The Potential of Zebrafish as a Model Organism for Improving the Translation of Genetic Anticancer Nanomedicines
Abstract
1. Nanotechnology Provides Innovative Approaches to Cancer Management
2. Genetic Nanomedicines and the Main Challenges for Their Translation to the Clinic
3. Zebrafish as a Model Species
4. Zebrafish Is Currently Being Used for the Development of Anticancer Therapeutics
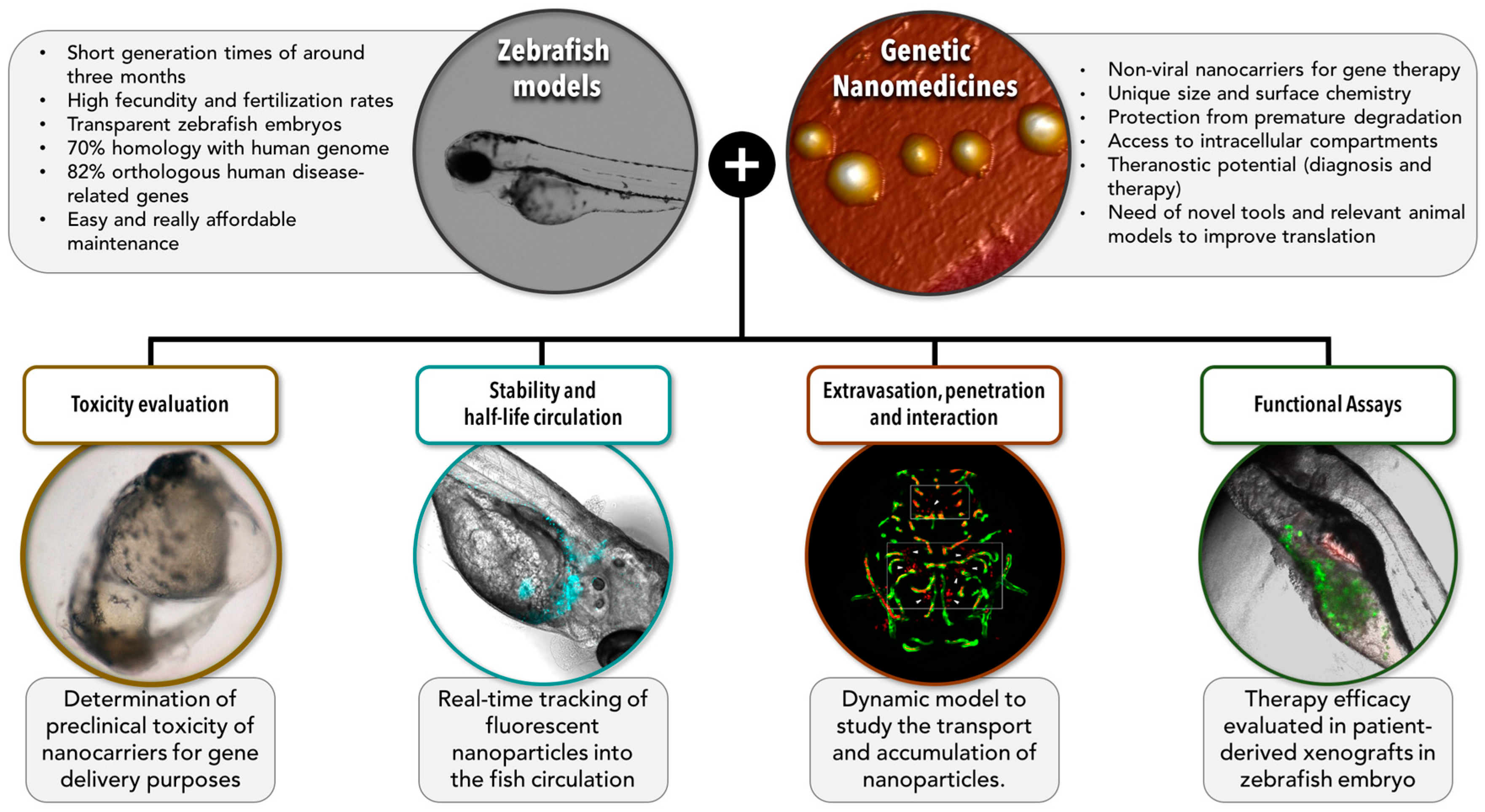
5. The Potential of Zebrafish for Increasing the Translation of Genetic Anticancer Nanomedicines: Barriers and Models
5.1. Toxicity
5.2. Stability and Half-Life While in Circulation
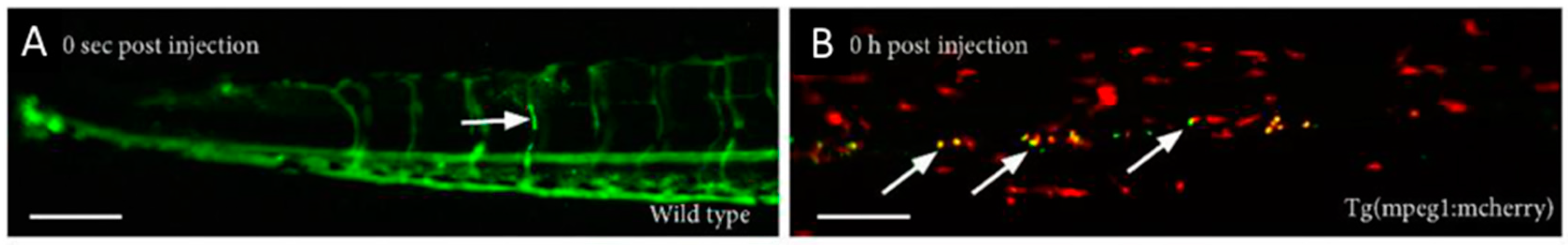
5.3. Extravasation, Penetration into the Tumor, and Interaction with the Target Cells
5.4. Functional Assays
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Swain, S.; Sahu, P.K.; Beg, S.; Babu, S.M. Nanoparticles for Cancer Targeting: Current and Future Directions. Curr. Drug Deliv. 2016, 13, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, T.L.; Hillaireau, H.; Vergnaud, J.; Fattal, E. Lipid-based nanosystems for CD44 targeting in cancer treatment: Recent significant advances, ongoing challenges and unmet needs. Nanomedicine 2016, 11, 1865–1887. [Google Scholar] [CrossRef] [PubMed]
- Thotakura, N.; Dadarwal, M.; Kumar, R.; Singh, B.; Sharma, G.; Kumar, P.; Katare, O.P.; Raza, K. Chitosan-palmitic acid based polymeric micelles as promising carrier for circumventing pharmacokinetic and drug delivery concerns of tamoxifen. Int. J. Biol. Macromol. 2017, 102, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hu, P.; Zheng, Q.; Tirelli, N.; Yang, X.; Wang, Z.; Wang, Y.; Tang, Q.; He, Y. Polymeric micelles with dual thermal and reactive oxygen species (ROS)-responsiveness for inflammatory cancer cell delivery. J. Nanobiotechnol. 2017, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ma, Z.; Zhang, Y.; Yang, C. Autophagy plays a dual role during intracellular siRNA delivery by lipoplex and polyplex nanoparticles. Acta Biomater. 2017, 58, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Crespo, D.; Jiménez, J.L.; Gómez, R.; De La Mata, F.J.; Majano, P.L.; Muñoz-Fernández, M.Á.; Gastaminza, P. Polyanionic carbosilane dendrimers prevent hepatitis C virus infection in cell culture. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Jia, T.; Sun, Z.; Lu, Y.; Gao, J.; Zou, H.; Xie, F.; Xu, H.; Sun, D.; Yu, Y.; Zhang, G. A dual brain-targeting curcumin-loaded polymersomes ameliorated cognitive dysfunction in intrahippocampal amyloid-β1–42-injected mice. Int. J. Nanomed. 2016, 11, 3765–3775. [Google Scholar] [CrossRef] [PubMed]
- Almhanna, K.; Wright, D.; Mercade, T.M.; Van Laethem, J.-L.; Gracian, A.C.; Guillen-Ponce, C.; Faris, J.; Lopez, C.M.; Hubner, R.A.; Bendell, J.; et al. A phase II study of antibody-drug conjugate, TAK-264 (MLN0264) in previously treated patients with advanced or metastatic pancreatic adenocarcinoma expressing guanylyl cyclase C. Investig. New Drugs 2017, 35, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Ao, M.; Zheng, X.; Li, N.; Xia, J.; Li, Y.; Li, D.; Hou, Z.; Qi, Z.; Chen, X.D. PEG–lipid–PLGA hybrid nanoparticles loaded with berberine–phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017, 24, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Raviña, M.; de la Fuente, M.; Correa, J.; Sousa-Herves, A.; Pinto, J.; Fernandez-Megia, E.; Riguera, R.; Sanchez, A.; Alonso, M.J. Core−Shell Dendriplexes with Sterically Induced Stoichiometry for Gene Delivery. Macromolecules 2010, 43, 6953–6961. [Google Scholar] [CrossRef]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Design of novel polysaccharidic nanostructures for gene delivery. Nanotechnology 2008, 19, 75105. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Csaba, N.; Garcia-Fuentes, M.; Alonso, M.J. Nanoparticles as protein and gene carriers to mucosal surfaces. Nanomedicine 2008, 3, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez-Gascón, A. Applications of lipid nanoparticles in gene therapy. Eur. J. Pharm. Biopharm. 