Regulatory Elements Located in the Upstream Region of the Rhizobium leguminosarum rosR Global Regulator Are Essential for Its Transcription and mRNA Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Conditions
2.2. DNA Methods and Sequence Analysis
2.3. Construction of rosR-lacZ Transcriptional Fusions Containing Changed Sequences of Regulatory Motifs
2.4. Construction of Plasmids Containing the Entire rosR Gene and Mutations in the Regulatory Motifs
2.5. RNA Isolation
2.6. Reverse Transcription and Quantitative Real-Time PCR
2.7. Determination of RNA Decay
2.8. β-Galactosidase Assay
2.9. Statistical Analysis
3. Results
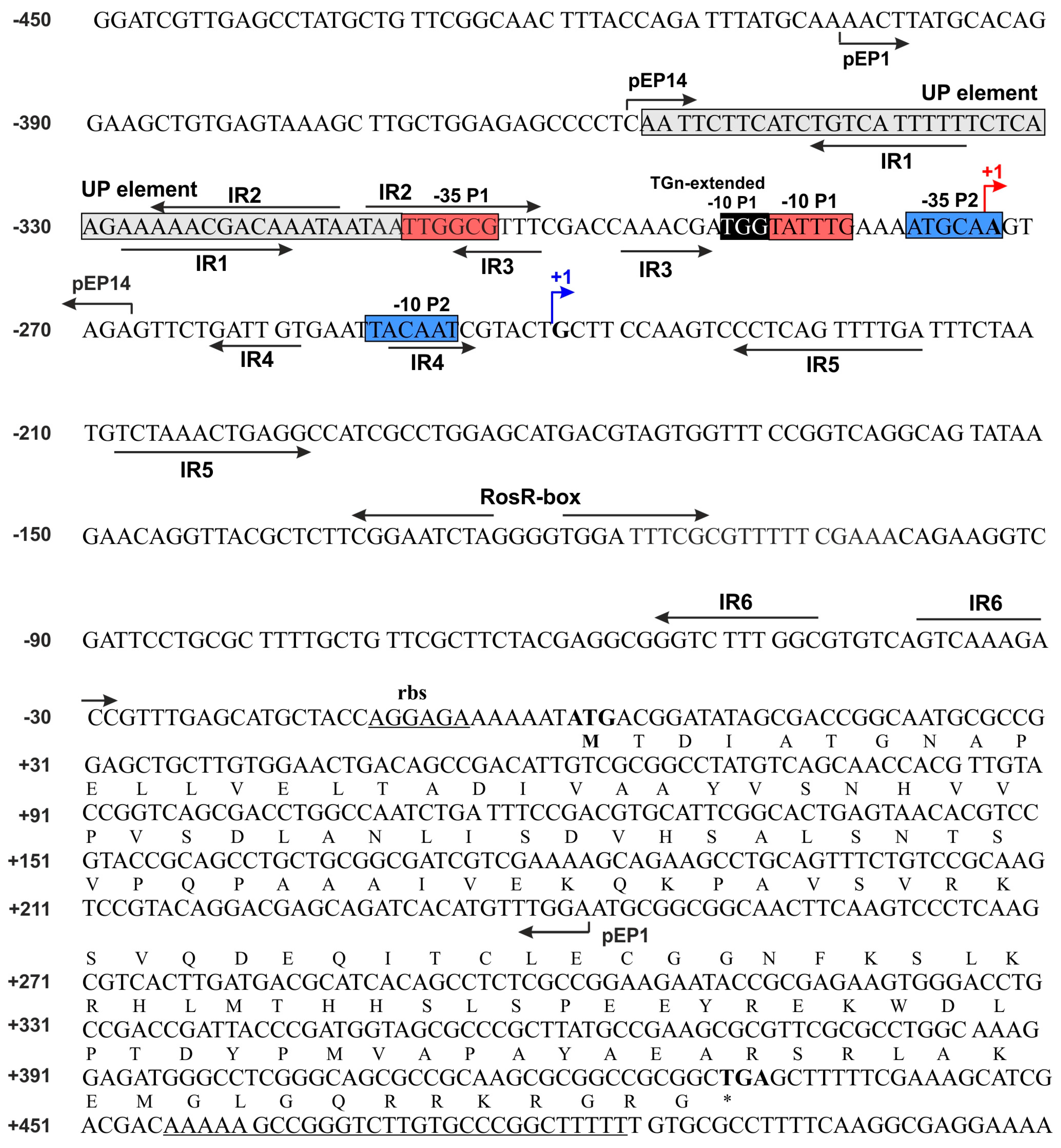
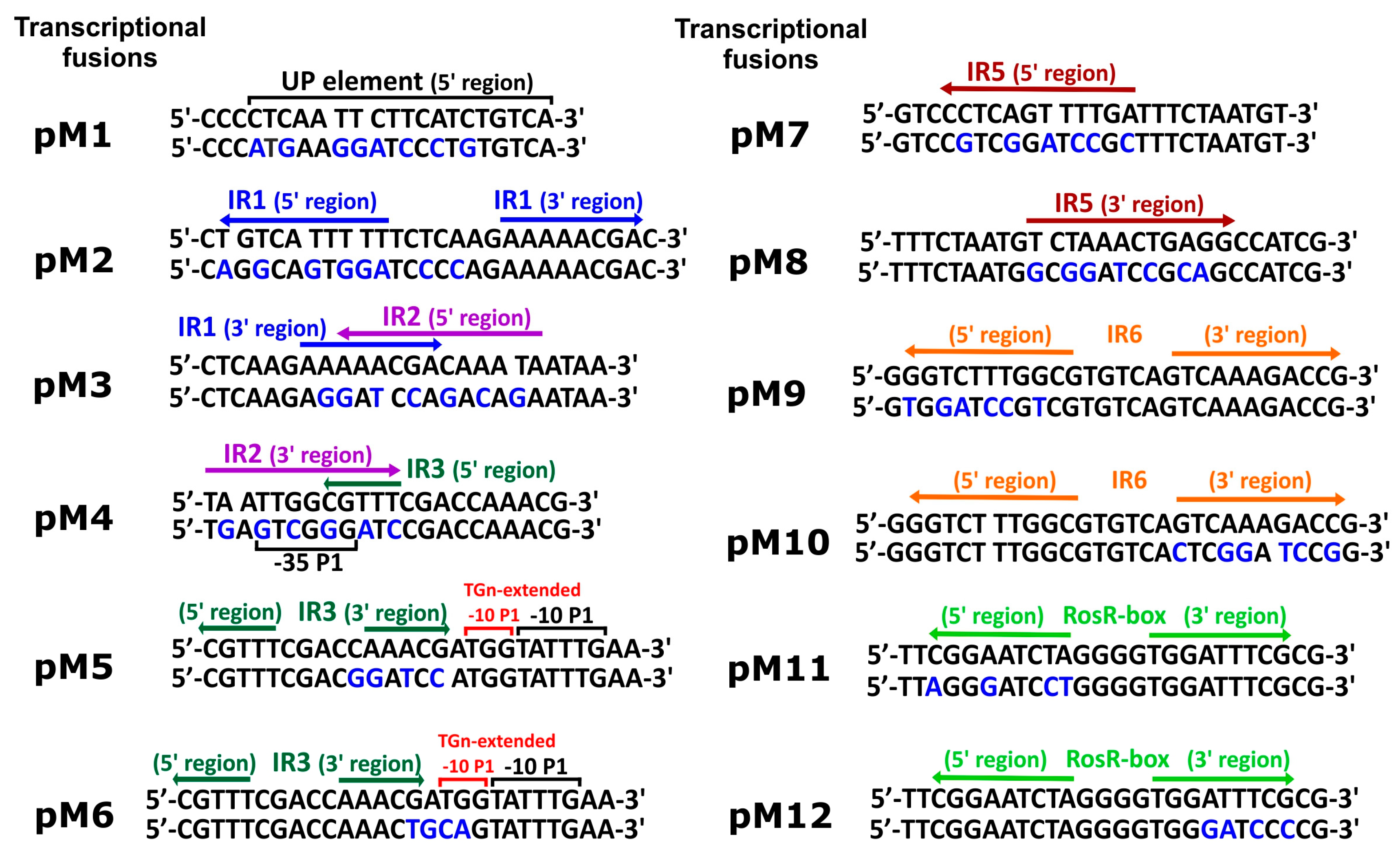
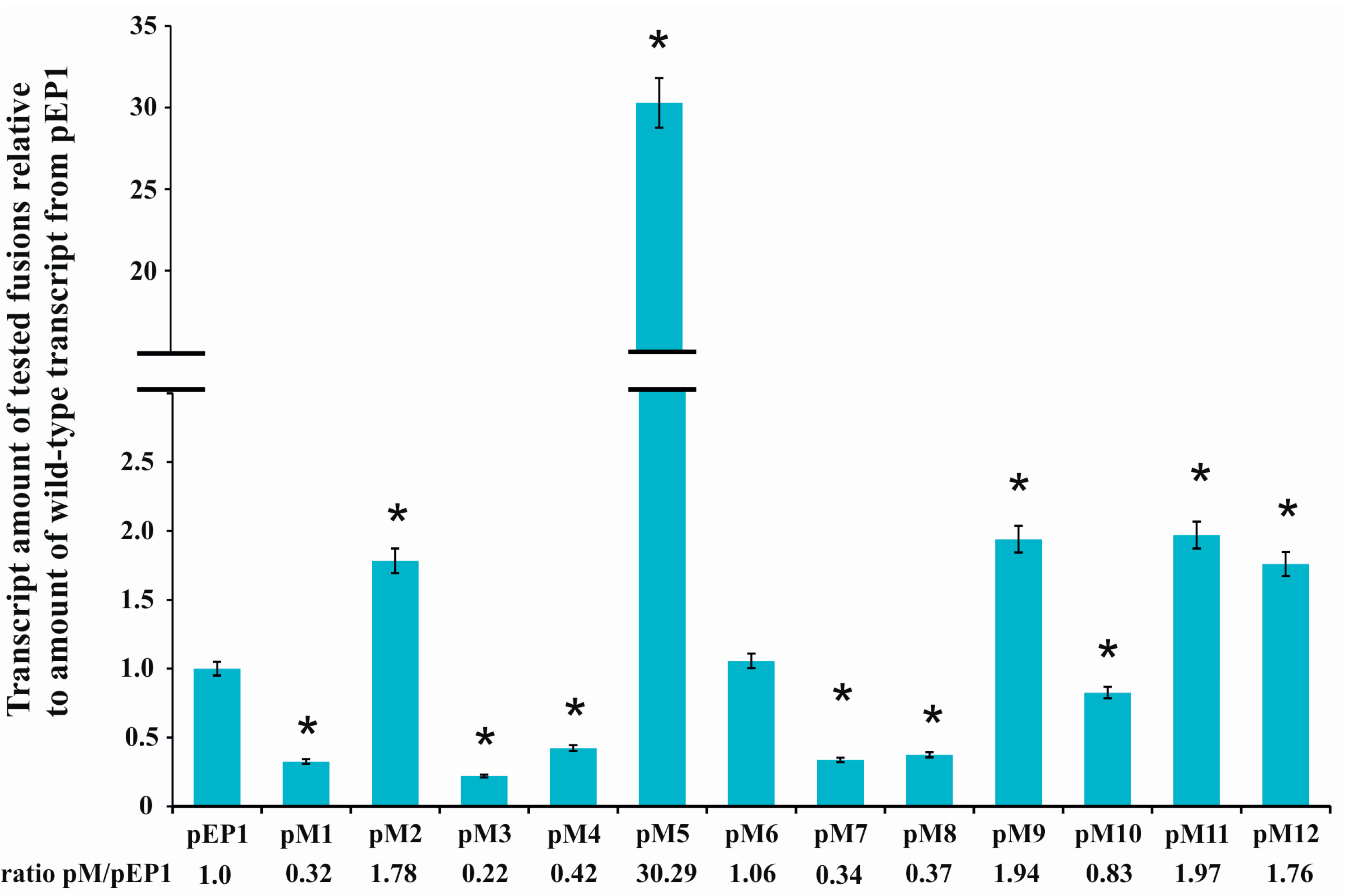
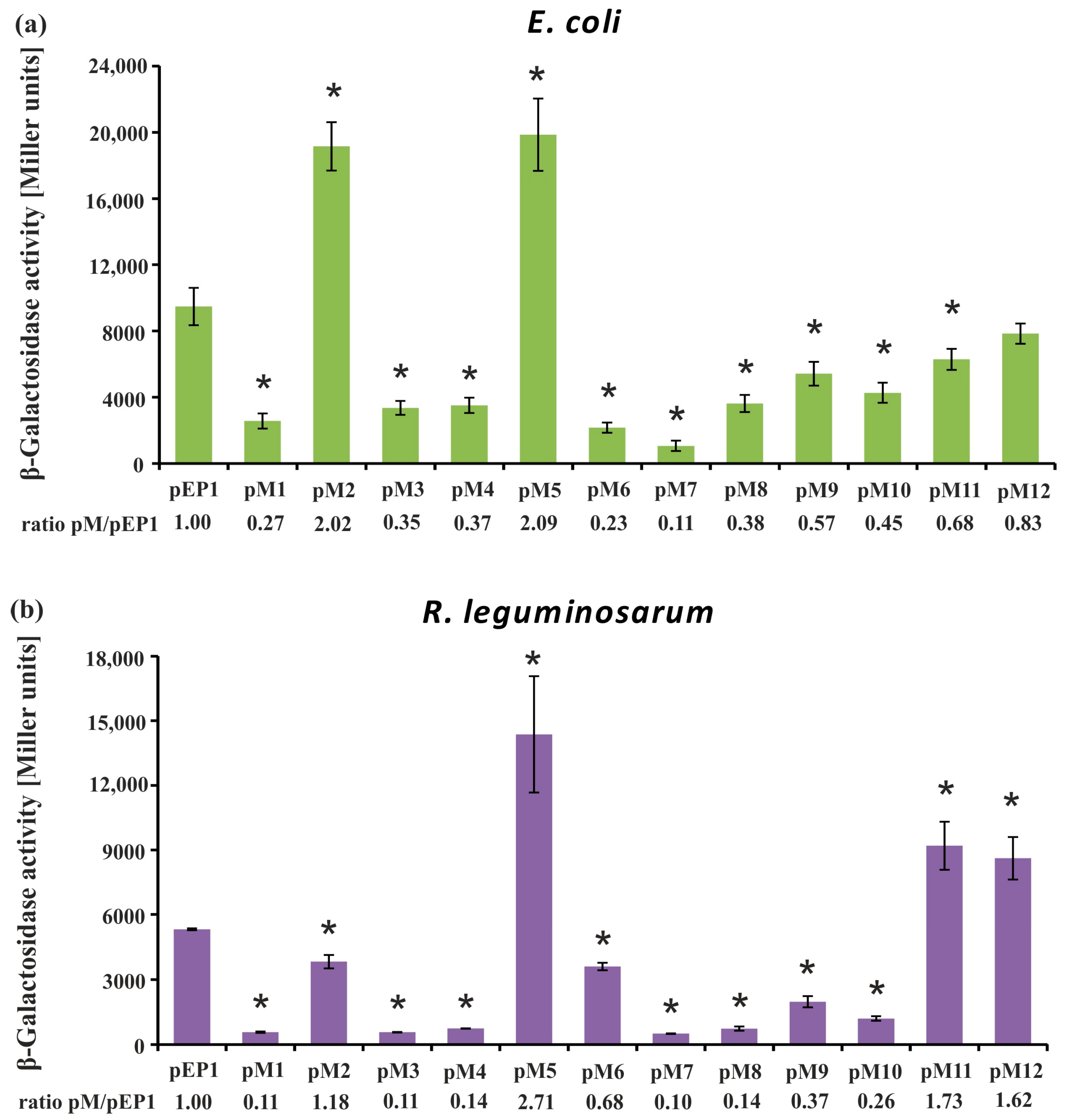
3.1. Directed-Mutagenesis and Functional Analysis of Regulatory Motifs Located in the rosR Upstream Region
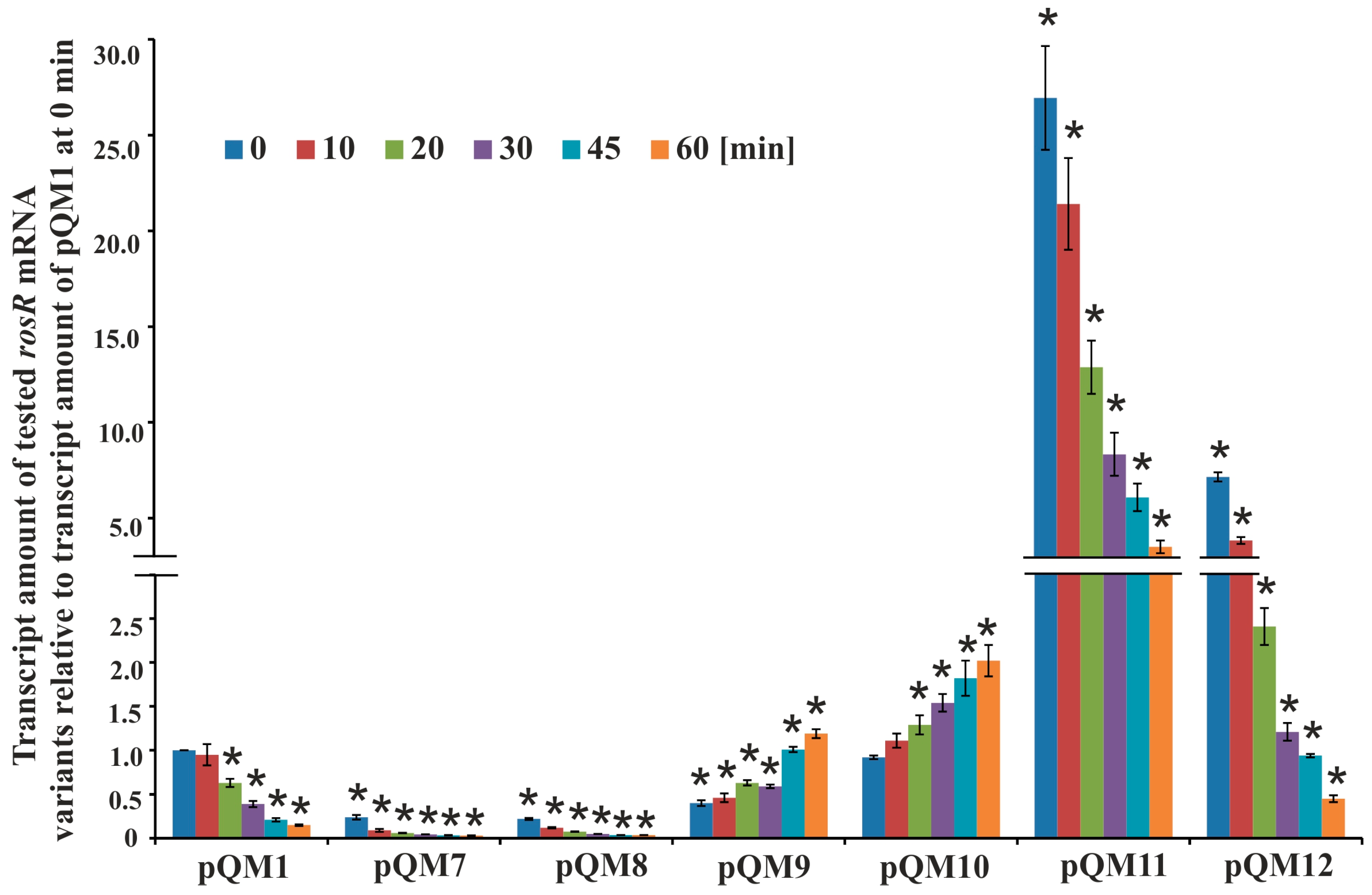
3.2. Determination of Secondary Structures of rosR Transcripts and Their Stability
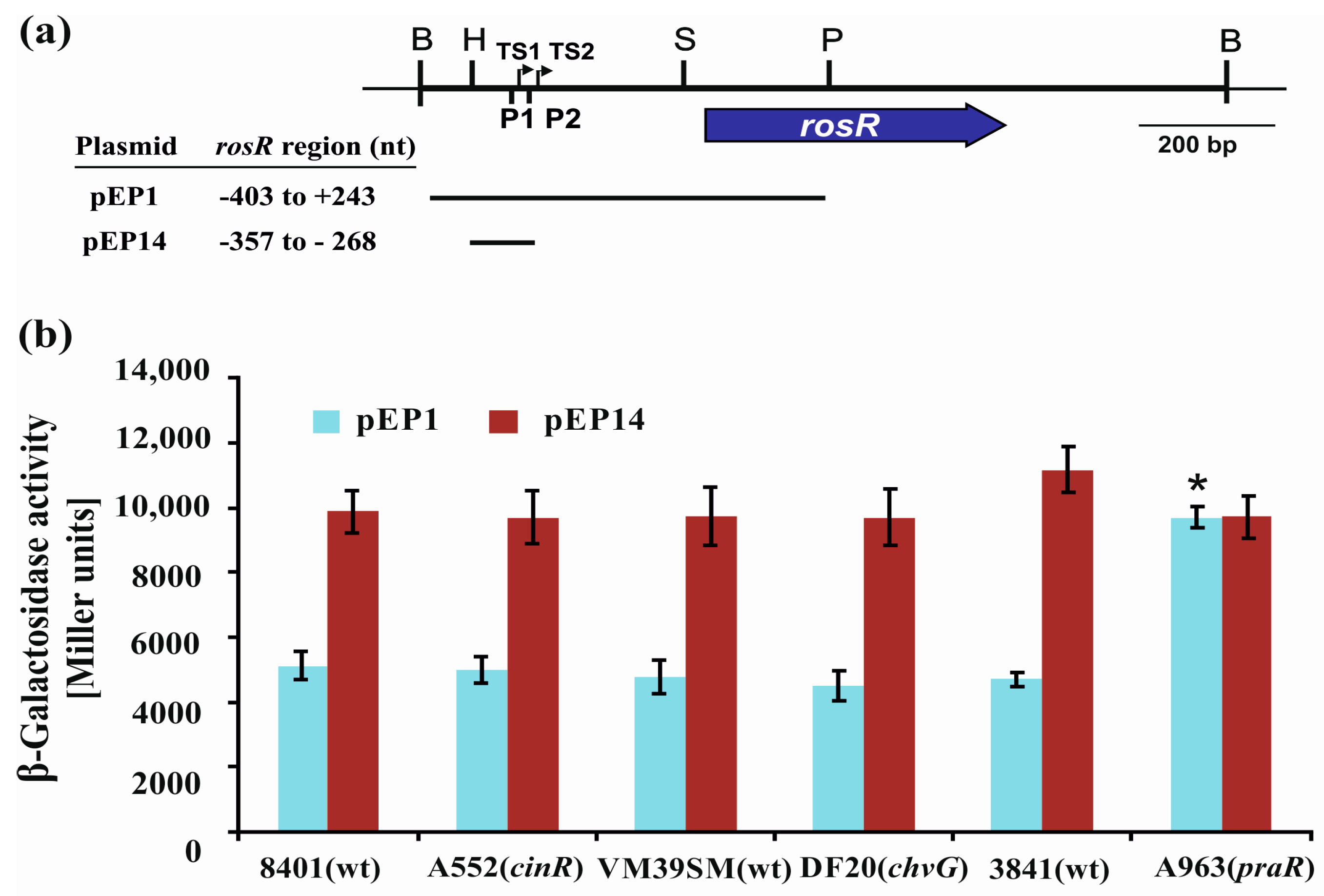
3.3. The Influence of CinR, PraR, and ChvG on the Expression of rosR
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Downie, J.A. The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots. FEMS Microbiol. Rev. 2010, 34, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Pini, F.; Galardini, M.; Bazzicalupo, M.; Mengoni, A. Plant-bacteria association and symbiosis: Are there common genomic traits in Alphaproteobacteria? Genes 2011, 2, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Jaszek, M.; Janczarek, M.; Kuczyński, K.; Piersiak, T.; Grzywnowicz, K. The response of the Rhizobium leguminosarum bv. trifolii wild-type and exopolysaccharide-deficient mutants to oxidative stress. Plant Soil 2014, 376, 75–94. [Google Scholar] [CrossRef]
- Rachwał, K.; Matczyńska, E.; Janczarek, M. Transcriptome profiling of a Rhizobium leguminosarum bv. trifolii rosR mutant reveals the role of the transcriptional regulator RosR in motility, synthesis of cell-surface components, and other cellular processes. BMC Genom. 2015, 16, 1–18. [Google Scholar] [CrossRef]
- Rachwał, K.; Boguszewska, A.; Kopcińska, J.; Karaś, M.; Tchórzewski, M.; Janczarek, M. The Regulatory protein RosR affects Rhizobium leguminosarum bv. trifolii protein profiles, cell surface properties, and symbiosis with clover. Front. Microbiol. 2016, 7, 1–21. [Google Scholar] [CrossRef]
- Janczarek, M.; Skorupska, A. The Rhizobium leguminosarum bv. trifolii RosR: Transcriptional regulator involved in exopolysaccharide production. Mol. Plant Microbe Interact. 2007, 20, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Shi, Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Cooley, M.B.; D’Souza, M.R.; Kado, C.I. The virC and virD operons of the Agrobacterium Ti plasmid are regulated by the ros chromosomal gene: Analysis of the cloned ros gene. J. Bacteriol. 1991, 173, 2608–2616. [Google Scholar] [CrossRef] [PubMed]
