP extracts but not for rural PM extract.
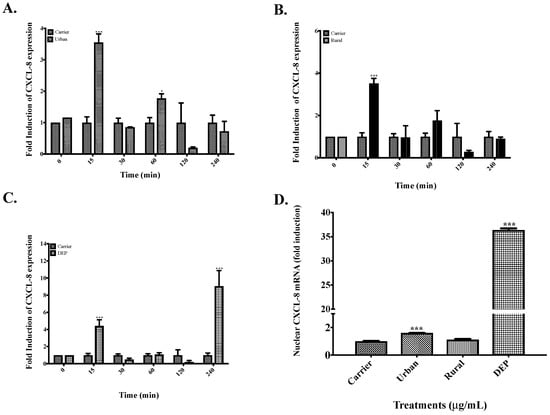
Figure 6.
Post-transcriptional control and nuclear expression of CXCL8 in cells exposed to organic extracts from rural PM2.5, urban PM2.5 and DEP. (A) Time course of cells exposed to 25 μg/mL of urban extract. (B) Time-course of cells exposed to 25 μg/mL of rural extract. (C) Time course of cells exposed to 10 μg/mL of DEP extract. (D) Induction of the transcription rate of CXCL8 transcript after 15 min of exposure to rural, urban, and DEP organic extracts. Values are expressed as the mean ± SEM (n = 3). * p ≤ 0.05, *** p ≤ 0.001 compared to carrier.
Since it is known that post-transcriptional regulation cause changes in mRNAs steady-state levels we proceeded to investigate if there are changes in the IL-6 and CXCL8 mRNA half-life. It is well known that steady-state levels depend on mRNA synthesis and mRNA degradation and changes in any of these two steps leads to overall changes in the mRNA level. In this study we used total RNA to assess the steady-state levels of IL-6 and CXCL8 in exposed cells. In order to calculate the mRNA half-lives of our samples we performed linear regressions on the fold expression time course data (Figures S1 and S2). To calculate the indirect half-lives we selected the time points from 15–120 min because previous studies in our lab showed that after 4 to 6 h of exposure there was an accumulation of IL-6 and CXCL8 transcripts, reaching a peak in transcription after 8 h for both mRNAs [32]. The calculated mRNA half-lives for both IL-6 and CXCL8 in exposed cells ranged between 59–76 min and 32–51 min, respectively (Table 1). The longest half-life time for IL-6 mRNA was observed in cells exposed to the urban extract.

Table 1.
Changes in mRNA Half-lives.
3.4. HuR Abundance
Since there was not an increase in de novo synthesis of IL-6 and CXCL8, as shown with the nuclear run-on assays, we explored the role of HuR at the time points with an increase in IL-6 and CXCL8 mRNA levels. HuR is an AREBP that stabilizes ARE-containing mRNA in the cytoplasm, including IL-6 and CXCL8. Therefore, to determine HuR cytoplasmic abundance at 15 min and 60 min a dot blot assay was performed. As showed in Figure 7A,B there was a significant increase in HuR abundance in cells exposed to urban PM extract at 15 min but not those exposed to rural PM and DEP organic extracts. At 60 min there was a significance decrease in HuR levels (Figure 7C,D).
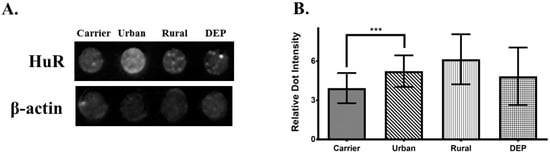
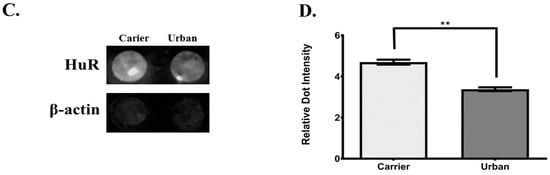
Figure 7.
