Influence of microRNAs and Long Non-Coding RNAs in Cancer Chemoresistance
Abstract
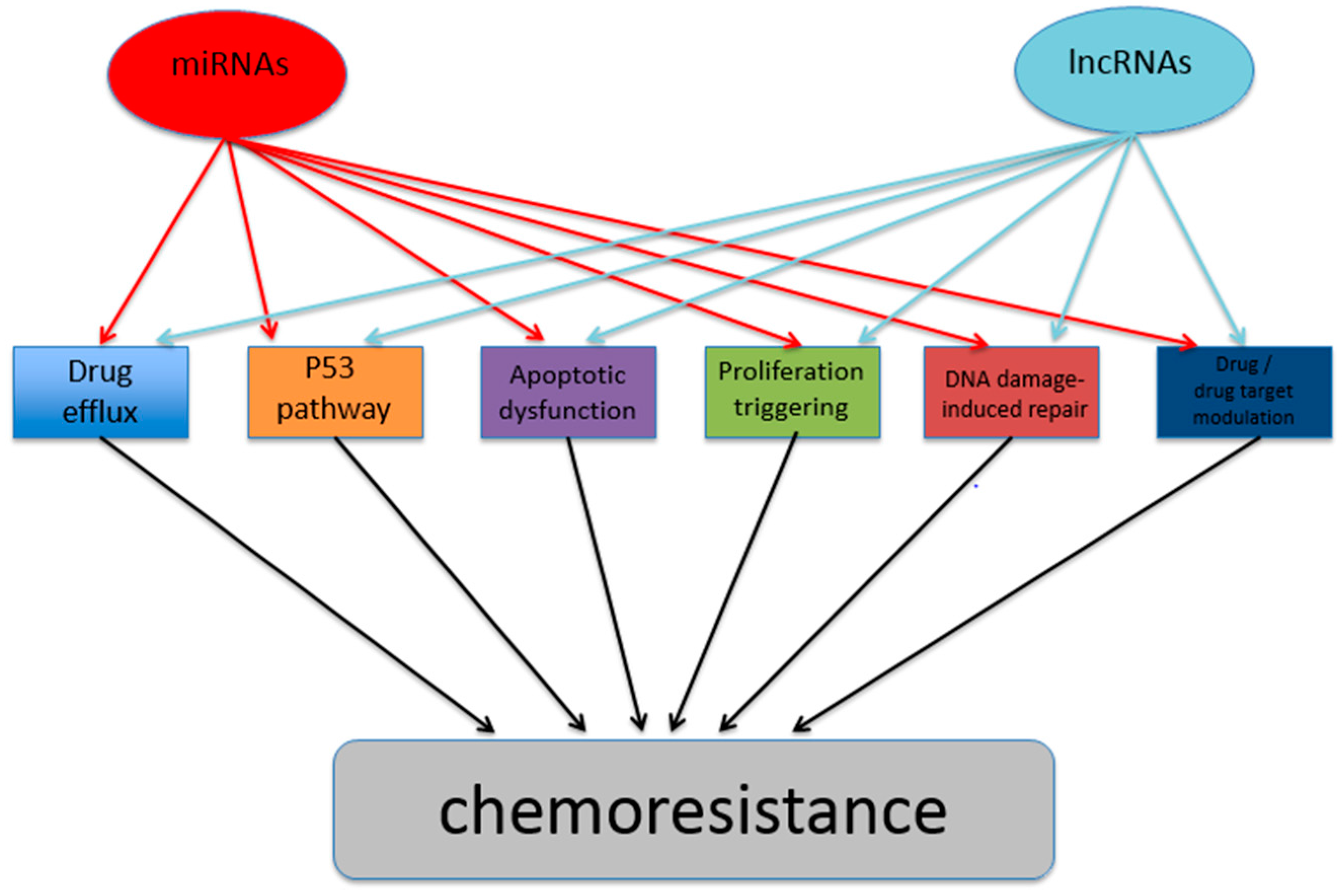
:1. Introduction
2. Cancer Chemoresistance Manifestations and Development Mechanisms
3. Influences of miRNAs in Cancer Chemoresistance
4. Influences of lncRNAs in Cancer Chemoresistance
5. Conclusions and Perspectives
Supplementary Materials
Conflicts of Interest
References
- Ayers, D.; Day, P.J. Unlocking the potential of RNA interference as a therapeutic tool. Malta Med. J. 2009, 21, 13–19. [Google Scholar]
- Ambros, V. microRNAs: Tiny regulators with great potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef]
- Ayers, D.; Baron, B.; Hunter, T. miRNA Influences in NRF2 Pathway Interactions within cancer models. J. Nucleic Acids 2015, 2015, 143636. [Google Scholar] [CrossRef] [PubMed]
- Stallings, R.L. MicroRNA involvement in the pathogenesis of neuroblastoma: Potential for microRNA mediated therapeutics. Curr. Pharm. Des. 2009, 15, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Ayers, D. Implications of miRNA-directed gene silencing in cancer. In RNAi Technology; Science Publishers: New York, NY, USA, 2011. [Google Scholar]
- Ayers, D. Long Non-Coding RNAs: Novel Emergent Biomarkers for Cancer Diagnostics. J. Cancer Res. Treat. 2013, 1, 31–35. [Google Scholar]
- Ayers, D.; Nasti, A. Utilisation of nanoparticle technology in cancer chemoresistance. J. Drug Deliv. 2012, 2012, 265691. [Google Scholar] [CrossRef]
- Lage, H. Gene Therapeutic Approaches to Overcome ABCB1-Mediated Drug Resistance. Recent Results Cancer Res. 2016, 209, 87–94. [Google Scholar]
- Schmitt, S.M.; Stefan, K.; Wiese, M. Pyrrolopyrimidine derivatives and purine analogs as novel activators of Multidrug Resistance-associated Protein 1 (MRP1, ABCC1). Biochim. Biophys. Acta 2017, 1859, 69–79. [Google Scholar] [CrossRef]
- Robey, R.W.; Massey, P.R.; Amiri-Kordestani, L.; Bates, S.E. ABC transporters: Unvalidated therapeutic targets in cancer and the CNS. Anticancer Agents Med. Chem. 2010, 10, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar]
- Colone, M.; Calcabrini, A.; Toccacieli, L.; Bozzuto, G.; Stringaro, A.; Gentile, M.; Cianfriglia, M.; Ciervo, A.; Caraglia, M.; Budillon, A.; et al. The multidrug transporter P-glycoprotein: A mediator of melanoma invasion? J. Investig. Dermatol. 2008, 128, 957–971. [Google Scholar] [CrossRef] [PubMed]
- MacLaine, N.J.; Hupp, T.R. How phosphorylation controls p53. Cell Cycle 2011, 10, 916–921. [Google Scholar] [CrossRef]
- Macchiarulo, A.; Giacchè, N.; Mancini, F.; Puxeddu, E.; Moretti, F.; Pellicciari, R. Alternative strategies for targeting mouse double minute 2 activity with small molecules: Novel patents on the horizon? Expert Opin. Ther. Pat. 2011, 21, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Mogi, A.; Kuwano, H. TP53 mutations in nonsmall cell lung cancer. J. Biomed. Biotechnol. 2011, 2011, 583929. [Google Scholar] [CrossRef] [PubMed]
- Plati, J.; Bucur, O.; Khosravi-Far, R. Apoptotic cell signaling in cancer progression and therapy. Integr. Biol. Quant. Biosci. Nano Macro 2011, 3, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Rolland, S.G.; Conradt, B. New role of the BCL2 family of proteins in the regulation of mitochondrial dynamics. Curr. Opin. Cell Biol. 2010, 22, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Camidge, D.R.; Ribeiro de Oliveira, M.; Bonomi, P.; Gandara, D.; Khaira, D.; Hann, C.L.; McKeegan, E.M.; Litvinovich, E.; Hemken, P.M.; et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J. Clin. Oncol. 2011, 29, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, R.; Xu, L.; Xie, S.; Dong, J.; Jing, Y. β-Elemene piperazine derivatives induce apoptosis in human leukemia cells through downregulation of c-FLIP and generation of ROS. PLoS ONE 2011, 6, e15843. [Google Scholar] [CrossRef] [PubMed]
- Avraham, R.; Yarden, Y. Feedback regulation of EGFR signalling: Decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 2011, 12, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.E.; Yeh, P.Y.; Lu, Y.S.; Lai, G.M.; Liao, C.M.; Gao, M.; Cheng, A.L. Basal levels and patterns of anticancer drug-induced activation of nuclear factor-kappaB (NF-κB), and its attenuation by tamoxifen, dexamethasone, and curcumin in carcinoma cells. Biochem. Pharmacol. 2002, 63, 1709–1716. [Google Scholar] [CrossRef]
- Olmos, Y.; Brosens, J.J.; Lam, E.W.-F. Interplay between SIRT proteins and tumour suppressor transcription factors in chemotherapeutic resistance of cancer. Drug Resist. Updat. 2011, 14, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Kothandapani, A.; Tillison, K.; Kalman-Maltese, V.; Patrick, S.M. Downregulation of XPF-ERCC1 enhances cisplatin efficacy in cancer cells. DNA Repair 2010, 9, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; An, J.Y.; Noh, S.H.; Shin, S.K.; Lee, Y.C.; Kim, H. High microsatellite instability predicts good prognosis in intestinal-type gastric cancers. J. Gastroenterol. Hepatol. 2011, 26, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.P.; Hamilton, T.C.; Schilder, R.J. Platinum resistance: The role of DNA repair pathways. Clin. Cancer Res. 2008, 14, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Assaraf, Y.G. Molecular basis of antifolate resistance. Cancer Metastasis Rev. 2007, 26, 153–181. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.; Esposito, M.T.; Gandillet, A.; Zeisig, B.B.; Griessinger, E.; Bonnet, D.; So, C.W.E. β-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell 2010, 18, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Singh, B.N.; Huang, Q.; Li, Z.; Gao, Y.; Mishra, P.; Hwa, Y.L.; Li, J.; Dowdy, S.C.; Jiang, S.-W. DNA hypermethylation as a chemotherapy target. Cell. Signal. 2011, 23, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Banerjee Mustafi, S.; Chakraborty, P.K.; Naz, S.; Dwivedi, S.K.D.; Street, M.; Basak, R.; Yang, D.; Ding, K.; Mukherjee, P.; Bhattacharya, R. MDR1 mediated chemoresistance: BMI1 and TIP60 in action. Biochim. Biophys. Acta 2016, 1859, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-R.; Ji, S.-Y.; Mia-Jan, K.; Cho, M.-Y. Chemoresistance of CD133(+) colon cancer may be related with increased survivin expression. Biochem. Biophys. Res. Commun. 2015, 463, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.-L.; Tang, Y.-B.; Song, F.-F.; Xu, L.; Ji, P.; Wang, S.-J.; Zhu, J.-M.; Zhang, Y.; Zhao, G.-P.; Wang, Y.; et al. DCTPP1 attenuates the sensitivity of human gastric cancer cells to 5-fluorouracil by up-regulating MDR1 expression epigenetically. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wei, X.; Lu, Y. Chaetominine reduces MRP1-mediated drug resistance via inhibiting PI3K/Akt/Nrf2 signaling pathway in K562/Adr human leukemia cells. Biochem. Biophys. Res. Commun. 2016, 473, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Wang, H.; Chen, T.; Chen, W.; Yang, L.; He, M.; Xu, S.; Wang, J. NOX1 mediates chemoresistance via HIF1α/MDR1 pathway in gallbladder cancer. Biochem. Biophys. Res. Commun. 2015, 468, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Gibb, E.A.; Brown, C.J.; Lam, W.L. The functional role of long non-coding RNA in human carcinomas. Mol. Cancer 2011, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Gibb, E.A.; Enfield, K.S.S.; Stewart, G.L.; Lonergan, K.M.; Chari, R.; Ng, R.T.; Zhang, L.; MacAulay, C.E.; Rosin, M.P.; Lam, W.L. Long non-coding RNAs are expressed in oral mucosa and altered in oral premalignant lesions. Oral Oncol. 2011, 47, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.C. Micro RNAs are complementary to 3’ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002, 30, 363–364. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.T.; Sharp, P.A. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 2002, 3, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Shamovsky, I.; Nudler, E. Gene control by large noncoding RNAs. Sci. STKE 2006, 2006, pe40. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Chetcuti, R.; Ayers, D. An Introspective Update on the Influence of miRNAs in Breast Carcinoma and Neuroblastoma Chemoresistance. Genet. Res. Int. 2014, 2014, 743050. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: MicroRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Croce, C.M. MicroRNAs as therapeutic targets in chemoresistance. Rev. Comment. Antimicrob. Anticancer Chemother. 2013, 16, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Van Peer, G.; Lefever, S.; Anckaert, J.; Beckers, A.; Rihani, A.; Van Goethem, A.; Volders, P.-J.; Zeka, F.; Ongenaert, M.; Mestdagh, P.; et al. miRBase Tracker: Keeping track of microRNA annotation changes. Database J. Biol. Databases Curation 2014, 2014, 3419–3420. [Google Scholar] [CrossRef] [PubMed]
- Amir, S.; Mabjeesh, N.J. microRNA expression profiles as decision-making biomarkers in the management of bladder cancer. Histol. Histopathol. 2017, 32, 107–119. [Google Scholar] [PubMed]
- Vinall, R.L.; Tepper, C.G.; Ripoll, A.A.Z.; Gandour-Edwards, R.F.; Durbin-Johnson, B.P.; Yap, S.A.; Ghosh, P.M.; deVere White, R.W. Decreased expression of let-7c is associated with non-response of muscle-invasive bladder cancer patients to neoadjuvant chemotherapy. Genes Cancer 2016, 7, 86–97. [Google Scholar] [PubMed]
- Kozinn, S.I.; Harty, N.J.; Delong, J.M.; Deliyiannis, C.; Logvinenko, T.; Summerhayes, I.C.; Libertino, J.A.; Holway, A.H.; Rieger-Christ, K.M. MicroRNA Profile to Predict Gemcitabine Resistance in Bladder Carcinoma Cell Lines. Genes Cancer 2013, 4, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Lv, L.; Li, Y.; Zhang, C.; Meng, F.; Pu, Y.; Xiao, J.; Qian, L.; Zhao, W.; Liu, Q.; et al. The miR-193a-3p regulated PSEN1 gene suppresses the multi-chemoresistance of bladder cancer. Biochim. Biophys. Acta 2015, 1852, 520–528. [Google Scholar] [CrossRef]
- Deng, H.; Lv, L.; Li, Y.; Zhang, C.; Meng, F.; Pu, Y.; Xiao, J.; Qian, L.; Zhao, W.; Liu, Q.; et al. miR-193a-3p regulates the multi-drug resistance of bladder cancer by targeting the LOXL4 gene and the oxidative stress pathway. Mol. Cancer 2014, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, H.; Lv, L.; Zhang, C.; Qian, L.; Xiao, J.; Zhao, W.; Liu, Q.; Zhang, D.; Wang, Y.; et al. The miR-193a-3p-regulated ING5 gene activates the DNA damage response pathway and inhibits multi-chemoresistance in bladder cancer. Oncotarget 2015, 6, 10195–10206. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-H.; Tao, Z.-H.; Zhang, J.; Li, T.; Ni, C.; Xie, J.; Zhang, J.-F.; Hu, X.-C. MiRNA-21 induces epithelial to mesenchymal transition and gemcitabine resistance via the PTEN/AKT pathway in breast cancer. Tumour Biol. 2016, 37, 7245–7254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Liu, P.; Chen, Q.; Situ, H.; Xie, T.; Zhang, J.; Peng, C.; Lin, Y.; Chen, J. MicroRNA-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 2014, 5, 7013–7026. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Guo, Y.-Q.; Tan, G.; Dong, L.; Cheng, L.; Li, K.-J.; Wang, Z.-Y.; Luo, H.-F. miR-125b regulates side population in breast cancer and confers a chemoresistant phenotype. J. Cell. Biochem. 2013, 114, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- He, D.-X.; Gu, X.-T.; Li, Y.-R.; Jiang, L.; Jin, J.; Ma, X. Methylation-regulated miR-149 modulates chemoresistance by targeting GlcNAc N-deacetylase/N-sulfotransferase-1 in human breast cancer. FEBS J. 2014, 281, 4718–4730. [Google Scholar] [CrossRef] [PubMed]