- Malgieri, G.; Russo, L.; Esposito, S.; Baglivo, I.; Zaccaro, L.; Pedone, E.M.; Di Blasio, B.; Isernia, C.; Pedone, P.V.; Fattorusso, R. The prokaryotic Cys2His2 zinc-finger adopts a novel fold as revealed by the NMR structure of Agrobacterium tumefaciens Ros DNA-binding domain. Proc. Natl. Acad. Sci. USA 2007, 104, 17341–17346. [Google Scholar] [CrossRef] [PubMed]
- Bittinger, M.A.; Milner, J.L.; Saville, B.J.; Handelsman, J. rosR, a determinant of nodulation competitiveness in Rhizobium etli. Mol. Plant Microbe Interact. 1997, 10, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.Y.; Archdeacon, J.; Kado, C.I. Agrobacterium transcriptional regulator Ros is a prokaryotic zinc finger protein that regulates the plant oncogene ipt. Proc. Natl. Acad. Sci. USA 1998, 95, 5293–5298. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Roxlau, A.; Weng, W.M.; Schmidt, M.; Quandt, J.; Niehaus, K.; Jording, D.; Arnold, W.; Pühler, A. Molecular analysis of the Rhizobium meliloti mucR gene regulating the biosynthesis of the exopolysaccharides succinoglycan and galactoglucan. Mol. Plant Microbe Interact. 1995, 8, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Jurado, S.; Alias-Villegas, C.; Navarro-Gómez, P.; Zehner, S.; Murdoch, P.D.; Rodríguez-Carvajal, M.A.; Soto, M.J.; Ollero, F.-J.; Ruiz-Sainz, J.E.; Göttfert, M.; et al. The Sinorhizobium fredii HH103 MucR1 global regulator is connected with the nod regulon and is required for efficient symbiosis with Lotus burttii and Glycine max cv. Williams. Mol. Plant Microbe Interact. 2016, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Wu, L.J.; Zhang, B.; Hu, Y.; Li, Y.; Zhang, X.X.; Guo, H.J.; Liu, L.X.; Chen, W.X.; Zhang, Z.; et al. MucR is required for transcriptional activation of conserved ion transporters to support nitrogen fixation of Sinorhizobium fredii in soybean nodules. Mol. Plant Microbe Interact. 2016, 29, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Ruberg, S.; Kuster, H.; Roxlau, A.A.; Keller, M.; Ivashina, T.; Cheng, H.P.; Walker, G.C.; Puhler, A. The 32-kilobase exp gene cluster of Rhizobium meliloti directing the biosynthesis of galactoglucan: Genetic organization and properties of the encoded gene products. J. Bacteriol. 1997, 179, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Bertram-Drogatz, P.A.; Rüberg, S.; Becker, A.; Pühler, A. The regulatory protein MucR binds to a short DNA region located upstream of the mucR coding region in Rhizobium meliloti. Mol. Gen. Genet. 1997, 254, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Bertram-Drogatz, P.A.; Quester, I.; Becker, A.; Pühler, A. The Sinorhizobium meliloti MucR protein, which is essential for the production of high-molecular-weight succinoglycan exopolysaccharide, binds to short DNA regions upstream of exoH and exoY. Mol. Gen. Genet. 1998, 257, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Jurado, S.; Rodríguez-Navarro, D.N.; Kawaharada, Y.; Perea, J.F.; Gil-Serrano, A.; Jin, H.; An, Q.; Rodríguez-Carvajal, M.A.; Andersen, S.U.; Sandal, N.; et al. Sinorhizobium fredii HH103 Invades Lotus burttii by crack entry in a nod factor-and surface polysaccharide-dependent manner. Mol. Plant Microbe Interact. 2016, 29, 925–937. [Google Scholar] [CrossRef] [PubMed]
