Novel Tetra-Primer ARMS-PCR Assays for Thiopurine Intolerance Susceptibility Mutations NUDT15 c.415C>T and TPMT c.719A>G (TPMT*3C) in East Asians
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples and DNA Extraction
2.2. ARMS-PCR Genotyping for NUDT15 c.415C>T and TPMT c.719A>G (TPMT*3C)
2.3. Validation of Genotypes by Sanger Sequencing
3. Results
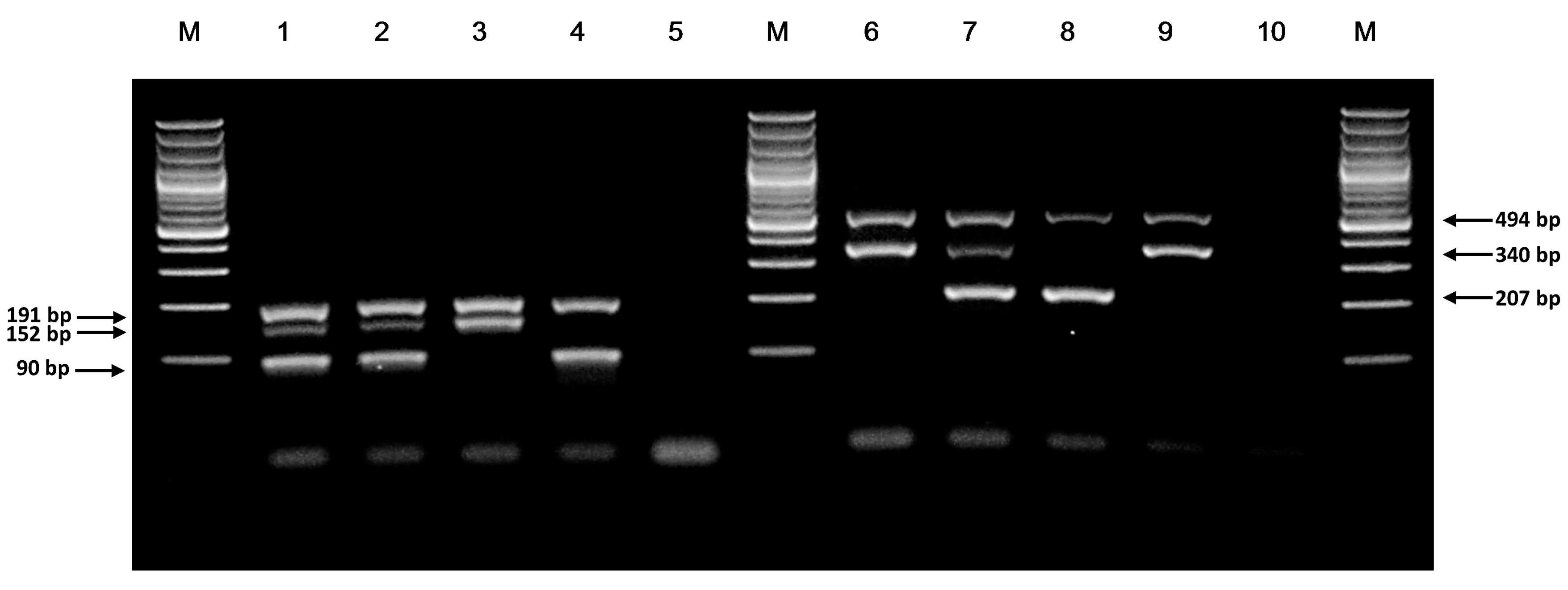
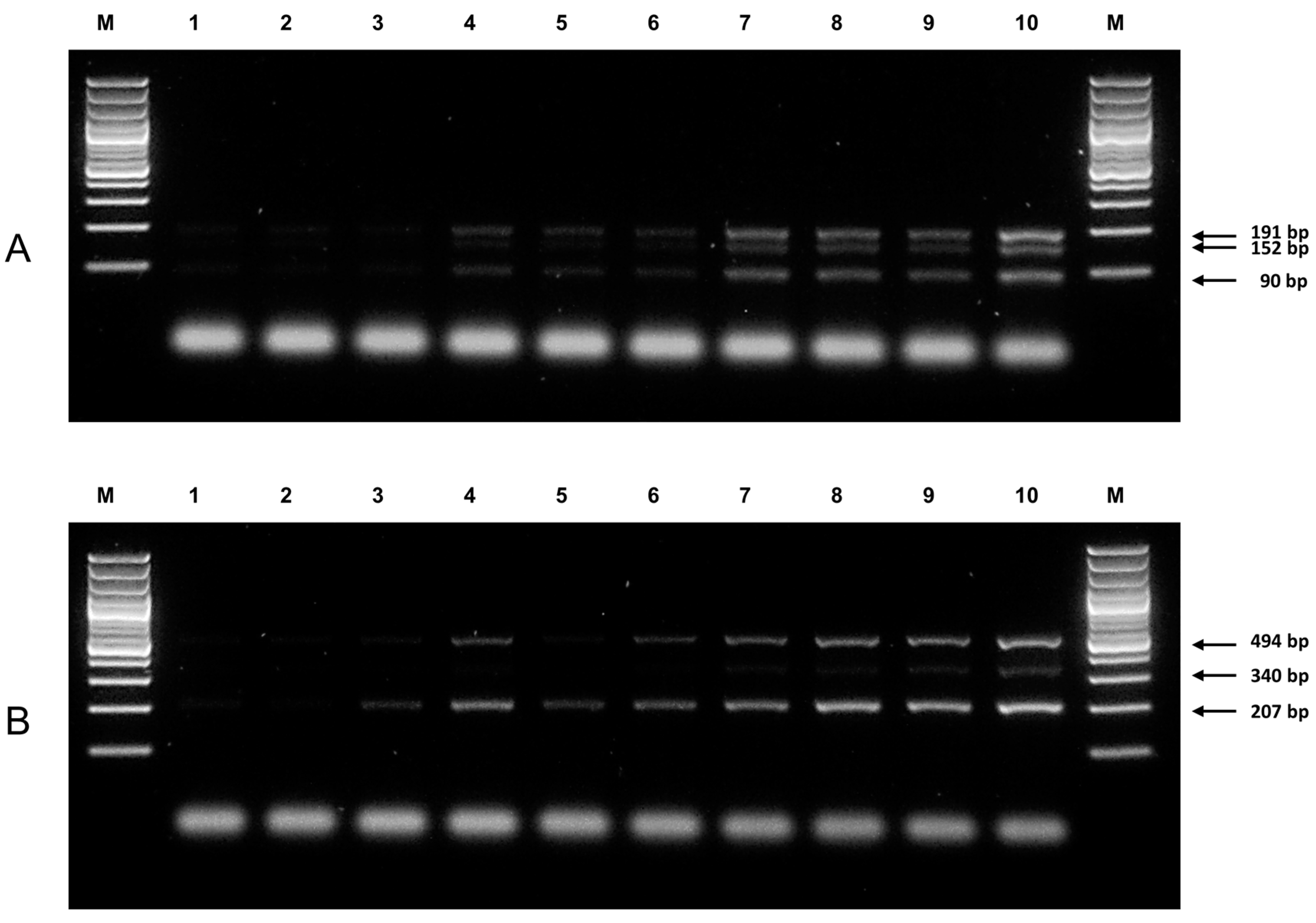
3.1. Genotypes of NUDT15 c.415C>T and TPMT*3C Variants by ARMS-PCR
3.2. Comparison of ARMS-PCR with Conventional Sanger Sequencing
3.3. Sensitivity and Specificity of ARMS-PCR Assay
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Escherich, G.; Richards, S.; Stork, L.C.; Vora, A.J. Childhood Acute Lymphoblastic Leukaemia Collaborative Group (CALLCG) Meta-analysis of randomised trials comparing thiopurines in childhood acute lymphoblastic leukaemia. Leukemia 2011, 25, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.-H.; Evans, W.E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 2006, 354, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Landier, W.; Yang, W.; Liu, C.; Hageman, L.; Cheng, C.; Pei, D.; Chen, Y.; Crews, K.R.; Kornegay, N.; et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kato, M.; Hasegawa, D.; Urayama, K.Y.; Nakadate, H.; Kondoh, K.; Nakamura, K.; Koh, K.; Komiyama, T.; Manabe, A. Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br. J. Haematol. 2015, 171, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Timmer, A.; Patton, P.H.; Chande, N.; McDonald, J.W.D.; MacDonald, J.K. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2016, CD000478. [Google Scholar] [CrossRef]
- Bär, F.; Sina, C.; Fellermann, K. Thiopurines in inflammatory bowel disease revisited. World J. Gastroenterol. 2013, 19, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.L.; Barclay, M.L. Update on thiopurine pharmacogenetics in inflammatory bowel disease. Pharmacogenomics 2015, 16, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, Y.; Naito, T.; Onodera, M.; Kuroha, M.; Kimura, T.; Shiga, H.; Endo, K.; Negoro, K.; Kinouchi, Y.; Shimosegawa, T. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenom. J. 2016, 16, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Earley, A.K.; Webster, A.C.; Schmid, C.H.; Balk, E.M.; Uhlig, K. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst. Rev. 2015, CD007746. [Google Scholar] [CrossRef]
- Aplenc, R.; Lange, B. Pharmacogenetic determinants of outcome in acute lymphoblastic leukaemia. Br. J. Haematol. 2004, 125, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Weinshilboum, R.M.; Sladek, S.L. Mercaptopurine pharmacogenetics: Monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am. J. Hum. Genet. 1980, 32, 651–662. [Google Scholar] [PubMed]
- Marshall, E. Preventing toxicity with a gene test. Science 2003, 302, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Gardner, E.E.; Sandborn, W.J.; Schmiegelow, K.; Pui, C.-H.; Yee, S.W.; Stein, C.M.; Carrillo, M.; Evans, W.E.; Klein, T.E. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin. Pharmacol. Ther. 2011, 89, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Gardner, E.E.; Sandborn, W.J.; Schmiegelow, K.; Pui, C.-H.; Yee, S.W.; Stein, C.M.; Carrillo, M.; Evans, W.E.; Hicks, J.K.; et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin. Pharmacol. Ther. 2013, 93, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, N.; Matsui, T.; Murakami, Y.; Ishihara, H.; Hisabe, T.; Nagahama, T.; Maki, S.; Beppu, T.; Takaki, Y.; Hirai, F.; et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2009, 24, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-K.; Hong, M.; Baek, J.; Choi, H.; Zhao, W.; Jung, Y.; Haritunians, T.; Ye, B.D.; Kim, K.-J.; Park, S.H.; et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat. Genet. 2014, 46, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Nishii, R.; Perez-Andreu, V.; Yang, W.; Klussmann, F.A.; Zhao, X.; Lin, T.