- He, D.-X.; Gu, X.-T.; Jiang, L.; Jin, J.; Ma, X. A methylation-based regulatory network for microRNA 320a in chemoresistant breast cancer. Mol. Pharmacol. 2014, 86, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Li, L.; Yang, S.; Wang, D.; Zhong, S.; Zhao, J.; Tang, J. MicroRNA-29a contributes to drug-resistance of breast cancer cells to adriamycin through PTEN/AKT/GSK3β signaling pathway. Gene 2016, 593, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Wei, Y.; Li, M.; Yu, S.; Ye, M.; Zhang, H.; Chen, S.; Liu, W.; Zhang, J. MiR-129-3p promotes docetaxel resistance of breast cancer cells via CP110 inhibition. Sci. Rep. 2015, 5, 15424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, D.-W.; Mao, L.; Zhang, J.; Jiang, L.-H.; Li, J.; Wu, Y.; Ji, H.; Chen, W.; Wang, J.; et al. MiR-139-5p inhibits the biological function of breast cancer cells by targeting Notch1 and mediates chemosensitivity to docetaxel. Biochem. Biophys. Res. Commun. 2015, 465, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Fu, Z.; Shi, M.; Xia, K.; Ji, C.; Xu, P.; Lv, M.; Pan, B.; Dai, L.; Xie, H. Systematic analysis of gene expression pattern in has-miR-760 overexpressed resistance of the MCF-7 human breast cancer cell to doxorubicin. Biomed. Pharmacother. Bioméd. Pharmacothér. 2015, 69, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.-G.; Song, C.-G.; Cao, Z.-G.; Xia, C.; Chen, D.-N.; Chen, L.; Li, S.; Qiao, F.; Ling, H.; Yao, L.; et al. Cytidine Deaminase Axis Modulated by miR-484 Differentially Regulates Cell Proliferation and Chemoresistance in Breast Cancer. Cancer Res. 2015, 75, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Masciarelli, S.; Fontemaggi, G.; Di Agostino, S.; Donzelli, S.; Carcarino, E.; Strano, S.; Blandino, G. Gain-of-function mutant p53 downregulates miR-223 contributing to chemoresistance of cultured tumor cells. Oncogene 2014, 33, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.-W.; Xing, A.-Y.; Xiang, S.; Shi, D.-B.; Liu, L.; Li, Y.-X.; Gao, P. Suppression of SPIN1-mediated PI3K-Akt pathway by miR-489 increases chemosensitivity in breast cancer. J. Pathol. 2016, 239, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Chen, L.; Sun, D.W.; Mao, C.; Chen, W.; Wu, J.Z.; Zhong, S.L.; Zhao, J.H.; Tang, J.H. β-elemene reverses chemoresistance of breast cancer via regulating MDR-related microRNA expression. Cell. Physiol. Biochem. 2014, 34, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, S.; Jia, W.; Deng, H.; Chen, K.; Zhu, L.; Yu, F.; Su, F. Reduced Let-7a Is Associated with Chemoresistance in Primary Breast Cancer. PLoS ONE 2015, 10, e0133643. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lv, X.; Wang, X.; Wang, B.; Shao, X.; Huang, Y.; Shi, L.; Chen, Z.; Huang, J.; Huang, P. MiR-181b promotes chemoresistance in breast cancer by regulating Bim expression. Oncol. Rep. 2016, 35, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-S.; Qiu, W.-S.; Yao, R.-Y.; Zhang, Q.; Zhuang, L.-K.; Zhou, F.; Sun, L.-B.; Yue, L. miR-141 confers docetaxel chemoresistance of breast cancer cells via regulation of EIF4E expression. Oncol. Rep. 2015, 33, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Miao, L.; Liu, M.; Li, C.; Yu, C.; Yan, H.; Yin, Y.; Wang, Y.; Qi, X.; Ren, J. miR-145 sensitizes breast cancer to doxorubicin by targeting multidrug resistance-associated protein-1. Oncotarget 2016, 7, 59714–59726. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, X.; Lv, M.; Chen, L.; Zhao, J.; Zhong, S.; Ji, M.; Hu, Q.; Luo, Z.; Wu, J.; et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS ONE 2014, 9, e95240. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, J.; Dalton, R.; Nwachukwu, C.; Huang, S.; Prat, A.; Yee, K.; Chang, Y.-F.; Huo, D.; Wen, Y.; Swanson, K.E.; et al. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat. Commun. 2013, 4, 1393. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Wang, Y.; Wang, C.-X.; Yin, M.; Liu, H.-L.; Chen, J.-P.; Han, J.-Q.; Wang, W.-B. MiRNA-155 mediates TAM resistance by modulating SOCS6-STAT3 signalling pathway in breast cancer. Am. J. Transl. Res. 2015, 7, 2115–2126. [Google Scholar] [PubMed]
- Hu, H.; Li, S.; Cui, X.; Lv, X.; Jiao, Y.; Yu, F.; Yao, H.; Song, E.; Chen, Y.; Wang, M.; Lin, L. The overexpression of hypomethylated miR-663 induces chemotherapy resistance in human breast cancer cells by targeting heparin sulfate proteoglycan 2 (HSPG2). J. Biol. Chem. 2013, 288, 10973–10985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Jiang, L.; He, M.; Bai, X.; Yu, L.; Wei, M. MiR-302a/b/c/d cooperatively sensitizes breast cancer cells to adriamycin via suppressing P-glycoprotein(P-gp) by targeting MAP/ERK kinase kinase 1 (MEKK1). J. Exp. Clin. Cancer Res. 2016, 35, 25. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Oak, P.S.; Wagner, E.; Roidl, A. miR-200c sensitizes breast cancer cells to doxorubicin treatment by decreasing TrkB and Bmi1 expression. PLoS ONE 2012, 7, e50469. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ke, G.; Han, D.; Liang, S.; Yang, G.; Wu, X. MicroRNA-181a enhances the chemoresistance of human cervical squamous cell carcinoma to cisplatin by targeting PRKCD. Exp. Cell Res. 2014, 320, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Cui, H.; Yu, H.; Ji, Q.; Kang, L.; Han, B.; Wang, J.; Dong, Q.; Li, Y.; Yan, Z.; et al. MiR-125a promotes paclitaxel sensitivity in cervical cancer through altering STAT3 expression. Oncogenesis 2016, 5, e197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, C.-P.; Zhang, Y.-F.; Nie, L. MicroRNA-100 resensitizes resistant chondrosarcoma cells to cisplatin through direct targeting of mTOR. Asian Pac. J. Cancer Prev. 2014, 15, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, Y.; Cai, S. Specific microRNAs as novel biomarkers for combination chemotherapy resistance detection of colon adenocarcinoma. Eur. J. Med. Res. 2015, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Zhou, X.; Song, L. Inhibition of lactate dehydrogenase A by microRNA-34a resensitizes colon cancer cells to 5-fluorouracil. Mol. Med. Rep. 2015, 11, 577–582. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xie, G.; Tong, J.; Peng, Y.; Huang, H.; Li, J.; Wang, N.; Liang, H. Overexpression of microRNA-122 re-sensitizes 5-FU-resistant colon cancer cells to 5-FU through the inhibition of PKM2 in vitro and in vivo. Cell Biochem. Biophys. 2014, 70, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Shi, H.; Ba, M.; Lin, S.; Tang, H.; Zeng, X.; Zhang, X. miR-409-3p sensitizes colon cancer cells to oxaliplatin by inhibiting Beclin-1-mediated autophagy. Int. J. Mol. Med. 2016, 37, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Dong, W.; Xie, C.; Wang, L.; Han, D.-L.; Wang, S.; Guo, H.-L.; Zhang, Z.-L. MicroRNA-494 sensitizes colon cancer cells to fluorouracil through regulation of DPYD. IUBMB Life 2015, 67, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Chen, Z. MiR-125a/b regulates the activation of cancer stem cells in paclitaxel-resistant colon cancer. Cancer Investig. 2013, 31, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-L.; Zhang, X.; Wang, L.-L.; Du, L.-T.; Yang, Y.-M.; Li, J.; Wang, C.-X. MicroRNA-218 is a prognostic indicator in colorectal cancer and enhances 5-fluorouracil-induced apoptosis by targeting BIRC5. Carcinogenesis 2015, 36, 1484–1493. [Google Scholar] [PubMed]
- Liu, Y.; Gao, S.; Chen, X.; Liu, M.; Mao, C.; Fang, X. Overexpression of miR-203 sensitizes paclitaxel (Taxol)-resistant colorectal cancer cells through targeting the salt-inducible kinase 2 (SIK2). Tumour Biol. 2016, 37, 12231–12239. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, F.; Zhang, X.-P. miR-203 enhances chemosensitivity to 5-fluorouracil by targeting thymidylate synthase in colorectal cancer. Oncol. Rep. 2015, 33, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cai, G.; Xu, Y.; Cai, S. The Plasma microRNA miR-1914* and -1915 Suppresses Chemoresistant in Colorectal Cancer Patients by Down-regulating NFIX. Curr. Mol. Med. 2016, 16, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liang, Y.; Shen, L.; Shen, L. MicroRNA-204 modulates colorectal cancer cell sensitivity in response to 5-fluorouracil-based treatment by targeting high mobility group protein A2. Biol. Open 2016, 5, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yin, Y.; Hu, Y.; Feng, Y.; Bian, Z.; Yao, S.; Li, M.; You, Q.; Huang, Z. miR-139-5p sensitizes colorectal cancer cells to 5-fluorouracil by targeting NOTCH-1. Pathol. Res. Pract. 2016, 212, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Eyking, A.; Reis, H.; Frank, M.; Gerken, G.; Schmid, K.W.; Cario, E. MiR-205 and MiR-373 Are Associated with Aggressive Human Mucinous Colorectal Cancer. PLoS ONE 2016, 11, e0156871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, X.; Miao, X.; Zhu, K.; Cui, S.; Meng, Q.; Sun, J.; Wang, T. MicroRNA-425-5p regulates chemoresistance in colorectal cancer cells via regulation of Programmed Cell Death 10. J. Cell. Mol. Med. 2016, 20, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.-J.; Cai, X.-J.; Li, S.-J. The Clinical Significance of MiR-429 as a Predictive Biomarker in Colorectal Cancer Patients Receiving 5-Fluorouracil Treatment. Med. Sci. Monit. 2016, 22, 3352–3361. [Google Scholar] [CrossRef]
