A Rare Form of Retinal Dystrophy Caused by Hypomorphic Nonsense Mutations in CEP290
Abstract
1. Introduction
2. Material and Methods
2.1. Clinical Examination
2.2. Genetic Testing
2.3. Sequence Analysis of the CEP290 Gene
2.4. Analysis of Hypomorphic Character of the c.4723A>T Variant
3. Results
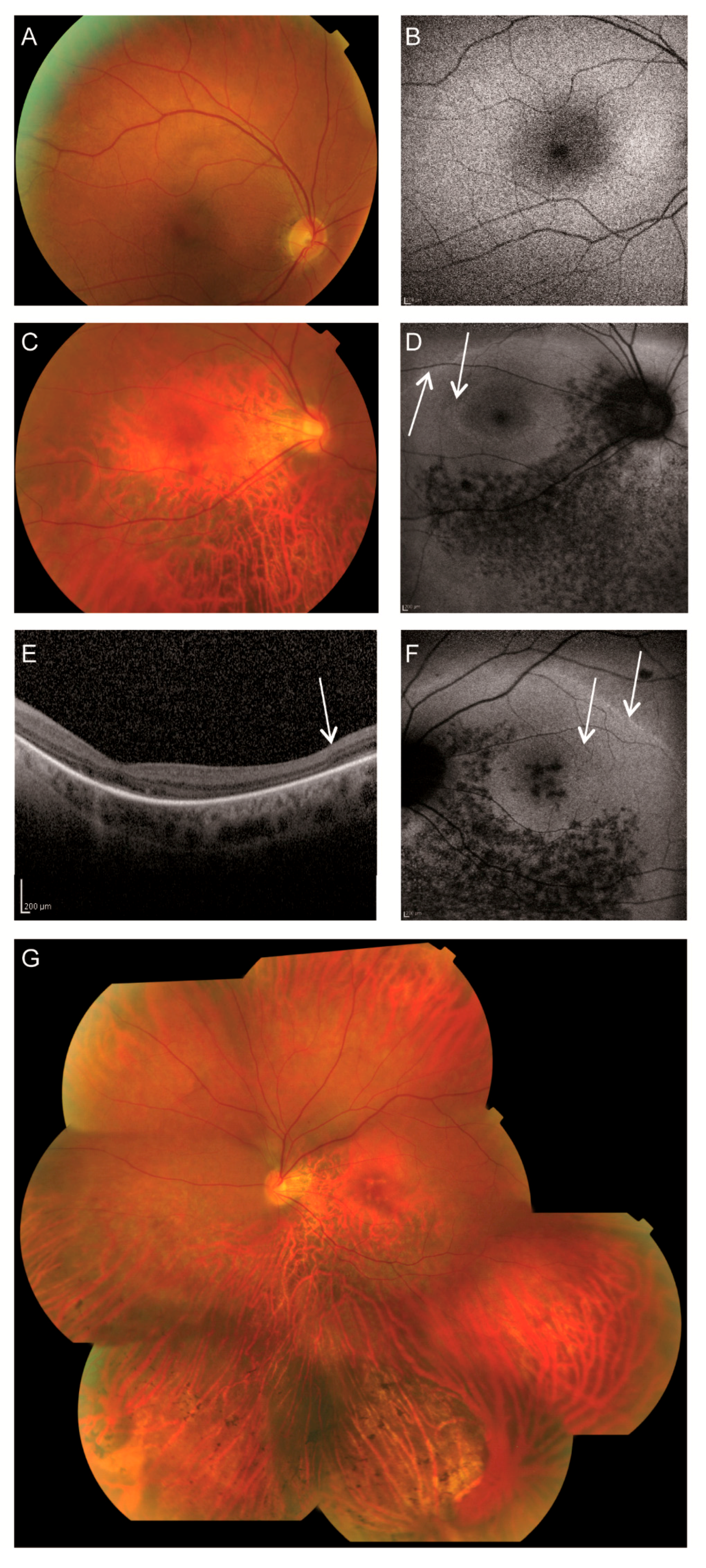
3.1. Clinical Findings
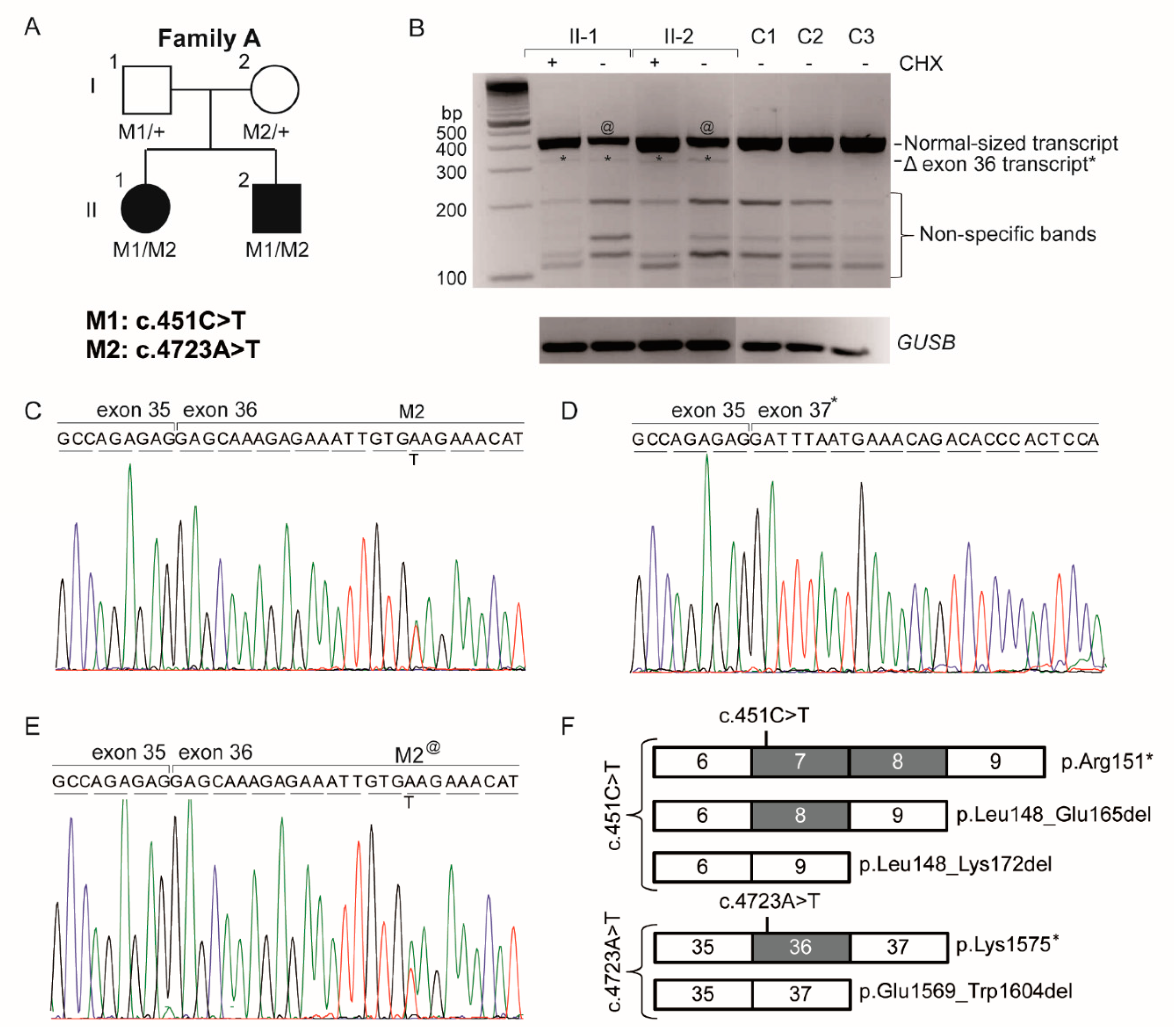
3.2. Genetic Analysis
3.3. Analysis of the Hypomorphic Character of c.4723A>T
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Craige, B.; Tsao, C.C.; Diener, D.R.; Hou, Y.; Lechtreck, K.F.; Rosenbaum, J.L.; Witman, G.B. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 2010, 190, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Barbelanne, M.; Song, J.; Ahmadzai, M.; Tsang, W.Y. Pathogenic NPHP5 mutations impair protein interaction with Cep290, a prerequisite for ciliogenesis. Hum. Mol. Genet. 2013, 22, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalo, F.R.; Corbit, K.C.; Sirerol-Piquer, M.S.; Ramaswami, G.; Otto, E.A.; Noriega, T.R.; Seol, A.D.; Robinson, J.F.; Bennett, C.L.; Josifova, D.J.; et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 2011, 43, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Den Hollander, A.I.; Koenekoop, R.K.; Yzer, S.; Lopez, I.; Arends, M.L.; Voesenek, K.E.; Zonneveld, M.N.; Strom, T.M.; Meitinger, T.; Brunner, H.G.; et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am. J. Hum. Genet. 2006, 79, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Khanna, H.; Hawes, N.; Jimeno, D.; He, S.; Lillo, C.; Parapuram, S.K.; Cheng, H.; Scott, A.; Hurd, R.E.; et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum. Mol. Genet. 2006, 15, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Littink, K.W.; Pott, J.W.; Collin, R.W.J.; Kroes, H.Y.; Verheij, J.B.; Blokland, E.A.; de Castro Miro, M.; Hoyng, C.B.; Klaver, C.C.W.; Koenekoop, R.K.; et al. A novel nonsense mutation in CEP290 induces exon skipping and leads to a relatively mild retinal phenotype. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3646–3652. [Google Scholar] [CrossRef] [PubMed]
- Sayer, J.A.; Otto, E.A.; O’Toole, J.F.; Nurnberg, G.; Kennedy, M.A.; Becker, C.; Hennies, H.C.; Helou, J.; Attanasio, M.; Fausett, B.V.; et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat. Genet. 2006, 38, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Baala, L.; Audollent, S.; Martinovic, J.; Ozilou, C.; Babron, M.C.; Sivanandamoorthy, S.; Saunier, S.; Salomon, R.; Gonzales, M.; Rattenberry, E.; et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am. J. Hum. Genet. 