- López-Baena, F.J.; Ruiz-Sainz, J.E.; Rodríguez-Carvajal, M.A.; Vinardell, J.-M. Bacterial molecular signals in the Sinorhizobium fredii-soybean symbiosis. Int. J. Mol. Sci. 2016, 17, 755. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Skorupska, A. Rhizobium leguminosarum bv. trifolii rosR gene expression is regulated by catabolic repression. FEMS Microbiol. Lett. 2009, 291, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Skorupska, A. Modulation of rosR expression and exopolysaccharide production in Rhizobium leguminosarum bv. trifolii by phosphate and clover root exudates. Int. J. Mol. Sci. 2011, 12, 4132–4155. [Google Scholar] [CrossRef] [PubMed]
- Ross, W.; Gosink, K.K.; Salomon, J.; Igarashi, K.; Zou, C.; Ishihama, A.; Severinov, K.; Gourse, R.L. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science 1993, 262, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Estrem, S.T.; Gaal, T.; Ross, W.; Gourse, R.L. Identification of an UP element consensus sequence for bacterial promoters. Proc. Natl. Acad. Sci. USA 1998, 95, 9761–9766. [Google Scholar] [CrossRef] [PubMed]
- Estrem, S.T.; Ross, W.; Gall, T.; Chen, Z.W.; Niu, W.; Ebright, R.H.; Gourse, R.L. Bacterial promoter architecture: Subsite structure of UP elements and interactions with the carboxy-terminal domain of the RNA polymerase α subunit. Genes Dev. 1999, 13, 2134–2147. [Google Scholar] [CrossRef] [PubMed]
- Ross, W.; Aiyar, S.E.; Salomon, J.; Gourse, R.L. Escherichia coli promoters with UP elements of different strengths: Modular structure of bacterial promoters. J. Bacteriol. 1998, 18, 5375–5383. [Google Scholar]
- Gourse, R.L.; Ross, W.; Gaal, T. UPs and downs in bacterial transcription initiation: The role of the alpha subunit of RNA polymerase in promoter recognition. Mol. Microbiol. 2000, 37, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Busby, S.J. More pieces in the promoter jigsaw: Recognition of -10 regions by alternative sigma factors. Mol. Microbiol. 2009, 72, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.W.; Beringer, J.E. Identification of the rhizobium strains in pea root nodules using genetic markers. J. Gen. Microbiol. 1975, 87, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Frederix, M.; Edwards, A.; McAnulla, C.; Downie, J.A. Co-ordination of quorum-sensing regulation in Rhizobium leguminosarum by induction of an anti-repressor. Mol. Microbiol. 2011, 81, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.W.; Hombrecher, G.; Johnston, A.B. Plasmid determined nodulation and nitrogen fixation abilities in Rhizobium phaseoli. Mol. Gen. Genet. 1982, 186, 449–452. [Google Scholar] [CrossRef]
- Lithgow, J.K.; Wilkinson, A.; Hardman, A.; Rodelas, B.; Wisniewski-Dyé, F.; Williams, P.; Downie, J.A. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol. Microbiol. 2000, 37, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Priefer, U.B. Genes involved in lipopolysaccharide production and symbiosis are clustered on the chromosome of Rhizobium leguminosarum biovar viciae VF39. J. Bacteriol. 1989, 171, 6161–6168. [Google Scholar] [CrossRef] [PubMed]
