Harnessing Rhizobia to Improve Heavy-Metal Phytoremediation by Legumes
Abstract
1. Introduction
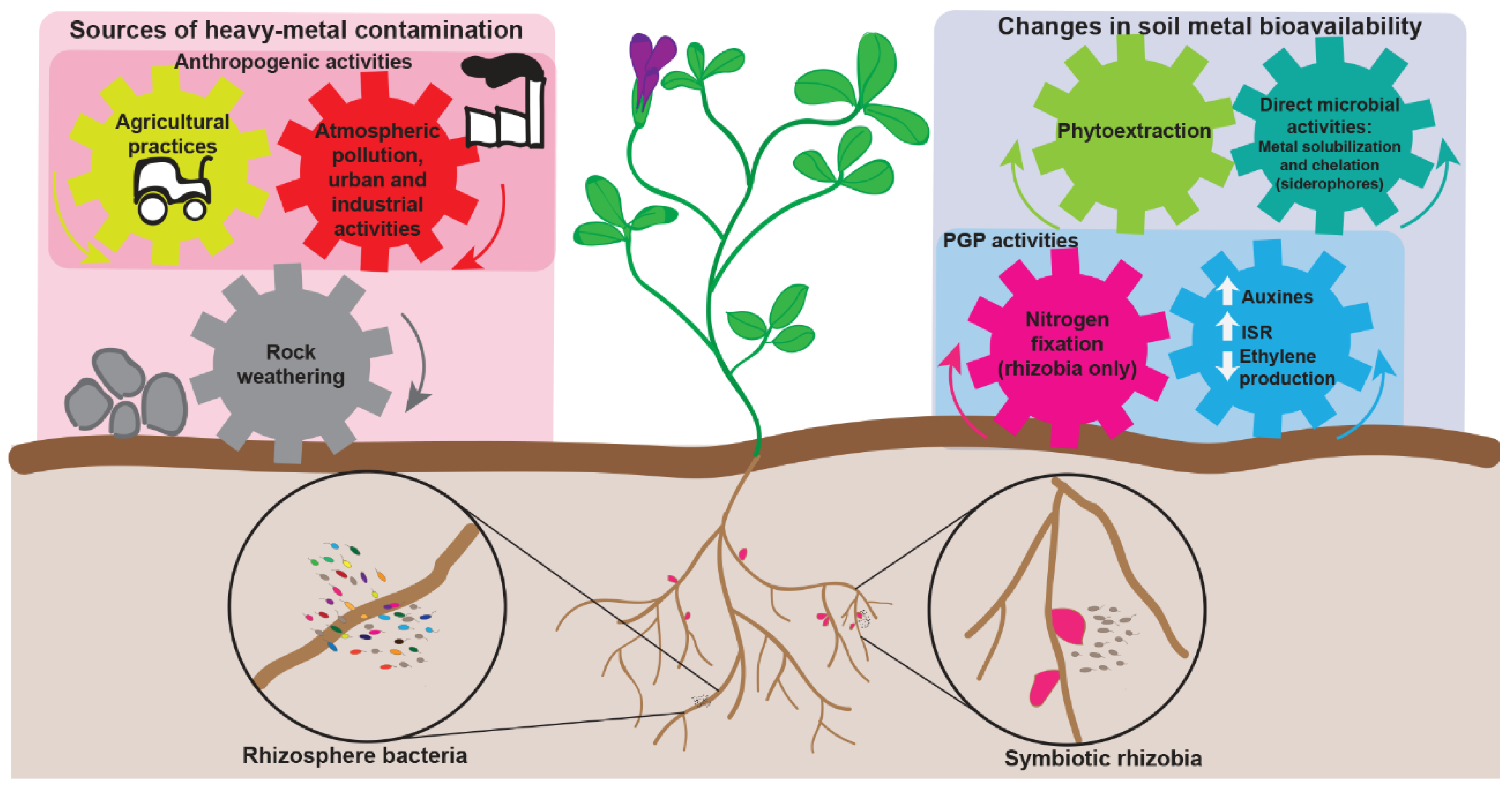
2. Legumes in Heavy-Metal Contaminated Areas
2.1. The Serpentine Vegetation: A Source of Legumes Evolved on Heavy-Metal Rich Soils
2.2. The Search for Heavy-Metal Tolerant Rhizobia and Their Use as Inoculants
3. Genetics and Genomics of Heavy-Metal Resistance in Symbiotic Rhizobia
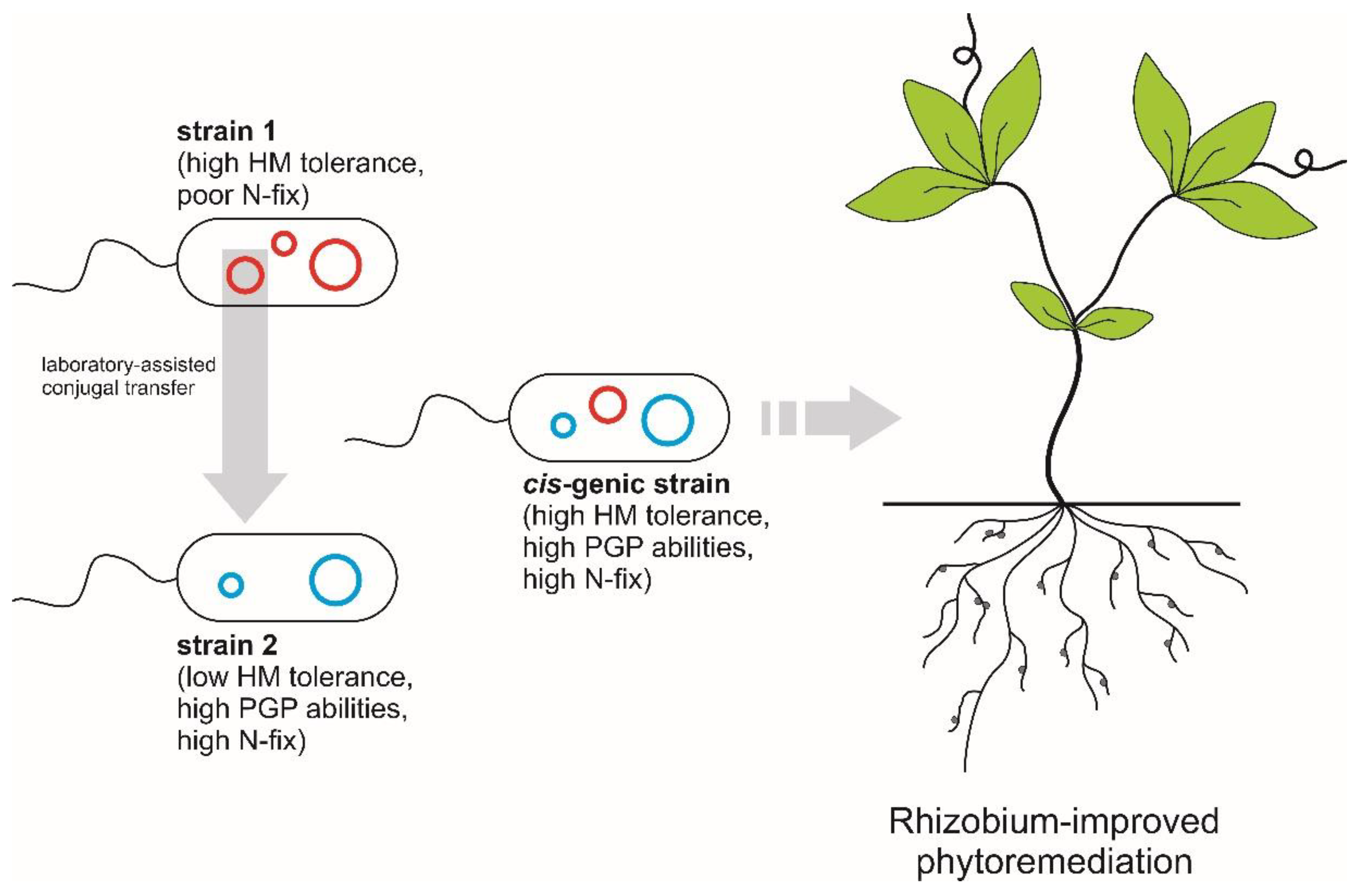
4. Genomic Manipulation Strategies for Improving Legume Phytoremediation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Müller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Münch, P.C.; Spaepen, S.; Remus-Emsermann, M.; et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364. [Google Scholar] [CrossRef] [PubMed]
- Mengoni, A.; Schat, H.; Vangronsveld, J. Plants as extreme environments? Ni-resistant bacteria and Ni-hyperaccumulators of serpentine flora. Plant Soil 2010, 331, 5–16. [Google Scholar] [CrossRef]
- Pini, F.; Frascella, A.; Santopolo, L.; Bazzicalupo, M.; Biondi, E.G.; Scotti, C.; Mengoni, A. Exploring the plant-associated bacterial communities in Medicago sativa L. BMC Microbiol. 2012, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J.I. Legume Nodulation: A Global Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 1444316397. [Google Scholar]
- Theis, K.R.; Dheilly, N.M.; Klassen, J.L.; Brucker, R.M.; Baines, J.F.; Bosch, T.C.G.; Cryan, J.F.; Gilbert, S.F.; Goodnight, C.J.; Lloyd, E.A.; et al. Getting the Hologenome Concept Right: An Eco-Evolutionary Framework for Hosts and Their Microbiomes. Msystems 2016, 1, e00028-16. [Google Scholar] [CrossRef] [PubMed]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014. [Google Scholar] [CrossRef]
- Lebrazi, S.; Fikri-Benbrahim, K. Rhizobium-Legume Symbioses: Heavy metal effects and principal approaches for bioremediation of contaminated soil. In Legumes for Soil Health and Sustainable Management; Springer: Heidelberg, Germany, 2018; pp. 205–233. ISBN 978-981-13-0252-7. [Google Scholar]
- Dary, M.; Chamber-Pérez, M.A.; Palomares, A.J.; Pajuelo, E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Glick, B.R. The Role of Plant Growth-Promoting Bacteria in Metal Phytoremediation. In Advanced in Microbial Physiology, 1st ed.; Elsevier Ltd.: New York, NY, USA, 2017; Volume 71, pp. 97–132. ISBN 0065-2911. [Google Scholar]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Weyens, N.; Lelie, D. Van Der; Taghavi, S.; Newman, L. Exploiting plant—Microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 2009, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.S.; Alvarez-Lopez, V.; Becerra-Castro, C.; Cabello-Conejo, M.; Prieto-Fernandez, A. Potential role of plant-associated bacteria in plant metal uptake and implications in phytotechnologies. In Advances in Botanical Research; Academic Press: London UK; New York, NY, USA, 2017; pp. 87–126. [Google Scholar]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization: A green approach to contaminant containment. Adv. Agron. 2011, 112, 145–204. [Google Scholar] [CrossRef]
- Mahieu, S.; Frérot, H.; Vidal, C.; Galiana, A.; Heulin, K.; Maure, L.; Brunel, B.; Lefèbvre, C.; Escarré, J.; Cleyet-Marel, J.-C. Anthyllis vulneraria/Mesorhizobium metallidurans, an efficient symbiotic nitrogen fixing association able to grow in mine tailings highly contaminated by Zn, Pb and Cd. Plant Soil 2011, 342, 405–417. [Google Scholar] [CrossRef]