Cytoplasmic abundance of ELAV-L protein 1 (HuR) after 15 min and 1 hr of exposure. (A) Dot blot of HuR and actin (loading control) after 15 min of exposure in cells treated with 25 μg/mL of both, rural and urban organic extracts and 10 μg/mL of DEP organic extract. (B) Densitometric analysis of cytoplasmic HuR dot blots from cells treated with DEP extract. (C) Dot blot of HuR and actin after 1 hr of exposure in cells treated with 25 μg/mL of urban organic extract. (D) Densitometric analysis of HuR dot blots from cells treated with the urban extract. Figure represents the average of three independent experiments (n = 3). Values are expressed as the mean ± SEM (n = 3). ** p ≤0.01, *** p ≤ 0.001 compared to carrier.
4. Discussion
Exposure to ambient PM has been shown to induce pro-inflammatory responses in the respiratory tract both in in vivo and in vitro models [11,33,34,35]. IL-6 and CXCL8 are commonly up-regulated in lung epithelial cells exposed to PM2.5 [7,9,10,11,35,36]. This is consistent with our previous studies exposing BEAS-2B cell line with different PM extracts from Puerto Rico [2,5,8,32,37]. In the current study, we observed significant increases in the secretion of IL-6 in cells exposed to both urban and rural PM2.5 organic extracts but only the rural extract induced the secretion CXCL8. In contrast, when our group previously tested the aqueous extracts only the PM from the urban site significantly induced CXCL8 [8] but polar and non-polar organic extracts from both sites did not [5]. However, we only measured the protein levels of CXCL8 in the cell supernatant and it has been recently reported that in cells exposed to PM2.5, CXCL8 can be sequestered in intracellular vesicles [38], which can explain our observations. In addition, differences in responses among sites and extracts could be explained by the chemical composition of the particles [5,8,26,39] as we have previously shown that both endotoxin and the metal composition play a crucial role in the pro-inflammatory responses induced by PM from Puerto Rico [32,37,40]. The PM samples used in the current study has been characterized for metal (i.e., As, Cd, Cu, Fe, Ni, Pb, V, Zn) [8,26], the elemental (EC) and organic carbon (OC) content [26], and polycyclic aromatic hydrocarbons (PAHs) [39]. Except for Fe, the levels of metals, EC, OC and PAHs were higher in PM from the urban site. In addition, endotoxin levels were higher in the urban extract (7.34 vs. <5.00 endotoxin units/mL). In contrast, the metal content in the organic extracts was similar for both sites [5].
It has been reported that PM can induce the production of pro-inflammatory mediators through different mechanisms [41,42] including the activation of: the inflammasome; different receptors including the TLRs and the Ah receptor among others; signaling cascades such as MAPKs; nuclear translocation of transcription factors such as NF-kB and AP-1; and microRNAs (miRNA) [43]. Most of the aforementioned mechanisms are involved in the transcriptional regulation of pro-inflammatory mediators, however both MAPKs and miRNA are also implicated in the post-transcriptional regulation of cytokines and chemokines. Since in this study we observed significant increases in the mRNA levels of IL-6 and CXCL8 mRNAs as early as 15 min, we evaluated whether the PM extracts had an effect in the post-transcriptional regulation of these pro-inflammatory mediators. In order to address this, we first examined the kinetics of IL-6 and CXCL8 mRNAs steady-state expression. All extracts had a significant effect in the steady-state levels of CXCL8 but only the urban extract had a significant effect in IL-6 steady-state levels. However, results from time-course experiments showed that the rural and urban PM extracts had an effect in the stabilization of both IL-6 and CXCL8 mRNAs as inferred from the substantial increase in their half-lives. In cells exposed to the urban PM extract, IL-6 half-life increased significantly from 14 min at basal levels to 76 min in exposed cells and the CXCL8 mRNA half-life from 30 to 49 min. The rural PM extract also induced similar changes in the mRNA half-lives of both IL-6 (t1/2 = 59.39) and CXCL8 (t1/2 = 50.73). The changes in the steady-state levels can be linked partially to the endotoxin levels present in both extracts because it is well known that LPS can promote both transcriptional and post-transcriptional control particularly altering the mRNA stability of ARE-containing