- Foreman, D.L.; Vanderlinde, E.M.; Bay, D.C.; Yost, C.K. Characterization of a gene family of outer membrane proteins (ropB) in Rhizobium leguminosarum bv. viciae VF39SM and the role of the sensor kinase ChvG in their regulation. J. Bacteriol. 2010, 192, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; pp. 11–26, 94–144, 162–186. ISBN 1936113422. [Google Scholar]
- Simon, R.; Quandt, J.; Klipp, W. New derivatives of transposon Tn5 suitable for mobilization of replicons, generation of operon fusions and induction of genes in Gram-negative bacteria. Gene 1989, 80, 161–169. [Google Scholar] [CrossRef]
- Spaink, H.P.; Okker, R.J.; Wijffelman, C.A.; Pees, E.; Lugtenberg, B.J. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol. Biol. 1987, 9, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Qiagen. Available online: https://www.qiagen.com/us/ (accessed on 16 October 2017).
- Vincent, J.M. A manual for the practical study of root nodule bacteria. In International Biological Program Handbook no. 15; Blackwell Scientific Publications, Ltd.: Oxford, UK; pp. 153–159. ISBN 0632064102.
- Brown, C.M.; Dilworth, M.J. Ammonia assimilation by rhizobium cultures and bacteroids. J. Gen. Microbiol. 1975, 86, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinde, E.M.; Yost, C.K. Mutation of the sensor kinase chvG in Rhizobium leguminosarum negatively impacts cellular metabolism, outer membrane stability, and symbiosis. J. Bacteriol. 2012, 194, 768–777. [Google Scholar] [CrossRef] [PubMed]
- AliBee-Multiple Alignment Program. Available online: http://www.genebee.msu.su/services/malign_reduced.html (accessed on 16 October 2017).
- Fuzznuc Program. Available online: http://emboss.ch.embnet.org?Pise (accessed on 16 October 2017).
- Zucker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- The UNAFold Web Server. Available online: http://www.bioinfo.rpi.edu/applications/mfold (accessed on 16 October 2017).
- Newbury, S.F.; Smith, N.H.; Robinson, E.C.; Hiles, I.D.; Higgins, C.F. Stabilization of translationally active mRNA by prokaryotic REP sequences. Cell 1987, 48, 297–310. [Google Scholar] [CrossRef]
- Wojda, I.; Taszłow, P. Heat shock affects host-pathogen interaction in Galleria mellonella infected with Bacillus thuringiensis. J. Insect Physiol. 2013, 59, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Pratte, B.S.; Thiel, T. Regulation of nitrogenase gene expression by transcript stability in the cyanobacterium Anabaena variabilis. J. Bacteriol. 2014, 196, 3609–3621. [Google Scholar] [CrossRef] [PubMed]
- Pratte, B.S.; Ungerer, J.; Thiel, T. Role of RNA secondary structure and processing in stability of the nifH1 transcript in the cyanobacterium Anabaena variabilis. J. Bacteriol. 2015, 197, 1408–1422. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.V.; Pontel, L.B.; Vescovi, E.G.; Soncini, F.C. Regulation of magnesium homeostasis in Salmonella: Mg2+ targets the mgtA transcript for degradation by RNase E. FEMS Microbiol. Lett. 2008, 280, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Gastebois, A.; Mathieu-Demazière, C.; Sorroche, F.; Masson-Boivin, C.; Batut, J.; Garnerone, A.M. transcriptomic insight in the control of legume root secondary infection by the Sinorhizobium meliloti transcriptional regulator Clr. Front. Microbiol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sippel, A.; Hartmann, G. Mode of action of rifampicin on the RNA polymerase reaction. Biochim. Biophys. Acta 1968, 157, 218–219. [Google Scholar] [CrossRef]
