Evolutionary Significance of Wolbachia-to-Animal Horizontal Gene Transfer: Female Sex Determination and the f Element in the Isopod Armadillidium vulgare
Abstract
1. Introduction
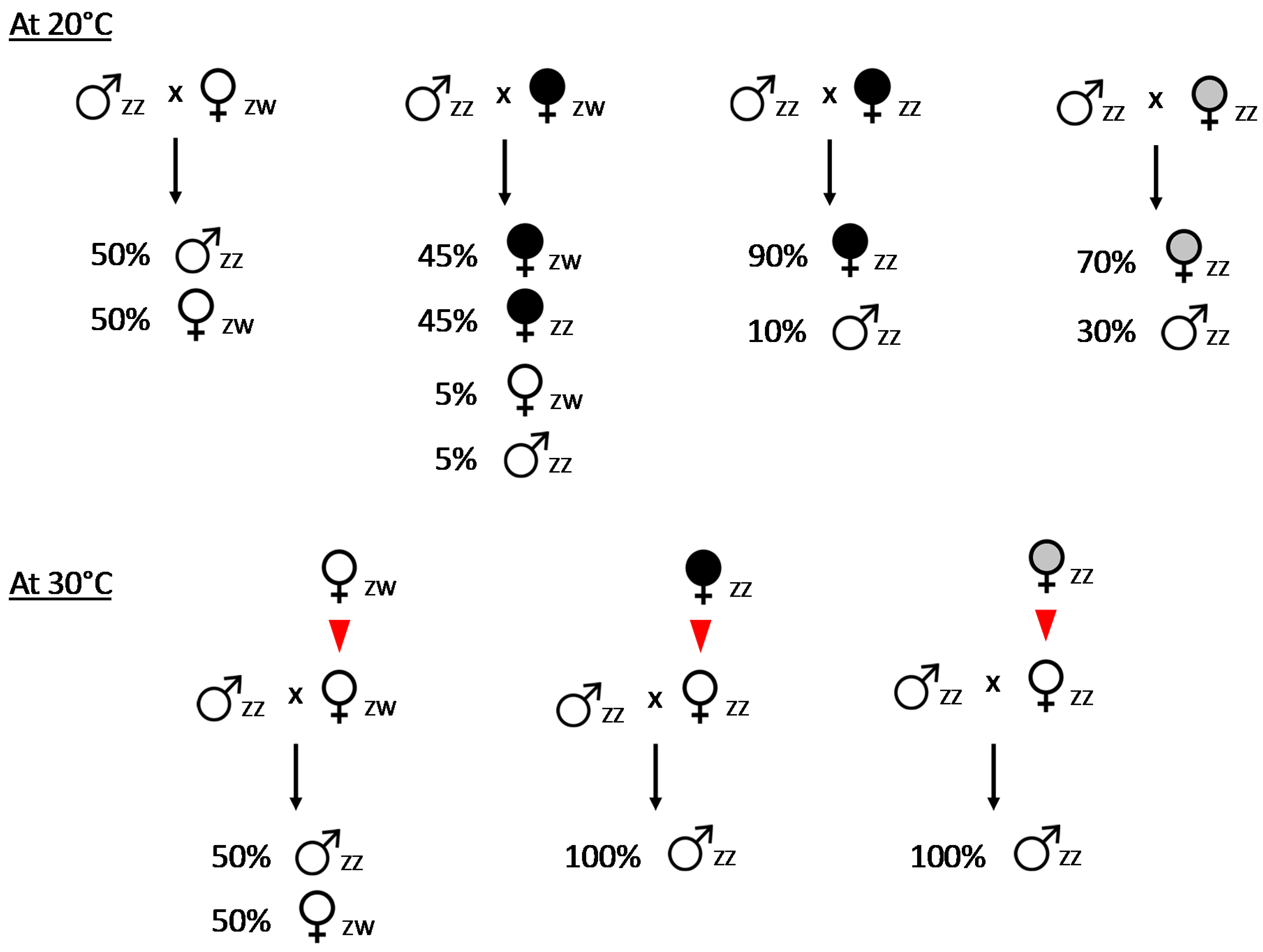
2. Feminizing Wolbachia and Sex Determination in Armadillidium vulgare
3. Characterization of the f Element of Armadillidium vulgare
4. Outstanding Questions Regarding the f Element
4.1. What Is the Molecular Genetic Basis of Feminization Induced by the f Element?
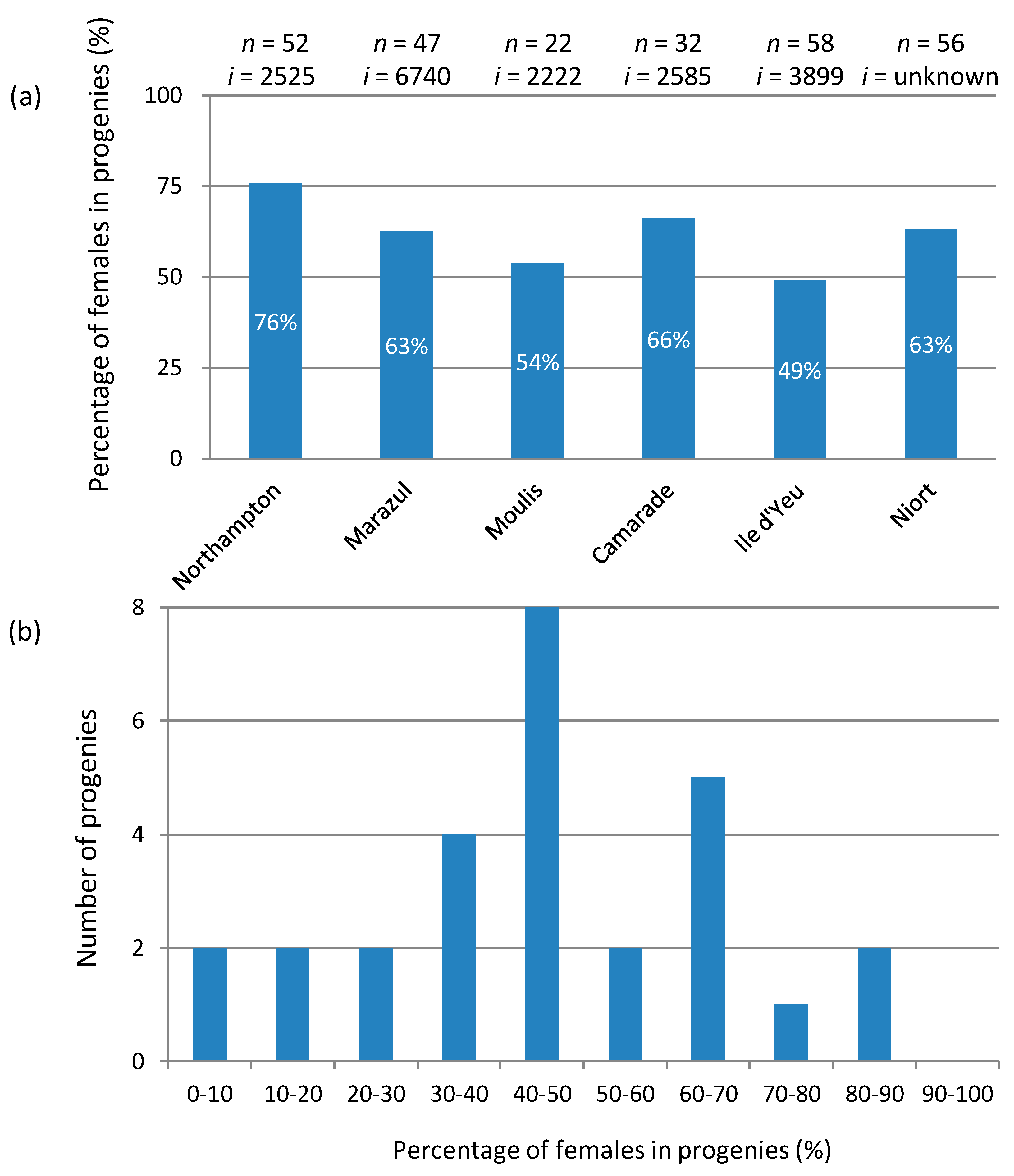
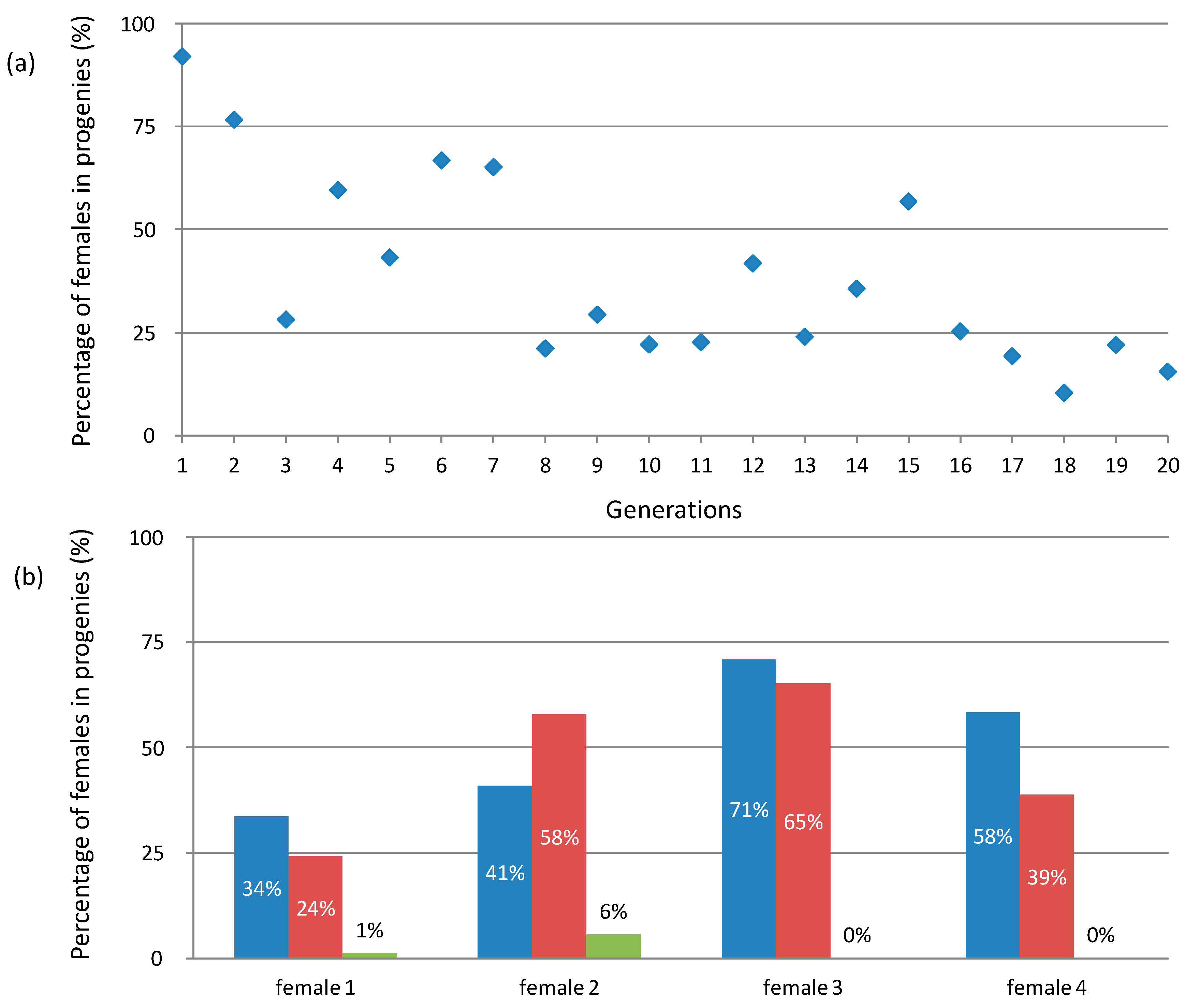
4.2. How to Explain the Unstable Inheritance of the f Element?
4.3. What Is the Population Dynamics of the f Element?
4.4. What Are the Patterns of Molecular Evolution of the f Element?
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ochman, H.; Lawrence, J.G.; Groisman, E.A. Lateral gene transfer and the nature of bacterial innovation. Nature 2000, 405, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Soucy, S.M.; Huang, J.L.; Gogarten, J.P. Horizontal gene transfer: Building the web of life. Nat. Rev. Genet. 2015, 16, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.O. Gene transfer and diversification of microbial eukaryotes. Annu. Rev. Microbiol. 2009, 63, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Keeling, P.J.; Palmer, J.D. Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 2008, 9, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Schaack, S.; Gilbert, C.; Feschotte, C. Promiscuous DNA: Horizontal transfer of transposable elements and why it matters for eukaryotic evolution. Trends Ecol. Evol. 2010, 25, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Dotto, B.R.; Carvalho, E.L.; Silva, A.F.; Silva, L.F.D.; Pinto, P.M.; Ortiz, M.F.; Wallau, G.L. HTT-DB: Horizontally transferred transposable elements database. Bioinformatics 2015, 31, 2915–2917. [Google Scholar] [CrossRef] [PubMed]
- Peccoud, J.; Loiseau, V.; Cordaux, R.; Gilbert, C. Massive horizontal transfer of transposable elements in insects. Proc. Natl. Acad. Sci. USA 2017, 114, 4721–4726. [Google Scholar] [CrossRef] [PubMed]
- Hazkani-Covo, E.; Zeller, R.M.; Martin, W. Molecular poltergeists: Mitochondrial DNA copies (numts) in sequenced nuclear genomes. PLoS Genet. 2010, 6, e1000834. [Google Scholar] [CrossRef] [PubMed]
- Dunning Hotopp, J.C. Horizontal gene transfer between bacteria and animals. Trends Genet. 2011, 27, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Boto, L. Horizontal gene transfer in the acquisition of novel traits by metazoans. Proc. R. Soc. B-Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [PubMed]
- Danchin, E.G.J.; Rosso, M.N.; Vieira, P.; de Almeida-Engler, J.; Coutinho, P.M.; Henrissat, B.; Abad, P. Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematodes. Proc. Natl. Acad. Sci. USA 2010, 107, 17651–17656. [Google Scholar] [CrossRef] [PubMed]