- Gadd, G.M. Accumulation and transformation of metals by microorganisms. In Biotechnology: Special Processes; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 10, pp. 226–264. ISBN 978-3-52-762093-7. [Google Scholar]
- Mastretta, C.; Taghavi, S.; Van Der Lelie, D.; Mengoni, A.; Galardi, F.; Gonnelli, C.; Barac, T.; Boulet, J.; Weyens, N.; Vangronsveld, J. Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. Int. J. Phytoremediat. 2009, 11, 251–267. [Google Scholar] [CrossRef]
- Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 147, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Novo, L.A.B.; Castro, P.M.L.; Alvarenga, P.; da Silva, E.F. Plant Growth–Promoting Rhizobacteria-Assisted phytoremediation of mine soils. In Bio-Geotechnologies for Mine Site Rehabilitation; Elsevier: New York, NY, USA, 2018; pp. 281–295. ISBN 978-0-12-812987-6. [Google Scholar]
- Teng, Y.; Wang, X.; Li, L.; Li, Z.; Luo, Y. Rhizobia and their bio-partners as novel drivers for functional remediation in contaminated soils. Front. Plant Sci. 2015, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, A.; Bazzicalupo, M.; Mengoni, A. Exploiting nitrogen-fixing rhizobial symbionts genetic resources for improving phytoremediation of contaminated soils. In Enhancing Cleanup of Environmental Pollutants; Springer: Heidelberg, Germany, 2017; Volume 1, pp. 275–288. ISBN 978-3-31-955426-6. [Google Scholar]
- González-Guerrero, M.; Matthiadis, A.; Saez, Á.; Long, T. Fixating on metals: New insights into the role of metals in nodulation and symbiotic nitrogen fixation. Front. Plant Sci. 2014, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Pini, F.; Spini, G.; Galardini, M.; Bazzicalupo, M.; Benedetti, A.; Chiancianesi, M.; Florio, A.; Lagomarsino, A.; Migliore, M.; Mocali, S.; et al. Molecular phylogeny of the nickel-resistance gene nreB and functional role in the nickel sensitive symbiotic nitrogen fixing bacterium Sinorhizobium meliloti. Plant Soil 2013, 377, 189–201. [Google Scholar] [CrossRef]
- Lavres, J.; Castro Franco, G.; de Sousa Câmara, G.M. Soybean seed treatment with nickel improves biological nitrogen fixation and urease activity. Front. Environ. Sci. 2016, 4, 37. [Google Scholar] [CrossRef]
- Ureta, A.-C.; Imperial, J.; Ruiz-Argüeso, T.; Palacios, J.M. Rhizobium leguminosarum biovar viciae symbiotic hydrogenase activity and processing are limited by the level of nickel in agricultural soils. Appl. Environ. Microbiol. 2005, 71, 7603–7606. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Taghavi, S.; Xie, P.; Orbach, M.J.; Alwathnani, H.A.; Rensing, C.; Wei, G. Phytoremediation of heavy and transition metals aided by legume-rhizobia symbiosis. Int. J. Phytoremediat. 2014, 16, 179–202. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Zaidi, A.; Khan, M.S.; Oves, M. Heavy metal toxicity to symbiotic nitrogen-fixing microorganism and host legumes. In Toxicity of Heavy Metals to Legumes and Bioremediation; Springer: Heidelberg, Germany, 2012; pp. 29–44. ISBN 3709107296. [Google Scholar]
- Doyle, J.J.; Luckow, M.A. The rest of the iceberg. Legume diversity and evolution in a phylogenetic context. Plant Physiol. 2003, 131, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.D.; Chadwick, M.J. The Restoration of Land: The Ecology and Reclamation of Derelict and Degraded Land; University of California Press: Berkeley, CA, USA, 1980; ISBN 0520039610. [Google Scholar]
- Reeves, R.D.; van der Ent, A.; Baker, A.J.M. Global distribution and ecology of hyperaccumulator plants. In Agromining: Farming for Metals; Springer: Heidelberg, Germany, 2018; pp. 75–92. ISBN 978-3-319-61898-2. [Google Scholar]
