Human Vascular Endothelial Growth Factor A165 Expression Induces the Mouse Model of Neovascular Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Changes in the Retinal Thickness and Vascularization
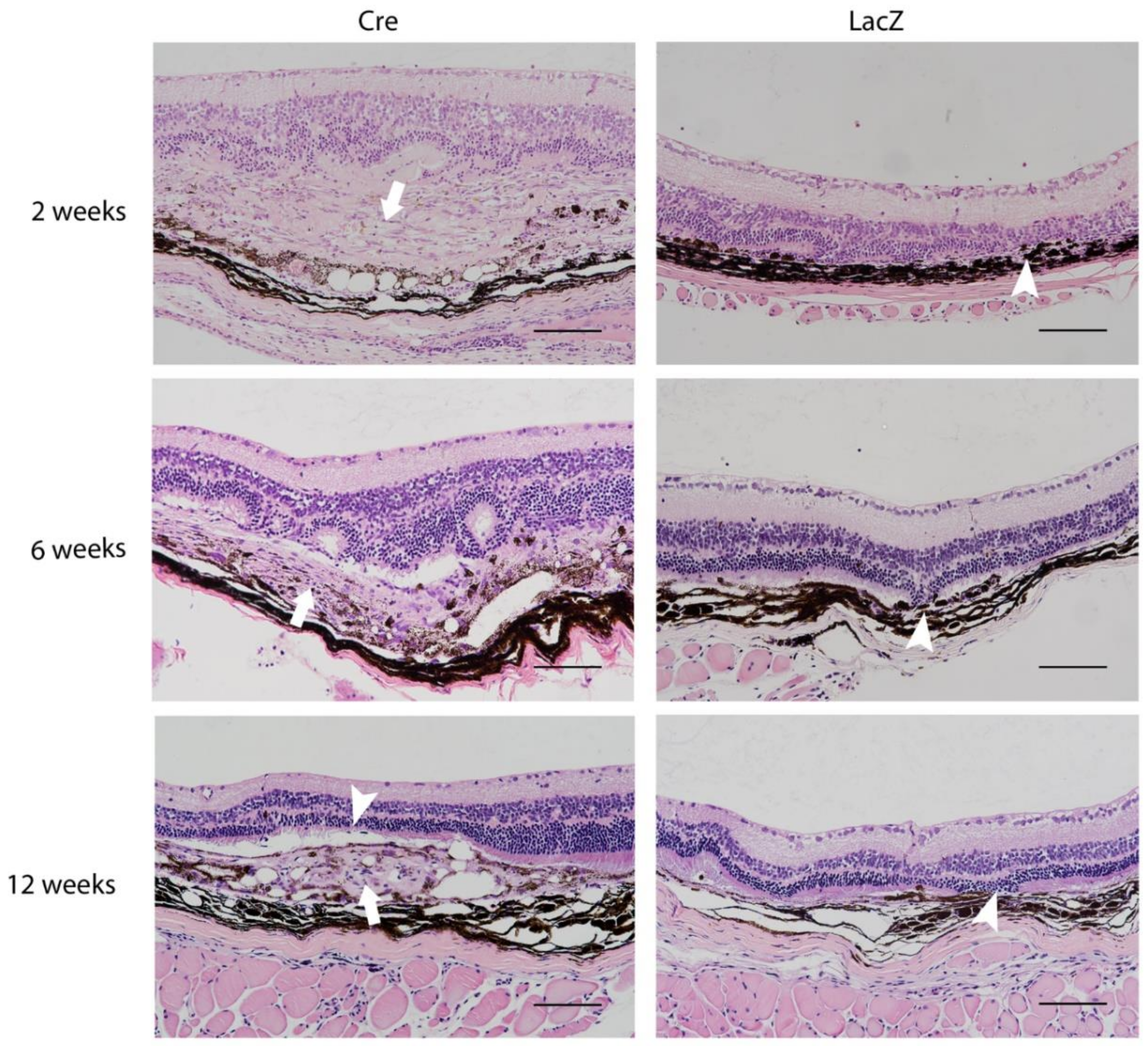
3.2. Histological Findings Related to Age-Related Macular Degeneration
3.3. Presence of Apoptotic Cells
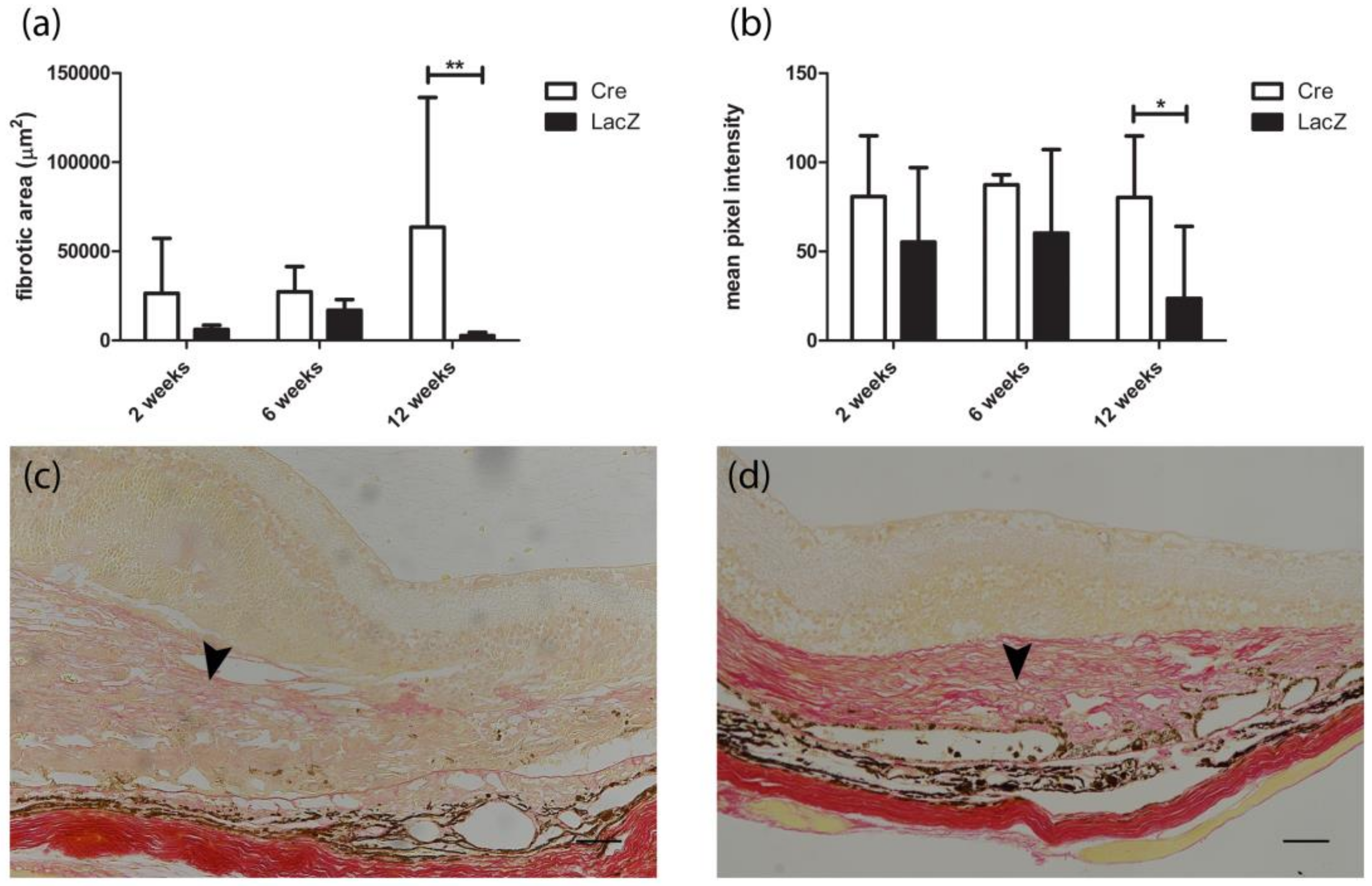
3.4. Development of Fibrovascular Membrane
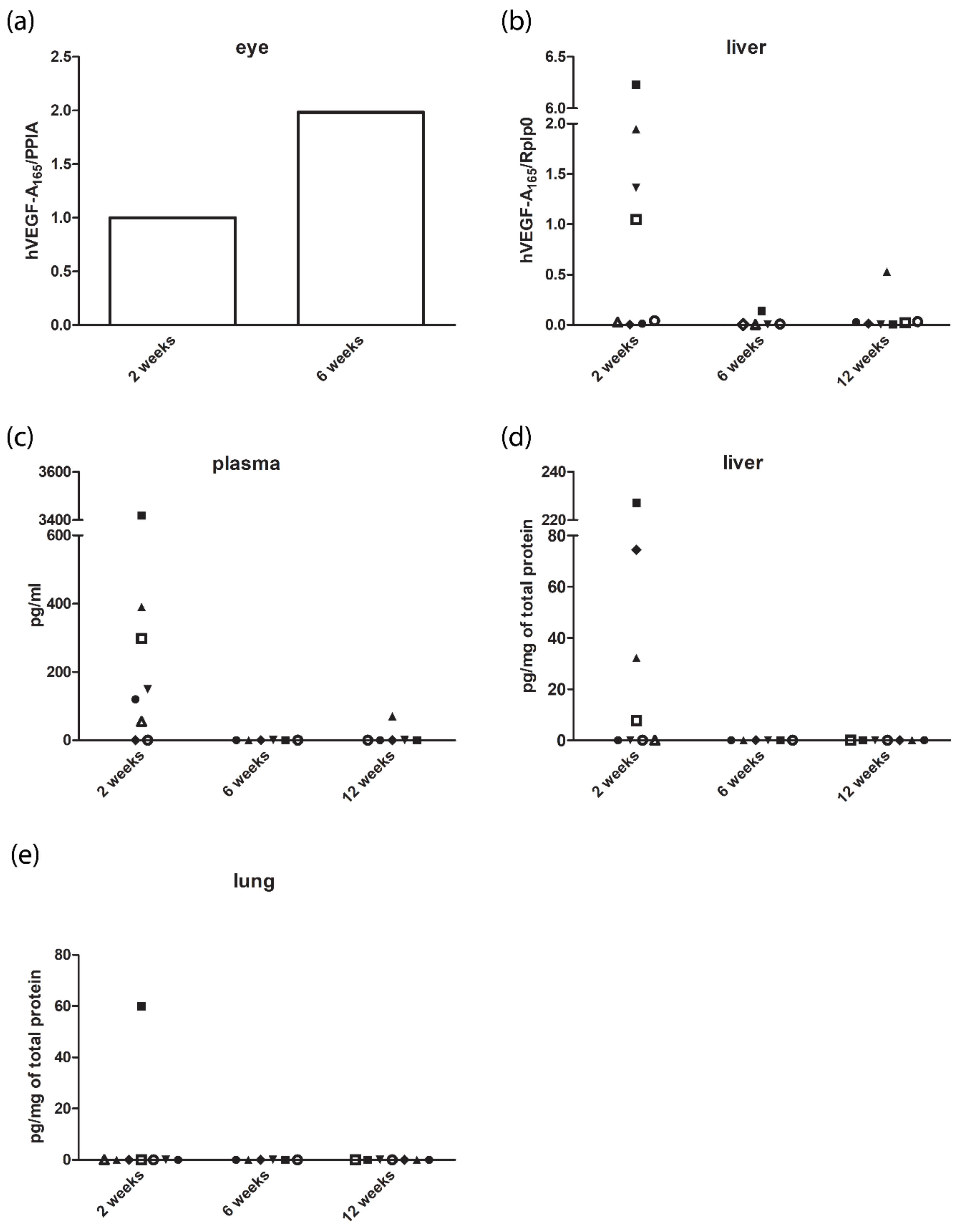
3.5. Expression Levels of Human Vascular Endothelial Growth Factor A165 in the Eye and Off-Targets
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Priority Eye Diseases. Available online: http://www.who.int/blindness/causes/priority/en/index7.html (accessed on 29 November 2017).
- Witmer, A.N.; Vrensen, G.F.J.M.; Van Noorden, C.J.F.; Schlingemann, R.O. Vascular endothelial growth factors and angiogenesis in eye disease. Prog. Retin. Eye Res. 2003, 22, 1–29. [Google Scholar] [CrossRef]
- Elizabeth Rakoczy, P.; Yu, M.J.T.; Nusinowitz, S.; Chang, B.; Heckenlively, J.R. Mouse models of age-related macular degeneration. Exp. Eye Res. 2006, 82, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S.; Bridges, C.; Katz, M.G.; Korpisalo, P. Angiogenic gene therapy in cardiovascular diseases: Dream or vision? Eur. Heart J. 2017, 38, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Anand, V.; Roy, S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J. Neuroimmune Pharmacol. 2014, 9, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.-P.; Chan, W.-M.; Liu, D.T.L.; Lai, T.Y.Y.; Choy, K.-W.; Pang, C.-P.; Lam, D.S.C. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am. J. Ophthalmol. 2006, 141, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Funk, M.; Karl, D.; Georgopoulos, M.; Benesch, T.; Sacu, S.; Polak, K.; Zlabinger, G.J.; Schmidt-Erfurth, U. Neovascular age-related macular degeneration: Intraocular cytokines and growth factors and the influence of therapy with ranibizumab. Ophthalmology 2009, 116, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Kliffen, M.; Sharma, H.S.; Mooy, C.M.; Kerkvliet, S.; de Jong, P.T. Increased expression of angiogenic growth factors in age-related maculopathy. Br. J. Ophthalmol. 1997, 81, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.F.; Sippy, B.D.; Lambert, H.M.; Thach, A.B.; Hinton, D.R. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1996, 37, 855–868. [Google Scholar]