- Steglich, C.; Lindell, D.; Futschik, M.; Rector, T.; Steen, R.; Chisholm, S.W. Short RNA half-lives in the slow-growing marine cyanobacterium Prochlorococcus. Genome Biol. 2010, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Frederix, M.; Edwards, A.; Swiderska, A.; Stanger, A.; Karunakaran, R.; Williams, A.; Abbruscato, P.; Sanchez-Contreras, M.; Poole, P.S.; Downie, J.A. Mutation of praR in Rhizobium leguminosarum enhances root biofilms, improving nodulation competitiveness by increased expression of attachment proteins. Mol. Microbiol. 2014, 93, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, S. Bacterial regulation: Global regulatory networks. Annu. Rev. Genet. 1984, 18, 415–444. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Rachwał, K.; Kopcińska, J. Genetic characterization of the Pss region and the role of PssS in exopolysaccharide production and symbiosis of Rhizobium leguminosarum bv. trifolii with clover. Plant Soil 2015, 396, 257–275. [Google Scholar] [CrossRef]
- Alon, U. Network motifs: Theory and experimental approaches. Nat. Rev. Genet. 2007, 8, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Chavez, I.; Angarica, V.E.; Collado-Vides, J.; Contreras-Moreira, B. The role of DNA-binding specificity in the evolution of bacterial regulatory networks. J. Mol. Biol. 2008, 379, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Balleza, E.; Lopez-Bojorquez, L.N.; Martinez-Antonio, A.; Resendis-Antonio, O.; Lozada-Chavez, I.; Balderas-Martinez, Y.I.; Encarnacion, S.; Collado-Vides, J. Regulation by transcription factors in bacteria: Beyond description. FEMS Microbiol. Rev. 2009, 33, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.F.; Busby, S.J. The regulation of bacterial transcription initiation. Nat. Rev. Microbiol. 2004, 2, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Aiyar, S.E.; Gourse, R.L.; Ross, W. Upstream A-tracts increase bacterial promoter activity through interactions with the RNA polymerase alpha subunit. Proc. Natl. Acad. Sci. USA 1998, 95, 14652–14657. [Google Scholar] [CrossRef] [PubMed]
- Leirmo, S.; Gourse, R.L. Factor-independent activation of Escherichia coli rRNA transcription. I. Kinetic analysis of the roles of the upstream activator region and supercoiling on transcription of the rrnB P1 promoter in vitro. J. Mol. Biol. 1991, 220, 555–568. [Google Scholar] [CrossRef]
- Husnain, S.I.; Thomas, M.S. The UP element is necessary but not sufficient for growth rate-dependent control of the Escherichia coli guaB promoter. J. Bacteriol. 2008, 190, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Banner, C.D.; Moran, C.P., Jr.; Losick, R. Deletion analysis of a complex promoter for a developmentally regulated gene from Bacillus subtilis. J. Mol. Biol. 1983, 168, 351–365. [Google Scholar] [CrossRef]