- Siemens, H.; Jackstadt, R.; Kaller, M.; Hermeking, H. Repression of c-Kit by p53 is mediated by miR-34 and is associated with reduced chemoresistance, migration and stemness. Oncotarget 2013, 4, 1399–1415. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Leung, W.W.; Ng, S.S.M. Exploiting a novel miR-519c-HuR-ABCG2 regulatory pathway to overcome chemoresistance in colorectal cancer. Exp. Cell Res. 2015, 338, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geng, L.; Talmon, G.; Wang, J. MicroRNA-520g confers drug resistance by regulating p21 expression in colorectal cancer. J. Biol. Chem. 2015, 290, 6215–6225. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Liao, D.; Wang, X.; Wu, Z.; Nie, J.; Bai, M.; Fu, X.; Mei, Q.; Han, W. Elevated microRNA-23a Expression Enhances the Chemoresistance of Colorectal Cancer Cells with Microsatellite Instability to 5-Fluorouracil by Directly Targeting ABCF1. Curr. Protein Pept. Sci. 2015, 16, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-A.; Kim, I.; Yoon, S.K.; Lee, E.K.; Kuh, H.-J. Indirect modulation of sensitivity to 5-fluorouracil by microRNA-96 in human colorectal cancer cells. Arch. Pharm. Res. 2015, 38, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talmon, G.; Wang, J. MicroRNA-587 antagonizes 5-FU-induced apoptosis and confers drug resistance by regulating PPP2R1B expression in colorectal cancer. Cell Death Dis. 2015, 6, e1845. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Yang, J.; Liu, C.; Zhou, P.; Xiao, L.; Zhang, K. MiR-218 inhibits HMGB1-mediated autophagy in endometrial carcinoma cells during chemotherapy. Int. J. Clin. Exp. Pathol. 2015, 8, 6617–6626. [Google Scholar] [PubMed]
- Iida, K.; Fukushi, J.-I.; Matsumoto, Y.; Oda, Y.; Takahashi, Y.; Fujiwara, T.; Fujiwara-Okada, Y.; Hatano, M.; Nabashima, A.; Kamura, S.; et al. miR-125b develops chemoresistance in Ewing sarcoma/primitive neuroectodermal tumor. Cancer Cell Int. 2013, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Zhao, X.; Wang, H.; Chen, W.; Xu, S.; Wang, W.; Shen, H.; Huang, S.; Wang, J. miR-145 sensitizes gallbladder cancer to cisplatin by regulating multidrug resistance associated protein 1. Tumour Biol. 2016, 37, 10553–10562. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wei, W.; Zhan, Z.; Xie, Y.; Xiao, Q. MiR-1284 modulates multidrug resistance of gastric cancer cells by targeting EIF4A1. Oncol. Rep. 2016, 35, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Qu, Y.; Xu, C.; Tang, Y. Upregulation of microRNA-375 increases the cisplatin-sensitivity of human gastric cancer cells by regulating ERBB2. Exp. Ther. Med. 2016, 11, 625–630. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, Z.; Shang, Y.; Jiang, X.; Dong, J.; Yu, P.; Nie, Y.; Zhao, Q. miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis. 2015, 6, e1766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, X.; Du, Y.; Huang, Z.; Zhu, J.; Xu, J.; Cheng, G.; Shu, Y.; Liu, P.; Zhu, W.; et al. miR-20a induces cisplatin resistance of a human gastric cancer cell line via targeting CYLD. Mol. Med. Rep. 2016, 14, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, M.; Lu, M.; Zhu, W.; Shu, Y.; Cao, P.; Liu, P. Regulation of microtubule-associated protein tau (MAPT) by miR-34c-5p determines the chemosensitivity of gastric cancer to paclitaxel. Cancer Chemother. Pharmacol. 2013, 71, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Song, X.; Li, X.; Xin, J.; Wang, S.; Yang, W.; Wang, J.; Wu, K.; Chen, X.; Liang, J.; et al. Noninvasive visualization of microRNA-16 in the chemoresistance of gastric cancer using a dual reporter gene imaging system. PLoS ONE 2013, 8, e61792. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.L.; Rodriguez-Cruz, V.; Rameshwar, P. High expression of miR-9 in CD133(+) glioblastoma cells in chemoresistance to temozolomide. J. Cancer Stem Cell Res. 2015, 3, e1003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wan, Y.; Xie, D.; Wang, Y.; Wei, J.; Yan, Q.; Lu, P.; Mo, L.; Xie, J.; Yang, S.; Qi, X. DNMT1 mediates chemosensitivity by reducing methylation of miRNA-20a promoter in glioma cells. Exp. Mol. Med. 2015, 47, e182. [Google Scholar] [CrossRef] [PubMed]
- Giunti, L.; da Ros, M.; Vinci, S.; Gelmini, S.; Iorio, A.L.; Buccoliero, A.M.; Cardellicchio, S.; Castiglione, F.; Genitori, L.; de Martino, M.; et al. Anti-miR21 oligonucleotide enhances chemosensitivity of T98G cell line to doxorubicin by inducing apoptosis. Am. J. Cancer Res. 2015, 5, 231–242. [Google Scholar] [PubMed]
- Chen, X.; Zhang, Y.; Shi, Y.; Lian, H.; Tu, H.; Han, S.; Peng, B.; Liu, W.; He, X. MiR-873 acts as a novel sensitizer of glioma cells to cisplatin by targeting Bcl-2. Int. J. Oncol. 2015, 47, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Sun, S.; Zhang, X.Q.; Zhang, P.D.; Ho, A.S.W.; Kiang, K.M.Y.; Fung, C.F.; Lui, W.M.; Leung, G.K.K. MicroRNA-210 and Endoplasmic Reticulum Chaperones in the Regulation of Chemoresistance in Glioblastoma. J. Cancer 2015, 6, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Stojcheva, N.; Schechtmann, G.; Sass, S.; Roth, P.; Florea, A.-M.; Stefanski, A.; Stühler, K.; Wolter, M.; Müller, N.S.; Theis, F.J.; et al. MicroRNA-138 promotes acquired alkylator resistance in glioblastoma by targeting the Bcl-2-interacting mediator BIM. Oncotarget 2016, 7, 12937–12950. [Google Scholar] [PubMed]
- Haemmig, S.; Baumgartner, U.; Glück, A.; Zbinden, S.; Tschan, M.P.; Kappeler, A.; Mariani, L.; Vajtai, I.; Vassella, E. miR-125b controls apoptosis and temozolomide resistance by targeting TNFAIP3 and NKIRAS2 in glioblastomas. Cell Death Dis. 2014, 5, e1279. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Bai, Y.; Qiu, S.; Zheng, L.; Huang, L.; Liu, T.; Wang, X.; Liu, Y.; Xu, N.; Yan, X.; et al. MiR-203 downregulation is responsible for chemoresistance in human glioblastoma by promoting epithelial-mesenchymal transition via SNAI2. Oncotarget 2015, 6, 8914–8928. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yan, K.; Fang, J.; Qu, Q.; Zhou, M.; Chen, F. Let-7b expression determines response to chemotherapy through the regulation of cyclin D1 in glioblastoma. J. Exp. Clin. Cancer Res. CR 2013, 32, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sai, K.; Chen, F.; Chen, Z. miR-181b modulates glioma cell sensitivity to temozolomide by targeting MEK1. Cancer Chemother. Pharmacol. 2013, 72, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Chen, Q.; Li, C.; Wang, L.; Qian, X.; Jiang, C.; Liu, X.; Wang, X.; Li, H.; Kang, C.; et al. MiR-124 governs glioma growth and angiogenesis and enhances chemosensitivity by targeting R-Ras and N-Ras. Neuro-Oncol. 2014, 16, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Berthois, Y.; Delfino, C.; Metellus, P.; Fina, F.; Nanni-Metellus, I.; Al Aswy, H.; Pirisi, V.; Ouafik, L.; Boudouresque, F. Differential expression of miR200a-3p and miR21 in grade II-III and grade IV gliomas: Evidence that miR200a-3p is regulated by O6-methylguanine methyltransferase and promotes temozolomide responsiveness. Cancer Biol. Ther. 2014, 15, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, Y.; Chen, B.; Lu, P.; Zhan, L.; Yu, Q.; Cao, K.; Li, Q. MiR-136 targets E2F1 to reverse cisplatin chemosensitivity in glioma cells. J. Neurooncol. 2014, 120, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Wong, G.; Shiina, M. Up-regulation of Histone Methyltransferase, DOT1L, by Matrix Hyaluronan Promotes MicroRNA-10 Expression Leading to Tumor Cell Invasion and Chemoresistance in Cancer Stem Cells from Head and Neck Squamous Cell Carcinoma. J. Biol. Chem. 2016, 291, 10571–10585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, J.; Chen, D.; Huang, J.; Feng, B.; Han, S.; Chen, Y.; Song, H.; De, W.; Zhu, Z.; et al. Aurora-A promotes chemoresistance in hepatocelluar carcinoma by targeting NF-kappaB/microRNA-21/PTEN signaling pathway. Oncotarget 2014, 5, 12916–12935. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, Q.; Gong, Z.; Zhou, L.; You, N.; Wang, S.; Li, X.; Li, J.; An, J.; Wang, D.; He, Y.; Dou, K. MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular carcinoma cells to sorafenib treatment. Technol. Cancer Res. Treat. 2014, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wang, R.; Zhou, L.; Zhu, Y.; Gong, J.; Zhuang, S.-M. MicroRNA-26b suppresses the NF-κB signaling and enhances the chemosensitivity of hepatocellular carcinoma cells by targeting TAK1 and TAB3. Mol. Cancer 2014, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Y.; Hou, Y.; Yang, Q.; Chen, S.; Wang, X.; Wang, Z.; Yang, Y.; Chen, C.; Wang, Z.; et al. The PDGF-D/miR-106a/Twist1 pathway orchestrates epithelial-mesenchymal transition in gemcitabine resistance hepatoma cells. Oncotarget 2015, 6, 7000–7010. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; An, Y.; Wang, Y.; Zhang, C.; Zhang, H.; Huang, C.; Jiang, H.; Wang, X.; Li, X. miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol. Rep. 2013, 29, 2019–2024. [Google Scholar] [PubMed]
- Jiang, J.-X.; Gao, S.; Pan, Y.-Z.; Yu, C.; Sun, C.-Y. Overexpression of microRNA-125b sensitizes human hepatocellular carcinoma cells to 5-fluorouracil through inhibition of glycolysis by targeting hexokinase II. Mol. Med. Rep. 2014, 10, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.-L.; Chen, Y.-B.; Zhang, W.-Y.; Yu, C.-H.; Zhu, D.-Q.; Jin, J. miR-145 regulates chemoresistance in hepatocellular carcinoma via epithelial mesenchymal transition. Cell. Mol. Biol. Noisy 2015, 61, 12–16. [Google Scholar]
- Shi, L.; Wu, L.; Chen, Z.; Yang, J.; Chen, X.; Yu, F.; Zheng, F.; Lin, X. MiR-141 Activates Nrf2-Dependent Antioxidant Pathway via Down-Regulating the Expression of Keap1 Conferring the Resistance of Hepatocellular Carcinoma Cells to 5-Fluorouracil. Cell. Physiol. Biochem. 2015, 35, 2333–2348. [Google Scholar] [CrossRef] [PubMed]
- Kishikawa, T.; Otsuka, M.; Tan, P.S.; Ohno, M.; Sun, X.; Yoshikawa, T.; Shibata, C.; Takata, A.; Kojima, K.; Takehana, K.; et al. Decreased miR122 in hepatocellular carcinoma leads to chemoresistance with increased arginine. Oncotarget 2015, 6, 8339–8352. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, Z.-G.; Wu, L.-L.; Zheng, J.-J.; Yang, J.-R.; Chen, X.-F.; Chen, Z.-Q.; Liu, C.-L.; Chi, S.-Y.; Zheng, J.-Y.; et al. miR-340 reverses cisplatin resistance of hepatocellular carcinoma cell lines by targeting Nrf2-dependent antioxidant pathway. Asian Pac. J. Cancer Prev. 2014, 15, 10439–10444. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Luo, M.; Qian, H.; Chen, W. Upregulated miR-182 increases drug resistance in cisplatin-treated HCC cell by regulating TP53INP1. Gene 2014, 538, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.M.; Xu, S.; Wang, W.G.; Chen, Y.; Mao, J.Y.; Tian, B.L. MicroRNA-215 is upregulated by treatment with Adriamycin and leads to the chemoresistance of hepatocellular carcinoma cells and tissues. Mol. Med. Rep. 2015, 12, 5274–5280. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-T.; He, X.; Mo, Y.-Y.; Beck, W.T. Transient resistance to DNA damaging agents is associated with expression of microRNAs-135b and -196b in human leukemia cell lines. Int. J. Biochem. Mol. Biol. 2016, 7, 27–47. [Google Scholar] [PubMed]