- Matsuoka, M.; Ogata, N.; Otsuji, T.; Nishimura, T.; Takahashi, K.; Matsumura, M. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2004, 88, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kvanta, A.; Algvere, P.V.; Berglin, L.; Seregard, S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1929–1934. [Google Scholar] [CrossRef]
- Amadio, M.; Govoni, S.; Pascale, A. Targeting VEGF in eye neovascularization: What’s new?: A comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacol. Res. 2016, 103, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age related macular degeneration. Mol. Asp. Med. 2012, 33, 487–509. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, P.; Kholová, I.; Mähönen, A.J.; Airenne, K.; Koota, S.; Mansukoski, H.; Närväinen, J.; Wirzenius, M.; Alhonen, L.; Jänne, J.; et al. Short and long-term effects of hVEGF-A165 in Cre-activated transgenic mice. PLoS ONE 2006, 1, e13. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.G.; ElShelmani, H.; Singh, M.K.; Mansergh, F.C.; Wride, M.A.; Padilla, M.; Keegan, D.; Hogg, R.E.; Ambati, B.K. Risk factors and biomarkers of age-related macular degeneration. Prog. Retin. Eye Res. 2016, 54, 64–102. [Google Scholar] [CrossRef] [PubMed]
- Petit, L.; Khanna, H.; Punzo, C. Advances in gene therapy for diseases of the eye. Hum. Gene Ther. 2016, 27, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Ueno, H.; Goto, Y.; Oshima, Y.; Ishibashi, T.; Inomata, H. A vitrectomy improves the transfection efficiency of adenoviral vector-mediated gene transfer to Müller cells. Gene Ther. 1998, 5, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Wilson, J.; Sun, D.; Forbes, B.; Maguire, A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2535–2542. [Google Scholar]
- Li, T.; Adamian, M.; Roof, D.J.; Berson, E.L.; Dryja, T.P.; Roessler, B.J.; Davidson, B.L. In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2543–2549. [Google Scholar]