- Busby, S.; Spassky, A.; Chan, B. RNA polymerase makes important contacts upstream from base pair -49 at the Escherichia coli galactose operon P1 promoter. Gene 1987, 53, 145–152. [Google Scholar] [CrossRef]
- Compan, I.; Touati, D. Anaerobic activation of arcA transcription in Escherichia coli: Roles of Fnr and ArcA. Mol. Microbiol. 1994, 11, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Shimizu, K. Transcriptional regulation of main metabolic pathways of cyoA, cydB, fnr, and fur gene knockout Escherichia coli in C-limited and N-limited aerobic continuous cultures. Microb. Cell Fact. 2011, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Emory, S.A.; Bouvet, P.; Belasco, J.G. A 5′-terminal stem-loop structure can stabilize mRNA in Escherichia coli. Genes Dev. 1992, 6, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Bensing, B.A.; Meyer, B.J.; Dunny, G.M. Sensitive detection of bacterial transcription initiation sites and differentiation from RNA processing sites in the pheromone-induced plasmid transfer system of Enterococcus faecalis. Proc. Natl. Acad. Sci. USA 1996, 93, 7794–7799. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, N.; Marland, E.; Yu, G.X.; Bhatnagar, S.; Lusk, R. Sentra, a database of signal transduction proteins. Nucl. Acids Res. 2002, 30, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Rui, S.; Tse-Dinh, Y.C. Topoisomerase function during bacterial responses to environmental challenge. Front. Biosci. 2003, 8, 256–263. [Google Scholar]
| Strains and Plasmids | Characteristics | Source or Reference |
|---|---|---|
| Rhizobium leguminosarum | ||
| Rt24.2 | Wild type, Rifr, Nxr | [6] |
| 3841 | Wild type, Smr | [28] |
| A963 | 3841 praR::Tn5, Kmr | [29] |
| 8401 | Wild type, Smr | [30] |
| A552 | 8401 cinR1::Tn5, Kmr | [31] |
| VF39SM | Wild type, Smr | [32] |
| DF20 | VF39SM chvG::Tn5, Kmr | [33] |
| Escherichia coli | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔ M15) hsdR17 recA1endA1gyrA96 thi-1 relA1 | [34] |
| S17-1 | 294, thi, RP4-2-Tc::Mu-Km::Tn7 | [35] |
| JM101 | supE thi-1 Δ(lac-proAB) F′ [traD36 proAB+ lacIq lacZΔM15] | [34] |
| Plasmids | ||
| pUC19 | Cloning and sequencing vector, Apr | [34] |
| pMP220 | IncP, mob, promoterless lacZ, Tcr | [36] |
| pQE-31 | ori ColE1, expression vector, Apr | [37] |
| pB31 | pUC19 carrying 1.17 kb BamHI fragment with Rt24.2 rosR | [6] |
| pPUC1 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the 5′-part of the UP element | This work |
| pPUC2 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the 3′-part of the UP element | This work |
| pPUC3 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the IR1 3′-part and IR2 5′-part | This work |
| pPUC4 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the P1-35, IR2 3′-part, and IR3 5′-part | This work |
| pPUC5 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the 3′-part of IR3 | This work |
| pPUC6 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the TGN-extended −10 motif | This work |