transcripts. Meanwhile exposure to DEP extract promoted the stabilization of IL-6 mRNA and significantly increased the steady-state level of CXCL8. The results from exposures to DEP extract strongly suggest that the increase in the steady-state levels of CXCL8 was due to an increase in the transcription rate and not a decrease in mRNA turnover. Therefore, because the results from this study showed an up-regulation of IL-6 and CXCL8 mRNAs a transcriptional contribution cannot be rejected. Thus, in order to differentiate between the transcriptional and post-transcriptional contribution in the accumulation of IL-6 and CXCL8 mRNAs, nuclear run-ons were performed to measure changes in the transcription rates of both transcripts. The rural organic extract did not show any change in the transcription rates, suggesting that the increase in CXCL8 mRNA steady-state levels after 15 min is due to post-transcriptional control mechanisms. In contrast, the urban organic extract showed an increase in the transcription rate for CXCL8 after 15 min but not after 60 min of exposure. Meanwhile in cells exposed to the DEP organic extract, there is a dramatic increase (>35 fold) in the transcription rate of CXCL8 after 15 min of exposure, strongly suggesting a transcriptional activation mediated by DEP extract. Moreover, the slight increase in steady-state CXCL8 mRNA at 60 min can be correlated to the cytoplasmic export of the de novo transcripts produced after 15 min of exposure. Overall, these results provide evidence that PM2.5 has a role in the post-transcriptional regulation of IL-6 and CXCL8.
Extensive evidence has shown that both IL-6 and CXCL8 mRNAs are post-transcriptionally regulated by the ARE-mediated decay (AMD). This regulatory mechanism buffers gene expression after production of mRNA as a response to cellular alterations or extracellular signals [44]. AMD regulation of IL-6 and CXCL8 may be mediated by different AREBPs that translocate from the nucleus to the cytoplasm once are activated. Since MAPKs can regulate the expression and function of AREBPs it might be possible that AMD is involved in post-transcriptional effects exerted by the PM extracts. Several studies indicate that phosphorylation is crucial to the cytoplasmic translocation of AREBPs and also to their mRNA binding activity [14,45,46,47,48]. Among the MAPKs, the role of p38 in post-transcriptional control is the most studied, particularly its role in the ARE-mediated pathway. Indirect evidence suggests a possible role for the involvement of these kinases in post-transcriptional regulation mechanisms in cells exposed to PM [49,50,51]. For example, Hashimoto et al., [50], reported an increase in phosphorylated p38 as early as 30 min which was sustained until 60 min. As HuR is one of the most studied AREBP that stabilize mRNAs [14] and our results showed that the organic extracts stabilized both IL-6 and CXCL8 mRNAs we investigated whether there was a cytoplasmic enrichment of HuR in cells exposed to the organic extracts. HuR levels were assessed by performing dot blots of cytoplasmic protein fractions of exposed BEAS-2B cells after 15 min and 60 min. Exposure to the urban organic extract resulted in a cytoplasmic enrichment of HuR only at 15 min of exposure, which coincided with the increase of IL-6 and CXCL8 mRNA levels during the time-course experiments. However, cells exposed to DEP and rural extracts did not show a significant increase in HuR cytoplasmic levels even though changes in the mRNA steady-state levels and half-lives were observed. To this extent, because there is no change in the transcription rates in cells exposed to the rural extracts, we can infer that other AREBPs and/or other post-transcriptional control mechanism are also involved.
5. Conclusions
In this study, we demonstrate urban and rural PM2.5 organic extracts from Puerto Rico have an effect on the post-transcriptional control of IL-6 and CXCL8. Additionally, this study presents for the first time the cytoplasmic enrichment of the AREBP HuR as a result of urban organic extract exposure, suggesting a possible involvement of the ARE-mediated decay during acute responses to PM. Moreover, we showed that urban PM and DEP extracts can modulate the transcriptional regulation of both IL-6 and CXCL8 inducing their de novo synthesis at short period of time.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4433/10/5/270/s1, Figure S1: IL-6 Linear Regression Data, Figure S2: CXCL-8 Linear Regression Data.