2016, 109, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.U.S. Food and Drug Administration approves paclitaxel protein-bound particles (Abraxane®) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. JOP 2013, 14, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.A.; Pytlik, R.; Kozak, T.; Chhanabhai, M.; Gascoyne, R.; Lu, B.; Deitcher, S.R.; Winter, J.N. Vincristine sulfate liposomes injection (Marqibo) in heavily pretreated patients with refractory aggressive non-Hodgkin lymphoma: Report of the pivotal phase 2 study. Cancer 2009, 115, 3475–3482. [Google Scholar] [CrossRef] [PubMed]
- DaunoXome approved. AIDS Patient Care STDS 1996, 10, 263.
- European Medicines Agency—Science Medicines Health. Available online: http://www.ema.europa.eu/ema/ (accessed on 24 November 2017).
- U.S. Food and Drug Administration Home Page. Available online: https://www.fda.gov/ (accessed on 24 November 2017).
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, R.; Estelrich, J.; Busquets, M. Liposomes Loaded with Hydrophobic Iron Oxide Nanoparticles: Suitable T2 Contrast Agents for MRI. Int. J. Mol. Sci. 2016, 17, 1209. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Guo, R.; Shi, X.; Allen, S.; Cao, Z.; Baker, J.R.; Wang, S.H. Modified Nanoemulsions with Iron Oxide for Magnetic Resonance Imaging. Nanomaterials 2016, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Dong, Z.; Cai, H.; Huang, K.; Liu, Y.; Fang, Z.; Li, X.; Luo, Y. Folate-targeted polymeric micelles loaded with superparamagnetic iron oxide as a contrast agent for magnetic resonance imaging of a human tongue cancer cell line. Mol. Med. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pratt, E.C.; Shaffer, T.M.; Grimm, J. Nanoparticles and radiotracers: Advances toward radionanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 872–890. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The future of cancer diagnosis and therapy. CA. Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Aalipour, A.; Vermesh, O.; Yu, J.H.; Gambhir, S.S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2017, 2, 17014. [Google Scholar] [CrossRef]
- Sneider, A.; VanDyke, D.; Paliwal, S.; Rai, P. Remotely Triggered Nano-Theranostics For Cancer Applications. Nanotheranostics 2017, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88. [Google Scholar] [CrossRef] [PubMed]
- Detappe, A.; Kunjachan, S.; Rottmann, J.; Robar, J.; Tsiamas, P.; Korideck, H.; Tillement, O.; Berbeco, R. AGuIX nanoparticles as a promising platform for image-guided radiation therapy. Cancer Nanotechnol. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Kotb, S.; Detappe, A.; Lux, F.; Appaix, F.; Barbier, E.L.; Tran, V.L.; Plissonneau, M.; Gehan, H.; Lefranc, F.; Rodriguez-Lafrasse, C.; et al. Gadolinium-based nanoparticles and radiation therapy for multiple brain melanoma metastases: Proof of concept before phase I trial. Theranostics 2016, 6, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Pahle, J.; Walther, W. Vectors and strategies for nonviral cancer gene therapy. Expert Opin. Biol. Ther. 2015, 16, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, X.; Zhu, D.; Wang, Y.; Zhang, Z.; Zhou, X.; Qiu, N.; Chen, X.; Shen, Y. Nonviral Cancer Gene Therapy: Delivery Cascade and Vector Nanoproperty Integration. Adv. Drug Deliv. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- FDA Press Announcements—FDA Approval Brings First Gene Therapy to the United States; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Novartis Receives First Ever FDA Approval for a CAR-T Cell Therapy, Kymriah(TM) (CTL019), for Children and Young Adults with B-cell ALL That Is Refractory or Has Relapsed at Least Twice; Novartis: Basel, Switzerland, 2017.
