Exploring Interactions between the Gut Microbiota and Social Behavior through Nutrition
Abstract
1. Introduction
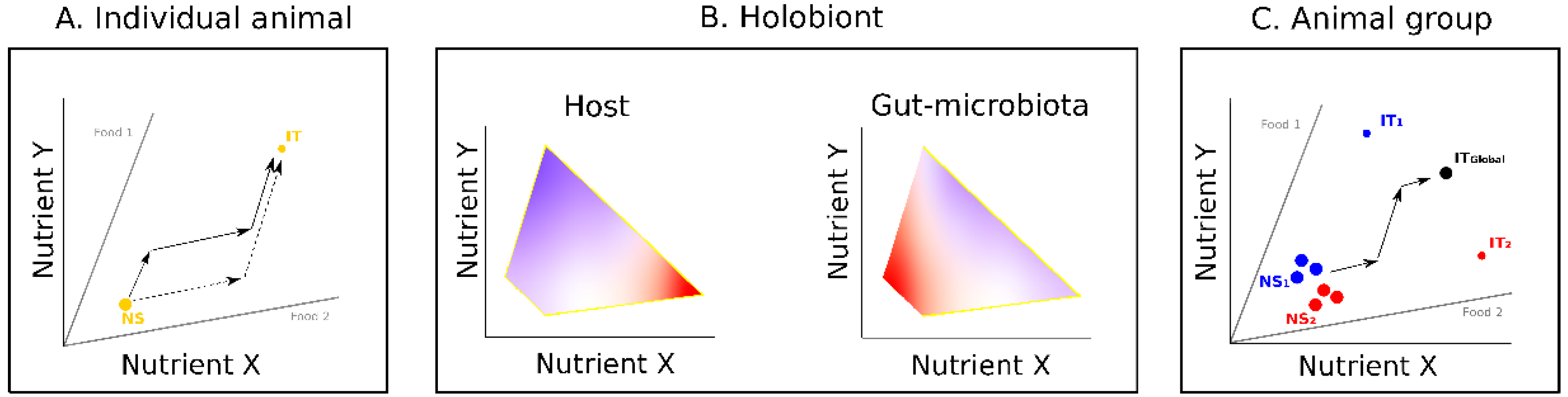
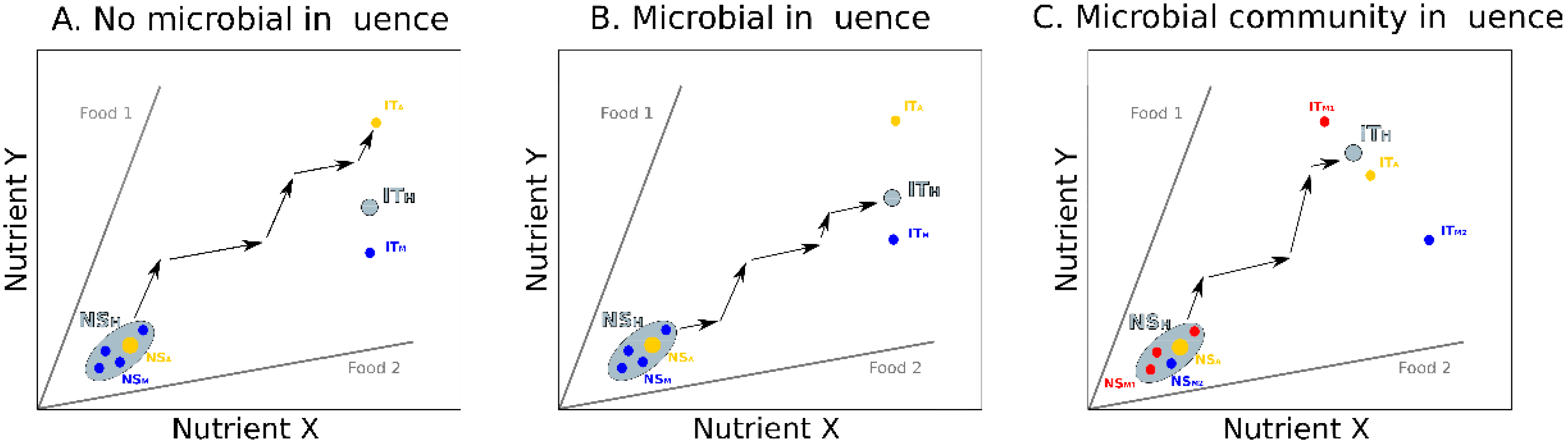
2. Influence of Gut Microbiota on Host Nutrition
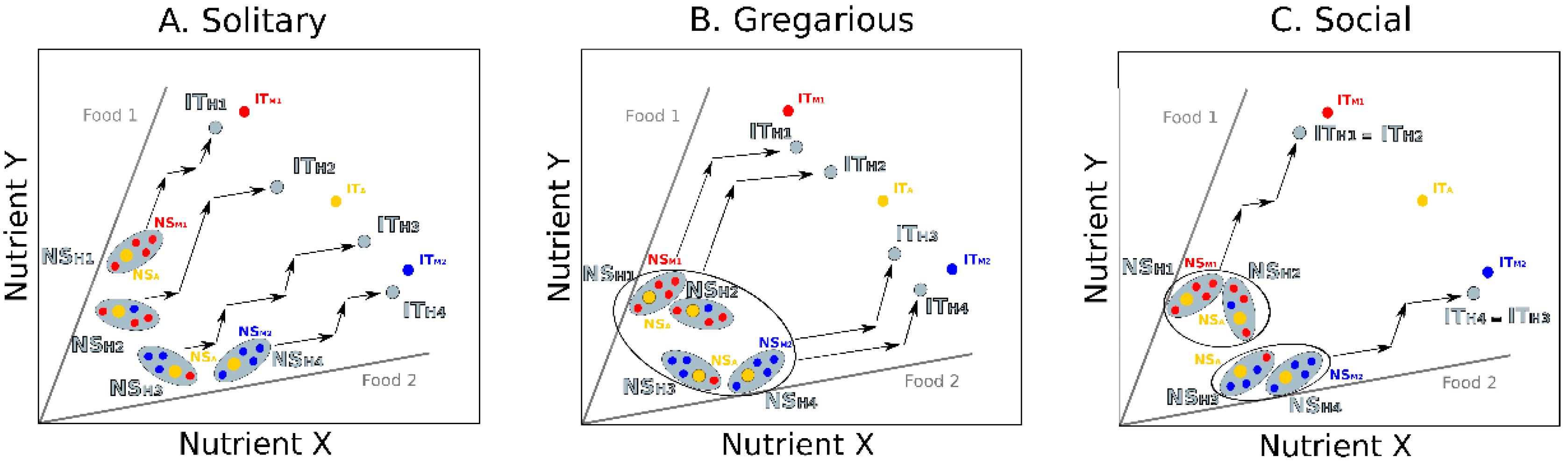
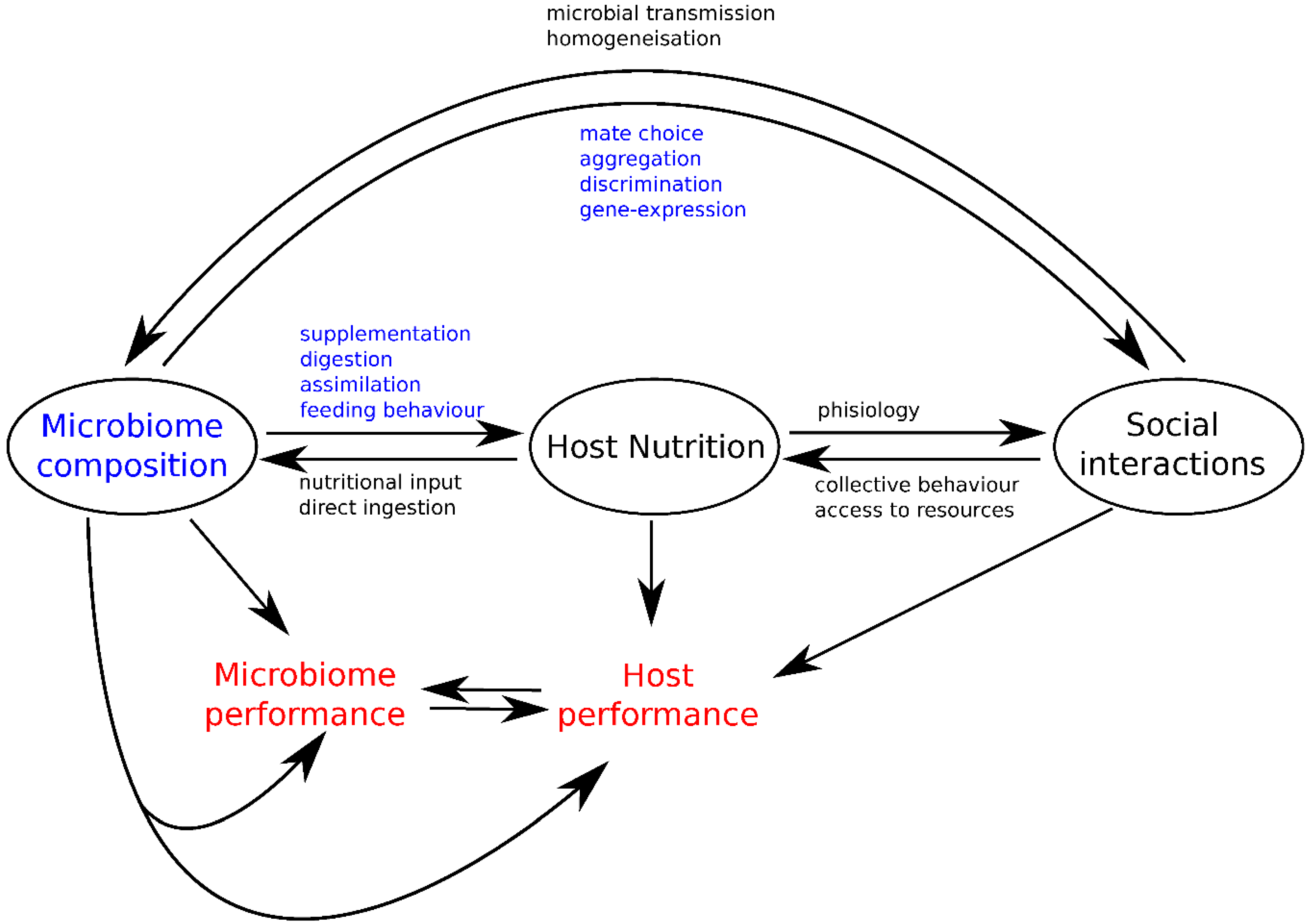
3. Integrating Gut Microbes, Host Nutrition and Social Behavior
4. Conclusions
Funding
Conflicts of Interest
References
- Margulis, L.; Fester, R. Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; MIT Press: Cambridge, MA, USA, 1991; ISBN 0-262-13269-9. [Google Scholar]
- Rohwer, F.; Seguritan, V.; Azam, F.; Knowlton, N. Diversity and distribution of coral-associated bacteria. Mar. Ecol. Prog. Ser. 2002, 243, 1–10. [Google Scholar] [CrossRef]
- Theis, K.R.; Dheilly, N.M.; Klassen, J.L.; Brucker, R.M.; Baines, J.F.; Bosch, T.C.; Cryan, J.F.; Gilbert, S.F.; Goodnight, C.J.; Lloyd, E.A. Getting the hologenome concept right: An eco-evolutionary framework for hosts and their microbiomes. Msystems 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Ezenwa, V.O.; Gerardo, N.M.; Inouye, D.W.; Medina, M.; Xavier, J.B. Animal behavior and the microbiome. Science 2012, 338, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Venu, I.; Durisko, Z.; Xu, J.; Dukas, R. Social attraction mediated by fruit flies’ microbiome. J. Exp. Biol. 2014, 217, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Wada-Katsumata, A.; Zurek, L.; Nalyanya, G.; Roelofs, W.L.; Zhang, A.; Schal, C. Gut bacteria mediate aggregation in the German cockroach. Proc. Natl. Acad. Sci. USA 2015, 112, 15678–15683. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Segal, D.; Ringo, J.M.; Hefetz, A.; Zilber-Rosenberg, I.; Rosenberg, E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2010, 107, 20051–20056. [Google Scholar] [CrossRef] [PubMed]
- Theis, K.R.; Venkataraman, A.; Dycus, J.A.; Koonter, K.D.; Schmitt-Matzen, E.N.; Wagner, A.P.; Holekamp, K.E.; Schmidt, T.M. Symbiotic bacteria appear to mediate hyena social odors. Proc. Natl. Acad. Sci. USA 2013, 110, 19832–19837. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K. Nestmate recognition mediated by intestinal bacteria in a termite, Reticulitermes speratus. Oikos 2001, 92, 20–26. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Münger, E.; Montiel-Castro, A.J.; Langhans, W.; Pacheco-López, G. Reciprocal Interactions Between Gut Microbiota and Host Social Behavior. Front. Integr. Neurosci. 