- Wybouw, N.; Pauchet, Y.; Heckel, D.G.; Van Leeuwen, T. Horizontal gene transfer contributes to the evolution of arthropod herbivory. Genome Biol. Evol. 2016, 8, 1785–1801. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Novelli, J.; Jiang, D.; Dailey, H.A.; Landmann, F.; Ford, L.; Taylor, M.J.; Carlow, C.K.S.; Kumar, S.; Foster, J.M.; et al. Interdomain lateral gene transfer of an essential ferrochelatase gene in human parasitic nematodes. Proc. Natl. Acad. Sci. USA 2013, 110, 7748–7753. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.; Daugherty, M.D.; Peterson, S.B.; Biboy, J.; Yang, Y.Y.; Jutras, B.L.; Fritz-Laylin, L.K.; Ferrin, M.A.; Harding, B.N.; Jacobs-Wagner, C.; et al. Transferred interbacterial antagonism genes augment eukaryotic innate immune function. Nature 2015, 518, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.A.; Funkhouser-Jones, L.J.; Brileya, K.; Reysenbach, A.-L.; Bordenstein, S.R. Antibacterial gene transfer across the tree of life. ELife 2014, 3, e04266. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Makepeace, B.L.; Gill, A.C. Wolbachia. In Rickettsiales; Thomas, S., Ed.; Springer International Publishing AG: Basel, Switzerland, 2016; pp. 465–512. [Google Scholar]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.A.; Araujo-Jnr, E.V.; Ahmed, M.Z.; Welch, J.J. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. B-Biol. Sci. 2015, 282. [Google Scholar] [CrossRef] [PubMed]
- Sicard, M.; Dittmer, J.; Greve, P.; Bouchon, D.; Braquart-Varnier, C. A host as an ecosystem: Wolbachia coping with environmental constraints. Environ. Microbiol. 2014, 16, 3583–3607. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Nikoh, N.; Ijichi, N.; Shimada, M.; Fukatsu, T. Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proc. Natl. Acad. Sci. USA 2002, 99, 14280–14285. [Google Scholar] [CrossRef] [PubMed]
- Fenn, K.; Conlon, C.; Jones, M.; Quail, M.A.; Holroyd, N.E.; Parkhill, J.; Blaxter, M. Phylogenetic relationships of the Wolbachia of nematodes and arthropods. PLoS Pathog. 2006, 2, e94. [Google Scholar] [CrossRef] [PubMed]
- Dunning Hotopp, J.C.; Clark, M.E.; Oliveira, D.C.S.G.; Foster, J.M.; Fischer, P.; Munoz Torres, M.C.; Giebel, J.D.; Kumar, N.; Ishmael, N.; Wang, S.; et al. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science 2007, 317, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Richards, S.; Desjardins, C.A.; Niehuis, O.; Gadau, J.; Colbourne, J.K.; Beukeboom, L.W.; Desplan, C.; Elsik, C.G.; Grimmelikhuijzen, C.J.P.; et al. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science 2010, 327, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Klasson, L.; Kumar, N.; Bromley, R.; Sieber, K.; Flowers, M.; Ott, S.H.; Tallon, L.J.; Andersson, S.G.E.; Hotopp, J.C.D. Extensive duplication of the Wolbachia DNA in chromosome four of Drosophila ananassae. BMC Genom. 2014, 15, 1097. [Google Scholar] [CrossRef] [PubMed]
- Funkhouser-Jones, L.J.; Sehnert, S.R.; Martínez-Rodríguez, P.; Toribio-Fernández, R.; Pita, M.; Bella, J.L.; Bordenstein, S.R. Wolbachia co-infection in a hybrid zone: Discovery of horizontal gene transfers from two Wolbachia supergroups into an animal genome. PeerJ 2015, 3, e1479. [Google Scholar] [CrossRef] [PubMed]