- Weng, H.; Huang, H.; Dong, B.; Zhao, P.; Zhou, H.; Qu, L. Inhibition of miR-17 and miR-20a by oridonin triggers apoptosis and reverses chemoresistance by derepressing BIM-S. Cancer Res. 2014, 74, 4409–4419. [Google Scholar] [CrossRef] [PubMed]
- Seca, H.; Lima, R.T.; Lopes-Rodrigues, V.; Guimaraes, J.E.; Almeida, G.M.; Vasconcelos, M.H. Targeting miR-21 induces autophagy and chemosensitivity of leukemia cells. Curr. Drug Targets 2013, 14, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-X.; Zheng, Z.; Xue, W.; Zhao, M.-Z.; Fei, X.-C.; Wu, L.-L.; Huang, L.-M.; Leboeuf, C.; Janin, A.; Wang, L.; et al. MicroRNA181a Is Overexpressed in T-Cell Leukemia/Lymphoma and Related to Chemoresistance. BioMed Res. Int. 2015, 2015, 197241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Y.; Song, X.; Zhou, H.; Li, N.; Miao, Y.; Jia, L. Upregulation of miR-181c inhibits chemoresistance by targeting ST8SIA4 in chronic myelocytic leukemia. Oncotarget 2016, 7, 60074–60086. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jacamo, R.; Konopleva, M.; Garzon, R.; Croce, C.; Andreeff, M. CXCR4 downregulation of let-7a drives chemoresistance in acute myeloid leukemia. J. Clin. Investig. 2013, 123, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Qu, Q.; Wang, G.; Zhou, H. Let-7c sensitizes acquired cisplatin-resistant A549 cells by targeting ABCC2 and Bcl-XL. Die Pharm. 2013, 68, 955–961. [Google Scholar]
- Li, W.; Wang, W.; Ding, M.; Zheng, X.; Ma, S.; Wang, X. MiR-1244 sensitizes the resistance of non-small cell lung cancer A549 cell to cisplatin. Cancer Cell Int. 2016, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Pu, X.; Wang, Q.; Cao, J.; Xu, F.; Xu, L.I.; Li, K. miR-96 induces cisplatin chemoresistance in non-small cell lung cancer cells by downregulating SAMD9. Oncol. Lett. 2016, 11, 945–952. [Google Scholar] [PubMed]
- Zhang, Z.; Zhang, L.; Yin, Z.-Y.; Fan, X.-L.; Hu, B.; Wang, L.-Q.; Zhang, D. miR-107 regulates cisplatin chemosensitivity of A549 non small cell lung cancer cell line by targeting cyclin dependent kinase 8. Int. J. Clin. Exp. Pathol. 2014, 7, 7236–7241. [Google Scholar] [PubMed]
- Chen, X.; Jiang, Y.; Huang, Z.; Li, D.; Chen, X.; Cao, M.; Meng, Q.; Pang, H.; Sun, L.; Zhao, Y.; et al. miRNA-378 reverses chemoresistance to cisplatin in lung adenocarcinoma cells by targeting secreted clusterin. Sci. Rep. 2016, 6, 19455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Y.; Wu, H.; Qi, K.; You, J.; Li, X.; Zu, L.; Pan, Z.; Wang, Y.; Li, Y.; et al. MiR-192 confers cisplatin resistance by targeting Bim in lung cancer. Chin. J. Lung Cancer 2014, 17, 384–390. [Google Scholar]
- Lei, L.; Huang, Y.; Gong, W. miR-205 promotes the growth, metastasis and chemoresistance of NSCLC cells by targeting PTEN. Oncol. Rep. 2013, 30, 2897–2902. [Google Scholar] [PubMed]
- Yang, Z.; Fang, S.; Di, Y.; Ying, W.; Tan, Y.; Gu, W. Modulation of NF-κB/miR-21/PTEN pathway sensitizes non-small cell lung cancer to cisplatin. PLoS ONE 2015, 10, e0121547. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Chen, Y.; Song, H.; Xu, Y.; Wang, R.; Chen, L. Mir-24-3p downregulation contributes to VP16-DDP resistance in small-cell lung cancer by targeting ATG4A. Oncotarget 2015, 6, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Dai, Y.; Wang, S.; Xing, X. MicroRNA-299-3p promotes the sensibility of lung cancer to doxorubicin through directly targeting ABCE1. Int. J. Clin. Exp. Pathol. 2015, 8, 10072–10081. [Google Scholar] [PubMed]
- Li, J.; Wang, Y.; Song, Y.; Fu, Z.; Yu, W. miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Mol. Cancer 2014, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wells, A.; Padilla, M.T.; Kato, K.; Kim, K.C.; Lin, Y. A signaling pathway consisting of miR-551b, catalase and MUC1 contributes to acquired apoptosis resistance and chemoresistance. Carcinogenesis 2014, 35, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Bai, Y.; Chen, Z.; Li, Y.; Luo, L.; Huang, J.; Yang, J.; Liao, H.; Guo, L. Downregulation of HOXA1 gene affects small cell lung cancer cell survival and chemoresistance under the regulation of miR-100. Eur. J. Cancer 2014, 50, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, W.; Bai, L.; Chen, W.; Padilla, M.T.; Lin, A.S.; Shi, S.; Wang, X.; Lin, Y. Receptor-interacting protein 1 increases chemoresistance by maintaining inhibitor of apoptosis protein levels and reducing reactive oxygen species through a microRNA-146a-mediated catalase pathway. J. Biol. Chem. 2014, 289, 5654–5663. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Wang, F.; Li, M.; Yu, Z.-S.; Hao, Y.; Chen, S. MicroRNA-182 modulates chemosensitivity of human non-small cell lung cancer to cisplatin by targeting PDCD4. Diagn. Pathol. 2014, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Y.; Cui, S.-Y.; Chen, Y.-T.; Song, H.-Z.; Huang, G.-C.; Feng, B.; Sun, M.; De, W.; Wang, R.; Chen, L.-B. MicroRNA-650 was a prognostic factor in human lung adenocarcinoma and confers the docetaxel chemoresistance of lung adenocarcinoma cells via regulating Bcl-2/Bax expression. PLoS ONE 2013, 8, e72615. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, L.-J.; Yang, Y.-C.; Wang, Z.-X.; Wang, R. MiR-224 promotes the chemoresistance of human lung adenocarcinoma cells to cisplatin via regulating G₁/S transition and apoptosis by targeting p21(WAF1/CIP1). Br. J. Cancer 2014, 111, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, J.; Zhang, K.; Pan, B.; Chen, J.; De, W.; Wang, R.; Chen, L. MicroRNA-451 induces epithelial-mesenchymal transition in docetaxel-resistant lung adenocarcinoma cells by targeting proto-oncogene c-Myc. Eur. J. Cancer Oxf. Engl. 1990 2014, 50, 3050–3067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, L.; Yao, Q.; Tao, Z. miR-15b regulates cisplatin resistance and metastasis by targeting PEBP4 in human lung adenocarcinoma cells. Cancer Gene Ther. 2015, 22, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Sui, C.; Meng, F.; Li, Y.; Jiang, Y. miR-148b reverses cisplatin-resistance in non-small cell cancer cells via negatively regulating DNA (cytosine-5)-methyltransferase 1(DNMT1) expression. J. Transl. Med. 2015, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Petanidis, S.; Kioseoglou, E.; Domvri, K.; Anestakis, D.; Zarogoulidis, K. MiR-205 and miR-218 expression is associated with carboplatin chemoresistance and regulation of apoptosis via Mcl-1 and Survivin in lung cancer cells. Cell. Signal. 2015, 27, 1576–1588. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Zhou, X.; Zhao, M.; Zhao, D.; Zhu, Z.; Li, S.; Yang, H. Down-regulation of microRNA-26b modulates non-small cell lung cancer cells chemoresistance and migration through the association of PTEN. Acta Biochim. Biophys. Sin. 2015, 47, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; He, Y.; Liu, H.-Q.; Wang, S.-B.; Zhao, B.-C.; Cheng, Y.-S. MicroRNA 192 regulates chemo-resistance of lung adenocarcinoma for gemcitabine and cisplatin combined therapy by targeting Bcl-2. Int. J. Clin. Exp. Med. 2015, 8, 12397–12403. [Google Scholar] [PubMed]
- Fujita, Y.; Yagishita, S.; Hagiwara, K.; Yoshioka, Y.; Kosaka, N.; Takeshita, F.; Fujiwara, T.; Tsuta, K.; Nokihara, H.; Tamura, T.; et al. The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, X.; Huang, J.; Peng, J.; Guo, L. miR-7 modulates chemoresistance of small cell lung cancer by repressing MRP1/ABCC1. Int. J. Exp. Pathol. 2015, 96, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, S.; He, W.; Padilla, M.T.; Zhang, L.; Wang, X.; Zhang, B.; Lin, Y. Retaining MKP1 expression and attenuating JNK-mediated apoptosis by RIP1 for cisplatin resistance through miR-940 inhibition. Oncotarget 2014, 5, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Feng, B.; Chen, Y.; Huang, G.; Wang, R.; Chen, L.; Song, H. MiR-200b regulates autophagy associated with chemoresistance in human lung adenocarcinoma. Oncotarget 2015, 6, 32805–32820. [Google Scholar] [PubMed]
- Shan, W.; Zhang, X.; Li, M.; Deng, F.; Zhang, J. Over expression of miR-200c suppresses invasion and restores methotrexate sensitivity in lung cancer A549 cells. Gene 2016, 593, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, Y.; Peng, J.; Liao, H.; Gao, H.; Guo, Y.; Guo, L. Overexpression of secretagogin inhibits cell apoptosis and induces chemoresistance in small cell lung cancer under the regulation of miR-494. Oncotarget 2014, 5, 7760–7775. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, S.; Choudhary, G.S.; Ebron, J.S.; Hill, B.T.; Vivekanathan, N.; Ting, A.H.; Radivoyevitch, T.; Smith, M.R.; Shukla, G.C.; Almasan, A. miR-377-dependent BCL-xL regulation drives chemotherapeutic resistance in B-cell lymphoid malignancies. Mol. Cancer 2015, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.X.; Gui, Y.X.; Na, W.N.; Chao, J.; Yang, X. Circulating microRNA-125b and microRNA-130a expression profiles predict chemoresistance to R-CHOP in diffuse large B-cell lymphoma patients. Oncol. Lett. 2016, 11, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-D.; Huang, T.-J.; Peng, L.-X.; Yang, C.-F.; Liu, R.-Y.; Huang, H.-B.; Chu, Q.-Q.; Yang, H.-J.; Huang, J.-L.; Zhu, Z.-Y.; et al. Epstein-Barr Virus_Encoded LMP1 upregulates microRNA-21 to promote the resistance of nasopharyngeal carcinoma cells to cisplatin-induced Apoptosis by suppressing PDCD4 and Fas-L. PLoS ONE 2013, 8, e78355. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cao, P.; He, D.; Han, S.; Zhou, J.; Tan, G.; Li, W.; Yu, F.; Yu, J.; Li, Z.; et al. MiR-634 sensitizes nasopharyngeal carcinoma cells to paclitaxel and inhibits cell growth both in vitro and in vivo. Int. J. Clin. Exp. Pathol. 2014, 7, 6784–6791. [Google Scholar] [PubMed]
- Phatak, P.; Byrnes, K.A.; Mansour, D.; Liu, L.; Cao, S.; Li, R.; Rao, J.N.; Turner, D.J.; Wang, J.-Y.; Donahue, J.M. Overexpression of miR-214-3p in esophageal squamous cancer cells enhances sensitivity to cisplatin by targeting survivin directly and indirectly through CUG-BP1. Oncogene 2016, 35, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Ichikawa, D.; Kawaguchi, T.; Miyamae, M.; Okajima, W.; Ohashi, T.; Imamura, T.; Kiuchi, J.; Konishi, H.; Shiozaki, A.; et al. Circulating miR-21 as an independent predictive biomarker for chemoresistance in esophageal squamous cell carcinoma. Am. J. Cancer Res. 2016, 6, 1511–1523. [Google Scholar] [PubMed]
- Meng, F.; Qian, L.; Lv, L.; Ding, B.; Zhou, G.; Cheng, X.; Niu, S.; Liang, Y. miR-193a-3p regulation of chemoradiation resistance in oesophageal cancer cells via the PSEN1 gene. Gene 2016, 579, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Miyata, H.; Sugimura, K.; Fukuda, S.; Kanemura, T.; Yamashita, K.; Miyazaki, Y.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; et al. miR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts. Carcinogenesis 2015, 36, 894–903. [Google Scholar] [PubMed]