2018, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Lax, S.; Smith, D.P.; Hampton-Marcell, J.; Owens, S.M.; Handley, K.M.; Scott, N.M.; Gibbons, S.M.; Larsen, P.; Shogan, B.D.; Weiss, S. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 2014, 345, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Ponton, F.; Wilson, K.; Cotter, S.C.; Raubenheimer, D.; Simpson, S.J. Nutritional immunology: A multi-dimensional approach. PLoS Pathog. 2011, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Feldhaar, H.; Straka, J.; Krischke, M.; Berthold, K.; Stoll, S.; Mueller, M.J.; Gross, R. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol. 2007, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Stengel, A.; Dearing, M.D. Inoculation of tannin-degrading bacteria into novel hosts increases performance on tannin-rich diets. Environ. Microbiol. 2016, 18, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Aphids and Their Symbiotic Bacteria Buchnera. Annu. Rev. Entomol. 1998, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Nalepa, C.A.; Bignell, D.E.; Bandi, C. Detritivory, coprophagy, and the evolution of digestive mutualisms in Dictyoptera. Insectes Sociaux 2001, 48, 194–201. [Google Scholar] [CrossRef]
- Wong, A.C.N.; Wang, Q.P.; Morimoto, J.; Senior, A.M.; Lihoreau, M.; Neely, G.G.; Simpson, S.J.; Ponton, F. Gut Microbiota Modifies Olfactory-Guided Microbial Preferences and Foraging Decisions in Drosophila. Curr. Biol. 2017, 27, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.A.; Lang, J.M.; Bhatnagar, S.; Eisen, J.A.; Kopp, A. Bacterial communities of diverse Drosophila species: Ecological context of a host-microbe model system. PLoS Genet. 2011, 7, e1002272. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, Y.; Koren, O.; Spor, A.; LeDuc, C.; Gutman, R.; Stombaugh, J.; Knight, R.; Ley, R.E.; Leibel, R.L. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity 2012, 20, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.J.; Chew, Y.V.; Colakoglu, F.; Cliff, J.B.; Klaassens, E.; Read, M.N.; Solon-Biet, S.M.; McMahon, A.C.; Cogger, V.C.; Ruohonen, K.; et al. Diet-Microbiome Interactions in Health Are Controlled by Intestinal Nitrogen Source Constraints. Cell Metab. 2017, 25, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.; Ridaura, V.; Faith, J. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Raubenheimer, D. A multi-level analysis of feeding behaviour: The geometry of nutritional decisions. Phil. Trans. R. Soc. Lond. B 1993, 342, 381–402. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity; Princeton University Press: Princeton, NJ, USA, 2012; ISBN 1-4008-4280-8. [Google Scholar]
- Wong, A.C.N.; Holmes, A.; Ponton, F.; Lihoreau, M.; Wilson, K.; Raubenheimer, D.; Simpson, S.J. Behavioral microbiomics: A multi-dimensional approach to microbial influence on behavior. Front. Microbiol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Steffan, S.A.; Chikaraishi, Y.; Currie, C.R.; Horn, H.; Gaines-Day, H.R.; Pauli, J.N.; Zalapa, J.E.; Ohkouchi, N. Microbes are trophic analogs of animals. Proc. Natl. Acad. Sci. USA 2015, 112, 15119–15124. [Google Scholar] [CrossRef] [PubMed]
- Lihoreau, M.; Buhl, J.; Charleston, M.A.; Sword, G.A.; Raubenheimer, D.; Simpson, S.J. Nutritional ecology beyond the individual: A conceptual framework for integrating nutrition and social interactions. Ecol. Lett. 2015, 18, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Lihoreau, M.; Buhl, J.; Charleston, M.A.; Sword, G.A.; Raubenheimer, D.; Simpson, S.J. Modelling nutrition across organizational levels: From individuals to superorganisms. J. Insect Physiol. 2014, 69, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Lihoreau, M.; Gómez-Moracho, T.; Pasquaretta, C.; Costa, J.T.; Buhl, J. Social nutrition: An emerging field in insect science. Curr. Opin. Insect Sci. 2018, 28, 73–80. [Google Scholar] [CrossRef]
- Buhl, J.; Sumpter, D.J.T.; Couzin, I.D.; Hale, J.J.; Despland, E.; Miller, E.R.; Simpson, S.J. From disorder to order in marching locusts. Science 2006, 312, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Dussutour, A.; Simpson, S.J. Communal nutrition in ants. Curr. Biol. 2009, 19, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Salomon, M.; Mayntz, D.; Lubin, Y. Colony nutrition skews reproduction in a social spider. Behav. Ecol. 2008, 19, 605–611. [Google Scholar] [CrossRef]
- Senior, A.M.; Nakagawa, S.; Lihoreau, M.; Simpson, S.J.; Raubenheimer, D. An overlooked consequence of dietary mixing: A varied diet reduces interindividual variance in fitness. Am. Nat. 2015, 186, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Senior, A.M.; Charleston, M.A.; Lihoreau, M.; Buhl, J.; Raubenheimer, D.; Simpson, S.J. Evolving nutritional strategies in the presence of competition: A geometric agent-based model. PLoS Comput. Biol. 2015, 11, e1004111. [Google Scholar] [CrossRef] [PubMed]