2007, 81, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, F.; Lefever, S.; Leroy, B.P.; De Baere, E. CEP290, a gene with many faces: Mutation overview and presentation of CEP290base. Hum. Mutat. 2010, 31, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Perrault, I.; Delphin, N.; Hanein, S.; Gerber, S.; Dufier, J.L.; Roche, O.; Defoort-Dhellemmes, S.; Dollfus, H.; Fazzi, E.; Munnich, A.; et al. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum. Mutat. 2007, 28, 416. [Google Scholar] [CrossRef] [PubMed]
- Van Lith, G.H.M. General cone dysfunction without achromatopsia. In 10th ISCERG Symposium; Documenta Ophthalmologica Proceedings Series; Springer: Dordrecht, The Netherlands, 1973; pp. 175–180. [Google Scholar]
- Vincent, A.; Wright, T.; Billingsley, G.; Westall, C.; Heon, E. Oligocone trichromacy is part of the spectrum of CNGA3-related cone system disorders. Ophthalmic Genet. 2011, 32, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.K.; Christoffersen, N.L.; Sander, B.; Edmund, C.; Larsen, M.; Grau, T.; Wissinger, B.; Kohl, S.; Rosenberg, T. Oligocone trichromacy: Clinical and molecular genetic investigations. Investig. Ophthalmol. Vis. Sci. 2010, 51, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, T.; Baumann, B.; Kohl, S.; Zrenner, E.; Jorgensen, A.L.; Wissinger, B. Variant phenotypes of incomplete achromatopsia in two cousins with GNAT2 gene mutations. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4256–4262. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, M.; Holder, G.E.; Bradshaw, K.; Hunt, D.M.; Mollon, J.D.; Moore, A.T. Oligocone trichromacy: A rare and unusual cone dysfunction syndrome. Br. J. Ophthalmol. 2004, 88, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, V.; Drumare, I.; Bouacha, I.; Puech, B.; Defoort-Dhellemmes, S. Long-term follow-up of two patients with oligocone trichromacy. Doc. Ophthalmol. 2015, 131, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Fulton, A.B.; Holder, G.E.; Miyake, Y.; Brigell, M.; Bach, M. ISCEV standard for full-field clinical electroretinography (2008 update). Doc. Ophthalmol. 2009, 118, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Littink, K.W.; van Genderen, M.M.; van Schooneveld, M.J.; Visser, L.; Riemslag, F.C.; Keunen, J.E.; Bakker, B.; Zonneveld, M.N.; den Hollander, A.I.; Cremers, F.P.; et al. A homozygous frameshift mutation in LRAT causes retinitis punctata albescens. Ophthalmology 2012, 119, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Frank, V.; den Hollander, A.I.; Bruchle, N.O.; Zonneveld, M.N.; Nurnberg, G.; Becker, C.; Du Bois, G.; Kendziorra, H.; Roosing, S.; Senderek, J.; et al. Mutations of the CEP290 gene encoding a centrosomal protein cause Meckel-Gruber syndrome. Hum. Mutat. 