- Wang, Y.; Zhao, Y.; Herbst, A.; Kalinski, T.; Qin, J.; Wang, X.; Jiang, Z.; Benedix, F.; Franke, S.; Wartman, T.; et al. miR-221 Mediates Chemoresistance of Esophageal Adenocarcinoma by Direct Targeting of DKK2 Expression. Ann. Surg. 2016, 264, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, J.; Huang, H.; Hou, J.; Zhang, B.; Wang, A. miR-181a-Twist1 pathway in the chemoresistance of tongue squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2013, 441, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Zhang, H.; Du, Y.; Tan, P. miR-23a promotes cisplatin chemoresistance and protects against cisplatin-induced apoptosis in tongue squamous cell carcinoma cells through Twist. Oncol. Rep. 2015, 33, 942–950. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, S.; Chen, Y.; Zhao, J.; Zhang, K.; Wang, J.; Chen, S. microRNA-143 is associated with the survival of ALDH1+CD133+ osteosarcoma cells and the chemoresistance of osteosarcoma. Exp. Biol. Med. 2015, 240, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Huo, L.; Li, K.; Wu, Y.; Hu, Z. Blocked autophagy by miR-101 enhances osteosarcoma cell chemosensitivity in vitro. Scientific World J. 2014, 2014, 794756. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, R.; Drago-Ferrante, R.; Pentimalli, F.; Di Marzo, D.; Forte, I.M.; D’Anneo, A.; Carlisi, D.; De Blasio, A.; Giuliano, M.; Tesoriere, G.; et al. MicroRNA-29b-1 impairs in vitro cell proliferation, self‑renewal and chemoresistance of human osteosarcoma 3AB-OS cancer stem cells. Int. J. Oncol. 2014, 45, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, Z.; Wu, S.; Zang, X.; Liu, M.; Shi, J. miR-33a is up-regulated in chemoresistant osteosarcoma and promotes osteosarcoma cell resistance to cisplatin by down-regulating TWIST. J. Exp. Clin. Cancer Res. 2014, 33, 12. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, H.; Xu, C.-X.; Bi, W.-Z.; Wang, Y. MiR-34c inhibits osteosarcoma metastasis and chemoresistance. Med. Oncol. Northwood Lond. Engl. 2014, 31, 972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, G.; Feng, S. MicroRNA-301a modulates doxorubicin resistance in osteosarcoma cells by targeting AMP-activated protein kinase alpha 1. Biochem. Biophys. Res. Commun. 2015, 459, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Chen, Y.; Liu, G.; Yang, X. miR-22 targets the 3’ UTR of HMGB1 and inhibits the HMGB1-associated autophagy in osteosarcoma cells during chemotherapy. Tumour Biol. 2014, 35, 6021–6028. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, H.; Xu, C.-X.; Sun, B.; Mao, Z.; Bi, W.-Z.; Wang, Y. miR-382 inhibits tumor growth and enhance chemosensitivity in osteosarcoma. Oncotarget 2014, 5, 9472–9483. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Calleja, L. R.; Baud’huin, M.; Quillard, T.; Heymann, D.; Lamoureux, F.; Ory, B. miRNA-193a-5p repression of p73 controls Cisplatin chemoresistance in primary bone tumors. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, S.; Wang, G.; Wu, X.; Ding, Y.; Guo, G.; Jiang, J.; Cui, S. Expression of miR-136 is associated with the primary cisplatin resistance of human epithelial ovarian cancer. Oncol. Rep. 2015, 33, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Sestito, R.; Cianfrocca, R.; Rosanò, L.; Tocci, P.; Semprucci, E.; Di Castro, V.; Caprara, V.; Ferrandina, G.; Sacconi, A.; Blandino, G.; et al. miR-30a inhibits endothelin A receptor and chemoresistance in ovarian carcinoma. Oncotarget 2016, 7, 4009–4023. [Google Scholar] [PubMed]
- Zhan, Y.; Xiang, F.; Wu, R.; Xu, J.; Ni, Z.; Jiang, J.; Kang, X. MiRNA-149 modulates chemosensitivity of ovarian cancer A2780 cells to paclitaxel by targeting MyD88. J. Ovarian Res. 2015, 8, 48. [Google Scholar] [CrossRef]
- Li, X.; Pan, Q.; Wan, X.; Mao, Y.; Lu, W.; Xie, X.; Cheng, X. Methylation-associated Has-miR-9 deregulation in paclitaxel- resistant epithelial ovarian carcinoma. BMC Cancer 2015, 15, 509. [Google Scholar] [CrossRef] [PubMed]
- Pink, R.C.; Samuel, P.; Massa, D.; Caley, D.P.; Brooks, S.A.; Carter, D.R.F. The passenger strand, miR-21-3p, plays a role in mediating cisplatin resistance in ovarian cancer cells. Gynecol. Oncol. 2015, 137, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Samuel, P.; Pink, R.C.; Caley, D.P.; Currie, J.M.S.; Brooks, S.A.; Carter, D.R.F. Over-expression of miR-31 or loss of KCNMA1 leads to increased cisplatin resistance in ovarian cancer cells. Tumour Biol. 2016, 37, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.K.; Watari, H.; Mitamura, T.; Mohamed, Z.; El-Khamisy, S.F.; Ohba, Y.; Sakuragi, N. P18/Stathmin1 is regulated by miR-31 in ovarian cancer in response to taxane. Oncoscience 2015, 2, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Sugio, A.; Iwasaki, M.; Habata, S.; Mariya, T.; Suzuki, M.; Osogami, H.; Tamate, M.; Tanaka, R.; Saito, T. BAG3 upregulates Mcl-1 through downregulation of miR-29b to induce anticancer drug resistance in ovarian cancer. Gynecol. Oncol. 2014, 134, 615–623. [Google Scholar] [CrossRef]
- Liu, N.; Zeng, J.; Zhang, X.; Yang, Q.; Liao, D.; Chen, G.; Wang, Y. Involvement of miR-200a in chemosensitivity regulation of ovarian cancer. Zhonghua Yi Xue Za Zhi 2014, 94, 2148–2151. [Google Scholar] [PubMed]
- Liu, G.; Yang, D.; Rupaimoole, R.; Pecot, C.V.; Sun, Y.; Mangala, L.S.; Li, X.; Ji, P.; Cogdell, D.; Hu, L.; et al. Augmentation of response to chemotherapy by microRNA-506 through regulation of RAD51 in serous ovarian cancers. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Weiner-Gorzel, K.; Dempsey, E.; Milewska, M.; McGoldrick, A.; Toh, V.; Walsh, A.; Lindsay, S.; Gubbins, L.; Cannon, A.; Sharpe, D.; et al. Overexpression of the microRNA miR-433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells. Cancer Med. 2015, 4, 745–758. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, H.; Yin, X.; Long, L.; Xie, C.; Liu, Y.; Hui, L.; Lin, X.; Fang, Y.; Cao, Y.; et al. miR-186 regulation of Twist1 and ovarian cancer sensitivity to cisplatin. Oncogene 2016, 35, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, M.; Wu, J.; Jie, Z.; Chang, S.; Shuang, T. The clinicopathological significance of miR-1307 in chemotherapy resistant epithelial ovarian cancer. J. Ovarian Res. 2015, 8, 23. [Google Scholar] [CrossRef]
- Zhao, H.; Bi, T.; Qu, Z.; Jiang, J.; Cui, S.; Wang, Y. Expression of miR-224-5p is associated with the original cisplatin resistance of ovarian papillary serous carcinoma. Oncol. Rep. 2014, 32, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, L.; Wang, H.; Yi, T.; Jia, X.; Chen, C.; Xu, P. MiR-130a and MiR-374a Function as Novel Regulators of Cisplatin Resistance in Human Ovarian Cancer A2780 Cells. PLoS ONE 2015, 10, e0128886. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Shi, H.; Ji, M.; Chen, C. MiR-106a targets Mcl-1 to suppress cisplatin resistance of ovarian cancer A2780 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Kim, T.H.; Kim, K.; Song, J.-A.; Jung, Y.J.; Jeong, J.-Y.; Lee, M.J.; Kim, Y.K.; Lee, D.H.; An, H.J. Dysregulation of miR-106a and miR-591 confers paclitaxel resistance to ovarian cancer. Br. J. Cancer 2013, 109, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yu, X.; Ding, Y.; Zhao, J.; Wang, G.; Wu, X.; Jiang, J.; Peng, C.; Guo, G.Z.; Cui, S. MiR-770-5p inhibits cisplatin chemoresistance in human ovarian cancer by targeting ERCC2. Oncotarget 2016, 7, 53254–53268. [Google Scholar] [CrossRef] [PubMed]
- Au Yeung, C.L.; Co, N.-N.; Tsuruga, T.; Yeung, T.-L.; Kwan, S.-Y.; Leung, C.S.; Li, Y.; Lu, E.S.; Kwan, K.; Wong, K.-K.; et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat. Commun. 2016, 7, 11150. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Siu, M.K.Y.; Liu, S.S.; Yam, J.W.P.; Ngan, H.Y.S.; Chan, D.W. Epigenetic silencing of microRNA-199b-5p is associated with acquired chemoresistance via activation of JAG1-Notch1 signaling in ovarian cancer. Oncotarget 2014, 5, 944–958. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Y.; Xie, C.; Yin, X.; Liu, Y.; Cao, Y.; Fang, Y.; Lin, X.; Xu, Y.; Xu, W.; et al. miR-145 sensitizes ovarian cancer cells to paclitaxel by targeting Sp1 and Cdk6. Int. J. Cancer 2014, 135, 1286–1296. [Google Scholar] [CrossRef]
- Cai, J.; Yang, C.; Yang, Q.; Ding, H.; Jia, J.; Guo, J.; Wang, J.; Wang, Z. Deregulation of let-7e in epithelial ovarian cancer promotes the development of resistance to cisplatin. Oncogenesis 2013, 2, e75. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yu, J.-J.; Xu, Q.; Wang, L.; Zheng, J.Z.; Liu, L.-Z.; Jiang, B.-H. Downregulation of ATG14 by EGR1-MIR152 sensitizes ovarian cancer cells to cisplatin-induced apoptosis by inhibiting cyto-protective autophagy. Autophagy 2015, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, H.; Wu, N.; Zhang, W.-J.; Shang, L.-X. Deregulation of miR-128 in ovarian cancer promotes cisplatin resistance. Int. J. Gynecol. Cancer 2014, 24, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, A.; Belletti, B.; Lovat, F.; Volinia, S.; Chiappetta, G.; Giglio, S.; Sonego, M.; Cirombella, R.; Onesti, E.C.; Pellegrini, P.; et al. A microRNA signature defines chemoresistance in ovarian cancer through modulation of angiogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 9845–9850. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.T.; Jeong, J.-Y.; Lee, M.-J.; Kim, K.-I.; Kim, T.-H.; Kwon, Y.; Lee, C.; Kim, O.J.; An, H.-J. MicroRNAs overexpressed in ovarian ALDH1-positive cells are associated with chemoresistance. J. Ovarian Res. 2013, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Frederick, P.J.; Green, H.N.; Huang, J.S.; Egger, M.E.; Frieboes, H.B.; Grizzle, W.E.; McNally, L.R. Chemoresistance in ovarian cancer linked to expression of microRNAs. Biotech. Histochem. 2013, 88, 403–409. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Guo, R.-D.; Guo, R.-M.; Sheng, W.; Yin, L.-R. MicroRNA-182 promotes cell growth, invasion, and chemoresistance by targeting programmed cell death 4 (PDCD4) in human ovarian carcinomas. J. Cell. Biochem. 2013, 114, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Huang, T.; Ding, Y.-C.; Yu, W.; Wang, Q.; Meng, B.; Luo, S.-X. MicroRNA-200c overexpression inhibits chemoresistance, invasion and colony formation of human pancreatic cancer stem cells. Int. J. Clin. Exp. Pathol. 2015, 8, 6533–6539. [Google Scholar] [PubMed]
- Liang, C.; Wang, Z.; Li, Y.-Y.; Yu, B.-H.; Zhang, F.; Li, H.-Y. miR-33a suppresses the nuclear translocation of β-catenin to enhance gemcitabine sensitivity in human pancreatic cancer cells. Tumour Biol. 2015, 36, 9395–9403. [Google Scholar] [CrossRef]