- Pajuelo, E.; Rodríguez-Llorente, I.D.; Lafuente, A.; Caviedes, M.Á. Legume–rhizobium symbioses as a tool for bioremediation of heavy metal polluted soils. In Biomanagement of Metal-Contaminated Soils; Springer: Heidelberg, Germany, 2011; pp. 95–123. ISBN 978-94-007-1914-9. [Google Scholar]
- Brooks, R.R. Serpentine and Its Vegetation: A Multidisciplinary Approach; Croom Helm: London, UK, 1987; ISBN 0709950632. [Google Scholar]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary ecology of plant adaptation to serpentine soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Words, K. Metal Hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Harrison, S.; Rajakaruna, N. Serpentine: The Evolution and Ecology of a Model System; University of California Press: Berkeley, CA, USA, 2011; ISBN 0520268350. [Google Scholar]
- Mengoni, A.; Mocali, S.; Surico, G.; Tegli, S.; Fani, R. Fluctuation of endophytic bacteria and phytoplasmosis in elm trees. Microbiol. Res. 2003, 158, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.B.; Coleman, R.G.; Harrison, S.P.; Keeler-Wolfe, T. Serpentine Geoecology of Western North America: Geology, Soils, and Vegetation; OUP: Oxfort, UK, 2007; ISBN 019516508X. [Google Scholar]
- Pustahija, F.; Brown, S.C.; Bogunić, F.; Bašić, N.; Muratović, E.; Ollier, S.; Hidalgo, O.; Bourge, M.; Stevanović, V.; Siljak-Yakovlev, S. Small genomes dominate in plants growing on serpentine soils in West Balkans, an exhaustive study of 8 habitats covering 308 taxa. Plant Soil 2013, 373, 427–453. [Google Scholar] [CrossRef]
- Selvi, F. Diversity, geographic variation and conservation of the serpentine flora of Tuscany (Italy). Biodivers. Conserv. 2007, 16, 1423–1439. [Google Scholar] [CrossRef]
- Chaintreuil, C.; Rigault, F.; Moulin, L.; Jaffré, T.; Fardoux, J.; Giraud, E.; Dreyfus, B.; Bailly, X. Nickel resistance determinants in Bradyrhizobium strains from nodules of the endemic New Caledonia legume Serianthes calycina. Appl. Environ. Microbiol. 2007, 73, 8018–8022. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Narasimha, M.; Prasad, V.; Freitas, H.; Ae, N. Biotechnological applications of serpentine soil bacteria for phytoremediation of trace metals. Crit. Rev. Biotechnol. 2009, 29, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Friesen, M.L. Widespread fitness alignment in the legume—Rhizobium symbiosis. New Phytol. 2012, 194, 1096–1111. [Google Scholar] [CrossRef] [PubMed]
- Grison, C.M.; Jackson, S.; Merlot, S.; Dobson, A.; Grison, C. Rhizobium metallidurans sp. nov., a symbiotic heavy metal resistant bacterium isolated from the anthyllis vulneraria Zn-hyperaccumulator. Int. J. Syst. Evol. Microbiol. 2015, 65, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Liao, B.; Li, J.T.; Mengoni, A.; Hu, M.; Luo, W.C.; Shu, W.S. Contrasting patterns of genetic divergence in two sympatric pseudo-metallophytes: Rumex acetosa L. and Commelina communis L. BMC Evol. Biol. 2012, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, R.; Maynaud, G.; Le Quéré, A.; Vidal, C.; Klonowska, A.; Yashiro, E.; Cleyet-Marel, J.-C.; Brunel, B. Ancient heavy metal contamination in soils as a driver of tolerant Anthyllis vulneraria rhizobial communities. Appl. Environ. Microbiol. 2016, 83, e01735-16. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; Chantreuil, C.; Berge, O.; Mauré, L.; Escarré, J.; Béna, G.; Brunel, B.; Cleyet-Marel, J.C. Mesorhizobium metallidurans sp. nov., a metal-resistant symbiont of Anthyllis vulneraria growing on metallicolous soil in Languedoc, France. Int. J. Syst. Evol. Microbiol. 2009, 59, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Sujkowska-Rybkowska, M.; Ważny, R. Metal resistant rhizobia and ultrastructure of Anthyllis vulneraria nodules from zinc and lead contaminated tailing in Poland. Int. J. Phytoremediat. 2018, 20, 709–720. [Google Scholar] [CrossRef] [PubMed]