2008, 29, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Maquat, L.E. A rule for termination-codon position within intron-containing genes: When nonsense affects RNA abundance. Trends Biochem. Sci. 1998, 23, 198–199. [Google Scholar] [CrossRef]
- ExAC. Available online: http://exac.broadinstitute.org/ (accessed on 29 June 2017).
- IGSR: The International Genome Sample Resource; 1000 Genomes. Available online: http://www.1000genomes.org/ (accessed on 29 June 2017).
- Michaelides, M.; Rha, J.; Dees, E.W.; Baraas, R.C.; Wagner-Schuman, M.L.; Mollon, J.D.; Dubis, A.M.; Andersen, M.K.; Rosenberg, T.; Larsen, M.; et al. Integrity of the cone photoreceptor mosaic in oligocone trichromacy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Hofer, H.; Carroll, J.; Neitz, J.; Neitz, M.; Williams, D.R. Organization of the human trichromatic cone mosaic. J. Neurosci. 2005, 25, 9669–9679. [Google Scholar] [CrossRef] [PubMed]
- Scholl, H.P.; Chong, N.H.; Robson, A.G.; Holder, G.E.; Moore, A.T.; Bird, A.C. Fundus autofluorescence in patients with Leber congenital amaurosis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2747–2752. [Google Scholar] [CrossRef] [PubMed]
- Escher, P.; Tran, H.V.; Vaclavik, V.; Borruat, F.X.; Schorderet, D.F.; Munier, F.L. Double concentric autofluorescence ring in NR2E3-p.G56R -linked autosomal dominant retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4754–4764. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Krishnaswami, S.R.; Gleeson, J.G. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum. Mol. Genet. 2008, 17, 3796–3805. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.Y.; Bossard, C.; Khanna, H.; Peranen, J.; Swaroop, A.; Malhotra, V.; Dynlacht, B.D. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev. Cell 2008, 15, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Cideciyan, A.V.; Aleman, T.S.; Jacobson, S.G.; Khanna, H.; Sumaroka, A.; Aguirre, G.K.; Schwartz, S.B.; Windsor, E.A.; He, S.; Chang, B.; et al. Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: Implications for therapy of Leber congenital amaurosis. Hum. Mutat. 2007, 28, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, S.; Shimauchi-Matsukawa, Y.; Tachibanaki, S.; Kawamura, S. Highly efficient retinal metabolism in cones. Proc. Natl. Acad. Sci. USA 2008, 105, 16051–16056. [Google Scholar] [CrossRef] [PubMed]