- Ueyama, K.; Mori, K.; Shoji, T.; Omata, H.; Gehlbach, P.L.; Brough, D.E.; Wei, L.L.; Yoneya, S. Ocular localization and transduction by adenoviral vectors are serotype-dependent and can be modified by inclusion of RGD fiber modifications. PLoS ONE 2014, 9, e108071. [Google Scholar] [CrossRef] [PubMed]
- Viegas, M.S.; Martins, T.C.; Seco, F.; do Carmo, A. An improved and cost-effective methodology for the reduction of autofluorescence in direct immunofluorescence studies on formalin-fixed paraffin-embedded tissues. Eur. J. Histochem. 2007, 51, 59–66. [Google Scholar] [PubMed]
- Grossniklaus, H.E.; Kang, S.J.; Berglin, L. Animal models of choroidal and retinal neovascularization. Prog. Retin. Eye Res. 2010, 29, 500–519. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, X.; Hirano, Y.; Tyagi, P.; Barabás, P.; Uehara, H.; Miya, T.R.; Singh, N.; Archer, B.; Qazi, Y.; et al. Targeted intraceptor nanoparticle therapy reduces angiogenesis and fibrosis in primate and murine macular degeneration. ACS Nano 2013, 7, 3264–3275. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Uehara, H.; Zhang, X.; Das, S.K.; Olsen, T.; Holt, D.; Simonis, J.M.; Jackman, K.; Singh, N.; Miya, T.R.; et al. Photoreceptor avascular privilege is shielded by soluble VEGF receptor-1. Elife 2013, 2, e00324. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Wang, Z.; Sun, Y.; Chen, J. Animal models of ocular angiogenesis: From development to pathologies. FASEB J. 2017, 31, 4665–4681. [Google Scholar] [CrossRef] [PubMed]
- Giani, A.; Thanos, A.; Roh, M.I.; Connolly, E.; Trichonas, G.; Kim, I.; Gragoudas, E.; Vavvas, D.; Miller, J.W. In vivo evaluation of laser-induced choroidal neovascularization using spectral-domain optical coherence tomography. Investig. Opthalmol. Vis. Sci. 2011, 52, 3880–3887. [Google Scholar] [CrossRef] [PubMed]
- Edelman, J.L.; Castro, M.R. Quantitative image analysis of laser-induced choroidal neovascularization in rat. Exp. Eye Res. 2000, 71, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Kannan, R.; Hinton, D.R. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp. Eye Res. 2016, 142, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Malek, G.; Johnson, L.V.; Mace, B.E.; Saloupis, P.; Schmechel, D.E.; Rickman, D.W.; Toth, C.A.; Sullivan, P.M.; Bowes Rickman, C. Apolipoprotein E allele-dependent pathogenesis: A model for age-related retinal degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 11900–11905. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Anand, A.; Fernandez, S.; Sakurai, E.; Lynn, B.C.; Kuziel, W.A.; Rollins, B.J.; Ambati, B.K. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat. Med. 2003, 9, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Miere, A.; Semoun, O.; Cohen, S.Y.; El Ameen, A.; Srour, M.; Jung, C.; Oubraham, H.; Querques, G.; Souied, E.H. Optical coherence tomography angiography features of subretinal fibrosis in age-related macular degeneration. Retina 2015, 35, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.; Toth, C.A.; Grunwald, J.E.; Jaffe, G.J.; Martin, D.F.; Fine, S.L.; Huang, J.; Ying, G.-S.; Hagstrom, S.A.; Winter, K.; et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014, 121, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.B.; Lund-Andersen, H.; Sander, B.; Larsen, M. Subfoveal fibrosis in eyes with neovascular age-related macular degeneration treated with intravitreal ranibizumab. Am. J. Ophthalmol. 2013, 156, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Rendahl, K.G.; Manning, W.C.; Quiroz, D.; Coyne, M.; Miller, S.S. AAV-mediated expression of vascular endothelial growth factor induces choroidal neovascularization in rat. Investig. Ophthalmol. Vis. Sci. 2003, 44, 781–790. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, L.; Li, Y.; Liu, Y.; Xiao, W.; Song, Y.; Luo, L.; Huang, D.; Yancopoulos, G.D.; Wiegand, S.J.; et al. A subretinal matrigel rat choroidal neovascularization (CNV) model and inhibition of CNV and associated inflammation and fibrosis by VEGF trap. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6009–6017. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jiang, A.; Liang, J.; Meng, H.; Chang, B.; Gao, H.; Qiao, X. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model’s retinal angiomatous proliferation. Investig. Opthalmol. Vis. Sci. 2008, 49, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.; Takeda, A.; Yoshimura, T.; Oshima, Y.; Sonoda, K.