- El Aafi, N.; Saidi, N.; Maltouf, A.F.; Perez-Palacios, P.; Dary, M.; Brhada, F.; Pajuelo, E. Prospecting metal-tolerant rhizobia for phytoremediation of mining soils from Morocco using Anthyllis vulneraria L. Environ. Sci. Pollut. Res. 2015, 22, 4500–4512. [Google Scholar] [CrossRef] [PubMed]
- Maynaud, G.; Willems, A.; Soussou, S.; Vidal, C.; Mauré, L.; Moulin, L.; Cleyet-Marel, J.-C.; Brunel, B. Molecular and phenotypic characterization of strains nodulating Anthyllis vulneraria in mine tailings, and proposal of Aminobacter anthyllidis sp. nov., the first definition of Aminobacter as legume-nodulating bacteria. Syst. Appl. Microbiol. 2012, 35, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.B.; Heeb, P.; Gris, C.; Timmers, T.; Batut, J.; Masson-boivin, C. Experimental evolution of a plant pathogen into a legume symbiont. PLoS Biol. 2010, 8. [Google Scholar] [CrossRef]
- Vamerali, T.; Bandiera, M.; Mosca, G. Field crops for phytoremediation of metal-contaminated land. A review. Environ. Chem. Lett. 2010, 8, 1–17. [Google Scholar] [CrossRef]
- Delgadillo, J.; Lafuente, A.; Doukkali, B.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Caviedes, M.A.; Pajuelo, E.; Rodríguez-Llorente, I.D. Improving legume nodulation and Cu rhizostabilization using a genetically modified rhizobia. Environ. Technol. 2015, 36, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Glick, B.R.; Duan, J.; Ding, S.; Tian, J.; McConkey, B.J.; Wei, G. Effects of 1-aminocyclopropane-1-carboxylate (ACC) deaminase-overproducing Sinorhizobium meliloti on plant growth and copper tolerance of Medicago lupulina. Plant Soil 2015, 70, 5891–5897. [Google Scholar] [CrossRef]
- Ghnaya, T.; Mnassri, M.; Ghabriche, R.; Wali, M.; Poschenrieder, C.; Lutts, S.; Abdelly, C. Nodulation by Sinorhizobium meliloti originated from a mining soil alleviates Cd toxicity and increases Cd-phytoextraction in Medicago sativa L. Front. Plant Sci. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zribi, K.; Nouairi, I.; Slama, I.; Talbi-Zribi, O.; Mhadhbi, H. Medicago sativa—Sinorhizobium meliloti Symbiosis Promotes the Bioaccumulation of Zinc in Nodulated Roots. Int. J. Phytoremediat. 2015, 17, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Saadani, O.; Fatnassi, I.C.; Chiboub, M.; Abdelkrim, S.; Barhoumi, F.; Jebara, M.; Jebara, S.H. In situ phytostabilisation capacity of three legumes and their associated Plant Growth Promoting Bacteria (PGPBs) in mine tailings of northern Tunisia. Ecotoxicol. Environ. Saf. 2016, 130, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Bianucci, E.; Godoy, A.; Furlan, A.; Peralta, J.M.; Hernández, L.E.; Carpena-Ruiz, R.O.; Castro, S. Arsenic toxicity in soybean alleviated by a symbiotic species of Bradyrhizobium. Symbiosis 2018, 74, 167–176. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.Q.; Yan, X.W.; Wei, G.H.; Zhang, J.H.; Fang, L.C. Rhizobium inoculation enhances copper tolerance by affecting copper uptake and regulating the ascorbate-glutathione cycle and phytochelatin biosynthesis-related gene expression in Medicago sativa seedlings. Ecotoxicol. Environ. Saf. 2018, 162, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Zribi, K.; Djébali, N.; Mrabet, M.; Khayat, N.; Smaoui, A.; Mlayah, A.; Aouani, M.E. Physiological responses to cadmium, copper, lead, and zinc of Sinorhizobium sp. strains nodulating Medicago sativa grown in Tunisian mining soils. Ann. Microbiol. 2012, 62, 1181–1188. [Google Scholar] [CrossRef]