Author Contributions
Conceptualization, E.R.-R. and L.B.M.; Methodology, E.R.-R.; Validation, E.R.-R. and A.O.-R.; Formal Analysis, E.R.-R., L.B.M. and B.J.-V.; Resources, B.J.-V.; Writing–Original Draft Preparation, E.R.-R.; Writing–Review & Editing, E.R.-R., L.B.M. and R.I.R.-C.; Supervision, L.B.M. and B.J.-V.; Project Administration, B.J.-V.; Funding Acquisition, B.J.-V.
Funding
This work was supported by NIH Grant R25GM061838 and NIH NCRR RCMI G12RR03051.
Acknowledgments
We like to give thanks to: (1) Wilfredo Delgado from University of Puerto Rico-Medical Sciences Campus/Biochemistry Department for his help and equipment facilitation (2) Jazmín Rodríguez from Bio Analytical Instruments for her support, (3) Solimar Díaz from Eppendorf for her help and technical support, (4) Carlos Santiago from Universidad Ana G. Mendez/School of Science and Technology for his help and equipment facilitation and (5) Mario Ortiz from University of Puerto Rico-Medical Sciences Campus/Biochemistry Department for all their help and support.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Backes, C.H.; Nelin, T.; Gorr, M.W.; Wold, L.E. Early life exposure to air pollution: How bad is it? Toxicol. Lett. 2013, 216, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cotto, R.; Ortíz-Martínez, M.G.; Rivera-Ramírez, E.; Méndez, L.B.; Dávila, J.C.; Jiménez-Vélez, B.D. African dust storms reaching Puerto Rican coast stimulate the secretion of IL-6 and IL-8 and cause cytotoxicity to human bronchial epithelial cells (BEAS-2B). Health 2013, 5, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef]
- Ferecatu, I.; Borot, M.C.; Bossard, C.; Leroux, M.; Boggetto, N.; Marano, F.; Baeza-Squiban, A.; Andreau, K. Polycyclic aromatic hydrocarbon components contribute to the mitochondria-antiapoptotic effect of fine particulate matter on human bronchial epithelial cells via the aryl hydrocarbon receptor. Part. Fibre Toxicol. 2010, 7, 18. [Google Scholar] [CrossRef]
- Fuentes-Mattei, E.; Rivera, E.; Gioda, A.; Sánchez-Rivera, D.; Román-Velázquez, R.; Jiménez-Vélez, B.D. Use of human bronchial epithelial cells (BEAS-2B) to study immunological markers resulting from exposure to PM2.5 organic extract from Puerto Rico. Toxicol. Appl. Pharmacol. 2009, 243, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Gioda, A.; Peréz, U.; Rosa, Z.; Jiménez-Vélez, B.D. Particulate Matter (PM10 and PM2.5) from different areas of Puerto Rico. Fresenius Environ. Bull. 2007, 16, 861–868. [Google Scholar]
- Mazzarella, G.; Ferraraccio, F.; Prati, M.V.; Annunziata, S.; Bianco, A.; Mezzogiorno, A.; Liguori, G.; Angelillo, I.F.; Cazzola, M. Effects of diesel exhaust particles on human lung epithelial cells: An in vitro study. Resp. Med. 2007, 101, 1155–1162. [Google Scholar] [CrossRef]
- Jiménez-Vélez, B.D.; Gioda, A.; Fuentes-Mattei, E. Organic and aqueous extracts from particulate matter (PM2.5) and their effect on immunological response of human bronchial epithelial cells BEAS-2B. Metal Ions Biol. Med. 2006, 9, 267–272. [Google Scholar]
- Hetland, R.B.; Cassee, F.R.; Lag, M.; Refsnes, M.; Dybing, E.; Schwarze, P.E. Cytokine release from alveolar macrophages exposed to ambient particulate matter: Heterogeneity in relation to size, city and season. Part. Fibre Toxicol. 2005, 2, 4–19. [Google Scholar] [CrossRef]
