Linking DNA Damage and Age-Related Promoter DNA Hyper-Methylation in the Intestine
Abstract
1. Introduction
2. Methods
2.1. 3D Multiscale Model of Epigenetic Aging of the Intestinal Epithelium
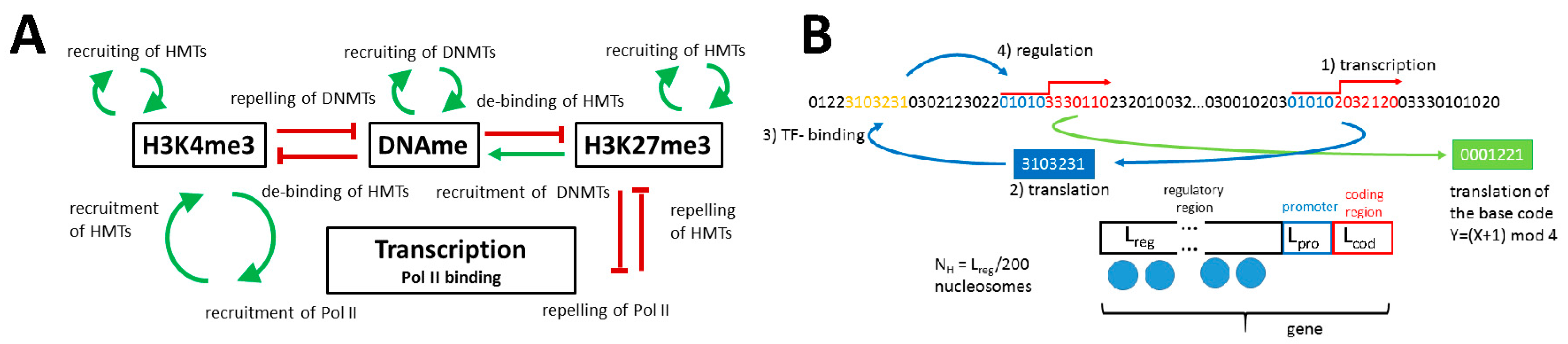
2.1.1. The Model of Epigenetic Regulation of Transcription
2.1.2. The 3D Individual Cell-Based Model of the Intestinal Crypt
2.2. Model of DNA Damage and Damage Repair in the Crypt
3. Results
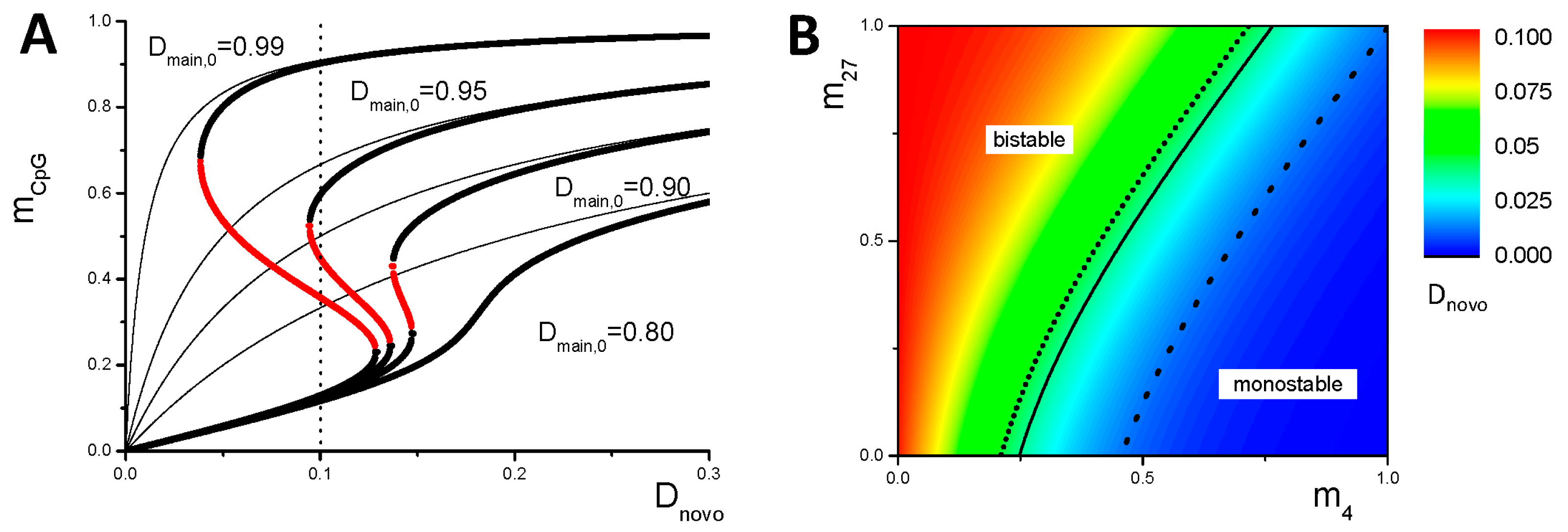
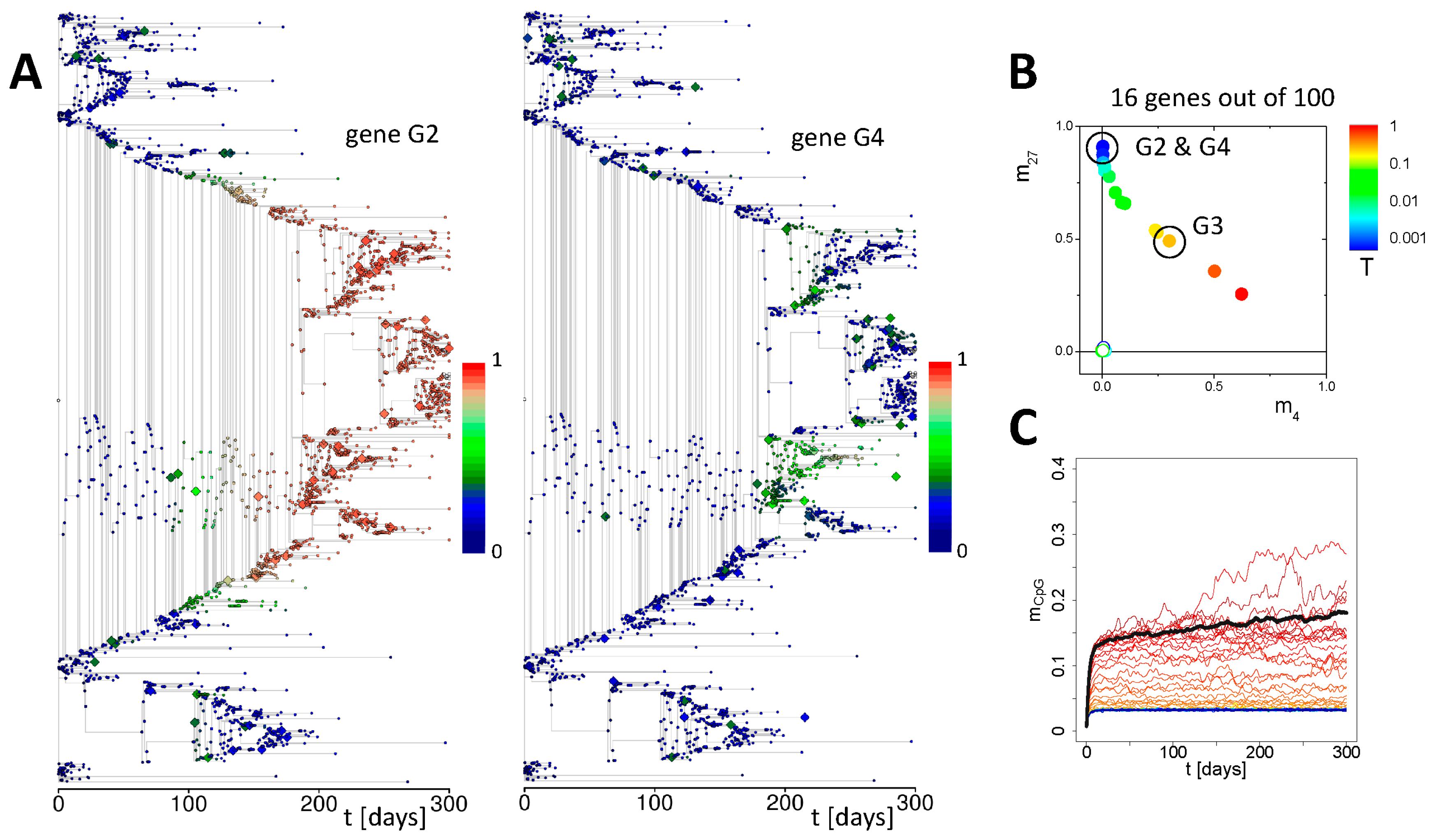
3.1. Maintenance of DNA Methylation with Positive Auto-Feedback
3.2. Simulation of Aging without Damage
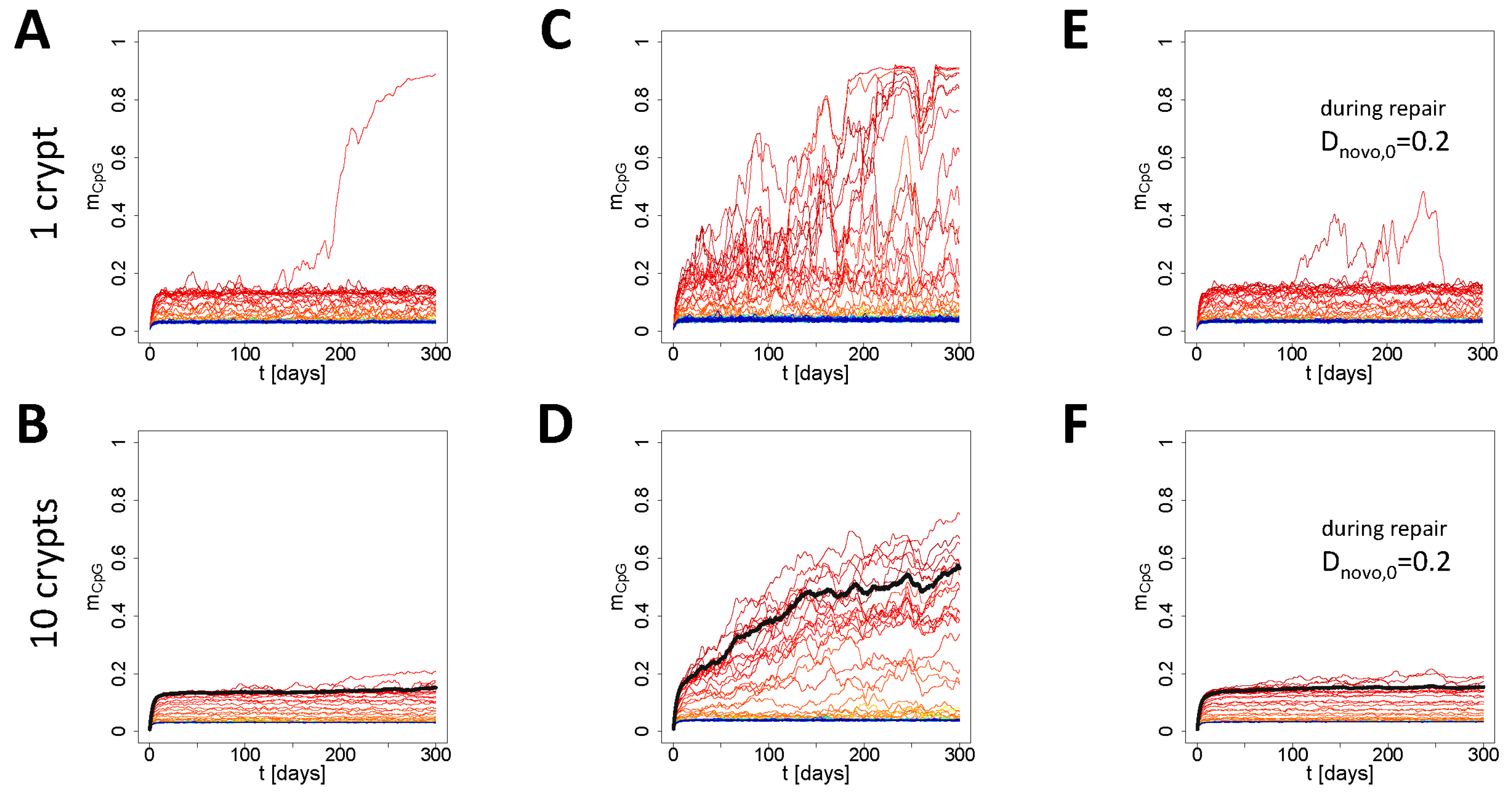
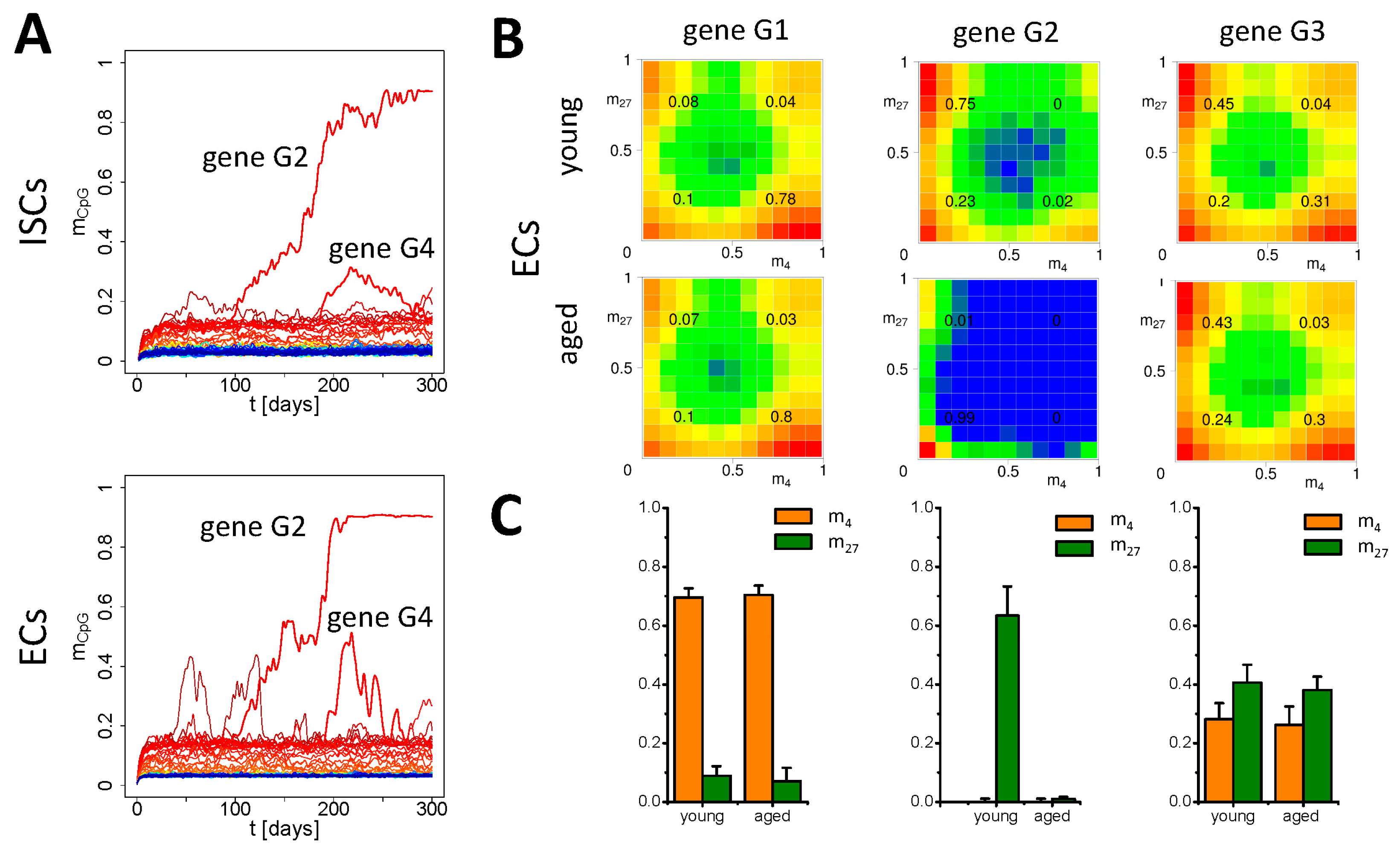
3.3. Simulation of Aging under Repeated DDR
4. Discussion
Acknowledgments
Author contributions
Conflicts of Interest
Abbreviations
| ISC | Intestinal stem cell |
| PC | Paneth cell |
| GC | Goblet cell |
| EC | Enterocyte |
| DSB | Double-strand break |
| DDR | DNA damage repair |
| HDM | Histone demethylases |
| HMT | Histone methyltransferase |
| DNMT | DNA methyltransferase |
Appendix A. Model Description
Appendix A1. Model of the Epigenetic Regulation
| Parameter | Value | Description |
|---|---|---|
| Histone modification | ||
| , | 10.0, 11.0 | ground enthalpy per bound HMT * |
| , | −5.0, −5.0 | free energy of CpG binding * |
| , | −0.8, −0.8 | free energy of histone binding * |
| , | 0.1, 0.1 | modification constant |
| , | 0.01 (0), 0.01 | de-modification constant |
| NH | 10 | number of cooperative nucleosomes |
| DNA methylation | ||
| Dmain,0 | 0.99 | probability of maintaining DNA methylation |
| Dnovo,0 | 0.1 (0.3) | probability of de novo DNA methylation |
| , | 6, 4 | interaction energy between HMTs and DNMTs * |
| Em,0 | 2 | ground enthalpy per bound maintenance DNMT * |
| Em,1 | −10 | free energy of methyl-CG binding * |
Appendix A2. Model of Transcriptional Regulation
| Parameter | Value | Description |
|---|---|---|
| Pmax | 100 | maximum promoter activity |
| δ | 0.1 | transcript degradation constant |
| εA | 1/−1 | free energy change of polymerase binding for activators/repressors * |