- Cioffi, M.; Trabulo, S.M.; Sanchez-Ripoll, Y.; Miranda-Lorenzo, I.; Lonardo, E.; Dorado, J.; Reis Vieira, C.; Ramirez, J.C.; Hidalgo, M.; Aicher, A.; et al. The miR-17-92 cluster counteracts quiescence and chemoresistance in a distinct subpopulation of pancreatic cancer stem cells. Gut 2015, 64, 1936–1948. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zou, D.; Wei, X.; Wang, L.; Zhang, Y.; Liu, S.; Si, Y.; Zhao, H.; Wang, F.; Yu, J.; et al. MiRNA-221-3p desensitizes pancreatic cancer cells to 5-fluorouracil by targeting RB1. Tumour Biol. 2016, 37, 16053–16063. [Google Scholar] [CrossRef]
- Hasegawa, S.; Eguchi, H.; Nagano, H.; Konno, M.; Tomimaru, Y.; Wada, H.; Hama, N.; Kawamoto, K.; Kobayashi, S.; Nishida, N.; et al. MicroRNA-1246 expression associated with CCNG2-mediated chemoresistance and stemness in pancreatic cancer. Br. J. Cancer 2014, 111, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Takiuchi, D.; Eguchi, H.; Nagano, H.; Iwagami, Y.; Tomimaru, Y.; Wada, H.; Kawamoto, K.; Kobayashi, S.; Marubashi, S.; Tanemura, M.; et al. Involvement of microRNA-181b in the gemcitabine resistance of pancreatic cancer cells. Pancreatology 2013, 13, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Zhu, S.; Zhang, J.; Yang, M.; Qin, Q.; Deng, S.; Wang, B.; Tian, K.; Liu, L.; et al. Ectopic expression of miR-494 inhibited the proliferation, invasion and chemoresistance of pancreatic cancer by regulating SIRT1 and c-Myc. Gene Ther. 2015, 22, 729–738. [Google Scholar] [CrossRef]
- Fan, P.; Liu, L.; Yin, Y.; Zhao, Z.; Zhang, Y.; Amponsah, P.S.; Xiao, X.; Bauer, N.; Abukiwan, A.; Nwaeburu, C.C.; et al. MicroRNA-101-3p reverses gemcitabine resistance by inhibition of ribonucleotide reductase M1 in pancreatic cancer. Cancer Lett. 2016, 373, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Dhayat, S.A.; Abdeen, B.; Köhler, G.; Senninger, N.; Haier, J.; Mardin, W.A. MicroRNA-100 and microRNA-21 as markers of survival and chemotherapy response in pancreatic ductal adenocarcinoma UICC stage II. Clin. Epigenetics 2015, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Dhayat, S.A.; Mardin, W.A.; Seggewiß, J.; Ströse, A.J.; Matuszcak, C.; Hummel, R.; Senninger, N.; Mees, S.T.; Haier, J. MicroRNA Profiling Implies New Markers of Gemcitabine Chemoresistance in Mutant p53 Pancreatic Ductal Adenocarcinoma. PLoS ONE 2015, 10, e0143755. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Hung, S.W.; Krentz, M.; Patel, D.; Lovin, D.; Manoharan, R.; Thomson, J.M.; Govindarajan, R. Differential processing of let-7a precursors influences RRM2 expression and chemosensitivity in pancreatic cancer: Role of LIN-28 and SET oncoprotein. PLoS ONE 2013, 8, e53436. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chitkara, D.; Kumar, V.; Behrman, S.W.; Mahato, R.I. miRNA profiling in pancreatic cancer and restoration of chemosensitivity. Cancer Lett. 2013, 334, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Li, W.; Yin, L.; Guo, S.; Xu, X.; Ouyang, Y.; Zhao, Z.; Liu, S.; Tian, Y.; et al. Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHK1/Akt/NF-κB signaling. Oncogene 2016, 35, 5501–5514. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Guan, H.; Mizokami, A.; Keller, E.T.; Xu, X.; Liu, X.; Tan, J.; Hu, L.; Lu, Y.; et al. Exosome-derived microRNAs contribute to prostate cancer chemoresistance. Int. J. Oncol. 2016, 49, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-A.; Kim, S.-W.; Nam, J.; Sung, E.-G.; Song, I.-H.; Kim, J.-Y.; Kwon, T.K.; Lee, T.-J. Inhibition of c-FLIPL expression by miRNA-708 increases the sensitivity of renal cancer cells to anti-cancer drugs. Oncotarget 2016, 7, 31832–31846. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Mo, R.; Ma, J.; Fan, J. let-7b and let-7c are determinants of intrinsic chemoresistance in renal cell carcinoma. World J. Surg. Oncol. 2015, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Mitsui, Y.; Fukuhara, S.; Gill, A.; Wong, D.K.; Yamamura, S.; Shahryari, V.; Tabatabai, Z.L.; Dahiya, R.; Shin, D.M.; et al. Loss of miR-200c up-regulates CYP1B1 and confers docetaxel resistance in renal cell carcinoma. Oncotarget 2015, 6, 7774–7787. [Google Scholar] [CrossRef] [PubMed]
- Ayers, D.; Mestdagh, P.; Van Maerken, T.; Vandesompele, J. Identification of miRNAs contributing to neuroblastoma chemoresistance. Comput. Struct. Biotechnol. J. 2015, 13, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Volders, P.-J.; Helsens, K.; Wang, X.; Menten, B.; Martens, L.; Gevaert, K.; Vandesompele, J.; Mestdagh, P. LNCipedia: A database for annotated human lncRNA transcript sequences and structures. Nucleic Acids Res. 2013, 41, D246–D251. [Google Scholar] [CrossRef] [PubMed]
- Volders, P.J.; Verheggen, K.; Menschaert, G.; Vandepoele, K.; Martens, L.; Vandesompele, J.; Mestdagh, P. An update on LNCipedia: A database for annotated human lncRNA sequences. Nucleic Acids Res. 2015, 43, 4363–4364. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Gao, H.; Tong, Y.; Yang, J.; Tang, R.; Niu, Y.; Li, M.; Guo, L. Long noncoding RNA-HOTAIR affects chemoresistance by regulating HOXA1 methylation in small cell lung cancer cells. Lab. Investig. 2016, 96, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, M.; Lu, K.; Liu, J.; Zhang, M.; Wu, W.; De, W.; Wang, Z.; Wang, R. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21(WAF1/CIP1) expression. PLoS ONE 2013, 8, e77293. [Google Scholar]
- Özeş, A.R.; Miller, D.F.; Özeş, O.N.; Fang, F.; Liu, Y.; Matei, D.; Huang, T.; Nephew, K.P. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene 2016, 35, 5350–5361. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Wu, K.; Zhao, Q.; Nie, Y.; Fan, D. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Mol. Cell. Biol. 2014, 34, 3182–3193. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Qu, X.; Li, W.; Zhong, X.; Li, P.; Yang, S.; Chen, X.; Shao, M.; Zhang, L. The long non-coding RNA, GAS5, enhances gefitinib-induced cell death in innate EGFR tyrosine kinase inhibitor-resistant lung adenocarcinoma cells with wide-type EGFR via downregulation of the IGF-1R expression. J. Hematol. Oncol. 2015, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Cai, W.; Ren, S.; Li, X.; Wang, Q.; Pan, H.; Zhao, M.; Li, J.; Zhang, Y.; Zhao, C.; et al. Long non-coding RNA UCA1 induces non-T790M acquired resistance to EGFR-TKIs by activating the AKT/mTOR pathway in EGFR-mutant non-small cell lung cancer. Oncotarget 2015, 6, 23582–23593. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, X.; Wu, W.; Xue, M.; Hou, H.; Zhai, W.; Chen, W. Long non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through CREB modulating miR-196a-5p in bladder cancer cells. Cancer Lett. 2016, 382, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shen, B.; Tan, M.; Mu, X.; Qin, Y.; Zhang, F.; Liu, Y. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J. 2014, 281, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- He, D.-X.; Zhang, G.-Y.; Gu, X.-T.; Mao, A.-Q.; Lu, C.-X.; Jin, J.; Liu, D.-Q.; Ma, X. Genome-wide profiling of long non-coding RNA expression patterns in anthracycline-resistant breast cancer cells. Int. J. Oncol. 2016, 49, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Huang, O.; Xie, Z.; Wu, S.; Zhang, X.; Shen, A.; Liu, H.; Chen, X.; Wu, J.; Lou, Y.; et al. A novel long non-coding RNA-ARA: Adriamycin resistance-associated. Biochem. Pharmacol. 2014, 87, 254–283. [Google Scholar] [CrossRef]
- Shi, S.-J.; Wang, L.-J.; Yu, B.; Li, Y.-H.; Jin, Y.; Bai, X.-Z. LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer. Oncotarget 2015, 6, 11652–11663. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhai, L.; Wang, H.; Liu, C.; Zhang, J.; Chen, W.; Wei, Q. Downregulation of LncRNA GAS5 causes trastuzumab resistance in breast cancer. Oncotarget 2016, 7, 27778–27786. [Google Scholar] [CrossRef] [PubMed]
- Schouten, P.C.; Vollebergh, M.A.; Opdam, M.; Jonkers, M.; Loden, M.; Wesseling, J.; Hauptmann, M.; Linn, S.C. High XIST and Low 53BP1 Expression Predict Poor Outcome after High-Dose Alkylating Chemotherapy in Patients with a BRCA1-like Breast Cancer. Mol. Cancer Ther. 2016, 15, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Redis, R.S.; Sieuwerts, A.M.; Look, M.P.; Tudoran, O.; Ivan, C.; Spizzo, R.; Zhang, X.; de Weerd, V.; Shimizu, M.; Ling, H.; et al. CCAT2, a novel long non-coding RNA in breast cancer: Expression study and clinical correlations. Oncotarget 2013, 4, 1748–1762. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, C.; Ku, J.-L.; Kim, W.; Yoon, S.K.; Kuh, H.-J.; Lee, J.-H.; Nam, S. W.; Lee, E.K. A long non-coding RNA snaR contributes to 5-fluorouracil resistance in human colon cancer cells. Mol. Cells 2014, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Cai, D.; Liu, C.; Fang, C.; Yan, D. Linc00152 Functions as a Competing Endogenous RNA to Confer Oxaliplatin Resistance and Holds Prognostic Values in Colon Cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, S.; Li, Y.; Zhang, W.; He, K.; Zhao, M.; Lin, H.; Li, D.; Zhang, H.; Zheng, Z.; et al. Decreased expression of LncRNA SLC25A25-AS1 promotes proliferation, chemoresistance, and EMT in colorectal cancer cells. Tumour Biol. 2016, 37, 14205–14215. [Google Scholar] [CrossRef] [PubMed]
- Hang, Q.; Sun, R.; Jiang, C.; Li, Y. Notch 1 promotes cisplatin-resistant gastric cancer formation by upregulating lncRNA AK022798 expression. Anticancer. Drugs 2015, 26, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bu, P.; Liu, L.; Zhang, X.; Li, J. Overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance. Biochem. Biophys. Res. Commun. 2015, 462, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yan, I.K.; Kogure, T.; Haga, H.; Patel, T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio 2014, 4, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, K.; Song, H.; Wang, R.; Chu, X.; Chen, L. Long noncoding RNA CCAT1 acts as an oncogene and promotes chemoresistance in docetaxel-resistant lung adenocarcinoma cells. Oncotarget 2016, 7, 62474–62489. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, H.; Hou, S.; Hu, B.; Liu, J.; Wang, J. The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell. PLoS ONE 2013, 8, e65309. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wan, L.; Lu, K.; Sun, M.; Pan, X.; Zhang, P.; Lu, B.; Liu, G.; Wang, Z. The long noncoding RNA MEG3 contributes to cisplatin resistance of human lung adenocarcinoma. PLoS ONE 2015, 10, e0114586. [Google Scholar] [CrossRef] [PubMed]