-H.; Ishibashi, T. A novel platelet-activating factor receptor antagonist inhibits choroidal neovascularization and subretinal fibrosis. PLoS ONE 2013, 8, e68173. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-J.; Sonoda, K.-H.; Oshima, Y.; Takeda, A.; Kohno, R.; Yamada, J.; Hamuro, J.; Yang, Y.; Notomi, S.; Hisatomi, T.; et al. Establishment of a new animal model of focal subretinal fibrosis that resembles disciform lesion in advanced age-related macular degeneration. Investig. Opthalmol. Vis. Sci. 2011, 52, 6089–6095. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Takeda, A.; Yoshimura, T.; Oshima, Y.; Sonoda, K.-H.; Ishibashi, T. IL-10 is significantly involved in HSP70-regulation of experimental subretinal fibrosis. PLoS ONE 2013, 8, e80288. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Sreekumar, P.G.; Spee, C.; Nazari, H.; Zhu, D.; Kannan, R.; Hinton, D.R. αB-crystallin regulates subretinal fibrosis by modulation of epithelial-mesenchymal transition. Am. J. Pathol. 2016, 186, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M. Fibrosis and diseases of the eye. J. Clin. Investig. 2007, 117, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Hellström, M.; Ruitenberg, M.J.; Pollett, M.A.; Ehlert, E.M.E.; Twisk, J.; Verhaagen, J.; Harvey, A.R. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene Ther. 2009, 16, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.-Q.; Aleman, T.S.; Dejneka, N.S.; Dudus, L.; Fisher, K.J.; Maguire, A.M.; Jacobson, S.G.; Bennett, J. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Mol. Ther. 2001, 4, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Provost, N.; Le Meur, G.; Weber, M.; Mendes-Madeira, A.; Podevin, G.; Cherel, Y.; Colle, M.-A.; Deschamps, J.-Y.; Moullier, P.; Rolling, F. Biodistribution of rAAV vectors following intraocular administration: Evidence for the presence and persistence of vector DNA in the optic nerve and in the brain. Mol. Ther. 2004, 11, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Lebherz, C.; Maguire, A.; Tang, W.; Bennett, J.; Wilson, J.M. Novel AAV serotypes for improved ocular gene transfer. J. Gene Med. 2008, 10, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Dudus, L.; Anand, V.; Acland, G.M.; Chen, S.J.; Wilson, J.M.; Fisher, K.J.; Maguire, A.M.; Bennett, J. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vis. Res. 1999, 39, 2545–2553. [Google Scholar] [CrossRef]