- Fan, M.; Xiao, X.; Guo, Y.; Zhang, J.; Wang, E.; Chen, W.; Lin, Y.; Wei, G. Enhanced phytoremdiation of Robinia pseudoacacia in heavy metal-contaminated soils with rhizobia and the associated bacterial community structure and function. Chemosphere 2018, 197, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Mergeay, M. Genomic context of metal response genes in Cupriavidus metallidurans with a focus on strain CH34. In Metal Response in Cupriavidus Metallidurans; Springer: Heidelberg, Germany, 2015; pp. 21–44. ISBN 978-3-319-20594-6. [Google Scholar]
- Rozycki, T. Von; Nies, Æ.D.H.; Alcaligenes, W.Á.; Ch, Á.Á.H. Cupriavidus metallidurans: Evolution of a metal-resistant bacterium. Anton. Leeuwenhoek 2008, 96, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Sanz, L.; Prieto, R.I.; Imperial, J.; Palacios, J.M.; Brito, B. Functional and expression analysis of the metal-inducible dmeRF system from Rhizobium leguminosarum bv. viciae. Appl. Environ. Microbiol. 2013, 79, 6414–6422. [Google Scholar] [CrossRef] [PubMed]
- Haney, C.J.; Grass, G.; Franke, S.; Rensing, C. New developments in the understanding of the cation diffusion facilitator family. J. Ind. Microbiol. Biotechnol. 2005, 32, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Zielazinski, E.L.; González-Guerrero, M.; Subramanian, P.; Stemmler, T.L.; Argüello, J.M.; Rosenzweig, A.C. Sinorhizobium meliloti Nia is a P1B-5-ATPase expressed in the nodule during plant symbiosis and is involved in Ni and Fe transport. Metallomics 2013, 5, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, M.; Wei, G. An omp gene enhances cell tolerance of Cu(II) in Sinorhizobium meliloti CCNWSX0020. World J. Microbiol. Biotechnol. 2013, 29, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, Z.; Liang, J.; Wei, Y.; Rensing, C.; Wei, G. Zinc resistance mechanisms of P 1B-type ATPases in Sinorhizobium meliloti CCNWSX0020. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Lu, M.; Jiao, S.; Gao, E.; Song, X.; Li, Z.; Hao, X.; Rensing, C.; Wei, G. Transcriptome response to heavy metals in Sinorhizobium meliloti CCNWSX0020 reveals new metal resistance determinants that also promote bioremediation by Medicago lupulina in metal contaminated soil. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C. CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 2001, 286, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Franke, S.; Grass, G.; Rensing, C.; Nies, D.H. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J. Bacteriol. 2003, 185, 3804–3812. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Su, C.-C.; Lei, H.-T.; Bolla, J.R.; Do, S.V.; Yu, E.W. Structure and mechanism of the tripartite CusCBA heavy-metal efflux complex. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Kahn, D.; David, M.; Domergue, O.; Daveran, M.L.; Ghai, J.; Hirsch, P.R.; Batut, J. Rhizobium meliloti fixGHI sequence predicts involvement of a specific cation pump in symbiotic nitrogen fixation. J. Bacteriol. 1989, 171, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Batut, J.; Terzaghi, B.; Gherardi, M.; Huguet, M.; Terzaghi, E.; Garnerone, A.M.; Boistard, P.; Huguet, T. Localization of a symbiotic fix region on Rhizobium meliloti pSym megaplasmid more than 200 kilobases from the nod-nif region. Mol. Gen. Genet. MGG 1985, 199, 232–239. [Google Scholar] [CrossRef]