- Slivac, I.; Guay, D.; Mangion, M.; Champeil, J.; Gaillet, B. Non-viral nucleic acid delivery methods. Expert Opin. Biol. Ther. 2017, 17, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Bottai, G.; Truffi, M.; Corsi, F.; Santarpia, L. Progress in nonviral gene therapy for breast cancer and what comes next? Expert Opin. Biol. Ther. 2017, 17, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, R.; Pichler, M.; Kanlikilicer, P.; Ivan, C.; Kahraman, N.; Aslan, B.; Oguztuzun, S. MicroRNA 603 acts as a tumor suppressor and inhibits triple- negative breast cancer tumorigenesis by targeting elongation factor 2 kinase. Oncotarget 2017, 8, 11641–11658. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, D.; Subramanian, K.; Sethuraman, S.; Krishnan, U.M. “Nano–in–nano” hybrid liposomes increase target specificity and gene silencing efficiency in breast cancer induced SCID mice. Eur. J. Pharm. Biopharm. 2017, 119, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, X.; Shao, R.; Xu, Y.; Gao, J.; Wenquan, L. VEGF siRNA delivered by polycation liposome- encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer. Int. J. Nanomed. 2017, 12, 6075–6088. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liao, J.Z.; Xiang, G.Y.; Zhao, P.X.; Ye, F.; Zhao, Q.; He, X.X. MiR-101 and doxorubicin codelivered by liposomes suppressing malignant properties of hepatocellular carcinoma. Cancer Med. 2017, 6, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Alonso, S.; Zapata-Benavides, P.; Chavez-Escamilla, A.K.; Manilla-Muñoz, E.; Zamora-Avila, D.E.; Franco-Molina, M.A.; Rodriguez-Padilla, C. WT1 shRNA delivery using transferrin-conjugated PEG liposomes in an in vivo model of melanoma. Exp. Ther. Med. 2016, 12, 3778–3784. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xu, B.; He, L.; Xia, S.; Chen, Y.; Zeng, J.; Liu, Y.; Li, S.; Tan, X.; Ren, K.; Yao, S.; Song, X. Pigment epithelial-derived factor gene loaded novel COOH-PEG-PLGA-COOHnanoparticles promoted tumor suppression by systemic administration. Int. J. Nanomed. 2016, 11, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Du, T.; Zhang, J.; Zhao, W.; Cheng, H.; Yang, Y.; Wu, Y.; Wang, C.; Men, K.; Gou, M. Efficient inhibition of ovarian cancer by degradable nanoparticle-delivered survivin T34A gene. Int. J. Nanomed. 2016, 11, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Deng, Y.; Shapiro, E.M.; Bortolomai, I.; Lopez, S.; Lin, K.; Bellone, S.; Cui, J.; Menderes, G.; Black, J.D.; et al. Dual-targeting nanoparticles for in vivo delivery of suicide genes to chemotherapy-resistant ovarian cancer cells. Mol. Cancer Ther. 2017, 16, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, X.; Zheng, S.; Wang, B.; Li, Y.; Zhao, C.; Muftuoglu, Y.; Chen, S.; Li, Y.; Yao, H.; Sun, H.; Mao, Q.; You, C.; Guo, G.; Wei, Y. Modified nanoparticle mediated IL-12 immunogene therapy for colon cancer. Nanomedicine 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.; Wu, Y.; Song, L.; Yang, X.; He, T.; Wang, N.; Yang, S.; Zeng, Y.; Wu, Q.; et al. Multifunctional Nucleus-targeting Nanoparticles with Ultra-high Gene Transfection Efficiency for In Vivo Gene Therapy. Theranostics 2017, 7, 1633–1649. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Zhang, K.; Tam, Y.Y.C.; Quick, J.; Tam, Y.K.; Lin, P.J.; Chen, S.; Liu, Y.; Nair, J.K.; Zlatev, I.; et al. A Glu-urea-Lys Ligand-conjugated Lipid Nanoparticle/siRNA System Inhibits Androgen Receptor Expression In Vivo. Mol. Ther. Nucleic Acids 2016, 5, e348. [Google Scholar] [CrossRef] [PubMed]
- D’Abundo, L.; Callegari, E.; Bresin, A.; Chillemi, A.; Elamin, B.K.; Guerriero, P.; Huang, X.; Saccenti, E.; Hussein, E.M.A.A.; Casciano, F.; et al. Anti-leukemic activity of microRNA-26a in a chronic lymphocytic leukemia mouse model. Oncogene 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Jones, M.C.; Santander-Ortega, M.J.; Mirenska, A.; Marimuthu, P.; Uchegbu, I.; Schätzlein, A. A nano-enabled cancer-specific ITCH RNAi chemotherapy booster for pancreatic cancer. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy—An overview. J. Clin. Diagnostic Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef] [PubMed]
- Home—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 24 November 2017).
- Shanker, M.; Jin, J.; Branch, C.D.; Miyamoto, S.; Grimm, E.A.; Roth, J.A.; Ramesh, R. Tumor suppressor gene-based nanotherapy: From test tube to the clinic. J. Drug Deliv. 2011, 2011, 465845. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef] [PubMed]
- McErlean, E.M.; McCrudden, C.M.; McCarthy, H.O. Delivery of nucleic acids for cancer gene therapy: Overcoming extra- and intra- cellular barriers. Ther. Deliv. 2016, 7, 619–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.; Peng, H.; Chen, Y.; Zhu, P.; Huang, Y. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv. Drug Deliv. Rev. 2015, 81, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Santander-Ortega, M.J.; de la Fuente, M.; Lozano, M.V.; Bekheet, M.E.; Progatzky, F.; Elouzi, A.; Uchegbu, I.F.; Schätzlein, A.G. Hydration forces as a tool for the optimization of core–shell nanoparticle vectors for cancer gene therapy. Soft Matter 2012, 8, 12080. [Google Scholar] [CrossRef]