- Senior, A.M.; Lihoreau, M.; Charleston, M.A.; Buhl, J.; Raubenheimer, D.; Simpson, S.J. Adaptive collective foraging in groups with conflicting nutritional needs. R. Soc. Open Sci. 2016, 3, 150638. [Google Scholar] [CrossRef] [PubMed]
- Poissonnier, L.-A.; Lihoreau, M.; Gomez-Moracho, T.; Dussutour, A.; Buhl, J. A theoretical exploration of dietary collective medication in social insects. J. Insect Physiol. 2018, 106, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Behmer, S.T. Animal behaviour: Feeding the superorganism. Curr. Biol. 2009, 19, R366–R368. [Google Scholar] [CrossRef] [PubMed]
- Dosmann, A.; Bahet, N.; Gordon, D.M. Experimental modulation of external microbiome affects nestmate recognition in harvester ants (Pogonomyrmex barbatus). PeerJ 2016, 4, e1566. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Tissot, M.; Cuevas, W.; Gordon, D.M. Harvester ants utilize cuticular hydrocarbons in nestmate recognition. J. Chem. Ecol. 2000, 26, 2245–2257. [Google Scholar] [CrossRef]
- Wagner, D.; Tissot, M.; Gordon, D. Task-related environment alters the cuticular hydrocarbon composition of harvester ants. J. Chem. Ecol. 2001, 27, 1805–1819. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.W.; Blomquist, G.J. Ecological, Behavioral, and Biochemical Aspects of Insect Hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Esponda, F.; Gordon, D.M. Distributed nestmate recognition in ants. Proc. R. Soc. B 2015, 282. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Heeb, P. Social and sexual behaviours aid transmission of bacteria in birds. Behav. Process. 2007, 74, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Castro, A.J.; González-Cervantes, R.M.; Bravo-Ruiseco, G.; Pacheco-López, G. The microbiota-gut-brain axis: Neurobehavioral correlates, health and sociality. Front. Integr. Neurosci. 2013, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.; Konturek, P.; Pawlik, T.; Brzozowski, T. Brain-gut axis and its role in the control of food intake. J. Physiol. Pharmacol. 2004, 55, 137–154. [Google Scholar] [PubMed]