- Stearns, G.; Evangelista, M.; Fadool, J.M.; Brockerhoff, S.E. A mutation in the cone-specific pde6 gene causes rapid cone photoreceptor degeneration in zebrafish. J. Neurosci. 2007, 27, 13866–13874. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, S.; Michalakis, S.; Claes, E.; Seeliger, M.W.; Humphries, P.; Biel, M.; Feigenspan, A. Synaptic plasticity in cnga3(−/−) mice: Cone bipolar cells react on the missing cone input and form ectopic synapses with rods. J. Neurosci. 2006, 26, 5248–5255. [Google Scholar] [CrossRef] [PubMed]
- Boudard, D.L.; Tanimoto, N.; Huber, G.; Beck, S.C.; Seeliger, M.W.; Hicks, D. Cone loss is delayed relative to rod loss during induced retinal degeneration in the diurnal cone-rich rodent Arvicanthis ansorgei. Neuroscience 2010, 169, 1815–1830. [Google Scholar] [CrossRef] [PubMed]
- Camacho, E.T.; Colon Velez, M.A.; Hernandez, D.J.; Rodriguez Bernier, U.; Van Laarhoven, J.; Wirkus, S. A mathematical model for photoreceptor interactions. J. Theor. Biol. 2010, 267, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.G.; Freed, M.A.; Sterling, P. Microcircuitry of the dark-adapted cat retina: Functional architecture of the rod-cone network. J. Neurosci. 1986, 6, 3505–3517. [Google Scholar] [PubMed]
- Bloomfield, S.A.; Dacheux, R.F. Rod vision: Pathways and processing in the mammalian retina. Prog. Retin. Eye Res. 2001, 20, 351–384. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Darrow, R.M.; Barsalou, L.; Kutty, R.K.; Wiggert, B. Susceptibility to retinal light damage in transgenic rats with rhodopsin mutations. Investig. Ophthalmol. Vis. Sci. 2003, 44, 486–492. [Google Scholar] [CrossRef]
- Naash, M.L.; Peachey, N.S.; Li, Z.Y.; Gryczan, C.C.; Goto, Y.; Blanks, J.; Milam, A.H.; Ripps, H. Light-induced acceleration of photoreceptor degeneration in transgenic mice expressing mutant rhodopsin. Investig. Ophthalmol. Vis. Sci. 1996, 37, 775–782. [Google Scholar]
- Novarino, G.; Akizu, N.; Gleeson, J.G. Modeling human disease in humans: The ciliopathies. Cell 2011, 147, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Drivas, T.G.; Wojno, A.P.; Tucker, B.A.; Stone, E.M.; Bennett, J. Basal exon skipping and genetic pleiotropy: A predictive model of disease pathogenesis. Sci. Transl. Med. 2015, 7, 291ra297. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, F.; Casteels, I.; Meire, F.; De Jaegere, S.; Hooghe, S.; van Regemorter, N.; Van Esch, H.; Matuleviciene, A.; Nunes, L.; Meersschaut, V.; et al. Genetic screening of LCA in Belgium: Predominance of CEP290 and identification of potential modifier alleles in AHI1 of CEP290-related phenotypes. Hum. Mutat. 2010, 31, E1709–E1766. [Google Scholar] [CrossRef] [PubMed]