- Mourtada-Maarabouni, M.; Williams, G.T. Role of GAS5 noncoding RNA in mediating the effects of rapamycin and its analogues on mantle cell lymphoma cells. Clin. Lymphoma Myeloma Leuk. 2014, 14, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Li, G.; Liu, C.; Cai, T.; Su, Z.; Wei, M.; She, L.; Tian, Y.; Qiu, Y.; Zhang, X.; et al. Next generation deep sequencing identified a novel lncRNA n375709 associated with paclitaxel resistance in nasopharyngeal carcinoma. Oncol. Rep. 2016, 36, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, M.; You, B.; Shi, S.; Shan, Y.; Bao, L.; You, Y. Long non-coding RNA ROR promotes proliferation, migration and chemoresistance of nasopharyngeal carcinoma. Cancer Sci. 2016, 107, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, W.; Li, G.; Sun, C.; Ren, Z.; Sheng, H.; Gao, H.; Wang, C.; Yu, H. High TUG1 expression is associated with chemotherapy resistance and poor prognosis in esophageal squamous cell carcinoma. Cancer Chemother. Pharmacol. 2016, 78, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Zheng, X.; Zhong, W.; Tian, X.; Yin, B.; Tian, K.; Zhang, W. Long non-coding RNA LINC00161 sensitises osteosarcoma cells to cisplatin-induced apoptosis by regulating the miR-645-IFIT2 axis. Cancer Lett. 2016, 382, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Zhu, K.-P.; Shen, G.-Q.; Zhu, Z.-S. A long non-coding RNA contributes to doxorubicin resistance of osteosarcoma. Tumour Biol. 2016, 37, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-P.; Zhang, C.-L.; Shen, G.-Q.; Zhu, Z.-S. Long noncoding RNA expression profiles of the doxorubicin-resistant human osteosarcoma cell line MG63/DXR and its parental cell line MG63 as ascertained by microarray analysis. Int. J. Clin. Exp. Pathol. 2015, 8, 8754–8773. [Google Scholar] [PubMed]
- Liu, E.; Liu, Z.; Zhou, Y.; Mi, R.; Wang, D. Overexpression of long non-coding RNA PVT1 in ovarian cancer cells promotes cisplatin resistance by regulating apoptotic pathways. Int. J. Clin. Exp. Med. 2015, 8, 20565–20572. [Google Scholar] [PubMed]
- Zhang, L.; Cao, X.; Zhang, L.; Zhang, X.; Sheng, H.; Tao, K. UCA1 overexpression predicts clinical outcome of patients with ovarian cancer receiving adjuvant chemotherapy. Cancer Chemother. Pharmacol. 2016, 77, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.I.; Wang, T.; Ren, C.; Liu, L.; Kong, D.; Jin, X.; Li, X.; Zhang, G. Downregulation of BC200 in ovarian cancer contributes to cancer cell proliferation and chemoresistance to carboplatin. Oncol. Lett. 2016, 11, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, X.; Zhou, Y.; Liu, Y.; Zhou, Q.; Ye, H.; Wang, Y.; Zeng, J.; Song, Y.; Gao, W.; et al. The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer. J. Transl. Med. 2015, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Chang, D.; Du, H.-Z.; Zhao, Y.-P. Genome-wide screen identifies PVT1 as a regulator of Gemcitabine sensitivity in human pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2011, 407, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Hu, H.; Han, T.; Yuan, C.; Wang, L.; Jin, Z.; Guo, Z.; Wang, L. Long noncoding RNA MALAT-1 enhances stem cell-like phenotypes in pancreatic cancer cells. Int. J. Mol. Sci. 2015, 16, 6677–6693. [Google Scholar] [CrossRef] [PubMed]
- Yacqub-Usman, K.; Pickard, M.R.; Williams, G.T. Reciprocal regulation of GAS5 lncRNA levels and mTOR inhibitor action in prostate cancer cells. Prostate 2015, 75, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-G.; Yang, M.-F.; Ren, Y.-Q.; Wu, C.-H.; Wang, L.-Q. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4362–4368. [Google Scholar] [PubMed]
| miRNA/s Involved (Species—Homo sapiens) | Accession (MIMAT) Number | Cancer Model | Affected Chemotherapeutic Drugs | Dysregulation Status | Effect on Chemo-Resistance Phenotype | Ref. |
|---|---|---|---|---|---|---|
| miR-34a-5p | 0000255 | bladder | neoadjuvant chemotherapy | nd | + | [44] |
| miR-100-5p | 0000098 | |||||
| miR-146b-5p | 0002809 | |||||
| miR-9-5p | 0000441 | |||||
| miR-193a-3p | 0000459 | |||||
| let-7c-5p | 0000064 | bladder | Platinum-based neoadjuvant chemotherapy | d | + | [45] |
| miR-1290 | 0005880 | bladder | gemcitabine | u | + | [46] |
| miR-138-5p | 0000430 | u | + | |||
| let-7i-5p | 0000415 | d | + | |||
| let-7b-5p | 0000063 | d | + | |||
| miR-193a-3p | 0000459 | bladder | MDR | u | + | [47,48,49] |
| miR-21-5p | 0000076 | breast | gemcitabine | u | + | [50] |
| miR-25-3p | 0000081 | breast | d | − | [51] | |
| miR-125b-5p | 0000423 | breast | u | + | [52] | |
| miR-149-5p | 0000450 | breast | d | + | [53] | |
| miR-320a | 0000510 | breast | d | + | [54] | |
| miR-29a-3p | 0000086 | breast | doxorubicin | u | + | [55] |
| miR-129-2-3p | 0004605 | breast | docetaxel | u | + | [56] |
| miR-139-5p | 0000250 | breast | docetaxel | d | + | [57] |
| miR-760 | 0004957 | breast | doxorubicin | u | + | [58] |
| miR-484 | 0002174 | breast | u | + | [59] | |
| miR-223-3p | 0000280 | breast | d | + | [60] | |
| miR-489-3p | 0002805 | breast | u | - | [61] | |
| miR-34a-5p | 0000255 | breast | doxorubicin docetaxel | u | − | [62] |
| miR-222-3p | 0000279 | d | − | |||
| miR-452-5p | 0001635 | d | − | |||
| miR-29a-3p | 0000086 | d | − | |||
| let-7a-5p | 0000062 | breast | epirubicin | d | + | [63] |
| miR-181b-5p | 0000257 | breast | doxorubicin | u | + | [64] |
| miR-141-3p | 0000432 | breast | docetaxel | u | + | [65] |
| miR-145-5p | 0000437 | breast | doxorubicin | u | − | [66] |
| miR-100-5p | 0000098 | breast | doxorubicin docetaxel | u | + | [67] |
| miR-222-3p | 0000279 | u | + | |||
| miR-30a-3p | 0000088 | u | + | |||
| miR-30a-5p | 0000087 | u | + | |||
| miR-30c-5p | 0000244 | breast | u | − | [68] | |
| miR-155-5p | 0000646 | breast | tamoxifen | u | + | [69] |
| miR-663a | 0003326 | breast | doxorubicin | u | + | [70] |
| miR-302a-3p | 0000684 | breast | doxorubicin | u | − | [71] |
| miR-302b-3p | 0000715 | u | − | |||
| miR-302c-3p | 0000717 | u | − | |||
| miR-302d-3p | 0000718 | u | − | |||
| miR-200c-3p | 0000617 | breast | doxorubicin | u | − | [72] |
| miR-181a-5p | 0000256 | cervical | cisplatin | u | + | [73] |
| miR-125a-5p | 0000443 | cervical | paclitaxel | u | − | [74] |
| miR-100-5p | 0000098 | chondrosarcoma | cisplatin | u | − | [75] |
| miR-4299 | 0016851 | colon | capecitabine oxaliplatin | d | − | [76] |
| miR-196b-5p | 0001080 | u | − | |||
| miR-34a-5p | 0000255 | colon | 5-fluorouracil | u | − | [77] |
| miR-122-5p | 0000421 | colon | 5-fluorouracil | u | − | [78] |
| miR-409-3p | 0001639 | colon | oxaliplatin | u | − | [79] |
| miR-223-3p | 0000280 | colon | d | + | [60] | |
| miR-494-3p | 0002816 | colon | 5-fluorouracil | u | − | [80] |
| miR-125a-5p | 0000443 | colon | paclitaxel | u | − | [81] |
| miR-125b-5p | 0000423 | |||||
| miR-218-5p | 0000275 | colorectal | 5-fluorouracil | u | − | [82] |
| miR-203a-3p | 0000264 | colorectal | paclitaxel 5-fluorouracil | u | − | [83,84] |
| miR-1914-3p | 0007890 | colorectal | capecitabine oxaliplatin | u | − | [85] |
| miR-1915-3p | 0007892 | u | − | |||
| miR-204-5p | 0000265 | colorectal | 5-fluorouracil | u | − | [86] |
| miR-139-5p | 0000250 | colorectal | 5-fluorouracil | u | − | [87] |
| miR-205-5p | 0000266 | colorectal | u | + | [88] | |
| miR-373-3p | 0000726 | u | + | |||
| miR-425-5p | 0003393 | colorectal | 5-fluorouracil oxaliplatin | u | + | [89] |
| miR-429 | 0001536 | colorectal | 5-fluorouracil | u | + | [90] |
| miR-34a-5p | 0000255 | colorectal | 5-fluorouracil | u | − | [91] |
| miR-519c-3p | 0002832 | colorectal | 5-fluorouracil irinotecan | d | + | [92] |
| miR-520g-3p | 0002858 | colorectal | 5-fluorouracil | u | + | [93] |
| miR-23a-3p | 0000078 | colorectal | 5-fluorouracil | u | + | [94] |
| miR-96-5p | 0000095 | colorectal | 5-fluorouracil | u | − | [95] |
| miR-587 | 0003253 | colorectal | 5-fluorouracil | u | + | [96] |
| miR-218-5p | 0000275 | endometrial | paclitaxel | u | − | [97] |
| miR-125b-5p | 0000423 | ewing sarcoma | doxorubicin | u | + | [98] |
| miR-145-5p | 0000437 | gallbladder | cisplatin | u | − | [99] |
| miR-1284 | 0005941 | gastric | vincristine | u | − | [100] |
| miR-375 | 0000728 | gastric | cisplatin | u | − | [101] |
| miR-23b-3p | 0000418 | gastric | MDR | u | − | [102] |
| miR-20a-5p | 0000075 | gastric | cisplatin | u | + | [103] |
| miR-34c-5p | 0000686 | gastric | paclitaxel | d | + | [104] |
| miR-16-5p | 0000069 | gastric | etoposide 5-fluorouracil | u | − | [105] |
| miR-9-5p | 0000441 | glioblastoma | temozolomide | u | + | [106] |
| miR-20a-5p | 0000075 | glioblastoma | temozolomide | u | − | [107] |
| miR-21-5p | 0000076 | glioblastoma | doxorubicin | u | + | [108] |
| miR-873-5p | 0004953 | glioblastoma | cisplatin | u | − | [109] |
| miR-210-3p | 0000267 | glioblastoma | temozolomide | u | − | [110] |
| miR-138-5p | 0000430 | glioblastoma | temozolomide | u | + | [111] |
| miR-125b-5p | 0000423 | glioblastoma | temozolomide | u | − | [112] |
| miR-203a-3p | 0000264 | glioblastoma | d | + | [113] | |
| let-7b-5p | 0000063 | glioblastoma | cisplatin | d | + | [114] |
| miR-181b-5p | 0000257 | glioma | temozolomide | u | − | [115] |
| miR-124-3p | 0000422 | glioma | temozolomide | u | − | [116] |
| miR-200a-3p | 0000682 | glioma | temozolomide | u | − | [117] |
| miR-136-5p | 0000448 | glioma | cisplatin | u | − | [118] |
| miR-10b-5p | 0000254 | head/neck squamous cell | cisplatin | u | + | [119] |
| miR-21-5p | 0000076 | hepatocellular | u | + | [120] | |
| miR-34a-5p | 0000255 | hepatocellular | sorafenib | u | − | [121] |
| miR-26b-5p | 0000083 | hepatocellular | doxorubicin | u | − | [122] |
| miR-106a-5p | 0000103 | hepatocellular | gemcitabine | d | + | [123] |
| miR-101-3p | 0000099 | hepatocellular | cisplatin | u | − | [124] |
| miR-125b-5p | 0000423 | hepatocellular | 5-fluorouracil | u | − | [125] |
| miR-145-5p | 0000437 | hepatocellular | doxorubicin | u | − | [126] |
| miR-141-3p | 0000432 | hepatocellular | 5-fluorouracil | u | + | [127] |
| miR-122-5p | 0000421 | hepatocellular | sorafenib | d | + | [128] |
| miR-340-5p | 0004692 | hepatocellular | cisplatin | u | − | [129] |
| miR-182-5p | 0000259 | hepatocellular | cisplatin | u | + | [130] |
| miR-215-5p | 0000272 | hepatocellular | doxorubicin | u | + | [131] |
| miR-135b-5p | 0000758 | leukaemia | genotoxic agent treatment (eg., etoposide, doxorubicin) | u | + | [132] |
| miR-196b-5p | 0001080 | u | + | |||
| miR-17-3p | 0000071 | leukaemia | d | − | [133] | |
| miR-17-5p | 0000070 | d | − | |||
| miR-20a-5p | 0000075 | d | − | |||
| miR-21-5p | 0000076 | leukaemia | etoposide, doxorubicin | d | − | [134] |
| miR-181a-5p | 0000256 | leukaemia | doxorubicin | u | + | [135] |