- Alcock, J.; Maley, C.C.; Aktipis, C.A. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays 2014, 36, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The intestinal microbiota in acute anorexia nervosa and during renourishment: Relationship to depression, anxiety, and eating disorder psychopathology. Psychosom. Med. 2015, 77, 969. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.A.; Swartz, T.D.; Sakar, Y.; Covasa, M. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS ONE 2012, 7, e39748. [Google Scholar] [CrossRef] [PubMed]
- Swartz, T.D.; Duca, F.; De Wouters, T.; Sakar, Y.; Covasa, M. Up-regulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. Br. J. Nutr. 2012, 107, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.R.; Schluter, J.; Coyte, K.Z.; Rakoff-Nahoum, S. The evolution of the host microbiome as an ecosystem on a leash. Nature 2017, 548, 43. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.H.; Foerster, S.; Wilson, M.L.; Pusey, A.E.; Hahn, B.H.; Ochman, H. Social behavior shapes the chimpanzee pan-microbiome. Sci. Adv. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.; Barreiro, L.B.; Burns, M.B.; Grenier, J.C.; Lynch, J.; Grieneisen, L.E.; Altmann, J.; Alberts, S.C.; Blekhman, R.; Archie, E.A. Social networks predict gut microbiome composition in wild baboons. eLife 2015, 2015, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Leclaire, S.; Nielsen, J.F.; Drea, C.M. Bacterial communities in meerkat anal scent secretions vary with host sex, age, and group membership. Behav. Ecol. 2014, 25, 996–1004. [Google Scholar] [CrossRef]
- Lucas, F.S.; Heeb, P. Environmental factors shape cloacal bacterial assemblages in great tit Parus major and blue tit P. caeruleus nestlings. J. Avian Biol. 2005, 36, 510–516. [Google Scholar] [CrossRef]
- White, J.; Mirleau, P.; Danchin, E.; Mulard, H.; Hatch, S.A.; Heeb, P.; Wagner, R.H. Sexually transmitted bacteria affect female cloacal assemblages in a wild bird. Ecol. Lett. 2010, 13, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Schmid-Hempel, P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc. Natl. Acad. Sci. USA 2011, 108, 19288–19292. [Google Scholar] [CrossRef] [PubMed]
- Bright, M.; Bulgheresi, S. A complex journey: Transmission of microbial symbionts. Nat. Rev. Microbiol. 2010, 8, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Florez, L.; Gerardo, N.; Kaltenpoth, M. An out-of-body experience: The extracellular dimension for the transmission of mutualistic bacteria in insects. Proc. R. Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef] [PubMed]