| Lreg | 2.000 bp | length of the regulatory region |
| Lpro | 5 bp | length of the promoter region |
| Lcod | 7 bp | length of the coding region |
Appendix A3. Crypt Model
| Symbol | Value | Parameter | References |
|---|---|---|---|
| Parameter of the cell model | |||
| V0 | 4/3π (5 µm)3 | minimal volume of an isolated cell | estimated |
| τ | 14 h | cell growth time | results in an effective cell cycle time ~24 h |
| E | 1 kPa | Young modulus | [51] |
| ν | 1/3 | Poisson ratio | |
| εc | 200 µN/m | cell-cell anchorage | |
| Vp | 0.88 V0 | threshold volume for contact inhibition | set |
| Parameter of the Basal Membrane model | |||
| z0 | 150 µm | length of the crypt | set, according to properties of the crypt shape [20] |
| r0 | 60 µm | crypt radius at the crypt-villus junction | |
| λ1 | 0.25 | shape parameter 1 | |
| λ2 | 0.1 | shape parameter 2 | |
| Ω | 0.95 | optimal cell-BM distance in cell radii | set |
| 35 10−12 Nm | maximum cell-knot interaction energy of PCs | set | |
| 5.5 10−12 Nm | maximum cell-knot interaction energy of all other cells | ensuring apoptosis rates < 5% [52] | |
| Parameter of crypt dynamics | |||
| ηc | 5 1010 Ns/m3 | friction constant for cell-cell friction | [51] |
| ηBM | 3.2 Ns/m | friction coefficient for cell-BM friction | fit: turnover |
| ηVO | 400 Ns/m | friction coefficient regarding volume changes | [51] |
| 7.5 nN | active migration force of PCs | fit: distribution of PCs | |
| 4.5 nN | active migration force of all other cells | fit: turnover and Brdu data | |
| Parameter of the lineage specification and differentiation model | |||
| P1 | −129 µm | position of the Wnt- threshold TPWnt for priming | fit: size of the PC compartment |
| P2 | −87.5 µm | position of the Wnt- threshold TDWnt for differentiation | fit: turnover and Brdu data |
| N1 | 3 | minimum number of Notch-ligand expressing neighbors in the niche | set: refers to TDNotch /LPPaneth and TDNotch/LPGoblet [20] |
| N2 | 1 | minimum number of Notch-ligand expressing neighbors outside the niche | |
| τP | 8 weeks | average PC lifespan with contact to SCs | [19] |
| τA | 12 h | max. PC lifespan without contact to SCs | |