- Riedl, M.; Díaz-Sánchez, D. Biology of Diesel Exhaust effects on respiratory function. J. Allergy. Clin. Immunol. 2005, 115, 221–228. [Google Scholar] [CrossRef]
- Alfaro-Moreno, E.; Martínez, L.; García-Cuellar, C.; Bonner, J.C.; Murray, C.; Rosas, I.; Ponce de León-Rosales, S.; Osornio-Vargas, A.R. Biologic effects induced in vitro by PM10 from three different zones of Mexico City. Environ. Health Perspect. 2002, 110, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Takizawa, H.; Takami, K.; Desaki, M.; Okazaki, H.; Kasama, T.; Kobayashi, K.; Yamamoto, K.; Nakahara, K.; Tanaka, M.; et al. Benzene-extracted components are important for the major activity of diesel exhaust particles: Effect on interleukin-8 gene expression in human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 2001, 24, 419–426. [Google Scholar] [CrossRef]
- Nemmar, A.; Holme, J.A.; Rosas, I.; Schwarze, P.E.; Alfaro-Moreno, E. Recent advances in Particulate Matter and Nanoparticle Toxicology: A review of the in vivo and in vitro studies. BioMed Res. Int. 2013, 1–22. [Google Scholar] [CrossRef]
- Hamilton, T.; Li, X.; Novotny, M.; Pavicic, P.G., Jr.; Datta, S.; Zhao, C.; Hartupee, J.; Sun, D. Cell type- and stimulus-specific mechanisms for post-transcriptional control of neutrophil chemokine gene expression. J. Leukoc. Biol. 2012, 91, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.; Jakymiw, A.; Van Tubergen, E.A.; D’Silva, N.J.; Kirkwood, K.L. Control of cytokine mRNA expression by RNA-binding proteins and micro RNAs. J. Dent. Res. 2012, 91, 651–658. [Google Scholar] [CrossRef]
- Joe, Y.; Kim, H.J.; Kim, S.; Chung, J.; Ko, M.S.; Lee, W.H.; Chang, K.C.; Park, J.W.; Chung, H.T. Tristetraprolin mediates anti-inflammatory effects of nicotine in lipopolisaccharide-stimulated macrophages. J. Biol. Chem. 2011, 286, 24735–24742. [Google Scholar] [CrossRef]
- Hao, S.; Baltimore, D. The stability of mRNA influences the temporal order of the induction of genes encoding inflammation molecules. Nat. Immunol. 2009, 10, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Shoenberg, D.R.; Maquat, L.E. Regulation of cytoplasmic mRNA decay. Nat. Rev. Genet. 2012, 13, 246–259. [Google Scholar] [CrossRef]
- Stumpo, D.J.; Lai, W.S.; Blackshear, P.J. Inflammation: Cytokines and RNA-based regulation. Wiley Interdiscip. Rev. RNA 2010, 1, 60–80. [Google Scholar] [CrossRef]
- Lal, A.; Mazan-Mamczarz, K.; Kawai, T.; Yang, X.; Martindale, J.L.; Gorospe, M. Concurrent versus individual binding of HuR and AUF-1 to common labile target mRNAs. EMBO J. 2004, 23, 3092–3112. [Google Scholar] [CrossRef]
- Wilusz, C.J.; Wormington, M.; Peltz, S.T. The Cap-to-tail guide to mRNA turnover. Nature Rev. Mol. Cell. Biol. 2001, 2, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Barreau, C.; Paillard, L.; Osborne, A.B. AU-rich elements and associated factors: Are there unifying principles. Nucl. Ac. Res. 2005, 33, 7138–7150. [Google Scholar] [CrossRef]
- Cheneval, D.; Kastelic, T.; Fuerst, P.; Parker, C. A review of methods to monitor the modulation of mRNA stability: A novel approach to drug discovery and therapeutic intervention. J. Biomol. Screen. 2010, 15, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Gratacós, F.M.; Brewer, G. The role of AUF1 in regulated mRNA decay. Wiley Interdiscip. Rev. RNA 2010, 1, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Moretto, N.; Bertolini, S.; Iadicicco, C.; Marchini, G.; Kaur, M.; Volpi, G.; Patacchini, R.; Singh, D.; Facchinetti, F. Cigarette smoke and its component acrolein augment IL-8/CXCL8 mRNA stability via p38 MAPK/MK2 signaling in human pulmonary cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L929–L938. [Google Scholar] [CrossRef] [PubMed]