- Nikoh, N.; Nakabachi, A. Aphids acquired symbiotic genes via lateral gene transfer. BMC Biol. 2009, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Husnik, F.; Nikoh, N.; Koga, R.; Ross, L.; Duncan, R.P.; Fujie, M.; Tanaka, M.; Satoh, N.; Bachtrog, D.; Wilson, A.C.C.; et al. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell 2013, 153, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, P.; Lu, Y.; Kumar, N.; Creasy, T.; Daugherty, S.; Chibucos, M.C.; Orvis, J.; Shetty, A.; Ott, S.; Flowers, M.; et al. Rapid transcriptome sequencing of an invasive pest, the brown marmorated stink bug Halyomorpha halys. BMC Genom. 2014, 15, 738. [Google Scholar] [CrossRef] [PubMed]
- Klasson, L.; Kambris, Z.; Cook, P.E.; Walker, T.; Sinkins, S.P. Horizontal gene transfer between Wolbachia and the mosquito Aedes aegypti. BMC Genom. 2009, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Woolfit, M.; Iturbe-Ormaetxe, I.; McGraw, E.A.; O’Neill, S.L. An ancient horizontal gene transfer between mosquito and the endosymbiotic bacterium Wolbachia pipientis. Mol. Biol. Evolut. 2009, 26, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Nikoh, N.; Tanaka, K.; Shibata, F.; Kondo, N.; Hizume, M.; Shimada, M.; Fukatsu, T. Wolbachia genome integrated in an insect chromosome: Evolution and fate of laterally transferred endosymbiont genes. Genome Res. 2008, 18, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Brelsfoard, C.; Tsiamis, G.; Falchetto, M.; Gomulski, L.M.; Telleria, E.; Alam, U.; Doudoumis, V.; Scolari, F.; Benoit, J.B.; Swain, M.; et al. Presence of extensive Wolbachia symbiont insertions discovered in the genome of its host Glossina morsitans morsitans. Plos Negl. Trop. Dis. 2014, 8, e2728. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, P.; Johnston, K.L.; Riley, D.R.; Kumar, N.; White, J.R.; Olarte, K.T.; Ott, S.; Tallon, L.J.; Foster, J.M.; Taylor, M.J.; et al. Extensively duplicated and transcriptionally active recent lateral gene transfer from a bacterial Wolbachia endosymbiont to its host filarial nematode Brugia malayi. BMC Genom. 2013, 14, 639. [Google Scholar] [CrossRef] [PubMed]
- McNulty, S.N.; Fischer, K.; Curtis, K.C.; Weil, G.J.; Brattig, N.W.; Fischer, P.U. Localization of Wolbachia-like gene transcripts and peptides in adult Onchocerca flexuosa worms indicates tissue specific expression. Parasites Vectors 2013, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Theze, J.; Chebbi, M.A.; Giraud, I.; Moumen, B.; Ernenwein, L.; Greve, P.; Gilbert, C.; Cordaux, R. Birth of a W sex chromosome by horizontal transfer of Wolbachia bacterial symbiont genome. Proc. Natl. Acad. Sci. USA 2016, 113, 15036–15041. [Google Scholar] [CrossRef] [PubMed]
- Cordaux, R.; Bouchon, D.; Greve, P. The impact of endosymbionts on the evolution of host sex-determination mechanisms. Trends Genet. 2011, 27, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, T.; Juchault, P.; Mocquard, J.P. The evolution of sex determination in isopod crustaceans. Bioessays 1997, 19, 409–416. [Google Scholar] [CrossRef]