- Romaniuk, K.; Dziewit, L.; Decewicz, P.; Mielnicki, S.; Radlinska, M.; Drewniak, L. Molecular characterization of the pSinB plasmid of the arsenite oxidizing, metallotolerant Sinorhizobium sp. M14—Insight into the heavy metal resistome of sinorhizobial extrachromosomal replicons. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [PubMed]
- Brokx, S.J.; Rothery, R.A.; Zhang, G.; Ng, D.P.; Weiner, J.H. Characterization of an Escherichia coli sulfite oxidase homologue reveals the role of a conserved active site cysteine in assembly and function. Biochemistry 2005, 44, 10339–10348. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.J.; Kappler, U. Sulfite oxidation in Sinorhizobium meliloti. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1787, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.S.; Chang, P.L.; Conow, C.A.; Dunham, J.P.; Friesen, M.L. Association mapping reveals novel serpentine adaptation gene clusters in a population of symbiotic Mesorhizobium. ISME J. 2017, 11, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, Z.; Hao, X.; Rensing, C.; Wei, G. Genes conferring copper resistance in Sinorhizobium meliloti CCNWSX0020 also promote the growth of Medicago lupulina in copper-contaminated soil. Appl. Environ. Microbiol. 2014, 80, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Xie, P.; Zhu, Y.-G.; Taghavi, S.; Wei, G.; Rensing, C. Copper tolerance mechanisms of Mesorhizobium amorphae and its role in aiding phytostabilization by Robinia pseudoacacia in copper contaminated soil. Environ. Sci. Technol. 2015, 49, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Maynaud, G.; Brunel, B.; Yashiro, E.; Mergeay, M.; Cleyet-Marel, J.C.; Le Quéré, A. CadA of Mesorhizobium metallidurans isolated from a zinc-rich mining soil is a PIB-2-type ATPase involved in cadmium and zinc resistance. Res. Microbiol. 2014, 165, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Sanz, L.; Brito, B.; Palacios, J. Analysis of metal tolerance in Rhizobium leguminosarum strains isolated from an ultramafic soil. FEMS Microbiol. Lett. 2018, 365, fny010. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Palacios, P.; Romero-Aguilar, A.; Delgadillo, J.; Doukkali, B.; Caviedes, M.A.; Rodríguez-Llorente, I.D.; Pajuelo, E. Double genetically modified symbiotic system for improved Cu phytostabilization in legume roots. Environ. Sci. Pollut. Res. 2017, 24, 14910–14923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Y.; Cao, T.; Chen, J.; Rosen, B.P.; Zhao, F.-J. Arsenic methylation by a genetically engineered Rhizobium-legume symbiont. Plant Soil 2017, 416, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Mohamad, O.A.; Deng, Z.; Liu, X.; Glick, B.R.; Wei, G. Rhizobial symbiosis effect on the growth, metal uptake, and antioxidant responses of Medicago lupulina under copper stress. Environ. Sci. Pollut. Res. 2015, 22, 12479–12489. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Valls, M.; De Lorenzo, V. Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol. Rev. 2002, 26, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Nies, D.H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 313–339. [Google Scholar] [CrossRef]