Appendix B. Additional Simulation Results
Appendix B1. Bistable DNA Methylation States

Appendix B2. Simulation of Aging

References
- Christensen, B.C.; Houseman, E.A.; Marsit, C.J.; Zheng, S.; Wrensch, M.R.; Wiemels, J.L.; Nelson, H.H.; Karagas, M.R.; Padbury, J.F.; Bueno, R.; et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009, 5, e1000602. [Google Scholar] [CrossRef] [PubMed]
- Beerman, I.; Bock, C.; Garrison, B.S.; Smith, Z.D.; Gu, H.; Meissner, A.; Rossi, D.J. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell 2013, 12, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [PubMed]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Weidner, C.I.; Lin, Q.; Koch, C.M.; Eisele, L.; Beier, F.; Ziegler, P.; Bauerschlag, D.O.; Jöckel, K.H.; Erbel, R.; Mühleisen, T.W.; et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014, 15, R24. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Ritz, B.R. Increased epigenetic age and granulocyte counts in the blood of Parkinson’s disease patients. Aging 2015, 7, 1130. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Levine, A.J. HIV-1 infection accelerates age according to the epigenetic clock. J. Infect. Dis. 2015, 212, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Teschendorff, A.E.; Menon, U.; Gentry-Maharaj, A.; Ramus, S.J.; Weisenberger, D.J.; Shen, H.; Campan, M.; Noushmehr, H.; Bell, C.G.; Maxwell, A.P.; et al. Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res. 2010, 20, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, Y.; Straussman, R.; Keshet, I.; Farkash, S.; Hecht, M.; Zimmerman, J.; Eden, E.; Yakhini, Z.; Ben-Shushan, E.; Reubinoff, B.E.; et al. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat. Genet. 2007, 39, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Rakyan, V.K.; Down, T.A.; Maslau, S.; Andrew, T.; Yang, T.P.; Beyan, H.; Whittaker, P.; McCann, O.T.; Finer, S.; Valdes, A.M.; et al. Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome Res. 2010, 20, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.E.; Mikkelsen, T.S.; Xie, X.; Kamal, M.; Huebert, D.J.; Cuff, J.; Fry, B.; Meissner, A.; Wernig, M.; Plath, K.; et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 2006, 125, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Przybilla, J.; Galle, J.; Rohlf, T. Is adult stem cell aging driven by conflicting modes of chromatin remodeling? Bioessays 2012, 34, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Przybilla, J.; Rohlf, T.; Loeffler, M.; Galle, J. Understanding epigenetic changes in aging stem cells—A computational model approach. Aging Cell 2014, 13, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Maegawa, S.; Hinkal, G.; Kim, H.S.; Shen, L.; Zhang, L.; Zhang, J.; Zhang, N.; Liang, S.; Donehower, L.A.; Issa, J.P. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 2010, 20, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Noreen, F.; Röösli, M.; Gaj, P.; Pietrzak, J.; Weis, S.; Urfer, P.; Regula, J.; Schär, P.; Truninger, K. Modulation of age- and cancer-associated DNA methylation change in the healthy colon by aspirin and lifestyle. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, H.; Ikegami, D.; Wakabayashi, M.; Niwa, T.; Kim, Y.J.; Ushijima, T. Induction of aberrant trimethylation of histone H3 lysine 27 by inflammation in mouse colonic epithelial cells. Carcinogenesis 2012, 33, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Coelho, R.; Grácio, D.; Dias, C.; Silva, M.; Peixoto, A.; Lopes, P.; Costa, C.; Teixeira, J.P.; Macedo, G.; et al. DNA damage and oxidative DNA damage in inflammatory bowel disease. J. Crohns Colitis 2016, 10, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Gursoy-Yuzugullu, O.; House, N.; Price, B.D. Patching Broken DNA: Nucleosome Dynamics and the Repair of DNA Breaks. J. Mol. Biol. 2016, 428, 1846–1860. [Google Scholar] [CrossRef] [PubMed]
- Thalheim, T.; Buske, P.; Przybilla, J.; Rother, K.; Loeffler, M.; Galle, J. Stem cell competition in the gut: Insights from multi-scale computational modelling. J. R. Soc. Interface 2016, 13, 20160218. [Google Scholar] [CrossRef] [PubMed]
- Buske, P.; Galle, J.; Barker, N.; Aust, G.; Clevers, H.; Loeffler, M. A comprehensive model of the spatio-temporal stem cell and tissue organisation in the intestinal crypt. PLoS Comput. Biol. 2011, 7, e1001045. [Google Scholar] [CrossRef] [PubMed]