- Rigaud, T. Inherited microorganisms and sex determination of arthropod hosts. In Influential Passengers: Inherited Microorganisms and Arthropod Reproduction; O’Neill, S.L., Hoffmann, A.A., Werren, J.H., Eds.; Oxford University Press: Oxford, UK, 1997; pp. 81–101. [Google Scholar]
- Bouchon, D.; Cordaux, R.; Grève, P. Feminizing Wolbachia and the evolution of sex determination in isopods. In Insect Symbiosis, Volume 3; Bourtzis, K., Miller, T., Eds.; Taylor and Francis Group LLC: Boca Raton, FL, USA, 2008; pp. 273–294. [Google Scholar]
- Hiroki, M.; Kato, Y.; Kamito, T.; Miura, K. Feminization of genetic males by a symbiotic bacterium in a butterfly, Eurema hecabe (Lepidoptera: Pieridae). Naturwissenschaften 2002, 89, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Narita, S.; Kageyama, D. Wolbachia-induced sex reversal in Lepidoptera. In Insect Symbiosis, Volume 3; Bourtzis, K., Miller, T., Eds.; Taylor and Francis Group LLC: Boca Raton, FL, USA, 2008; pp. 295–319. [Google Scholar]
- Negri, I.; Pellecchia, M.; Mazzoglio, P.J.; Patetta, A.; Alma, A. Feminizing Wolbachia in Zyginidia pullula (Insecta, Hemiptera), a leafhopper with an XX/X0 sex-determination system. Proc. Biol. Sci. 2006, 273, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Pannebakker, B.A.; van de Zande, L.; Schwander, T.; Wertheim, B.; Beukeboom, L.W. Diploid males support a two-step mechanism of endosymbiont-induced thelytoky in a parasitoid wasp. BMC Evol. Biol. 2015, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Juchault, P.; Legrand, J.J. Mise en évidence d’un micro-organisme intracytoplasmique symbiote de l’oniscoïde Armadillidium vulgare Latr., dont la présence accompagne l’intersexualité ou la féminisation total des mâles génétiques de la lignée thélygène. C. R. Acad. Sci. Paris 1973, 276, 2213–2216. [Google Scholar]
- Rigaud, T.; Souty-Grosset, C.; Raimond, R.; Mocquard, J.P.; Juchault, P. Feminizing endocytobiosis in the terrestrial crustacean Armadillidium vulgare Latr. (Isopoda): Recent acquisitions. Endocytobiosis Cell Res. 1991, 7, 259–273. [Google Scholar]
- Cordaux, R.; Michel-Salzat, A.; Frelon-Raimond, M.; Rigaud, T.; Bouchon, D. Evidence for a new feminizing Wolbachia strain in the isopod Armadillidium vulgare: Evolutionary implications. Heredity 2004, 93, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Juchault, P.; Legrand, J.J. Croisement de néo-mâles experimentaux chez Armadillidium vulgare Latr. (Crustace, Isopode, Oniscoide). Mise en evidence d’une hétérogamétie femelle. C. R. Acad. Sci. Paris 1972, 274, 1387–1389. [Google Scholar]
- Juchault, P.; Martin, G.; Legrand, J.J. Induction par la température d’une physiologie mâle chez les néo-femelles et les intersexués du crustacé oniscoïde Armadillidium vulgare Latr. Hébergeant un bactéroïde à action féminisante. Int. J. Inv. Reprod. 1980, 2, 223–235. [Google Scholar] [CrossRef]