- Drewniak, L.; Dziewit, L.; Ciezkowska, M.; Gawor, J.; Gromadka, R.; Sklodowska, A. Structural and functional genomics of plasmid pSinA of Sinorhizobium sp. M14 encoding genes for the arsenite oxidation and arsenic resistance. J. Biotechnol. 2013, 164, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Drewniak, L.; Matlakowska, R.; Sklodowska, A. Arsenite and arsenate metabolism of Sinorhizobium sp. M14 living in the extreme environment of the Zloty Stok gold mine. Geomicrobiol. J. 2008, 25, 363–370. [Google Scholar] [CrossRef]
- DiCenzo, G.C.; Finan, T.M. The divided bacterial genome: Structure, function, and evolution. Microbiol. Mol. Biol. Rev. 2017, 81, e00019-17. [Google Scholar] [CrossRef] [PubMed]
- DiCenzo, G.C.; Benedict, A.B.; Fondi, M.; Walker, G.C.; Finan, T.M.; Mengoni, A.; Griffitts, J.S. Robustness encoded across essential and accessory replicons of the ecologically versatile bacterium Sinorhizobium meliloti. PLoS Genet. 2018, 14, e1007357. [Google Scholar] [CrossRef] [PubMed]
- DiCenzo, G.C.; Wellappili, D.; Golding, G.B.; Finan, T.M. Inter-replicon gene flow contributes to transcriptional integration in the Sinorhizobium meliloti multipartite genome. G3 Genes Genomes Genet. 2018, 8, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Landeta, C.; Dávalos, A.; Cevallos, M.Á.; Geiger, O.; Brom, S.; Romero, D. Plasmids with a chromosome-like role in Rhizobia. J. Bacteriol. 2011, 193, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- DiCenzo, G.C.; Checcucci, A.; Bazzicalupo, M.; Mengoni, A.; Viti, C.; Dziewit, L.; Finan, T.M.; Galardini, M.; Fondi, M. Metabolic modelling reveals the specialization of secondary replicons for niche adaptation in Sinorhizobium meliloti. Nat. Commun. 2016, 7, 12219. [Google Scholar] [CrossRef] [PubMed]
- Galardini, M.; Brilli, M.; Spini, G.; Rossi, M.; Roncaglia, B.; Bani, A.; Chiancianesi, M.; Moretto, M.; Engelen, K.; Bacci, G.; et al. Evolution of intra-specific regulatory networks in a multipartite bacterial genome. PLoS Comput. Biol. 2015, 11, e1004478. [Google Scholar] [CrossRef] [PubMed]
- Galardini, M.; Pini, F.; Bazzicalupo, M.; Biondi, E.G.; Mengoni, A. Replicon-dependent bacterial genome evolution: The case of Sinorhizobium meliloti. Mol. Biol. 2013, 5, 542–558. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, A.; diCenzo, G.C.; Ghini, V.; Bazzicalupo, M.; Beker, A.; Decorosi, F.; Dohlemann, J.; Fagorzi, C.; Finan, T.M.; Fondi, M.; et al. Creation and multi-omics characterization of a genomically hybrid strain in the nitrogen-fixing symbiotic bacterium Sinorhizobium meliloti. bioRxiv 2018. [Google Scholar] [CrossRef]
- Checcucci, A.; DiCenzo, G.C.; Bazzicalupo, M.; Mengoni, A. Trade, diplomacy, and warfare: The quest for elite rhizobia inoculant strains. Front. Microbiol. 2017, 8, 2207. [Google Scholar] [CrossRef] [PubMed]
- Van Opijnen, T.; Camilli, A. Transposon insertion sequencing: A new tool for systems-level analysis of microorganisms. Nat. Rev. Microbiol. 2013, 11, 435–442. [Google Scholar] [CrossRef] [PubMed]
| Legume Species | Heavy-Metals in the Soil | Rhizobium Inoculant | Co-Inoculation with Other PGPR? | Evidence for Stimulation of Rhizosphere Microbiota | Type of Study | Effect | Reference |
|---|---|---|---|---|---|---|---|
| Glycine max | As | Bradyrhizobium sp. Per 3.61 | No | NA | Lab scale (pot) | Reduce translocation factor | [59] |
| Lupinus luteus | Cu, Cd, Pb | Bradyrhizobium sp. 750 | Yes | NA | In situ | Increased metal accumulation in root | [10] |