- Thalheim, T.; Herberg, M.; Loeffler, M.; Galle, J. The Regulatory Capacity of Bivalent Genes-A Theoretical Approach. Int. J. Mol. Sci. 2017, 18, 1069. [Google Scholar] [CrossRef] [PubMed]
- Buratowski, S.; Kim, T. The Role of Cotranscriptional Histone Methylations. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2010; Volume 75, pp. 95–102. [Google Scholar]
- Vermeulen, M.; Mulder, K.W.; Denissov, S.; Pijnappel, W.W.; van Schaik, F.M.; Varier, R.A.; Baltissen, M.P.; Stunnenberg, H.G.; Mann, M.; Timmers, H.T. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell 2007, 131, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Jermann, P.; Hoerner, L.; Burger, L.; Schübeler, D. Short sequences can efficiently recruit histone H3 lysine 27 trimethylation in the absence of enhancer activity and DNA methylation. PNAS 2014, 111, E3415–E3421. [Google Scholar] [CrossRef] [PubMed]
- Pasini, D.; Malatesta, M.; Jung, H.R.; Walfridsson, J.; Willer, A.; Olsson, L.; Skotte, J.; Wutz, A.; Porse, B.; Jensen, O.N.; et al. Characterization of an antagonistic switch between histone H3 lysine 27 methylation and acetylation in the transcriptional regulation of Polycomb group target genes. Nucleic Acids Res. 2010, 38, 4958–4969. [Google Scholar] [CrossRef] [PubMed]
- Alabert, C.; Barth, T.K.; Reverón-Gómez, N.; Sidoli, S.; Schmidt, A.; Jensen, O.N.; Imhof, A.; Groth, A. Two distinct modes for propagation of histone PTMs across the cell cycle. Genes Dev. 2015, 29, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.K.; Qiu, C.; Bernstein, E.; Li, K.; Jia, D.; Yang, Z.; Erdjument-Bromage, H.; Tempst, P.; Lin, S.P.; Allis, C.D.; et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 2007, 448, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.P.; Skene, P.J.; Selfridge, J.; Clouaire, T.; Guy, J.; Webb, S.; Kerr, A.R.; Deaton, A.; Andrews, R.; James, K.D.; et al. CpG islands influence chromatin structure via the CpG-binding protein Cfp1. Nature 2010, 464, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Rush, M.; Appanah, R.; Lee, S.; Lam, L.L.; Goyal, P.; Lorincz, M.C. Targeting of EZH2 to a defined genomic site is sufficient for recruitment of Dnmt3a but not de novo DNA methylation. Epigenetics 2009, 4, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.; Wirth, H.; Galle, J. Gene expression density profiles characterize modes of genomic regulation: Theory and experiment. J. Biotechnol. 2010, 149, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Qian, X.; Shen, J.; Wang, Y.; Li, X.; Liu, R.; Xia, Y.; Chen, Q.; Peng, G.; Lin, S.Y.; Lu, Z. Local generation of fumarate promotes DNA repair through inhibition of histone H3 demethylation. Nat. Cell Biol. 2015, 17, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Cloos, P.A.; Christensen, J.; Agger, K.; Helin, K. Erasing the methyl mark: Histone demethylases at the center of cellular differentiation and disease. Genes Dev. 2008, 22, 1115–1140. [Google Scholar] [CrossRef] [PubMed]
- Boulard, M.; Edwards, J.R.; Bestor, T.H. FBXL10 protects Polycomb-bound genes from hypermethylation. Nat. Genet. 2015, 47, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Sontag, L.B.; Lorincz, M.C.; Luebeck, E.G. Dynamics, stability and inheritance of somatic DNA methylation imprints. J. Theor. Biol. 2006, 242, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Sormani, G.; Haerter, J.O.; Lövkvist, C.; Sneppen, K. Stabilization of epigenetic states of CpG islands by local cooperation. Mol. Biosyst. 2016, 12, 2142–2146. [Google Scholar] [CrossRef] [PubMed]
- Luebeck, E.G.; Curtius, K.; Hazelton, W.D.; Maden, S.; Yu, M.; Thota, P.N.; Patil, D.T.; Chak, A.; Willis, J.E.; Grady, W.M. Identification of a key role of widespread epigenetic drift in Barrett’s esophagus and esophageal adenocarcinoma. Clin. Epigenetics 2017, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Laget, S.; Miotto, B.; Chin, H.G.; Estève, P.O.; Roberts, R.J.; Pradhan, S.; Defossez, P.A. MBD4 cooperates with DNMT1 to mediate methyl-DNA repression and protects mammalian cells from oxidative stress. Epigenetics 2014, 9, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Li, M.; Wu, H.; Jia, S.; Zhang, N.; Dai, Y.; Zhao, M.; Lu, Q. Down-regulation