- Acevedo Figueroa, D.; Rodríguez-Sierra, C.J.; Jiménez-Vélez, B.J. Concentrations of Ni and V, other heavy metals, arsenic, elemental and organic carbon in atmospheric fine particles (PM2.5) from Puerto Rico. Toxicol. Ind. Health 2006, 22, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1981, 24, 119–124. [Google Scholar] [CrossRef]
- Graham, J.R.; Hendershott, M.C.; Terragni, J.; Cooper, G.M. mRNA degradation plays a significant role in the program of gene expression regulated by phosphatidylinositol 3-kinase signaling. Mol. Cell Biol. 2010, 30, 5295–5305. [Google Scholar] [CrossRef]
- Chen, C.-Y.A.; Shyu, A.-B. AU-rich elements: Characterization and importance in mRNA degradation. Trends Biochem. Sci. 1995, 20, 465–470. [Google Scholar] [CrossRef]
- Patrone, G.; Puppo, F.; Cusano, R.; Scaranari, M.; Ceccherini, I.; Puliti, A.; Ravazzolo, R. Nuclear run-on assay using biotin labeling, magnetic bead capture and analysis by fluorescence-based RT-PCR. Bio. Tech. 2000, 29, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.K.; WenGe, Y. Electrophoretic mobility shift assay for the detection of specific DNA–protein complex in nuclear extracts from the cultured cells and frozen autopsy human brain tissue. Brain Res. Protoc. 2000, 5, 257–265. [Google Scholar] [CrossRef]
- Rodríguez-Cotto, R.; Ortíz-Martínez, M.G.; Jiménez-Vélez, B.D. Organic extracts from African dust storms stimulate oxidative stress and induce inflammatory responses in human lung cells through Nrf2 but not NF-kB. Envirol. Toxicol. Pharmacol. 2015, 39, 845–856. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Air Quality Criteria for Particulate Matter, Vol. I.; National Center for Environmental Assessment RTP Office: Research Triangle Park, NC, USA, 2004.
- Veranth, J.M.; Reilly, C.A.; Veranth, M.M.; Moss, T.A.; Langelier, C.R.; Lanza, D.L.; Yost, G.S. Inflammatory cytokines and cell death in BEAS-2B lung cells treated with soil dust, lipopolysaccharide, and surface-modified particles. Toxicol. Sci. 2004, 82, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Hayashi, S.; Hogg, J.C.; Vincent, R.; Van Eeden, S.F. Particulate Matter induces cytokine expression in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001, 25, 265–271. [Google Scholar] [CrossRef]
- Monn, C.; Becker, S. Cytotoxicity and induction of Pro-inflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10-2.5) in outdoor and indoor air. Toxicol. Appl. Pharmacol. 1999, 155, 245–252. [Google Scholar] [CrossRef]
- Ortiz Martínez, M.G.; Rivera, E.; Méndez, L.; Jiménez Vélez, B.D. Role of chemical and biological constituents of PM10 from Saharan Dust in the exacerbation of asthma in Puerto Rico. In Biodiversity Science for Humanity; Theophanides, M., Theophanides, T., Eds.; Athens Institute for Education and Research (ATINER): Athens, Greece, 2010; pp. 101–118. [Google Scholar]
- Longhin, E.; Holme, J.A.; Gualtieri, M.; Camatini, M.; Øvrevik, J. Milan winter fine particulate matter (wPM2.5) induces IL-6 and IL-8 synthesis in human bronchial BEAS-2B cells, but specifically impairs IL-8 release. Toxicol. Vitro 2018, 52, 365–373. [Google Scholar] [CrossRef]