- Schmack, I.; Berglin, L.; Nie, X.; Wen, J.; Kang, S.J.; Marcus, A.I.; Yang, H.; Lynn, M.J.; Kapp, J.A.; Grossniklaus, H.E. Modulation of choroidal neovascularization by subretinal injection of retinal pigment epithelium and polystyrene microbeads. Mol. Vis. 2009, 15, 146–161. [Google Scholar] [PubMed]
- Kalesnykas, G.; Kokki, E.; Alasaarela, L.; Lesch, H.P.; Tuulos, T.; Kinnunen, K.; Uusitalo, H.; Airenne, K.; Yla-Herttuala, S. Comparative study of adeno-associated virus, adenovirus, baculovirus and lentivirus vectors for gene therapy of the eyes. Curr. Gene Ther. 2017, 17, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Verderber, L.; Johnson, W.; Mucke, L.; Sarthy, V. Differential regulation of a glial fibrillary acidic protein-LacZ transgene in retinal astrocytes and Müller cells. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1137–1143. [Google Scholar]
- Skeie, J.M.; Mullins, R.F. Macrophages in neovascular age-related macular degeneration: Friends or foes? Eye 2009, 23, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Ling, J.X.; Wallace, T.M.; Dithmar, S.; Lawson, D.H.; Cohen, C.; Elner, V.M.; Elner, S.G.; Sternberg, P. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol. Vis. 2002, 8, 119–126. [Google Scholar] [PubMed]
- Cherepanoff, S.; McMenamin, P.; Gillies, M.C.; Kettle, E.; Sarks, S.H. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br. J. Ophthalmol. 2010, 94, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Copland, D.A.; Horie, S.; Wu, W.-K.; Chen, M.; Xu, Y.; Paul Morgan, B.; Mack, M.; Xu, H.; Nicholson, L.B.; et al. Myeloid cells expressing VEGF and arginase-1 following uptake of damaged retinal pigment epithelium suggests potential mechanism that drives the onset of choroidal angiogenesis in mice. PLoS ONE 2013, 8, e72935. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Lundh von Leithner, P.; Izumi-Nagai, K.; Hosking, B.; Chang, B.; Hurd, R.; Adamson, P.; Adamis, A.P.; Foxton, R.H.; Ng, Y.S.; et al. Spontaneous CNV in a novel mutant mouse is associated with early VEGF-A-driven angiogenesis and late-stage focal edema, neural cell loss, and dysfunction. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3709–3719. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, U.F.O.; Robbie, S.; Munro, P.M.G.; Barker, S.E.; Duran, Y.; Luong, V.; Fitzke, F.W.; Bainbridge, J.W.B.; Ali, R.R.; MacLaren, R.E. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5934–5943. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, U.F.O.; Carvalho, L.S.; Robbie, S.J.; Cowing, J.A.; Duran, Y.; Munro, P.M.G.; Bainbridge, J.W.B.; Ali, R.R. Ccl2, Cx3cr1 and Ccl2/Cx3cr1 chemokine deficiencies are not sufficient to cause age-related retinal degeneration. Exp. Eye Res. 2013, 107, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M.; Manivannan, A.; Lois, N.; Forrester, J.V. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell 2008, 7, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, H.R.; Mclenachan, S.; Humphries, T.; Kezic, J.M.; Chen, X.; Ruitenberg, M.J.; Mcmenamin, P.G. Accumulation of murine subretinal macrophages: Effects of age, pigmentation and CX3CR1. Neurobiol. Aging 2012, 33, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, P.; Cowing, J.A.; Cristante, E.; Liyanage, S.E.; Ribeiro, J.; Duran, Y.; Abelleira Hervas, L.; Carvalho, L.S.; Bainbridge, J.W.B.; Luhmann, U.F.O.; et al. Cd59a deficiency in mice leads to preferential innate immune activation in the retinal pigment epithelium–choroid with age. Neurobiol. Aging 2015, 36, 2637–2648. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Tzekov, R.; Tang, S.; Kaushal, S. Accumulation and autofluorescence of phagocytized rod outer segment material in macrophages and microglial cells. Mol. Vis. 2012, 18, 103–113. [Google Scholar] [PubMed]
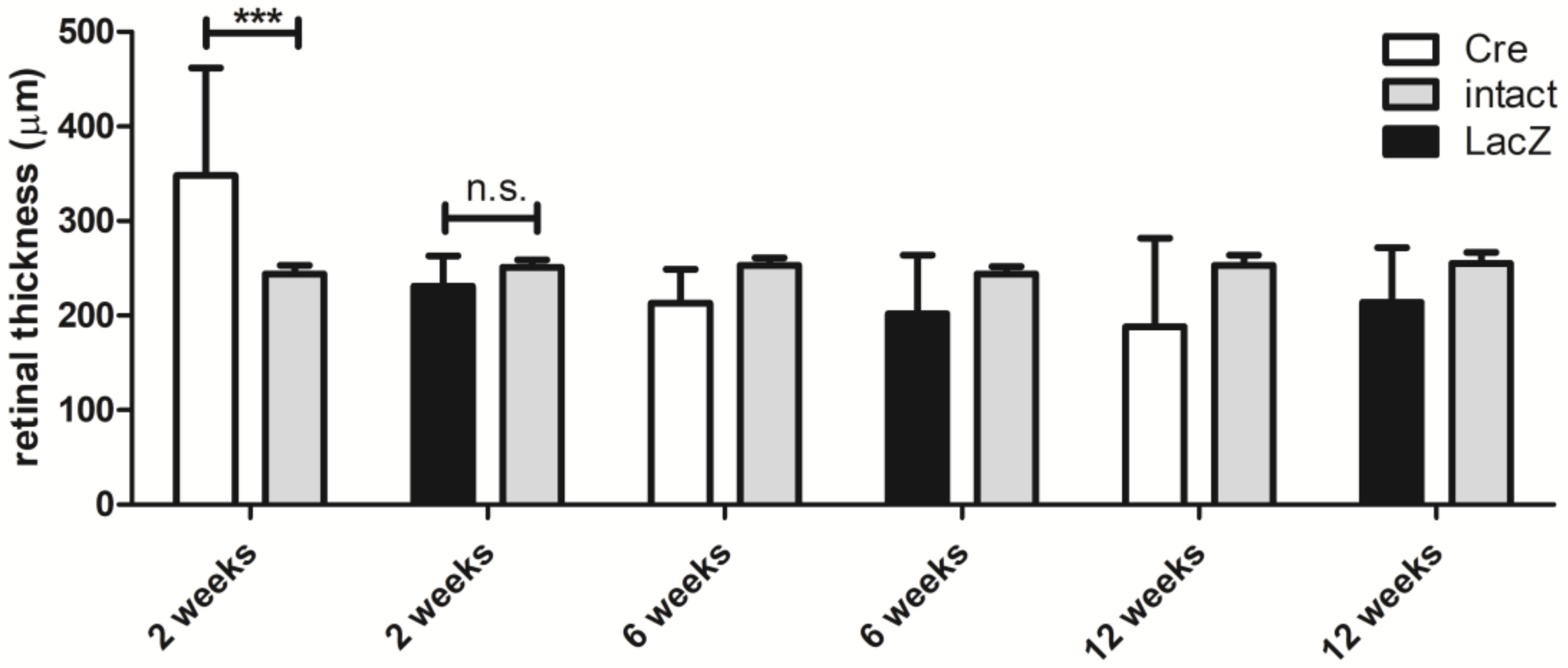

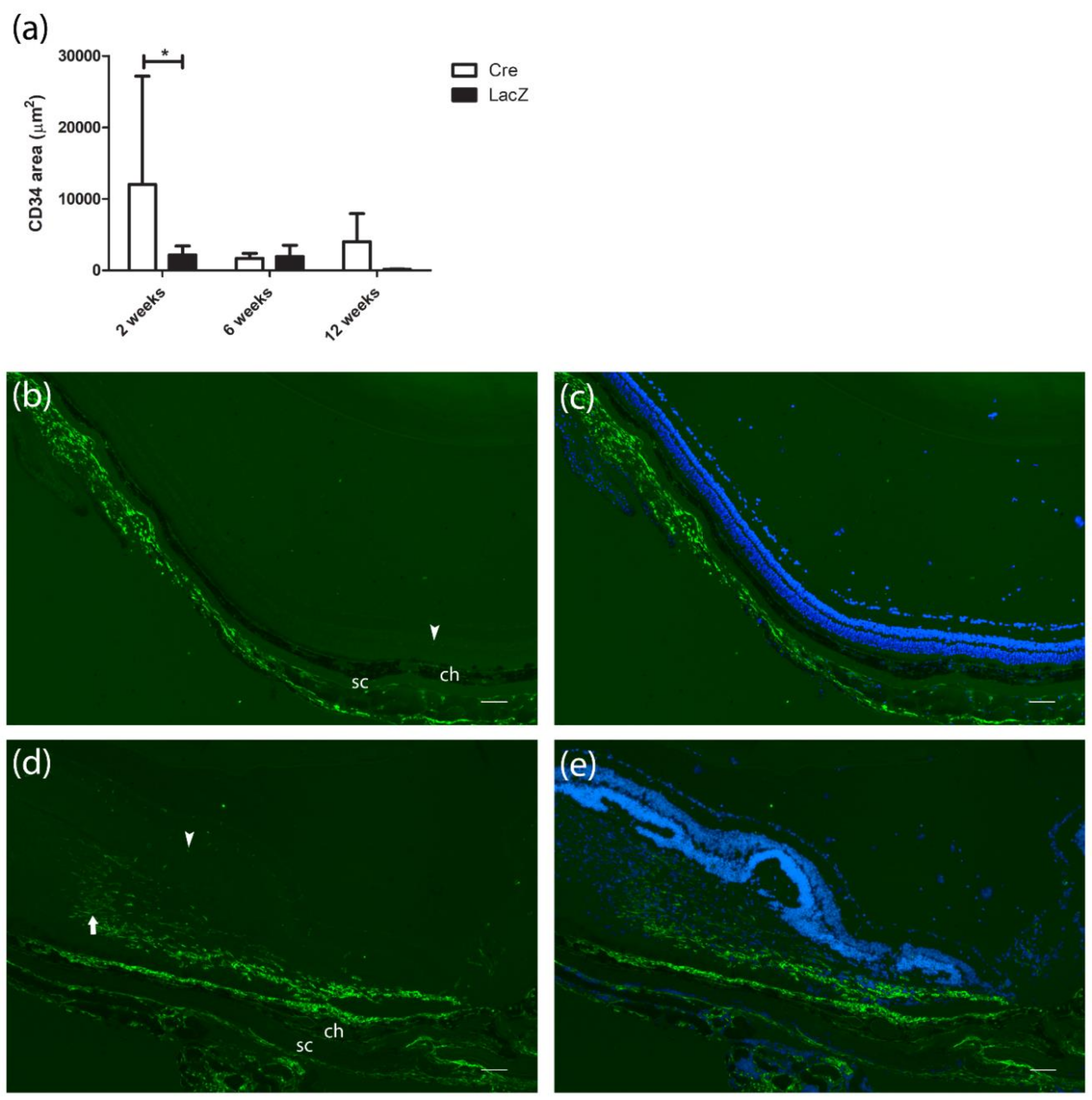


| Baseline | 2 Weeks | Baseline | 2 Weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cre | CRT | CRT | FA | CD34 Area | LacZ | CRT | CRT | FA | CD34 Area | ||
| mouse 1 | 241 | 255 | 2 | 106 | mouse 9 | 243 | 194 | 1 | 272 | ||