of MBD4 contributes to hypomethylation and overexpression of CD70 in CD4+ T cells in systemic lupus erythematosus. Clin. Epigenetics 2017, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Kazakevych, J.; Sayols, S.; Messner, B.; Krienke, C.; Soshnikova, N. Dynamic changes in chromatin states during specification and differentiation of adult intestinal stem cells. Nucleic Acids Res. 2017, 45, 5770–5784. [Google Scholar] [CrossRef] [PubMed]
- Snippert, H.J.; van der Flier, L.G.; Sato, T.; van Es, J.H.; van den Born, M.; Kroon-Veenboer, C.; Barker, N.; Klein, A.M.; van Rheenen, J.; Simons, B.D.; et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010, 143, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- O’Hagan, H.M.; Mohammad, H.P.; Baylin, S.B. Double strand breaks can initiate gene silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island. PLoS Genet 2008, 4, e1000155. [Google Scholar] [CrossRef] [PubMed]
- Przybilla, J.; Hopp, L.; Lübbert, M.; Loeffler, M.; Galle, J. Targeting DNA hypermethylation: Computational modeling of DNA demethylation treatment of acute myeloid leukemia. Epigenetics 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Franzen, J.; Zirkel, A.; Blake, J.; Rath, B.; Benes, V.; Papantonis, A.; Wagner, W. Senescence-associated DNA methylation is stochastically acquired in subpopulations of mesenchymal stem cells. Aging Cell 2017, 16, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Teodoridis, J.M.; Hardie, C.; Brown, R. CpG island methylator phenotype (CIMP) in cancer: Causes and implications. Cancer Lett. 2008, 268, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Ferk, F.; Sterneder, S.; Setayesh, T.; Kepcija, T.; Roth, S.; Noorizadeh, R.; Greunz, M.; Rebhan, I.; Wagner, K.H.; et al. Vitamin E modifies high-fat diet-induced increase of DNA strand breaks, and changes in expression and DNA methylation of Dnmt1 and MLH1 in C57BL/6J Male Mice. Nutrients 2017, 9, 607. [Google Scholar] [CrossRef] [PubMed]