- Alvarez-Avilés, O.; Cuadra-Rodríguez, L.; González-Illán, F.; Quiñones-González, J.; Rosario, O. Optimization of a novel method for the organic chemical characterization of atmospheric aerosols based on microwave-assisted extraction combined with stir bar sorptive extraction. Ana. Chim. Acta. 2007, 597, 273–281. [Google Scholar] [CrossRef]
- Ortiz-Martínez, M.G.; Rodríguez-Cotto, R.I.; Ortiz-Rivera, M.A.; Pluguez-Turull, C.W.; Jiménez-Vélez, B.D. Linking endotoxins, African Dust PM10 and asthma in an urban and rural Environment of Puerto Rico. Mediators Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, Q.; Liu, T. Toxicity research of PM2.5 compositions in vitro. Int. J. Environ. Res. Public Health 2017, 14, 232. [Google Scholar] [CrossRef]
- Øvrevik, J.; Refsnes, M.; Låg, M.; Holme, J.A.; Schwarze, P.E. Activation of proinflammatory responses in cell of the airway mucosa by Particulate Matter: Oxidant- and non-oxidant mediated triggering mechanisms. Biomolecules 2015, 5, 1399–1440. [Google Scholar] [CrossRef] [PubMed]
- Longhin, E.; Capasso, L.; Battaglia, C.; Proverbio, M.C.; Cosentino, C.; Cifola, I.; Mangano, E.; Camatini, M.; Gualtieri, M. Integrative transcriptomic and protein analysis of human bronchial BEAS-2B exposed to seasonal urban particulate matter. Environ. Pollut. 2016, 209, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Bak, R.O.; Mikkelsen, J.G. Regulation of cytokines by small RNAs during skin inflammation. J. Biomed. Sci. 2010, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Khabar, K.S.A. Post-transcriptional control during chronic inflammation and cancer: A focus on AU-rich elements. Cell. Mol. Life Sci. 2010, 67, 2937–2955. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Vignere, C.Z.; Levitan, E.S. AUF-1 is upregulated by angiotensin II to destabilize cardiac Kv4.3 channel mRNA. J. Mol. Cell. Cardiol. 2008, 45, 832–838. [Google Scholar] [CrossRef][Green Version]
- David, P.S.; Tanveer, R.; Port, J.D. FRET-detectable interactions between the ARE binding proteins, HuR and AUF-1. RNA 2007, 13, 1453–1468. [Google Scholar] [CrossRef]
- Dean, J.L.E.; Sully, G.; Clark, A.R.; Saklatvala, J. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilization. Cell. Signal. 2004, 16, 1113–1121. [Google Scholar] [CrossRef]
- Kim, Y.M.; Reed, W.; Lenz, A.G.; Jaspers, I.; Silbajoris, R.; Nick, H.S.; Samet, J.M. Ultrafine carbon particles induce interleukin-8 gene transcription and p38 MAPK activation in normal human bronchial epithelial cells. AM. J. Physiol. Lung Cell. Mol. Pysiol. 2005, 288, L432–L441. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Gon, Y.; Takeshita, I.; Matsumoto, K.; Jibiki, I.; Takizawa, H.; Kudoh, S.; Horie, T. Diesel Exhaust Particles activate p38 MAP kinase to produce interleukin 8 and RANTES by human bronchial epithelial cells and N-Acetylcysteine attenuates p38 MAP kinase activation. Am. J. Respir. Crit. Care Med. 2000, 161, 280–285. [Google Scholar] [CrossRef]
- Takizawa, H.; Abe, S.; Ohtoshi, T.; Kawasaki, K.; Desaki, M.; Sugawara, I.; Hashimoto, S.; Azuma, A.; Nakahara, K.; Kudoh, S. Diesel exhaust particles up-regulate expression of intercellular adhesion molecule-1 (ICAM-1) in human bronchial epithelial cells. Clin. Exp. Immun. 2000, 120, 356–362. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).