| pPUC7 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the 5′-part of IR5 | This work |
| pPUC8 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the 3′-part of IR5 | This work |
| pPUC9 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the 5′- part of IR6 | This work |
| pPUC10 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the 3′-part of IR6 | This work |
| pPUC11 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the 5′-part of the RosR-box | This work |
| pPUC12 | pUC19 carrying 647 bp EcoRI-XbaI fragment (rosR region from −403 to +243 bp) with a changed sequence in the 3′-part of the RosR-box | This work |
| pEP1 | pMP220 carrying the −403 to +243 bp rosR upstream region | [6] |
| pEP14 | pMP220 carrying the −358 to −268 bp rosR upstream region | [20] |
| pM1 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC1 | This work |
| pM2 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC2 | This work |
| pM3 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC3 | This work |
| pM4 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC4 | This work |
| pM5 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC5 | This work |
| pM6 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC6 | This work |
| pM7 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC7 | This work |
| pM8 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC8 | This work |
| pM9 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC9 | This work |
| pM10 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC10 | This work |
| pM11 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC11 | This work |
| pM12 | pMP220 carrying 647 bp EcoRI-XbaI fragment of pPUC12 | This work |
| pQM1 | pQE-31 carrying 0.8 kb EcoRI-HindIII fragment with the wild-type rosR | This work |
| pQM7 | pQE-31 carrying 0.8 kb EcoRI-HindIII fragment with rosR mutated in IR5 5′ | This work |
| pQM8 | pQE-31 carrying 0.8 kb EcoRI-HindIII fragment with rosR mutated in IR5 3′ | This work |
| pQM9 | pQE-31 carrying 0.8 kb EcoRI-HindIII fragment with rosR mutated in IR6 5′ | This work |
| pQM10 | pQE-31 carrying 0.8 kb EcoRI-HindIII fragment with rosR mutated in IR6 3′ | This work |
| pQM11 | pQE-31 carrying 0.8 kb EcoRI-HindIII fragment with rosR mutated in the 5′-part of the RosR-box | This work |
| pQM12 | pQE-31 carrying 0.8 kb EcoRI-HindIII fragment with rosR mutated in the 3′-part of the RosR-box | This work |
| Primer | Sequence (5′–3′) 1 | Source or Reference |
|---|---|---|
| EB1 | TGACAGATGGATCCTTCATGGGCT | This work |
| EB2 | CCCTCAAGGATCCCTGTGTCATTT | This work |
| EB3 | TTCTTGGGATCCACTGCCTGATGAA | This work |
| EB4 | GTCATTGGATCCCCAGAAAAACGA | This work |
| EB5 | ATTTGTGGATCCTCGTGAGAAAAAA | This work |
| EB6 | CTCAAGAGGATCCAGACAGAATAA | This work |
| EB7 | TTGGTCGGATCCCGACTCATTATTT | This work |
| EB8 | AATTGGGGATCCGACCAAACGAT | This work |
| EB9 | TCAAATACTGCAGTTTGGTCGAAACG | This work |
| EB10 | GACCAAACTGCAGTATTTGAAAATGCAAG | This work |
| EB11 | TACCATGGATCCGTCGAAACGCCA | This work |
| EB12 | GTTTCGACGGATCCATGGTATTTGAA | This work |