- Santander-Ortega, M.J.; de la Fuente, M.; Lozano, M.V.; Tsui, M.L.; Bolton, K.; Uchegbu, I.F.; Schätzlein, A.G. Optimisation of synthetic vector systems for cancer gene therapy—The role of the excess of cationic dendrimer under physiological conditions. Curr. Top. Med. Chem. 2014, 14, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Raviña, M.; Sousa-Herves, A.; Correa, J.; Riguera, R.; Fernandez-Megia, E.; Sánchez, A.; Alonso, M.J. Exploring the efficiency of gallic acid-based dendrimers and their block copolymers with PEG as gene carriers. Nanomedicine 2012, 7, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Santoriello, C.; Zon, L.I. Science in medicine Hooked! Modeling human disease in zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Ablain, J.; Zon, L.I. Of fish and men: Using zebrafish to fight human diseases. Trends Cell Biol. 2013, 23, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Giannaccini, M.; Cuschieri, A.; Dente, L.; Raffa, V. Non-mammalian vertebrate embryos as models in nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.; Torroja, C.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.B.; Westerfield, M. Zebrafish models in translational research: Tipping the scales toward advancements in human health. Dis. Model. Mech. 2014, 7, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jang, G.H.; Byun, C.H.; Jeun, M.; Searson, P.C.; Lee, K.H. Zebrafish models for functional and toxicological screening of nanoscale drug delivery systems: Promoting preclinical applications. Biosci. Rep. 2017, 37, BSR20170199. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Benard, E.L.; Kanwal, Z.; Stockhammer, O.W.; van der Vaart, M.; Zakrzewska, A.; Spaink, H.P.; Meijer, A.H. Infectious Disease Modeling and Innate Immune Function in Zebrafish Embryos, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 105, ISBN 9780123813206. [Google Scholar]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef]
- Li, F.; Zhang, S.; Wang, Z.; Li, H. Genes of the adaptive immune system are expressed early in zebrafish larval development following lipopolysaccharide stimulation. Chin. J. Oceanol. Limnol. 2011, 29, 326–333. [Google Scholar] [CrossRef]
- Bill, B.R.; Petzold, A.M.; Clark, K.J.; Schimmenti, L.A.; Ekker, S.C. A Primer for Morpholino Use in Zebrafish. Zebrafish 2009, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Bedell, V.M.; Westcot, S.E.; Ekker, S.C. Lessons from morpholino-based screening in zebrafish. Brief. Funct. Genom. 2011, 10, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Timme-Laragy, A.R.; Karchner, S.I.; Hahn, M.E. Developmental Toxicology. Methods Mol Biol. 2012, 889, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Irion, U.; Krauss, J.; Nusslein-Volhard, C. Precise and efficient genome editing in zebrafish using the CRISPR/Cas9 system. Development 2014, 141, 4827–4830. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.N.; Davey, C.F.; Whitebirch, A.C.; Miller, A.C.; Moens, C.B. Rapid Reverse Genetic Screening Using CRISPR in Zebrafish. Nat. Methods 2015, 12, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, L.; Page-McCaw, P.S.; Chen, W. Zebrafish Genome Engineering Using the CRISPR–Cas9 System. Trends Genet. 2016, 32, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Albadri, S.; Del Bene, F.; Revenu, C. Genome editing using CRISPR/Cas9-based knock-in approaches in zebrafish. Methods 2017, 121–122, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Haldi, M.; Ton, C.; Seng, W.L.; McGrath, P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 2006, 9, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Zon, L.I. Zebrafish tumor assays: The state of transplantation. Zebrafish 2009, 6, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Drabsch, Y.; Snaar-Jagalska, B.E.; Ten Dijke, P. Fish tales: The use of zebrafish xenograft human cancer cell models. Histol. Histopathol. 2017, 32, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Idilli, A.; Precazzini, F.; Mione, M.; Anelli, V. Zebrafish in Translational Cancer Research: Insight into Leukemia, Melanoma, Glioma and Endocrine Tumor Biology. Genes 2017, 8, 236. [Google Scholar] [CrossRef]
- Wyatt, R.A.; Trieu, N.P.V.; Crawford, B.D. Zebrafish Xenograft: An Evolutionary Experiment in Tumour Biology. Genes 2017, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Roel, M.; Rubiolo, J.A.; Botana, L.M.; Guerra-Varela, J.; Cabezas-Sainz, P.; Sanchez, L.; Silva, S.B.L.; Thomas, O.P.; Silva, S.B.L.; et al. Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget 2016, 7, 83071–83087. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.M.; May, R.C.; Wheeler, R.T. Zebrafish: A see-through host and a fluorescent toolbox to probe host-pathogen interaction. PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Fenaroli, F.; Westmoreland, D.; Benjaminsen, J.; Kolstad, T.; Skjeldal, F.M.; Meijer, A.H.; Van Der Vaart, M.; Ulanova, L.; Roos, N. Nanoparticles as Drug Delivery System against Tuberculosis in Zebrafish Embryos: Direct Visualization and treatment. ACS Nano 2014, 8, 7014–7026. [Google Scholar] [CrossRef]
- Teijeiro-Valiño, C.; Yebra-Pimentel, E.; Guerra-Varela, J.; Csaba, N.; Alonso, M.J.; Sánchez, L. Assessment of the permeability and toxicity of polymeric nanocapsules using the zebrafish model. Nanomedicine 2017, 12, 2069–2082. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lin, S.; Rallo, R.; Zhao, Y.; Damoiseaux, R.; Xia, T.; Lin, S.; Nel, A.; Cohen, Y. Automated phenotype recognition for zebrafish embryo based in vivo high throughput toxicity screening of engineered nano-materials. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lin, S.; Zhao, Y.; Nel, A.E. Zebrafish: An in vivo model for nano EHS studies. Small 2013, 9, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent Adult Zebrafish as a Tool for In Vivo Transplantation Analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Rose, K.; Zon, L. Zebrafish cancer: The state of the art and the path forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Henderson, R.E.; Garraway, L.A.; Zon, L.I. Long-term drug administration in the adult zebrafish using oral gavage for cancer preclinical studies. Dis. Model. Mech. 2016, 9, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Moore, J.C.; Ignatius, M.S.; Tenente, I.M.; Hayes, M.N.; Garcia, E.G.; Torres Yordán, N.; Bourque, C.; He, S.; Blackburn, J.S.; et al. Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nat. Commun. 2016, 7, 10358. [Google Scholar] [CrossRef] [PubMed]
- EFSA Opinion of the Scientific Panel on Animal Health and Welfare on a request from the Commission related to the aspects of the biology and welfare of animals used for experimental and other scientific purposes (EFSA-Q-2004-105). EFSA J. 2005, 292, 1–46.