- Walia, S.; Fishman, G.A.; Jacobson, S.G.; Aleman, T.S.; Koenekoop, R.K.; Traboulsi, E.I.; Weleber, R.G.; Pennesi, M.E.; Heon, E.; Drack, A.; et al. Visual acuity in patients with Leber’s congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology 2010, 117, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Brancati, F.; Barrano, G.; Silhavy, J.L.; Marsh, S.E.; Travaglini, L.; Bielas, S.L.; Amorini, M.; Zablocka, D.; Kayserili, H.; Al-Gazali, L.; et al. CEP290 mutations are frequently identified in the oculo-renal form of joubert syndrome-related disorders. Am. J. Hum. Genet. 2007, 81, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Halbritter, J.; Diaz, K.; Chaki, M.; Porath, J.D.; Tarrier, B.; Fu, C.; Innis, J.L.; Allen, S.J.; Lyons, R.H.; Stefanidis, C.J.; et al. High-throughput mutation analysis in patients with a nephronophthisis-associated ciliopathy applying multiplexed barcoded array-based PCR amplification and next-generation sequencing. J. Med. Genet. 2012, 49, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.M. Leber congenital amaurosis - a model for efficient genetic testing of heterogeneous disorders: LVIX Edward Jackson memorial lecture. Am. J. Ophthalmol. 2007, 144, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Papon, J.F.; Perrault, I.; Coste, A.; Louis, B.; Gerard, X.; Hanein, S.; Fares-Taie, L.; Gerber, S.; Defoort-Dhellemmes, S.; Vojtek, A.M.; et al. Abnormal respiratory cilia in non-syndromic Leber congenital amaurosis with CEP290 mutations. J. Med. Genet. 2010, 47, 829–834. [Google Scholar] [CrossRef] [PubMed]
| Patient ID | II-1 | II-2 |
|---|---|---|
| Sex | Female | Male |
| Age at diagnosis | 3 | 2 |
| Age recent examination | 46 | 45 |
| Nystagmus | Present | Present |
| Visual acuity RE | 20/100 | 20/80 |
| Visual acuity LE | 20/100 | 20/160 |
| Refraction | RE: +1.25–2.25 × 19; LE: −0.25–3.00 × 170 | RE: −14.25–1.75 × 5; LE: −10.25–2.25 × 155 |
| Lens | Clear | Clear |
| Fundus | Pink optic discs, mildly attenuated arterioles, coarse-grained aspect RPE posterior pole and mid-periphery superior quadrants, faintly recognizable foveola reflex BE with subtle indication for bull’s eye-like maculopathy, RPE atrophy in far periphery of superior quadrants, RPE atrophy in mid- and far periphery of inferior quadrants with scarce bone-spicule pigmentations | Pink, myopic optic discs, mildly attenuated arterioles, subtle RPE alterations macula RE, ring-shaped atrophy surrounding the fovea LE, faintly recognizable foveola reflex BE, perimacular RPE atrophy, mild RPE changes superior quadrants, RPE atrophy inferior quadrants with bone-spicule pigmentations |