- Caspi-Fluger, A.; Inbar, M.; Mozes-Daube, N.; Katzir, N.; Portnoy, V.; Belausov, E.; Hunter, M.S.; Zchori-Fein, E. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc. R. Soc. B Biol. Sci. 2012, 279, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.P. Access to mutualistic endosymbiotic microbes: An underappreciated benefit of group living. Behav. Ecol. Sociobiol. 2008, 62, 479–497. [Google Scholar] [CrossRef]
- Barber, I.; Wright, H.A. How strong are familiarity preferences in shoaling fish? Anim. Behav. 2001, 61, 975–979. [Google Scholar] [CrossRef]
- Wiszniewski, J.; Lusseau, D.; Möller, L.M. Female bisexual kinship ties maintain social cohesion in a dolphin network. Anim. Behav. 2010, 80, 895–904. [Google Scholar] [CrossRef]
- Kurvers, R.H.J.M.; Adamczyk, V.M.A.P.; Kraus, R.H.S.; Hoffman, J.I.; van Wieren, S.E.; van der Jeugd, H.P.; Amos, W.; Prins, H.H.T.; Jonker, R.M. Contrasting context dependence of familiarity and kinship in animal social networks. Anim. Behav. 2013, 1–9. [Google Scholar] [CrossRef]
- Nalepa, C.A. Nourishment and the origin of termite eusociality. In Nourishment and Evolution in Insect Societies; Westview Press: Boulder, CO, USA, 1994; pp. 57–104. [Google Scholar]
- Moran, N.A.; Hansen, A.K.; Powell, J.E.; Sabree, Z.L. Distinctive gut microbiota of honey bees assessed using deep sampling from individual worker bees. PLoS ONE 2012, 7, e36393. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Fruciano, C.; Hildebrand, F.; Al Toufalilia, H.; Balfour, N.J.; Bork, P.; Engel, P.; Ratnieks, F.L.W.; Hughes, W.O.H. Gut microbiota composition is associated with environmental landscape in honey bees. Ecol. Evol. 2018, 8, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.O. The Insect Societies; Harvard University Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Simpson, S.J.; Raubenheimer, D.; Charleston, M.A.; Clissold, F.J. Modelling nutritional interactions: From individuals to communities. Trends Ecol. Evol. 2010, 25, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Clissold, F.J.; Lihoreau, M.; Ponton, F.; Wilder, S.M.; Raubenheimer, D. Recent Advances in the Integrative Nutrition of Arthropods. Annu. Rev. Entomol. 2015, 60, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Moloney, G.M.; Ryan, F.J.; Hoban, A.E.; Bastiaanssen, T.F.; Shanahan, F.; Clarke, G.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Social interaction-induced activation of RNA splicing in the amygdala of microbiome-deficient mice. eLife 2018, 7, e33070. [Google Scholar] [CrossRef] [PubMed]