- Juchault, P.; Mocquard, J.P. Effets de la température sur le sex ratio de la descendance et la physiologie sexuelle des femelles d’Armadillidium vulgare Latr. (Crustacea, Oniscidea) hébergeant une bactérie féminisante. C. R. Acad. Sci. Paris 1988, 306, 321–324. [Google Scholar]
- Rigaud, T.; Juchault, P.; Mocquard, J.P. Experimental-study of temperature effects on the sex-ratio of broods in terrestrial crustacea Armadillidium vulgare Latr. possible implications in natural populations. J. Evolut. Biol. 1991, 4, 603–617. [Google Scholar] [CrossRef]
- Legrand, J.J.; Legrand-Hamelin, E.; Juchault, P. Sex determination in Crustacea. Biol. Rev. 1987, 62, 439–470. [Google Scholar] [CrossRef]
- Martin, G.; Sorokine, O.; Moniatte, M.; Bulet, P.; Hetru, C.; Van Dorsselaer, A. The structure of a glycosylated protein hormone responsible for sex determination in the isopod, Armadillidium vulgare. Eur. J. Biochem. 1999, 262, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Legrand, J.J.; Juchault, P. Rôle de bactéries symbiotiques dans l’intersexualité, la monogénie et la spéciation chez des crustacés oniscoïdes. Boll Zool. 1986, 53, 161–172. [Google Scholar] [CrossRef]
- Rigaud, T.; Juchault, P. Sterile intersexuality in an isopod induced by the interaction between a bacterium (Wolbachia) and the environment. Can. J. Zool. 1998, 76, 493–499. [Google Scholar]
- Taylor, D.R. Evolutionary consequences of cytoplasmic sex ratio distorters. Evolut. Ecol. 1990, 4, 235–248. [Google Scholar] [CrossRef]
- Ferdy, J.B.; Liu, N.; Sicard, M. Transmission modes and the evolution of feminizing symbionts. J. Evolut. Biol. 2016, 29, 2395–2409. [Google Scholar] [CrossRef] [PubMed]
- Juchault, P.; Legrand, J.J.; Mocquard, J.P. Contribution à l’étude qualitative et quantitative des facteurs contrôlant le sexe dans les populations du crustacé isopode terrestre Armadillidum vulgare Latreille. I. La population de niort (deux sèvres). Arch. Zool. Exp. Gen. 1980, 121, 3–27. [Google Scholar]
- Juchault, P.; Rigaud, T.; Mocquard, J.P. Evolution of sex determination and sex-ratio variability in wild populations of Armadillidium vulgare (Latr) (Crustacea, Isopoda)—A case-study in conflict-resolution. Acta Oecol.-Int. J. Ecol. 1993, 14, 547–562. [Google Scholar]
- Juchault, P.; Legrand, J.J. Modification de la sex ratio dans les croisements entre différentes populations du crustacé oniscoïde Armadllidium vulgare Latr. Notion de déterminisme polygénique et épigénétique du sexe. Arch. Zool. Exp. Gen. 1976, 117, 81–93. [Google Scholar]
- Legrand, J.J.; Juchault, P. Nouvelles donnees sur le determinisme genetique et epigenetique de la monogenie chez le crustace isopode terrestre Armadillidium vulgare Latr. Genet. Sel. Evol. 1984, 16, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Juchault, P.; Rigaud, T.; Mocquard, J.P. Evolution of sex-determining mechanisms in a wild population of Armadillidium vulgare Latr. (Crustacea, Isopoda)—Competition between 2 feminizing parasitic sex factors. Heredity 1992, 69, 382–390. [Google Scholar] [CrossRef]