| Fundus autofluorescence | Relatively normal | Double hyperautofluorescent ring, hypoautofluorescent spots along and inferior to the inferior vascular arcade BE, and in macula LE |
| OCT | Failed | No discernible photoreceptor complexes at the macula, but present at the peripheral part of the scan. |
| Color vision (Panel D-15) | Saturated: normal Desaturated: minor errors RE, multiple errors LE, no specific axis | RE: de- and saturated: multiple errors mainly in tritan axis LE: failed |
| Visual field (Goldmann) | Radius < 100 | Altitudinal defect BE, partially including the center RE, central scotoma LE |
| ERG Dark adapted | Mildly reduced isolated rod responses with significantly reduced ‘mixed’ responses | Significantly reduced isolated rod and ‘mixed’ responses |
| ERG Light adapted | Non-recordable | Non-recordable |
| Miscellaneous | Anorexia, depressions, followed by the diagnosis of schizofrenia at age 29 | None |
| First Allele | Second Allele | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample ID | Diagnosis | DNA Variant | Predicted Protein Variant | Predicted Proteins Based on RNA Study | DNA Variant | Predicted Protein Variant | Predicted Proteins Based on RNA Study | Ref. |
| Compound heterozygous | ||||||||
| Family A | OT | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] # | c.451C>T | p.(Arg151*) | p.[Arg151*, Leu148_Glu165del, Leu148_Lys172del] | This study |
| 809 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.1709C>G | p.(Ser570*) | ND | [10] |
| LCA-6 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.2991+1655A>G | p.(Cys998*) | p.[Cys998*, =] $ | [41] |
| LCA-7 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.2991+1655A>G | p.(Cys998*) | p.[Cys998*, =] | [41] |
| LCA-8 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.2991+1655A>G | p.(Cys998*) | p.[Cys998*, =] | [41] |
| LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.2991+1655A>G | p.(Cys998*) | p.[Cys998*, =] | [42] | |
| LCA-24 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4696G>C | p.(Ala1556Pro) | ND | [41] |
| COR031/CORS1 | CORS | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4393C>T | p.(Arg1465*) | ND | [10,43] |
| SLS-2 | SLSN | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4393C>T | p.(Arg1465*) | ND | [41] |
| SLS-3 | SLSN | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4393C>T | p.(Arg1465*) | ND | [41] |