| BX13 | TAGAAATCGGATCCGACGGACTTGG | This work |
| BX14 | GTCCCTCGGATCCGCTTTCTAATGT | This work |
| BX15 | TGGCCTCGGATCCGCCATTAGAAA | This work |
| BX16 | TAATGTCGGATCCGCAGCCATCG | This work |
| BX17 | ACCCCTGGATCCCTAAGAGCGTAA | This work |
| BX18 | TCTTCGGGATCCTGGGGTGGATTT | This work |
| BX19 | AAACGCGGATCCCTCCCCTAGATT | This work |
| BX20 | GGGTGGGATCCCCGTTTTTCGAAA | This work |
| BX21 | AAACGGGATCCGAGTGACACGCCA | This work |
| BX22 | TCAGTCGGATCCGGTTTGAGCATG | This work |
| BX23 | ACGCCGGATCCACGCCTCGTAGAA | This work |
| BX24 | AGGCGGGGATCCGTCGTGTCAGT | This work |
| EP1 | ATGCAAGAATTCTGCACAGGAAGC | [6] |
| RR1 | CGCATTCTAGACATGTGATCTGCT | [6] |
| EP3 | GGTATTTGGAATTCCAAGTAGAGTTCT | [6] |
| rosR-P | AAAGCAGAAGCCTGCAGTTTCTGT | This work |
| rosR-H | TCCTGACAAGCTTCATCGAGATTA | This work |
| rosR4-Fw | GCGACCTGGCCAATCTGATTTC | This work |
| rosR4-Rv | CTGCAGGCTTCTGCTTTTCGAC | This work |
| recA2-Fw | GGCGAGGGTGTTTCCAAGAC | This work |
| recA2-Rv | GACGCTGGCTGTTATAGGAGAAC | This work |
| 16SEc-F1 | CCATGCCGCGTGTATGAAGAAG | This work |
| 16SEc-R1 | TCTGCGGGTAACGTCAATGAGC | This work |
| pssAG1f | CGCACATGCGAAAGATTTGCTGCG | This work |
| pssA2r | CCAGATCGAGGAATTCCCGACGTA | This work |
| pssY5f | GTCGTCGATGACGATGCGGCTGTT | This work |
| pssY5r | GAAACTATGTGCTTCCCATGTCATCG | This work |
| Type of Transcripts | ΔG of Secondary Structures of rosR Transcripts (kcal/mol) | |
|---|---|---|
| 766 nt Long Transcript | 733 nt Long Transcript | |
| Wild type (control) | −348.89 | −334.87 |
| Mutation in the 5′-part of IR5 | −347.84 | −331.50 |
| Mutation in the 3′-part of IR5 | −334.05 | −330.29 |
| Mutation in the 5′-part of the RosR-box | −347.00 | −333.25 |
| Mutation in the 3′-part of the RosR-box | −348.92 | −334.90 |
| Mutation in the 5′-part of IR6 | −345.92 | −332.24 |
| Mutation in the 3′-part of IR6 | −346.72 | −331.38 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachwał, K.; Lipa, P.; Wojda, I.; Vinardell, J.-M.; Janczarek, M. Regulatory Elements Located in the Upstream Region of the Rhizobium leguminosarum rosR Global Regulator Are Essential for Its Transcription and mRNA Stability. Genes 2017, 8, 388. https://doi.org/10.3390/genes8120388
Rachwał K, Lipa P, Wojda I, Vinardell J-M, Janczarek M. Regulatory Elements Located in the Upstream Region of the Rhizobium leguminosarum rosR Global Regulator Are Essential for Its Transcription and mRNA Stability. Genes. 2017; 8(12):388. https://doi.org/10.3390/genes8120388
Chicago/Turabian StyleRachwał, Kamila, Paulina Lipa, Iwona Wojda, José-María Vinardell, and Monika Janczarek. 2017. "Regulatory Elements Located in the Upstream Region of the Rhizobium leguminosarum rosR Global Regulator Are Essential for Its Transcription and mRNA Stability" Genes 8, no. 12: 388. https://doi.org/10.3390/genes8120388
APA StyleRachwał, K., Lipa, P., Wojda, I., Vinardell, J.-M., & Janczarek, M. (2017). Regulatory Elements Located in the Upstream Region of the Rhizobium leguminosarum rosR Global Regulator Are Essential for Its Transcription and mRNA Stability. Genes, 8(12), 388. https://doi.org/10.3390/genes8120388