- Juchault, P.; Legrand, J.J. Contribution à l’étude qualitative et quantitative des facteurs contrôlant le sexe dans les populations du crustacé isopode terrestre Armadillidum vulgare Latreille. III Populations n’hébergeant pas le facteur féminisant f (bactéroïde intracytoplasmique). Arch. Zool. Exp. Gen. 1981, 122, 117–131. [Google Scholar]
- Juchault, P.; Legrand, J.J. Etude génétique de l’intersexualité des mâles à ouvertures géntiales femelles chez l’oniscoïde Armadillidium vulgare Latr.: Interprétation et modalités de la transmission héréditaire. C. R. Soc. Biol. 1976, 170, 429–433. [Google Scholar]
- Rigaud, T.; Juchault, P. Conflict between feminizing sex ratio distorters and an autosomal masculinizing gene in the terrestrial isopod Armadillidium vulgare Latr. Genetics 1993, 133, 247–252. [Google Scholar] [PubMed]
- Legrand, J.J.; Juchault, P. Le déterminisme de l’intersexualité chez les crustacés isopodes terrestres; corrélation entre intersexualité et monogénie. CR Acad. Sci. Paris 1969, 268, 1647–1649. [Google Scholar]
- Juchault, P.; Mocquard, J.P. Transfer of a parasitic sex factor to the nuclear genome of the host - a hypothesis on the evolution of sex-determining mechanisms in the terrestrial isopod Armadillidium vulgare Latr. J. Evolut. Biol. 1993, 6, 511–528. [Google Scholar] [CrossRef]
- Bergero, R.; Charlesworth, D. The evolution of restricted recombination in sex chromosomes. Trends Ecol. Evol. 2009, 24, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Chilton, M.D.; Drummond, M.H.; Merio, D.J.; Sciaky, D.; Montoya, A.L.; Gordon, M.P.; Nester, E.W. Stable incorporation of plasmid DNA into higher plant cells: The molecular basis of crown gall tumorigenesis. Cell 1977, 11, 263–271. [Google Scholar] [CrossRef]
- Sagi, A.; Manor, R.; Ventura, T. Gene silencing in Crustaceans: From basic research to biotechnologies. Genes 2013, 4, 620–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hastings, M.H.; Green, E.W.; Tauber, E.; Sladek, M.; Webster, S.G.; Kyriacou, C.P.; Wilcockson, D.C. Dissociation of circadian and circatidal timekeeping in the marine crustacean Eurydice pulchra. Curr. Biol. 2013, 23, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Serano, J.M.; Jarvis, E.; Bruce, H.S.; Wang, J.; Ray, S.; Barker, C.A.; O’Connell, L.C.; Patel, N.H. CRISPR/Cas9 mutagenesis reveals versatile roles of HOX genes in crustacean limb specification and evolution. Curr. Biol. 2016, 26, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Troadec, C.; Boualem, A.; Rajab, M.; Fernandez, R.; Morin, H.; Pitrat, M.; Dogimont, C.; Bendahmane, A. A transposon-induced epigenetic change leads to sex determination in melon. Nature 2009, 461, 1135–1237. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Beukeboom, L.W. Sex determination, sex ratios, and genetic conflict. Annu. Rev. Ecol. Syst. 1998, 29, 233–261. [Google Scholar] [CrossRef]
- Beukeboom, L.W.; Perrin, N. The Evolution of Sex Determination; Oxford University Press: Oxford, UK, 2014; p. 222. [Google Scholar]
- Jaenike, J. Sex chromosome meiotic drive. Annu. Rev. Ecol. Syst. 2001, 32, 25–49. [Google Scholar] [CrossRef]