| F283-21 | SLSN | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.1984C>T | p.(Gln662*) | ND | [44] |
| A3100-21 | SLSN | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.1987A>T | p.(Lys663*) | ND | [44] |
| A1210-21 | SLSN | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.3802C>T | p.(Gln1268*) | ND | [44] |
| F118-21 | SLSN | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4452_4455delAGAA | p.(Lys1484Asnfs*4) | ND | [44] |
| A1712-21 | SLSN | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.1189+1A>G | p.(?) | ND | [44] |
| Homozygous | ||||||||
| 1 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | [46] |
| 2 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | [46] |
| 738 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | [10] |
| 848 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | [10] |
| 258 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | [10] |
| 419 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | [10] |
| LEP | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | [10] |
| LCA-25 | LCA | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | [41] |
| 623 | JBTS+retina | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | c.4723A>T | p.(Lys1575*) | p.[Lys1575*, Glu1569_Trp1604del] | [10] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roosing, S.; Cremers, F.P.M.; Riemslag, F.C.C.; Zonneveld-Vrieling, M.N.; Talsma, H.E.; Klessens-Godfroy, F.J.M.; Den Hollander, A.I.; Van den Born, L.I. A Rare Form of Retinal Dystrophy Caused by Hypomorphic Nonsense Mutations in CEP290. Genes 2017, 8, 208. https://doi.org/10.3390/genes8080208
Roosing S, Cremers FPM, Riemslag FCC, Zonneveld-Vrieling MN, Talsma HE, Klessens-Godfroy FJM, Den Hollander AI, Van den Born LI. A Rare Form of Retinal Dystrophy Caused by Hypomorphic Nonsense Mutations in CEP290. Genes. 2017; 8(8):208. https://doi.org/10.3390/genes8080208
Chicago/Turabian StyleRoosing, Susanne, Frans P. M. Cremers, Frans C. C. Riemslag, Marijke N. Zonneveld-Vrieling, Herman E. Talsma, Francoise J. M. Klessens-Godfroy, Anneke I. Den Hollander, and L. Ingeborgh Van den Born. 2017. "A Rare Form of Retinal Dystrophy Caused by Hypomorphic Nonsense Mutations in CEP290" Genes 8, no. 8: 208. https://doi.org/10.3390/genes8080208
APA StyleRoosing, S., Cremers, F. P. M., Riemslag, F. C. C., Zonneveld-Vrieling, M. N., Talsma, H. E., Klessens-Godfroy, F. J. M., Den Hollander, A. I., & Van den Born, L. I. (2017). A Rare Form of Retinal Dystrophy Caused by Hypomorphic Nonsense Mutations in CEP290. Genes, 8(8), 208. https://doi.org/10.3390/genes8080208