- Elgar, M.A. Predator vigilance and group size in mammals and birds: A critical review of the empirical evidence. Biol. Rev. Camb. Philos. Soc. 1989, 64, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Kurvers, R.H.J.M.; van Oers, K.; Nolet, B.A.; Jonker, R.M.; van Wieren, S.E.; Prins, H.H.T.; Ydenberg, R.C. Personality predicts the use of social information. Ecol. Lett. 2010, 13, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Lihoreau, M.; Charleston, M.A.; Senior, A.M.; Clissold, F.J.; Raubenheimer, D.; Simpson, S.J.; Buhl, J. Collective foraging in spatially complex nutritional environments. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160238. [Google Scholar] [CrossRef] [PubMed]
- Storelli, G.; Defaye, A.; Erkosar, B.; Hols, P.; Royet, J.; Leulier, F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011, 14, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Callens, M.; Macke, E.; Muylaert, K.; Bossier, P.; Lievens, B.; Waud, M.; Decaestecker, E. Food availability affects the strength of mutualistic host-microbiota interactions in Daphnia magna. ISME J. 2016, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; Raubenheimer, D.; Rothman, J.M.; Clarke, D.; Swedell, L. 30 Days in the life: Daily nutrient balancing in a wild chacma baboon. PLoS ONE 2013, 8, e70383. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, D.; Machovsky-Capuska, G.E.; Chapman, C.A.; Rothman, J.M. Geometry of nutrition in field studies: An illustration using wild primates. Oecologia 2015, 177, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, M.N.; Zonana, D.M.; Safran, R.J. An integrative view of the signaling phenotype: Dynamic links between signals, physiology, behavior and social context. Curr. Zool. 2014, 60, 739–754. [Google Scholar] [CrossRef]