- Russell, W.M.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen Publishing: London, UK, 1959. [Google Scholar]
- Stern, H.M.; Zon, L.I. Cancer genetics and drug discovery in the zebrafish. Nat. Rev. Cancer 2003, 3, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Goessling, W.; North, T.E.; Zon, L.I. New waves of discovery: Modeling cancer in zebrafish. J. Clin. Oncol. 2007, 25, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Deveau, A.P.; Bentley, V.L.; Berman, J.N. Using zebrafish models of leukemia to streamline drug screening and discovery. Exp. Hematol. 2017, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Rooijen, E.; Fazio, M.; Zon, L.I. From fish bowl to bedside: The power of zebrafish to unravel melanoma pathogenesis and discover new therapeutics. Pigment Cell Melanoma Res. 2017, 30, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Huang, J.; Ye, J. A fresh look at zebrafish from the perspective of cancer research. J. Exp. Clin. Cancer Res. 2015, 34, 80. [Google Scholar] [CrossRef] [PubMed]
- Lenis-Rojas, O.A.; Fernandes, A.R.; Roma-Rodrigues, C.; Baptista, P.V.; Marques, F.; Pérez-Fernández, D.; Guerra-Varela, J.; Sánchez, L.; Vázquez-García, D.; Torres, M.L.; et al. Heteroleptic mononuclear compounds of ruthenium(ii): synthesis, structural analyses, in vitro antitumor activity and in vivo toxicity on zebrafish embryos. Dalton Trans. 2016, 45, 19127–19140. [Google Scholar] [CrossRef] [PubMed]
- Lenis-Rojas, O.A.; Roma-Rodrigues, C.; Fernandes, A.R.; Marques, F.; Pérez-Fernández, D.; Guerra-Varela, J.; Sánchez, L.; Vázquez-García, D.; López-Torres, M.; Fernández, A.; et al. Dinuclear RuII(bipy)2 Derivatives: Structural, Biological, and in Vivo Zebrafish Toxicity Evaluation. Inorg. Chem. 2017, 56, 7127–7144. [Google Scholar] [CrossRef] [PubMed]
- Penas, C.; Sánchez, M.I.; Guerra-Varela, J.; Sanchez, L.; Vázquez, M.E.; Mascareñas, J.L. Light-Controlled Cellular Internalization and Cytotoxicity of Nucleic Acid-Binding Agents: Studies in Vitro and in Zebrafish Embryos. ChemBioChem 2016, 17, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.S.; Langenau, D.M. Zebrafish as a model to assess cancer heterogeneity, progression and relapse. Dis. Model. Mech. 2014, 7, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Tat, J.; Liu, M.; Wen, X.Y. Zebrafish cancer and metastasis models for in vivo drug discovery. Drug Discov. Today Technol. 2013, 10, e83–e89. [Google Scholar] [CrossRef] [PubMed]
- Veinotte, C.J.; Dellaire, G.; Berman, J.N. Hooking the big one: The potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. Dis. Model Mech. 2014, 7, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, S.; Butler, P.; Goldsmith, P.; Waldron, G.; Gardner, I.; Golder, Z.; Richards, F.M.; Kimber, G.; Roach, A.; Alderton, W.; et al. Zebrafish based assays for the assessment of cardiac, visual and gut function—potential safety screens for early drug discovery. J. Pharmacol. Toxicol. Methods 2008, 58, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kemadjou, J.R.; Zinsmeister, C.; Bauer, M.; Legradi, J.; Müller, F.; Pankratz, M.; Jäkel, J.; Strähle, U. Transcriptional profiling reveals barcode-like toxicogenomic responses in the zebrafish embryo. Genome Biol. 2007, 8, R227. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.O.; Fan, C.Y.; Ramabhadran, R. Cellular stress response pathway system as a sentinel ensemble in toxicological screening. Toxicol. Sci. 2009, 111, 202–225. [Google Scholar] [CrossRef] [PubMed]
- Barbazuk, W.B.; Korf, I.; Kadavi, C.; Heyen, J.; Tate, S.; Wun, E.; Bedell, J.A.; McPherson, J.D.; Johnson, S.L. The synthenic relationship of the zebrafish and human genomes. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Huiting, L.N.; Laroche, F.; Feng, H. The Zebrafish as a Tool to Cancer Drug Discovery. Austin J. Pharmacol. Ther. 2015, 3, 1069. [Google Scholar] [PubMed]
- Lee, L.M.J.; Seftor, E.A.; Bonde, G.; Cornell, R.A.; Hendrix, M.J.C. The fate of human malignant melanoma cells transplanted into zebrafish embryos: Assessment of migration and cell division in the absence of tumor formation. Dev. Dyn. 2005, 233, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.J.; Weiss, F.U.; Vlecken, D.H.; Nitsche, C.; Bakkers, J.; Lagendijk, A.K.; Partecke, L.I.; Heidecke, C.-D.; Lerch, M.M.; Bagowski, C.P. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 2009, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Davis, S.; Tereshchenko, I.; Budak-alpdogan, T.; Zhong, H.; Stein, M.N.; Kim, I.Y.; Dipaola, R.S.; Bertino, J.R.; Sabaawy, H.E. Enrichment of human prostate cancer cells with tumor initiating properties in mouse and zebrafish xenografts by differential adhesion. Prostate 2014, 74, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shimada, Y.; Kuroyanagi, J.; Umemoto, N.; Nishimura, Y.; Tanaka, T. Quantitative phenotyping-based in vivo chemical screening in a zebrafish model of leukemia stem cell xenotransplantation. PLoS ONE 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.K.; Schiavone, K.; Tazzyman, S.; Heymann, D.; Chico, T.J.A. Zebrafish xenograft models of cancer and metastasis for drug discovery. Expert Opin. Drug Discov. 2017, 12, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Mort, R.L.; Jackson, I.J.; Patton, E.E. The melanocyte lineage in development and disease. Development 2015, 142, 1387. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ross, J.L.; Cowell, J.K.; Teng, Y. Future Medicinal Chemistry. Future Med. Chem. 2015, 7, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Tanguay, R.L. Integrating zebrafish toxicology and nanoscience for safer product development. Green Chem. 2013, 15, 872. [Google Scholar] [CrossRef] [PubMed]