| miR-181c-5p | 0000258 | leukaemia | chronic myelocytic leukaemia | u | − | [136] |
| let-7a-5p | 0000062 | leukaemia | cytarabine | d | + | [137] |
| let-7c-5p | 0000064 | lung | cisplatin | u | − | [138] |
| miR-1244 | 0005896 | lung | cisplatin | u | − | [139] |
| miR-96-5p | 0000095 | lung | cisplatin | u | + | [140] |
| miR-107 | 0000104 | lung | cisplatin | u | − | [141] |
| miR-378a-3p | 0000732 | lung | cisplatin | u | − | [142] |
| miR-192-5p | 0000222 | lung | cisplatin | u | + | [143] |
| miR-205-5p | 0000266 | lung | u | + | [144] | |
| miR-21-5p | 0000076 | lung | cisplatin | d | − | [145] |
| miR-24-3p | 0000080 | lung | etoposide, cisplatin | d | + | [146] |
| miR-299-3p | 0000687 | lung | doxorubicin | u | − | [147] |
| miR-27a-3p | 0000084 | lung | cisplatin | u | − | [148] |
| miR-551a | 0003214 | lung | u | + | [149] | |
| miR-100-5p | 0000098 | lung | u | + | [150] | |
| miR-146a-5p | 0000449 | lung | cisplatin | u | + | [151] |
| miR-182 | (sequence not listed in paper) | lung | cisplatin | u | + | [152] |
| miR-650 | 0003320 | lung | docetaxel | u | + | [153] |
| miR-224-5p | 0000281 | lung | cisplatin | u | + | [154] |
| miR-451a | 0001631 | lung | docetaxel | u | − | [155] |
| miR-15b-5p | 0000417 | lung | cisplatin | u | − | [156] |
| miR-148b-3p | 0000759 | lung | cisplatin | u | − | [157] |
| miR-205-5p | 0000266 | lung | carboplatin | u | + | [158] |
| miR-218-5p | 0000275 | u | + | |||
| miR-26b-5p | 0000083 | lung | d | − | [159] | |
| miR-192-5p | 0000222 | lung | gemcitabine, cisplatin | u | − | [160] |
| miR-197-3p | 0000227 | lung | platinum-based | d | + | [161] |
| miR-7-5p | 0000252 | lung | u | − | [162] | |
| miR-940 | 0004983 | lung | cisplatin | d | + | [163] |
| miR-200b-3p | 0000318 | lung | docetaxel | u | − | [164] |
| miR-200c-3p | 0000617 | lung | methotrexate | u | − | [165] |
| miR-494-3p | 0002816 | lung | u | − | [166] | |
| miR-377-3p | 0000730 | lymphoma (b-cell) | venetoclax | u | + | [167] |
| miR-125b-5p | 0000423 | lymphoma (b-cell) | cyclophosphamide, doxorubicin, vincristine | u | + | [168] |
| miR-130a-3p | 0000425 | u | + | |||
| miR-21-5p | 0000076 | nasopharyngeal | cisplatin | u | + | [169] |
| miR-634 | 0003304 | nasopharyngeal | paclitaxel | u | − | [170] |
| miR-214-3p | 0000271 | oesophageal (squamous cell) | cisplatin | u | − | [171] |
| miR-21-5p | 0000076 | oesophageal (squamous cell) | 5-fluorouracil cisplatin (circulating miRnas) | u | + | [172] |
| miR-193a-3p | 0000459 | oesophageal | chemoradiation | u | − | [173] |
| miR-27a-3p | 0000084 | oesophageal | cisplatin | u | + | [174] |
| miR-221-3p | 0000278 | oesophageal | 5-fluorouracil | u | + | [175] |
| miR-181a-5p | 0000256 | oral squamous cell | cisplatin | u | − | [176] |
| miR-23a-3p | 0000078 | oral squamous cell | cisplatin | u | + | [177] |
| miR-143-3p | 0000435 | osteosarcoma | doxorubicin | d | + | [178] |
| miR-101-3p | 0000099 | osteosarcoma | cisplatin doxorubicin methotrexate | u | − | [179] |
| miR-29b-1 | MI00000105 (precursor) | osteosarcoma | u | − | [180] | |
| miR-33a-5p | 0000091 | osteosarcoma | cisplatin | u | + | [181] |
| miR-34c-5p | 0000686 | osteosarcoma | u | − | [182] | |
| miR-301a-3p | 0000688 | osteosarcoma | doxorubicin | u | + | [183] |
| miR-22-3p | 0000077 | osteosarcoma | u | − | [184] | |
| miR-382-5p | 0000737 | osteosarcoma | u | − | [185] | |
| miR-193a-5p | 0004614 | osteo-/ewing sarcoma | cisplatin | u | − | [186] |
| miR-136-5p | 0000448 | ovarian | cisplatin | u | + | [187] |
| miR-30a-5p | 0000087 | ovarian | cisplatin | u | − | [188] |
| miR-149-5p | 0000450 | ovarian | paclitaxel | d | + | [189] |
| miR-9-5p | 0000441 | ovarian | paclitaxel | d | + | [190] |
| miR-21-3p | 0004494 | ovarian | cisplatin | u | + | [191] |
| miR-31-5p | 0000089 | ovarian | cisplatin | u | + | [192] |
| miR-31-5p | 0000089 | ovarian | taxane | u | − | [193] |
| miR-29b-3p | 0000100 | ovarian | paclitaxel | d | + | [194] |
| miR-200a-3p | 0000682 | ovarian | paclitaxel | u | − | [195] |
| miR-506-3p | 0002878 | ovarian | cisplatin olaparib | u | − | [196] |
| miR-433-3p | 0001627 | ovarian | paclitaxel | u | + | [197] |
| miR-186-5p | 0000456 | ovarian | cisplatin | u | − | [198] |
| miR-1307-3p | 0005951 | ovarian | u | + | [199] | |
| miR-224-5p | 0000281 | ovarian | cisplatin | u | + | [200] |
| miR-130a-3p | 0000425 | ovarian | cisplatin | u | − | [201] |
| miR-374a-5p | 0000727 | u | − | |||
| miR-106a-5p | 0000103 | ovarian | cisplatin | u | − | [202] |
| miR-106a-5p | 0000103 | ovarian | paclitaxel | u | + | [203] |
| miR-591 | 0003259 | d | + | |||
| miR-770-5p | 0003948 | ovarian | cisplatin | u | − | [204] |
| miR-21-5p | 0000076 | ovarian | paclitaxel; exosome-driven | u | + | [205] |
| miR-199b-5p | 0000263 | ovarian | cisplatin | d | + | [206] |
| miR-145-5p | 0000437 | ovarian | paclitaxel | u | − | [207] |
| let-7e-5p | 0000066 | ovarian | cisplatin | d | + | [208] |
| miR-152-3p | 0000438 | ovarian | cisplatin | u | − | [209] |
| miR-128-3p | 0000424 | ovarian | cisplatin | d | + | [210] |
| miR-484 | 0002174 | ovarian | d | + | [211] | |
| miR-642a-5p | 0003312 | d | + | |||
| miR-217 | 0000274 | d | + | |||
| miR-23a-3p | 0000078 | ovarian | u | + | [212] | |
| miR-27b-3p | 0000419 | u | + | |||
| miR-424-5p | 0001341 | u | + | |||
| miR-503-5p | 0002874 | u | + | |||
| miR-21-5p | 0000076 | ovarian | carboplatin paclitaxel | u | + | [213] |
| miR-214-3p | 0000271 | u | + | |||
| miR-182-5p | 0000259 | ovarian | cisplatin paclitaxel | u | + | [214] |
| miR-200c-3p | 0000617 | pancreatic | u | - | [215] | |
| miR-33a-5p | 0000091 | pancreatic | gemcitabine | u | - | [216] |
| miR-17-92 cluster | pancreatic | d | + | [217] | ||
| miR-221-3p | 0000278 | pancreatic | 5-fluorouracil | u | + | [218] |
| miR-1246 | 0005898 | pancreatic | u | + | [219] | |
| miR-181b-5p | 0000257 | pancreatic | gemcitabine | u | + | [220] |
| miR-494-3p | 0002816 | pancreatic | u | − | [221] | |
| miR-101-3p | 0000099 | pancreatic | gemcitabine | u | − | [222] |
| miR-100-5p | 0000098 | pancreatic (ductal) | gemcitabine | u | + | [223,224] |
| miR-21-5p | 0000076 | u | + | |||
| miR-99a-5p | 0000097 | u | + | |||
| miR-125b-5p | 0000423 | u | + | |||
| miR-138-5p | 0000430 | u | + | |||
| miR-210-3p | 0000267 | u | + | |||
| miR-31-3p | 0004504 | d | + | |||
| miR-330-3p | 0000751 | d | + | |||
| miR-378-5p | 0000731 | d | + | |||
| let-7a-5p | 0000062 | pancreatic | gemcitabine | u | − | [225] |
| miR-205-5p | 0000266 | pancreatic | gemcitabine | u | − | [226] |
| miR-506-3p | 0002878 | pancreatic | d | + | [227] | |
| miR-3176 | 0015053 | prostate | paclitaxel | d | + | [228] |
| miR-141-3p | 0000432 | d | + | |||
| miR-5004-5p | 0021027 | d | + | |||
| miR-16-5p | 0000069 | d | + | |||
| miR-3915 | 0018189 | d | + | |||
| miR-488-3p | 0004763 | d | + | |||
| miR-23c | 0018000 | d | + | |||
| miR-3673 | 0018096 | d | + | |||
| miR-3654 | 0018074 | d | + | |||
| miR-32-5p | 0000090 | u | + | |||
| miR-606 | 0003274 | u | + | |||
| miR-381-3p | 0000736 | u | + | |||
| miR-429 | 0001536 | u | + | |||
| miR-708 | 0004926 | renal | doxorubicin | u | − | [229] |
| let-7b-5p | 0000063 | renal | 5-fluorouracil | u | − | [230] |
| let-7c-5p | 0000064 | u | − | |||
| miR-200c-3p | 0000617 | renal | docetaxel | d | + | [231] |
| lncRNA/s involved (Species—Homo sapiens) | gene ID (LNCipedia.org—Where Applicable) | Cancer Model | Affected Chemo-Therapy Drugs | Dysregulation Status | Effect on Chemo-Resistance Phenotype | Ref. |
|---|---|---|---|---|---|---|
| UCA1 | UCA1 | bladder | cisplatin, gemcitabine | u | + | [242,243] |
| NONHSAT028712 | lnc-DGKA-1 | breast | doxorubicin | u | + | [244] |
| NONHSAT057282 | lnc-RP11-677O4.1.1-7 | u | + | |||
| NONHSAG023333 | lnc-TXNDC2-7 | u | + | |||
| ARA | lnc-ALG13-7 | breast | doxorubicin | u | + | [245] |
| ATB | lncRNA-AL589182 | breast | trastuzumab | u | + | [246] |
| GAS5 | GAS5 | breast | trastuzumab | d | + | [247] |
| XIST | XIST | breast | alkylating agents | u | + | [248] |
| 53BP1 | Lnc-TP53BP1-1 | d | + | |||
| CCAT2 | lnc-POU5F1B-8 | breast | 5-fluorouracil | u | + | [249] |
| snaR | lnc-BSPH1-1/2 | colon | 5-fluorouracil | u | + | [250] |
| LINC00152 | LINC00152 | colon | oxaliplatin | u | + | [251] |
| SLC25A25-AS1 | SLC25A25-AS1 | colorectal | d | + | [252] | |
| MRUL | (NR_024549) | gastric | MDR | u | + | [239] |
| AK022798 | lnc-TRAF3IP3-3 | gastric | cisplatin | u | + | [253] |
| PVT1 | PVT1 | gastric | MDR | u | + | [254] |
| LINC-ROR | LINC-ROR | hepatocellular | sorafenib, doxorubicin | u | + | [255] |
| CCAT1 | lnc-TMEM75-3 | lung | docetaxel | u | + | [256] |
| AK126698 | (LINC00969) | lung | cisplatin | d | + | [257] |
| HOTAIR | HOTAIR | lung | MDR | u | + | [236,237] |
| GAS5 | GAS5 | lung | EGFR-tyrosine kinase inhibitors | u | − | [240] |
| UCA1 | UCA1 | lung | EGFR-tyrosine kinase inhibitors | u | + | [241] |
| MEG3 | MEG3 | lung | cisplatin | u | + | [258] |
| GAS5 | GAS5 | lymphoma (mantle cell) | mTOR inhibitors | u | − | [259] |
| N375709 | (lnc-SRCIN1-1) | nasopharyngeal | paclitaxel | d | − | [260] |
| LINC-ROR | LINC-ROR | nasopharyngeal | u | + | [261] | |
| TUG1 | TUG1 | oesophageal | u | + | [262] | |
| LINC00161 | LINC00161 | osteosarcoma | cisplatin | u | − | [263] |
| ODRUL | FOXC2-AS1 | osteosarcoma | doxorubicin | u | + | [264] |
| ODRUL | FOXC2-AS1 | osteosarcoma | doxorubicin | u | + | [265] |
| HOTAIR | HOTAIR | ovarian | platinum-based drugs | u | + | [238] |
| PVT1 | PVT1 | ovarian | cisplatin | u | + | [266] |
| UCA1 | UCA1 | ovarian | u | + | [267] | |
| HOTTIP | BCYRN1 | ovarian | carboplatin | d | + | [268] |
| HOTTIP | BCYRN1 | pancreatic | gemcitabine | u | + | [269] |
| PVT1 | PVT1 | pancreatic | gemcitabine | u | + | [270] |
| MALAT-1 | MALAT1 | pancreatic | u | + | [271] | |
| GAS5 | GAS5 | prostate | mTOR inhibitors | u | − | [272] |
| UCA1 | UCA1 | breast | tamoxifen | u | + | [273] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayers, D.; Vandesompele, J. Influence of microRNAs and Long Non-Coding RNAs in Cancer Chemoresistance. Genes 2017, 8, 95. https://doi.org/10.3390/genes8030095
Ayers D, Vandesompele J. Influence of microRNAs and Long Non-Coding RNAs in Cancer Chemoresistance. Genes. 2017; 8(3):95. https://doi.org/10.3390/genes8030095
Chicago/Turabian StyleAyers, Duncan, and Jo Vandesompele. 2017. "Influence of microRNAs and Long Non-Coding RNAs in Cancer Chemoresistance" Genes 8, no. 3: 95. https://doi.org/10.3390/genes8030095
APA StyleAyers, D., & Vandesompele, J. (2017). Influence of microRNAs and Long Non-Coding RNAs in Cancer Chemoresistance. Genes, 8(3), 95. https://doi.org/10.3390/genes8030095