- Senior, A.M.; Lihoreau, M.; Buhl, J.; Raubenheimer, D.; Simpson, S.J. Social network analysis and nutritional behavior: An integrated modeling approach. Front. Psychol. 2016, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, J.N.; Berdahl, A.; Riehl, C.; Pinter-Wollman, N.; Moeller, H.V.; Pringle, E.G.; Aplin, L.M.; Robinson, E.J.; Grilli, J.; Yeh, P. Social tipping points in animal societies. Proc. R. Soc. B 2018, 285, 20181282. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, M.B. Foraging strategies of Drosophila melanogaster: A chromosomal analysis. Behav. Genet. 1980, 10, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Pasquaretta, C.; Battesti, M.; Klenschi, E.; Bousquet, C.A.; Sueur, C.; Mery, F. How social network structure affects decision-making in Drosophila melanogaster. Proc. Biol. Sci. 2016, 283. [Google Scholar] [CrossRef] [PubMed]
- Kort, R.; Caspers, M.; van de Graaf, A.; van Egmond, W.; Keijser, B.; Roeselers, G. Shaping the oral microbiota through intimate kissing. Microbiome 2014, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, A.; Aizpurua, O.; Bohmann, K.; Zepeda-Mendoza, M.L.; Gilbert, M.T.P. Do vertebrate gut metagenomes confer rapid ecological adaptation? Trends Ecol. Evol. 2016, 31, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621. [Google Scholar] [CrossRef] [PubMed]
- Delsuc, F.; Metcalf, J.L.; Wegener Parfrey, L.; Song, S.J.; González, A.; Knight, R. Convergence of gut microbiomes in myrmecophagous mammals. Mol. Ecol. 2014, 23, 1301–1317. [Google Scholar] [CrossRef] [PubMed]
- Roggenbuck, M.; Schnell, I.B.; Blom, N.; Bælum, J.; Bertelsen, M.F.; Sicheritz-Pontén, T.; Sørensen, S.J.; Gilbert, M.T.P.; Graves, G.R.; Hansen, L.H. The microbiome of New World vultures. Nat. Commun. 2014, 5, 5498. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquaretta, C.; Gómez-Moracho, T.; Heeb, P.; Lihoreau, M. Exploring Interactions between the Gut Microbiota and Social Behavior through Nutrition. Genes 2018, 9, 534. https://doi.org/10.3390/genes9110534
Pasquaretta C, Gómez-Moracho T, Heeb P, Lihoreau M. Exploring Interactions between the Gut Microbiota and Social Behavior through Nutrition. Genes. 2018; 9(11):534. https://doi.org/10.3390/genes9110534
Chicago/Turabian StylePasquaretta, Cristian, Tamara Gómez-Moracho, Philipp Heeb, and Mathieu Lihoreau. 2018. "Exploring Interactions between the Gut Microbiota and Social Behavior through Nutrition" Genes 9, no. 11: 534. https://doi.org/10.3390/genes9110534
APA StylePasquaretta, C., Gómez-Moracho, T., Heeb, P., & Lihoreau, M. (2018). Exploring Interactions between the Gut Microbiota and Social Behavior through Nutrition. Genes, 9(11), 534. https://doi.org/10.3390/genes9110534