- Harper, B.; Thomas, D.; Chikkagoudar, S.; Baker, N.; Tang, K.; Heredia-Langner, A.; Lins, R.; Harper, S. Comparative hazard analysis and toxicological modeling of diverse nanomaterials using the embryonic zebrafish (EZ) metric of toxicity. J. Nanoparticle Res. 2015, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Cho, H.J.; Choi, M.; Lee, W.S.; Chung, B.H.; Lee, J.S. In vivo toxicity assessment of angiogenesis and the live distribution of nano-graphene oxide and its PEGylated derivatives using the developing zebrafish embryo. Carbon N. Y. 2015, 93, 431–440. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, X.; Chen, T.; Yi, X.; Wang, R.; Zhao, H.; Lee, S.M.Y.; Wang, X.; Zheng, Y. Zebrafish as a visual and dynamic model to study the transport of nanosized drug delivery systems across the biological barriers. Coll. Surf. B Biointerfaces 2017, 156, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Grossen, P.; Detampel, P.; Siegfried, S.; Witzigmann, D.; Huwyler, J. Zebrafish as an early stage screening tool to study the systemic circulation of nanoparticulate drug delivery systems in vivo. J. Control. Release 2017, 264, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Evensen, L.; Johansen, P.L.; Koster, G.; Zhu, K.; Herfindal, L.; Speth, M.; Fenaroli, F.; Hildahl, J.; Bagherifam, S.; Tulotta, C.; Prasmickaite, L.; Mælandsmo, G.M.; Snaar-Jagalska, E.; Griffiths, G. Zebrafish as a model system for characterization of nanoparticles against cancer. Nanoscale 2016, 8, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Wehmas, L.C.; Tanguay, R.L.; Punnoose, A.; Greenwood, J.A. Developing a Novel Embryo–Larval Zebrafish Xenograft Assay to Prioritize Human Glioblastoma Therapeutics. Zebrafish 2016, 13, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shimada, Y.; Olsthoorn, R.C.L.; Snaar-Jagalska, B.E.; Spaink, H.P.; Kros, A. Application of Coiled Coil Peptides in Liposomal Anticancer Drug Delivery Using a Zebrafish Xenograft Model. ACS Nano 2016, 10, 7428–7435. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Jin, Q.; Zeng, J.; Yu, T.; Chen, Y.; Li, S.; Gong, D.; He, L.; Tan, X.; Yang, L.; He, G.; Wu, J.; Song, X. Combined Tumor- and Neovascular-“Dual Targeting” Gene/Chemo-Therapy Suppresses Tumor Growth and Angiogenesis. ACS Appl. Mater. Interfaces 2016, 8, 25753–25769. [Google Scholar] [CrossRef] [PubMed]
- Aldrian, G.; Vaissière, A.; Konate, K.; Seisel, Q.; Vivès, E.; Fernandez, F.; Viguier, V.; Genevois, C.; Couillaud, F.; Démèné, H.; et al. PEGylation rate influences peptide-based nanoparticles mediated siRNA delivery in vitro and in vivo. J. Control. Release 2017, 256, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.; Carvalho, L.; Silva, J.; Saúde, L.; Fernandes, A.; Baptista, P. Gold Nanobeacons for Tracking Gene Silencing in Zebrafish. Nanomaterials 2017, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Wang, W.; Lei, D.; Yin, Y.; Ren, P.; Chen, J.; Yin, T.; Wang, B.; Wang, G.; Wang, Y. Penetration of blood-brain barrier and antitumor activity and nerve repair in glioma by doxorubicin-loaded monosialoganglioside micelles system. Int. J. Nanomed. 2017, 12, 4879–4889. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Annangi, B.; Rubio, L.; Marcos, R.; Dorn, M.; Merker, C.; Estrela-Lopis, I.; Cimpan, M.R.; Ibrahim, M.; Cimpan, E.; et al. High throughput toxicity screening and intracellular detection of nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Brand, W.; Noorlander, C.W.; Giannakou, C.; Park, M.V.D.Z.; Vandebriel, R.J.; Bosselaers, I.E.M. Nanomedicinal products: A survey on specific toxicity and side effects. Int. J. Nanomed. 2017, 12, 6107–6129. [Google Scholar] [CrossRef] [PubMed]
- Giannakou, C.; Park, M.V.D.Z.; De Jong, W.H.; Van Loveren, H.; Vandebriel, R.J.; Geertsma, R.E. A comparison of immunotoxic effects of nanomedicinal products with regulatory immunotoxicity testing requirements. Int. J. Nanomed. 2016, 11, 2935–2952. [Google Scholar] [CrossRef] [PubMed]
- Omidi, Y.; Hollins, A.J.; Drayton, R.M.; Akhtar, S. Polypropylenimine dendrimer-induced gene expression changes: The effect of complexation with DNA, dendrimer generation and cell type. J. Drug Target. 2005, 13, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Di Giorgio, M.L. Toxicogenomics to improve comprehension of the mechanisms underlying responses of in vitro and in vivo systems to nanomaterials: A review. Curr. Genom. 2008, 9, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Taratula, O.; Garbuzenko, O.B.; Patil, M.L.; Savla, R.; Zhang, M.; Minko, T. Genotoxicity of different nanocarriers: Possible modifications for the delivery of nucleic acids. Curr. Drug Discov. Technol. 2013, 10, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Anchordoquy, T.J.; Barenholz, Y.; Boraschi, D.; Chorny, M.; Decuzzi, P.; Dobrovolskaia, M.A.; Farhangrazi, Z.S.; Farrell, D.; Gabizon, A.; Ghandehari, H.; et al. Mechanisms and Barriers in Cancer Nanomedicine: Addressing Challenges, Looking for Solutions. ACS Nano 2017, 11, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Guideline, T.T.; Guideline, O. OECD Guidelines for the Testing of Chemicals; Oecd/Ocde 220; Organisation for Economic Co-operation and Development: Paris, France, 2004; pp. 1–22. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2013; pp. 1–22. [Google Scholar] [CrossRef]
- Fent, K.; Weisbrod, C.J.; Wirth-Heller, A.; Pieles, U. Assessment of uptake and toxicity of fluorescent silica nanoparticles in zebrafish (Danio rerio) early life stages. Aquat. Toxicol. 2010, 100, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.R.; Noyes, P.D.; Tanguay, R.L. Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 2016, 161, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.J.; Zhao, X.; Thistle, M.E.; Maccormack, T.J.; Clark, R.J.; Ma, G.; Martinez-Rubi, Y.; Simard, B.; Loo, J.S.C.; Veinot, J.G.C.; Goss, G.G. Mechanistic insights into the effect of nanoparticles on zebrafish hatch. Nanotoxicology 2014, 8, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Sukardi, H.; Ung, C.Y.; Gong, Z.; Lam, S.H. Incorporating zebrafish omics into chemical biology and toxicology. Zebrafish 2010, 7, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Hussainzada, N.; Lewis, J.A.; Baer, C.E.; Ippolito, D.L.; Jackson, D.A.; Stallings, J.D. Whole adult organism transcriptional profiling of acute metal exposures in male zebrafish. BMC Pharmacol. Toxicol. 2014, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, B.; Ladhar, C.; Cambier, S.; Treguer-Delapierre, M.; Brèthes, D.; Bourdineaud, J.-P. Impact of dietary gold nanoparticles in zebrafish at very low contamination pressure: The role of size, concentration and exposure time. Nanotoxicology 2012, 6, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Segura-Aguilar, J.; Kostrzewa, R.M. Neurotoxins and neurotoxicity mechanisms. An overview. Neurotox. Res. 2006, 10, 263–287. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, R.C.; Hunter, D.L.; Irons, T.D.; Padilla, S. Locomotion and Behavioral Toxicity in Larval Zebrafish: Background, Methods, and Data. In Zebrafish; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 151–164. ISBN 9781118102138. [Google Scholar]