| Medicago lupulina | Cu | Sinorhizobium meliloti CCNWSX0020 | No | NA | In vitro (pot) | Increased plant growth and copper tolerance | [55] |
| Medicago sativa | Cu | Sinorhizobium meliloti CCNWSX0020 | No | NA | In vitro | Increased tolerance of seedlings | [60] |
| Medicago sativa | Cd | Sinorhizobium meliloti (from contaminated soil [61]) | No | NA | Lab scale (pot) | Increased Cd phytoextraction | [56] |
| Medicago sativa | Zn | Sinorhizobium meliloti (from contaminated soil [61]) | No | NA | Lab scale (pot with sterile sand) | Increased Zn accumulation in root | [57] |
| Medicago truncatula | Cu | Sinorhizobium medicae MA11 (genetically modified with copAB genes) | No | NA | In vitro | Increased metal accumulation in root | [54] |
| Robinia pseudoacacia | Cd, Zn, Pb | Mesorhizobium loti HZ76 | No | Yes | Lab scale (pot) | Increased growth of the plant | [62] |
| Sulla conoraria | Cu, Zn, Pb | Rhizobium sullae | Yes | NA | In situ | Increased soil Zn stabilization | [58] |
| Vicia faba | Cu, Zn, Pb | Rhizobium sp. CCNWSX0481 | Yes | NA | In situ | Increased soil Cu stabilization | [58] |
| Strain | Host Plant | Isolation Site | Method of Identification | Gene(s) | Metal(s) Tolerance | Reference |
|---|---|---|---|---|---|---|
| Bradhyrhizobium spp. | Serianthes calycina | Serpentine (New Caledonia) | PCR amplification, site-directed mutagenesis | cnr/nre systems | Co, Ni | [42] |
| Mesorhizobium spp. | Acmispon wrangelianus | Serpentine (California) | Association mapping | Various | Ni | [79] |
| Mesorhizobium metallidurans | Antyllis vulneraria | Zinc mine (France) | Cosmid library | cadA (PIB-2-type ATPase) | Zn, Cd | [82] |
| Sinorhizobium meliloti 1021 | Medicago sativa | Laboratory strain | Site-directed gene deletion | nreB (SMa1641) | Ni | [25] |
| Sinorhizobium meliloti 1021 | Medicago sativa | Laboratory strain | Tn5 insertion, biochemical characterization | SMa1163 (P1B-5-ATPase) | Ni, Fe | [67] |
| Sinorhizobium meliloti CCNWSX0020 | Medicago lupulina | Mine tailings (China) | Site-directed gene deletion and transcriptomics | P1B-type ATPases and others | Cu, Zn | [69,70] |
| Rhizobium leguminosarum bv. viciae UPM1137 | Pisum sativum | Serpentine (Italy) | Transposon mutagenesis | 14 loci (gene annotation corresponds to Rlv 3841 genome): RL2862, RL2436, RL2322, pRL110066, RL1351, RL4539, pRL90287, RL4188, RL2793, RL2100, RL0615, RL1589, pRL110071, RL1553 | Ni, Co | [83] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagorzi, C.; Checcucci, A.; DiCenzo, G.C.; Debiec-Andrzejewska, K.; Dziewit, L.; Pini, F.; Mengoni, A. Harnessing Rhizobia to Improve Heavy-Metal Phytoremediation by Legumes. Genes 2018, 9, 542. https://doi.org/10.3390/genes9110542
Fagorzi C, Checcucci A, DiCenzo GC, Debiec-Andrzejewska K, Dziewit L, Pini F, Mengoni A. Harnessing Rhizobia to Improve Heavy-Metal Phytoremediation by Legumes. Genes. 2018; 9(11):542. https://doi.org/10.3390/genes9110542
Chicago/Turabian StyleFagorzi, Camilla, Alice Checcucci, George C. DiCenzo, Klaudia Debiec-Andrzejewska, Lukasz Dziewit, Francesco Pini, and Alessio Mengoni. 2018. "Harnessing Rhizobia to Improve Heavy-Metal Phytoremediation by Legumes" Genes 9, no. 11: 542. https://doi.org/10.3390/genes9110542
APA StyleFagorzi, C., Checcucci, A., DiCenzo, G. C., Debiec-Andrzejewska, K., Dziewit, L., Pini, F., & Mengoni, A. (2018). Harnessing Rhizobia to Improve Heavy-Metal Phytoremediation by Legumes. Genes, 9(11), 542. https://doi.org/10.3390/genes9110542