- Janzer, A.; Stamm, K.; Becker, A.; Zimmer, A.; Buettner, R.; Kirfel, J. The H3K4me3 histone demethylase Fbxl10 is a regulator of chemokine expression, cellular morphology, and the metabolome of fibroblasts. J. Biol. Chem. 2012, 287, 30984–30992. [Google Scholar] [CrossRef] [PubMed]
- Vilenchik, M.M.; Knudson, A.G. Endogenous DNA double-strand breaks: Production, fidelity of repair, and induction of cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 12871–12876. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.; Steiner, L.; Przybilla, J.; Rohlf, T.; Prohaska, S.; Galle, J. Transcriptional regulation by histone modifications: Towards a theory of chromatin re-organization during stem cell differentiation. Phys. Biol. 2013, 10, 026006. [Google Scholar] [CrossRef] [PubMed]
- Galle, J.; Loeffler, M.; Drasdo, D. Modeling the effect of deregulated proliferation and apoptosis on the growth dynamics of epithelial cell populations in vitro. Biophys. J. 2005, 88, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Marshman, E.; Ottewell, P.D.; Potten, C.S.; Watson, A.J. Caspase activation during spontaneous and radiation-induced apoptosis in the murine intestine. J. Pathol. 2001, 195, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Reinhardt, R.; Jeltsch, A. Accuracy of DNA methylation pattern preservation by the Dnmt1 methyltransferase. Nucleic Acids Res. 2006, 34, 1182–1188. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thalheim, T.; Herberg, M.; Galle, J. Linking DNA Damage and Age-Related Promoter DNA Hyper-Methylation in the Intestine. Genes 2018, 9, 17. https://doi.org/10.3390/genes9010017
Thalheim T, Herberg M, Galle J. Linking DNA Damage and Age-Related Promoter DNA Hyper-Methylation in the Intestine. Genes. 2018; 9(1):17. https://doi.org/10.3390/genes9010017
Chicago/Turabian StyleThalheim, Torsten, Maria Herberg, and Joerg Galle. 2018. "Linking DNA Damage and Age-Related Promoter DNA Hyper-Methylation in the Intestine" Genes 9, no. 1: 17. https://doi.org/10.3390/genes9010017
APA StyleThalheim, T., Herberg, M., & Galle, J. (2018). Linking DNA Damage and Age-Related Promoter DNA Hyper-Methylation in the Intestine. Genes, 9(1), 17. https://doi.org/10.3390/genes9010017