- Truong, L.; Saili, K.S.; Miller, J.M.; Hutchison, J.E.; Tanguay, R.L. Persistent adult zebrafish behavioral deficits results from acute embryonic exposure to gold nanoparticles. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Usenko, C.Y.; Harper, S.L.; Tanguay, R.L. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon N. Y. 2007, 45, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Vibe, C.B.; Fenaroli, F.; Pires, D.; Wilson, S.R.; Bogoeva, V.; Kalluru, R.; Speth, M.; Anes, E.; Griffiths, G.; Hildahl, J. Thioridazine in PLGA nanoparticles reduces toxicity and improves rifampicin therapy against mycobacterial infection in zebrafish. Nanotoxicology 2016, 10, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Zhang, Z.; Zhang, W.; Bao, L.; Xu, C.; Zhang, H. Enantioselective developmental toxicity and immunotoxicity of pyraclofos toward zebrafish (Danio rerio). Aquat. Toxicol. 2015, 159, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Dong, X.; Zhang, Z.; Yang, M.; Wu, X.; Liu, H.; Lao, Q.; Li, C. Assessment of immunotoxicity of dibutyl phthalate using live zebrafish embryos. Fish Shellfish Immunol. 2015, 45, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Daroczi, B.; Kari, G.; McAleer, M.F.; Wolf, J.C.; Rodeck, U.; Dicker, A.P. In vivo Radioprotection by the Fullerene Nanoparticle DF-1 as Assessed in a Zebrafish Model. Clin. Cancer Res. 2006, 12, 7086–7091. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Wang, L.; Su, M.; Zhao, X.; Hu, R.; Yu, X.; Hong, J.; Liu, D.; Xu, B.; Zhu, Y.; Wang, H.; Hong, F. Mechanism of TiO2 nanoparticle-induced neurotoxicity in zebrafish (D anio rerio). Environ. Toxicol. 2016, 31, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, X.; Zhang, X.; Zhao, Z.; Liu, H.; George, R.; Wilson-Rawls, J.; Chang, Y.; Chen, Y. Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemosphere 2011, 83, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.H.; Lee, K.Y.; Choi, J.; Kim, S.H.; Lee, K.H. Multifaceted toxicity assessment of catalyst composites in transgenic zebrafish embryos. Environ. Pollut. 2016, 216, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhao, Y.; Xia, T.; Meng, H.; Ji, Z.; Liu, R.; George, S.; Xiong, S.; Wang, X.; Zhang, H.; et al. High content screening in zebrafish speeds up hazard ranking of transition metal oxide nanoparticles. ACS Nano 2011, 5, 7284–7295. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Ichihara, G.; Shimada, Y.; Tada-Oikawa, S.; Kuroyanagi, J.; Zhang, B.; Suzuki, Y.; Sehsah, R.; Kato, M.; Tanaka, T.; et al. Copper Oxide Nanoparticles Reduce Vasculogenesis in Transgenic Zebrafish Through Down-Regulation of Vascular Endothelial Growth Factor Expression and Induction of Apoptosis. J. Nanosci. Nanotechnol. 2015, 15, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ilan, O.; Louis, K.M.; Yang, S.P.; Pedersen, J.A.; Hamers, R.J.; Peterson, R.E.; Heideman, W. Titanium dioxide nanoparticles produce phototoxicity in the developing zebrafish. Nanotoxicology 2012, 6, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, K.; Sun, X.; Dong, Q.; Huang, C.; Wang, H.; Guo, M.; Cui, X. Toxicological effect of MPA–CdSe QDs exposure on zebrafish embryo and larvae. Chemosphere 2012, 89, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.R.; Huttenlocher, A. Neutrophils in the Tumor Microenvironment. Trends Immunol. 2016, 37, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.E.J.; O’Neill, C.L.; O’Doherty, T.M.; Medina, R.J.; Stitt, A.W. The role of immune-related myeloid cells in angiogenesis. Immunobiology 2013, 218, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, S.A.; Loynes, C.A.; Trushell, D.M.I.; Elworthy, S.; Ingham, P.W.; Whyte, M.K.B. A transgenic zebrafish model of neutrophilic inflammation. Blood 2006, 108, 3976–3978. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Hu, H.; Li, Q.; Jiang, L.; Zou, Y.; Wang, Y.; Sun, Z. Combined toxicity of silica nanoparticles and methylmercury on cardiovascular system in zebrafish (Danio rerio) embryos. Environ. Toxicol. Pharmacol. 2016, 44, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Boushehri, M.A.S.; Lamprecht, A. Nanoparticles as drug carriers: Current issues with in vitro testing. Nanomedicine 2015, 10, 3213–3230. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Lee, B.J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Pawar, R.S.; Pandey, R.S.; Madan, J.; Pawar, S.; Lakshmi, P.K.; Sudheesh, M.S. In-vitro in-vivo correlation (IVIVC) in nanomedicine: Is protein corona the missing link? Biotechnol. Adv. 2017, 35, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.; Maddux, B.L.S.; Hutchison, J.E.; Usenko, C.; Tanguay, R. Biodistribution and Toxicity of Nanomaterials In Vivo: Effects of Composition, Size, Surface Functionalization and Route of Exposure. Nanotech 2007, 2, 666–669. [Google Scholar]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Torrice, M. Does Nanomedicine Have a Delivery Problem? ACS Publ. 2016. [Google Scholar] [CrossRef] [PubMed]
- Muntimadugu, E.; Kommineni, N.; Khan, W. Exploring the Potential of Nanotherapeutics in Targeting Tumor Microenvironment for Cancer Therapy. Pharmacol. Res. 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Jain, R.K. Design considerations for nanotherapeutics in oncology. Nanomedicine 2015, 11, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Kuninty, P.R.; Schnittert, J.; Storm, G.; Prakash, J. MicroRNA Targeting to Modulate Tumor Microenvironment. Front. Oncol. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kirchberger, S.; Sturtzel, C.; Pascoal, S.; Distel, M. Quo natas, Danio?—Recent Progress in Modeling Cancer in Zebrafish. Front. Oncol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Z.; Zhang, X.M.; Nakamura, M.; Sun, M.; Hartman, J.; Harris, R.A.; Sun, Y.; Cao, Y. Novel mechanism of macrophage-mediated metastasis revealed in a zebrafish model of tumor development. Cancer Res. 2015, 75, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Rouhi, P.; Jensen, L.D.; Cao, Z.; Hosaka, K.; Länne, T.; Wahlberg, E.; Steffensen, J.F.; Cao, Y. Hypoxia-induced metastasis model in embryonic zebrafish. Nat. Protoc. 2010, 5, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Stoletov, K.; Montel, V.; Lester, R.D.; Gonias, S.L.; Klemke, R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 17406–17411. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, W.; Burrello, C.; de Lange, M.J.; van der Velden, P.A.; Jochemsen, A.G.; Jager, M.J.; Snaar-Jagalska, B.E. Embryonic Zebrafish: Different Phenotypes after Injection of Human Uveal Melanoma Cells. Ocul. Oncol. Pathol. 2015, 1, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shimada, Y.; Kuroyanagi, J.; Nishimura, Y.; Umemoto, N.; Nomoto, T.; Shintou, T.; Miyazaki, T.; Tanaka, T. Zebrafish xenotransplantation model for cancer stem-like cell study and high-throughput screening of inhibitors. Tumor Biol. 2014, 35, 11861–11869. [Google Scholar] [CrossRef] [PubMed]