- Burt, A.; Trivers, R. Genes in Conflict; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Lindholm, A.K.; Dyer, K.A.; Firman, R.C.; Fishman, L.; Forstmeier, W.; Holman, L.; Johannesson, H.; Knief, U.; Kokko, H.; Larracuente, A.M.; et al. The ecology and evolutionary dynamics of meiotic drive. Trends Ecol. Evol. 2016, 31, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.; Cook, J.M.; Kageyama, D.; Riegler, M. Double trouble: Combined action of meiotic drive and Wolbachia feminization in Eurema butterflies. Biol. Lett. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.P.M.; Sharbel, T.F.; Beukeboom, L.W. B-chromosome evolution. Philos. Trans. R. Soc. Lond. Ser. B-Biol. Sci. 2000, 355, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Artault, J.C. Contribution à L’étude des Garnitures Chromosomiques Chez Quelques Crustacés Isopodes. Ph.D. Thesis, University of Poitiers, Poitiers, France, 1977. [Google Scholar]
- Bailly-Bechet, M.; Martins-Simoes, P.; Szollosi, G.; Mialdea, G.; Sagot, M.F.; Charlat, S. How long does Wolbachia remain on board? Mol. Biol. Evol. 2017, 34, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Bertin, A.; Caubet, Y.; Rigaud, T. Sexual selection in an isopod with Wolbachia-induced sex reversal: Males prefer real females. J. Evolut. Biol. 2001, 14, 388–394. [Google Scholar] [CrossRef]
- Rigaud, T.; Moreau, M. A cost of Wolbachia-induced sex reversal and female-biased sex ratios: Decrease in female fertility after sperm depletion in a terrestrial isopod. Proc. R. Soc. B-Biol. Sci. 2004, 271, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Braquart-Varnier, C.; Lachat, M.; Herbiniere, J.; Johnson, M.; Caubet, Y.; Bouchon, D.; Sicard, M. Wolbachia mediate variation of host immunocompetence. PLoS ONE 2008, 3, e3286. [Google Scholar] [CrossRef] [PubMed]
- Sicard, M.; Chevalier, F.; De Vlechouver, M.; Bouchon, D.; Greve, P.; Braquart-Varnier, C. Variations of immune parameters in terrestrial isopods: A matter of gender, aging and Wolbachia. Naturwissenschaften 2010, 97, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.; Richard, F.J. Intra-cellular bacterial infections affect learning and memory capacities of an invertebrate. Front. Zool. 2015, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Braquart-Varnier, C.; Altinli, M.; Pigeault, R.; Chevalier, F.D.; Greve, P.; Bouchon, D.; Sicard, M. The mutualistic side of Wolbachia-isopod interactions: Wolbachia mediated protection against pathogenic intracellular bacteria. Front. Microbiol. 2015, 6, 1388. [Google Scholar] [CrossRef] [PubMed]
- Juchault, P.; Legrand, J.J. Contribution à l’étude qualitative et quantitative des facteurs contrôlant le sexe dans les populations du crustacé isopode terrestre Armadillidum vulgare Latreille. II Populations hébergeant le facteur féminisant f (bactéroïde intracytoplasmique). Arch. Zool. Exp. Gen. 1981, 122, 65–74. [Google Scholar]