-N.; Hoshitsuki, K.; Nersting, J.; Kihira, K.; et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat. Genet. 2016, 48, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Chiengthong, K.; Ittiwut, C.; Muensri, S.; Sophonphan, J.; Sosothikul, D.; Seksan, P.; Suppipat, K.; Suphapeetiporn, K.; Shotelersuk, V. NUDT15 c.415C>T increases risk of 6-mercaptopurine induced myelosuppression during maintenance therapy in children with acute lymphoblastic leukemia. Haematologica 2016, 101, e24–e26. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.-C.; Yang, C.-P.; Liu, H.-C.; Jaing, T.-H.; Chen, S.-H.; Hung, I.-J.; Yeh, T.-C.; Lin, T.-H.; Lai, C.-L.; Lai, C.-Y.; et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenom. J. 2016, 16, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-T.; Choi, R.; Won, H.-H.; Choe, Y.H.; Kang, B.; Lee, K.; Koo, H.H.; Yoo, K.H.; Kim, Y.-H.; Lee, S.-Y. NUDT15 genotype distributions in the Korean population. Pharmacogenet. Genom. 2017, 27, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, B.K.; Fell, J.M.E.; Beattie, R.M.; Mitton, S.G.; Wilson, D.C.; Jenkins, H. IBD Working Group of the British Society of Paediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the management of inflammatory bowel disease in children in the United Kingdom. J. Pediatr. Gastroenterol. Nutr. 2010, 50 (Suppl. 1), S1–S13. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Andreoletti, G.; Ashton, J.J.; Batra, A.; Afzal, N.A.; Gao, Y.; Williams, A.P.; Beattie, R.M.; Ennis, S. Genes implicated in thiopurine-induced toxicity: Comparing TPMT enzyme activity with clinical phenotype and exome data in a paediatric IBD cohort. Sci. Rep. 2016, 6, 34658. [Google Scholar] [CrossRef] [PubMed]
- Clinical Pharmacogenetics Implementation Consortium CPIC® Guideline for Thiopurines and TPMT. Available online: https://cpicpgx.org/guidelines/guideline-for-thiopurines-and-tpmt/ (accessed on 18 October 2017).