- Eguiara, A.; Holgado, O.; Beloqui, I.; Abalde, L.; Sanchez, Y.; Callol, C.; Martin, A.G. Xenografts in zebrafish embryos as a rapid functional assay for breast cancer stem-like cell identification. Cell Cycle 2011, 10, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, X.; Ding, T.W.; Gong, Z. Enhanced angiogenesis, hypoxia and neutrophil recruitment during Myc-induced liver tumorigenesis in zebrafish. Sci. Rep. 2016, 6, 31952. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.S.; Delk, N.A.; Lukianova-hleb, E.Y.; Hafner, J.H.; Lapotko, D.O. The in vivo performance of plasmonic nanobubbles as cell theranostic agents in zebrafish hosting prostate cancer xenografts. Biomaterials 2010, 31, 7567–7574. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.S.; Makhija, D.T.; Goel, P.N.; Shah, S.M.; Nikam, Y.; Gude, R.P.; Jagtap, A.G.; Nagarsenker, M.S. Docetaxel in cationic lipid nanocapsules for enhanced in vivo activity. Pharm. Dev. Technol. 2016, 21, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Deng, S.; Wu, Q.; Xiang, M.; Wei, X.; Li, L.; Gao, X.; Wang, B.; Sun, L.; Chen, Y.; et al. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials 2013, 34, 1413–1432. [Google Scholar] [CrossRef] [PubMed]
- Naber, H.P.H.; Drabsch, Y.; Snaar-Jagalska, B.E.; ten Dijke, P.; van Laar, T. Snail and Slug, key regulators of TGF-β-induced EMT, are sufficient for the induction of single-cell invasion. Biochem. Biophys. Res. Commun. 2013, 435, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Cui, W.; Gu, A.; Xu, C.; Yu, S.C.; Li, T.T.; Cui, Y.H.; Zhang, X.; Bian, X.W. A Novel Zebrafish Xenotransplantation Model for Study of Glioma Stem Cell Invasion. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lamers, G.E.M.; Beenakker, J.W.M.; Cui, C.; Ghotra, V.P.S.; Danen, E.H.J.; Meijer, A.H.; Spaink, H.P.; Snaar-Jagalska, B.E. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J. Pathol. 2012, 227, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Tobia, C.; Gariano, G.; De Sena, G.; Presta, M. Zebrafish embryo as a tool to study tumor/endothelial cell cross-talk. Biochim. Biophys. Acta 2013, 1832, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Hu, H.; Feng, L.; Yang, X.; Sun, Z. Silica nanoparticles inhibit macrophage activity and angiogenesis via VEGFR2-mediated MAPK signaling pathway in zebrafish embryos. Chemosphere 2017, 183, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Van Ham, T.J.; Mapes, J.; Kokel, D.; Peterson, R.T. Live imaging of apoptotic cells in zebrafish. FASEB J. 2010, 24, 4336–4342. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Blechinger, S.R.; Evans, T.G.; Tang, P.T.; Kuwada, J.Y.; Warren, J.T., Jr.; Krone, P.H. The heat-inducible zebrafish hsp70 gene is expressed during normal lens development under non-stress conditions. Mech. Dev. 2002, 112, 213–215. [Google Scholar] [CrossRef]
| Nanocarrier | Gene Vector | Target | Indication | Administration Route | Ref |
|---|---|---|---|---|---|
| Liposomes | miRNA | Restoration of oncossuppressor | Breast cancer | Tail vein | [40] |
| siRNA | EpCAM silencing | Breast cancer | Tumor adjacent | [41] | |
| siRNA | Anti-angiogenesis | Breast cancer | Intratumoral | [42] | |
| miRNA | Restoration of oncosuppressor | Hepatocellular carcinoma | Intratumoral | [43] | |
| shRNA | WT1 silencing | Melanoma | Tail vein | [44] | |
| Polymeric nanoparticles | pDNA | Anti-angiogenesis | Colon cancer | Tail vein | [45] |
| pDNA | Induce apoptosis | Ovarian cancer | Intraperitoneal | [46] | |
| pDNA | Suicide gene therapy | Ovarian cancer | Intraperitoneal | [47] | |
| pDNA | Immunotherapy | Colorectal cancer | Intratumoral | [48] | |
| pDNA | Suicide gene therapy | Colon cancer | Intratumoral | [49] | |
| Lipid nanoparticles | siRNA | Androgen receptor silencing | Prostate cancer | Tail vein | [50] |
| miRNA | Restoration of microRNA-26a | Lymphocytic leukemia | Intraperitoneal | [51] | |
| Dendrimers | si/shRNA | ITCH silencing | Pancreatic cancer | Tail vein | [52] |
| Model | Features | Application | Ref |
|---|---|---|---|
| Wild type | From nature, with pigmentation according to sex, without fluorescence | Toxicity, biodistribution, xenograft | [194] |
| Flk-1:eGFP | Fluorescent vascular system | Toxicity, biodistribution, xenograft, angiogenesis, extravasation, half-life circulation, metastasis | [107,130] |
| Fli-1:eGFP | [107,127,162] | ||
| Gata1:DsRed | [107] | ||
| Nacre/fli1:eGFP | [163] | ||
| Casper fli | Without pigmentation (transparent) and fluorescent vascular system | [91] | |
| Casper | Without pigmentation (transparent) | Toxicity, biodistribution, xenograft, metastasis | [91] |
| ARE:eGFP | Fluorescence of reactive oxygen species (ROS) | Toxicity | [162] |
| Cmlc2:eGFP | Fluorescence in the heart | Cardiotoxicity | [167] |
| Mpo:GFP | Fluorescent neutrophils | Interaction, half-life circulation, immuno response | [167] |
| Mpeg1:mcherry | Fluorescent macrophages | [127] | |
| Hsp70:eGFP | Fluorescence of the protein HSP70 stress product | Toxicity | [195] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Lovera, C.; Vázquez-Ríos, A.; Guerra-Varela, J.; Sánchez, L.; De la Fuente, M. The Potential of Zebrafish as a Model Organism for Improving the Translation of Genetic Anticancer Nanomedicines. Genes 2017, 8, 349. https://doi.org/10.3390/genes8120349
Gutiérrez-Lovera C, Vázquez-Ríos A, Guerra-Varela J, Sánchez L, De la Fuente M. The Potential of Zebrafish as a Model Organism for Improving the Translation of Genetic Anticancer Nanomedicines. Genes. 2017; 8(12):349. https://doi.org/10.3390/genes8120349
Chicago/Turabian StyleGutiérrez-Lovera, C, AJ Vázquez-Ríos, J Guerra-Varela, L Sánchez, and M De la Fuente. 2017. "The Potential of Zebrafish as a Model Organism for Improving the Translation of Genetic Anticancer Nanomedicines" Genes 8, no. 12: 349. https://doi.org/10.3390/genes8120349
APA StyleGutiérrez-Lovera, C., Vázquez-Ríos, A., Guerra-Varela, J., Sánchez, L., & De la Fuente, M. (2017). The Potential of Zebrafish as a Model Organism for Improving the Translation of Genetic Anticancer Nanomedicines. Genes, 8(12), 349. https://doi.org/10.3390/genes8120349