- Cordaux, R.; Pichon, S.; Hatira, H.B.; Doublet, V.; Greve, P.; Marcade, I.; Braquart-Varnier, C.; Souty-Grosset, C.; Charfi-Cheikhrouha, F.; Bouchon, D. Widespread Wolbachia infection in terrestrial isopods and other crustaceans. ZooKeys 2012, 176, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Bachtrog, D. Y-chromosome evolution: Emerging insights into processes of Y-chromosome degeneration. Nat. Rev. Genet. 2013, 14, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Caubet, Y.; Hatcher, M.J.; Mocquard, J.P.; Rigaud, T. Genetic conflict and changes in heterogametic mechanisms of sex determination. J. Evol. Biol. 2000, 13, 766–777. [Google Scholar] [CrossRef]
- Bouchon, D.; Rigaud, T.; Juchault, P. Evidence for widespread Wolbachia infection in isopod crustaceans: Molecular identification and host feminization. Proc. Biol. Sci. 1998, 265, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Juchault, P.; Rigaud, T. Evidence for female heterogamety in two terrestrial crustaceans and the problem of sex chromosome evolution in isopods. Heredity 1995, 75, 466–471. [Google Scholar] [CrossRef]
- Becking, T.; Giraud, I.; Raimond, M.; Moumen, B.; Chandler, C.; Cordaux, R.; Gilbert, C. Diversity and evolution of sex determination systems in terrestrial isopods. Sci. Rep. 2017, 7, 1084. [Google Scholar] [CrossRef] [PubMed]

| Biological Feature | Feminizing Wolbachia | f Element |
|---|---|---|
| Conversion of genetic males into phenotypic females | Yes | Yes |
| Presence of cytoplasmic microorganisms | Yes (bacteria) | No |
| Thermosensitivity | Yes | Yes |
| Mendelian inheritance | No | No |
| Mode of inheritance | Exclusively maternal | Mainly maternal, occasionally paternal |
| Stability of sex ratio bias in progenies | Yes (80–90% females) | No (60–70% females on average, but ranges from 0% to 100% females) |
| Presence of intersex individuals in progenies | Yes (up to 25%) | Rare (<1%) |
| Experimental reversal into males by implantation of androgenic gland | Failure | Success |
| Epistasis with masculinizing M gene | Wolbachia > M gene | M gene > f element |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordaux, R.; Gilbert, C. Evolutionary Significance of Wolbachia-to-Animal Horizontal Gene Transfer: Female Sex Determination and the f Element in the Isopod Armadillidium vulgare. Genes 2017, 8, 186. https://doi.org/10.3390/genes8070186
Cordaux R, Gilbert C. Evolutionary Significance of Wolbachia-to-Animal Horizontal Gene Transfer: Female Sex Determination and the f Element in the Isopod Armadillidium vulgare. Genes. 2017; 8(7):186. https://doi.org/10.3390/genes8070186
Chicago/Turabian StyleCordaux, Richard, and Clément Gilbert. 2017. "Evolutionary Significance of Wolbachia-to-Animal Horizontal Gene Transfer: Female Sex Determination and the f Element in the Isopod Armadillidium vulgare" Genes 8, no. 7: 186. https://doi.org/10.3390/genes8070186
APA StyleCordaux, R., & Gilbert, C. (2017). Evolutionary Significance of Wolbachia-to-Animal Horizontal Gene Transfer: Female Sex Determination and the f Element in the Isopod Armadillidium vulgare. Genes, 8(7), 186. https://doi.org/10.3390/genes8070186