- Fong, W.-Y.; Ho, C.-C.; Poon, W.-T. Comparison of Direct Sequencing, Real-Time PCR-High Resolution Melt (PCR-HRM) and PCR-Restriction Fragment Length Polymorphism (PCR-RFLP) Analysis for Genotyping of Common Thiopurine Intolerant Variant Alleles NUDT15 c.415C>T and TPMT c.719A>G (TPMT*3C). Diagnostics 2017, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-C.; Tai, S.-M.; Lee, E.C.-N.; Mak, T.S.-H.; Liu, T.K.-T.; Tang, V.W.-L.; Poon, W.-T. Rapid Identification of Pathogenic Variants in Two Cases of Charcot-Marie-Tooth Disease by Gene-Panel Sequencing. Int. J. Mol. Sci. 2017, 18, 770. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M. The amplification refractory mutation system. Methods Mol. Med. 1998, 16, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Dhillon, S.; Ke, X.; Collins, A.R.; Day, I.N.M. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001, 29, e88. [Google Scholar] [CrossRef] [PubMed]
- Oender, K.; Lanschuetzer, C.M.; Laimer, M.; Klausegger, A.; Paulweber, B.; Kofler, B.; Hintner, H.; Bauer, J.W. Introducing a fast and simple PCR-RFLP analysis for the detection of mutant thiopurine S-methyltransferase alleles TPMT*3A and TPMT*3C. J. Eur. Acad. Dermatol. Venereol. JEADV 2006, 20, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Weise, A.; Prause, S.; Klemm, M.; Eidens, M.; Luchi, M.; Forst, T.; Pfützner, A.; Weber, M.M. Development and validation of a rapid and reliable method for TPMT genotyping using real-time PCR. Clin. Lab. 2012, 58, 959–971. [Google Scholar] [PubMed]
- Skrzypczak-Zielinska, M.; Borun, P.; Bartkowiak-Kaczmarek, A.; Zakerska-Banaszak, O.; Walczak, M.; Dobrowolska, A.; Kurzawski, M.; Waszak, M.; Lipinski, D.; Plawski, A.; et al. A Simple Method for TPMT and ITPA Genotyping Using Multiplex HRMA for Patients Treated with Thiopurine Drugs. Mol. Diagn. Ther. 2016, 20, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Etlik, O.; Koksal, V.; Arican-Baris, S.T.; Baris, I. Development and validation of a cost-effective in-house method, tetra-primer ARMS PCR assay, in genotyping of seven clinically important point mutations. Mol. Cell. Probes 2011, 25, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.L.; Barclay, M.L.; Gearry, R.B.; Kennedy, M.A. A multiplexed allele-specific polymerase chain reaction assay for the detection of common thiopurine S-methyltransferase (TPMT) mutations. Clin. Chim. Acta Int. J. Clin. Chem. 2004, 341, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.R.; de Oliveira, B.M.; Viana, M.B.; Murao, M.; Romanha, A.J. Thiopurine S-methyltransferase (TPMT) gene polymorphism in Brazilian children with acute lymphoblastic leukemia: Association with clinical and laboratory data. Ther. Drug Monit. 2008, 30, 700–704. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hoskins, J.M.; McLeod, H.L. Copy number variants in pharmacogenetic genes. Trends Mol. Med. 2011, 17, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.; Marinaki, A.M.; Lambooy, L.H.; Duley, J.A.; Shobowale-Bakre, M.; De Abreu, R.A. Pitfalls in the determination of mutant alleles of the thiopurine methyltransferase gene. Leukemia 2001, 15, 1792–1793. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.D.; Chan, K.C.A. Setting up a polymerase chain reaction laboratory. Methods Mol. Biol. Clifton NJ 2006, 336, 11–18. [Google Scholar] [CrossRef]
- Pont-Kingdon, G.; Gedge, F.; Wooderchak-Donahue, W.; Schrijver, I.; Weck, K.E.; Kant, J.A.; Oglesbee, D.; Bayrak-Toydemir, P.; Lyon, E. Biochemical and Molecular Genetic Resource Committee of the College of American Pathologists Design and analytical validation of clinical DNA sequencing assays. Arch. Pathol. Lab. Med. 2012, 136, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Medrano, R.F.V.; de Oliveira, C.A. Guidelines for the Tetra-Primer ARMS–PCR Technique Development. Mol. Biotechnol. 2014, 56, 599–608. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence | Tm (°C) 1 | Expected Product Size (bp) | Final Concentration (µM) 2 |
|---|---|---|---|---|
| NUDT15 c.415C>T genotyping | ||||
| N-OF | 5′-CCCAAATAAACACCCTTTGTTTTCTGT-3′ | 55.2 | 191 | 0.18 |
| N-OR | 5′-CCTTTGTATCCCACCAGATGGTTC-3′ | 57.4 | 191 | 0.18 |
| N-WT | 5′-GGACCAGCTTTTCTGGGGACTAC-3′ | 58.8 | 90 | 0.82 |
| N-MT | 5′-GGATCATAGCCTTGTTCTTTTAAACAATA-3′ | 54.4 | 152 | 0.82 |
| TPMT c.719A>G (TPMT*3C) genotyping | ||||
| T-OF | 5′-CACCCAGCCAATTTTGAGTA-3′ | 49.7 | 494 | 0.1 |
| T-OR | 5′-CAGGTAACACATGCTGATTGG-3′ | 52.4 | 494 | 0.1 |
| T-MT | 5′-ATGTCTCATTTACTTTTCTGTAAGTACAC-3′ | 54.4 | 207 | 0.9 |
| T-WT | 5′-TTGACTGTCTTTTTGAAAAGTTCTA-3′ | 49.5 | 340 | 0.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.-C.; Fong, W.-Y.; Lee, Y.-H.; Poon, W.-T. Novel Tetra-Primer ARMS-PCR Assays for Thiopurine Intolerance Susceptibility Mutations NUDT15 c.415C>T and TPMT c.719A>G (TPMT*3C) in East Asians. Genes 2017, 8, 285. https://doi.org/10.3390/genes8100285
Ho C-C, Fong W-Y, Lee Y-H, Poon W-T. Novel Tetra-Primer ARMS-PCR Assays for Thiopurine Intolerance Susceptibility Mutations NUDT15 c.415C>T and TPMT c.719A>G (TPMT*3C) in East Asians. Genes. 2017; 8(10):285. https://doi.org/10.3390/genes8100285
Chicago/Turabian StyleHo, Chi-Chun, Wai-Ying Fong, Yuen-Hon Lee, and Wing-Tat Poon. 2017. "Novel Tetra-Primer ARMS-PCR Assays for Thiopurine Intolerance Susceptibility Mutations NUDT15 c.415C>T and TPMT c.719A>G (TPMT*3C) in East Asians" Genes 8, no. 10: 285. https://doi.org/10.3390/genes8100285
APA StyleHo, C.-C., Fong, W.-Y., Lee, Y.-H., & Poon, W.-T. (2017). Novel Tetra-Primer ARMS-PCR Assays for Thiopurine Intolerance Susceptibility Mutations NUDT15 c.415C>T and TPMT c.719A>G (TPMT*3C) in East Asians. Genes, 8(10), 285. https://doi.org/10.3390/genes8100285