| mouse 2 | 250 | 585 | 4 | n/a | mouse 10 | 253 | 235 | 2 | 530 | ||
| mouse 3 | 261 | 380 | 4 | 10350 | mouse 11 | 248 | 185 | 3 | 603 | ||
| mouse 4 | 250 | 267 | 2 | 1067 | mouse 12 | 254 | 255 | 1 | 37 | ||
| mouse 5 | 240 | 310 | 4 | 41797 | mouse 13 | 252 | n/a | n/a | n/a | ||
| mouse 6 | 234 | 301 | 4 | 21304 | mouse 14 | 250 | 205 | 3 | 6776 | ||
| mouse 7 | 244 | 256 | 1 | 423 | mouse 15 | 270 | 272 | 1 | 171 | ||
| mouse 8 | 232 | 430 | 4 | 8952 | mouse 16 | 241 | 248 | 1 | 8741 | ||
| mouse 17 | 253 | 254 | 1 | 220 | |||||||
| mean ± SD | 244 ± 9.5 | 348 ± 114 | 3.1±1.2 | 12000 ± 15174 | mean ± SD | 251 ± 8.5 | 231 ± 32.2 | 1.6 ± 0.9 | 2169 ± 3495 |
| Characteristic | 2 Weeks | 6 Weeks | 12 Weeks |
|---|---|---|---|
| Subretinal swelling | ++ | ND | ND |
| Macrophage infiltration | ++ | + | + |
| Photoreceptor loss | +++ | +++ | +++ |
| Drusen-like deposit * | ND | + | + |
| GFAP activation | +++ | ++ | +++ |
| ONL atrophy or loss | ++ | +++ | ++ |
| INL atrophy | + | + | + |
| Infiltrating cells in the vitreous ¶ | 5/8 | 1/6 | 1/8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokki, E.; Karttunen, T.; Olsson, V.; Kinnunen, K.; Ylä-Herttuala, S. Human Vascular Endothelial Growth Factor A165 Expression Induces the Mouse Model of Neovascular Age-Related Macular Degeneration. Genes 2018, 9, 438. https://doi.org/10.3390/genes9090438
Kokki E, Karttunen T, Olsson V, Kinnunen K, Ylä-Herttuala S. Human Vascular Endothelial Growth Factor A165 Expression Induces the Mouse Model of Neovascular Age-Related Macular Degeneration. Genes. 2018; 9(9):438. https://doi.org/10.3390/genes9090438
Chicago/Turabian StyleKokki, Emmi, Tommi Karttunen, Venla Olsson, Kati Kinnunen, and Seppo Ylä-Herttuala. 2018. "Human Vascular Endothelial Growth Factor A165 Expression Induces the Mouse Model of Neovascular Age-Related Macular Degeneration" Genes 9, no. 9: 438. https://doi.org/10.3390/genes9090438
APA StyleKokki, E., Karttunen, T., Olsson, V., Kinnunen, K., & Ylä-Herttuala, S. (2018). Human Vascular Endothelial Growth Factor A165 Expression Induces the Mouse Model of Neovascular Age-Related Macular Degeneration. Genes, 9(9), 438. https://doi.org/10.3390/genes9090438






