Therapeutic Targeting of Telomerase
Abstract
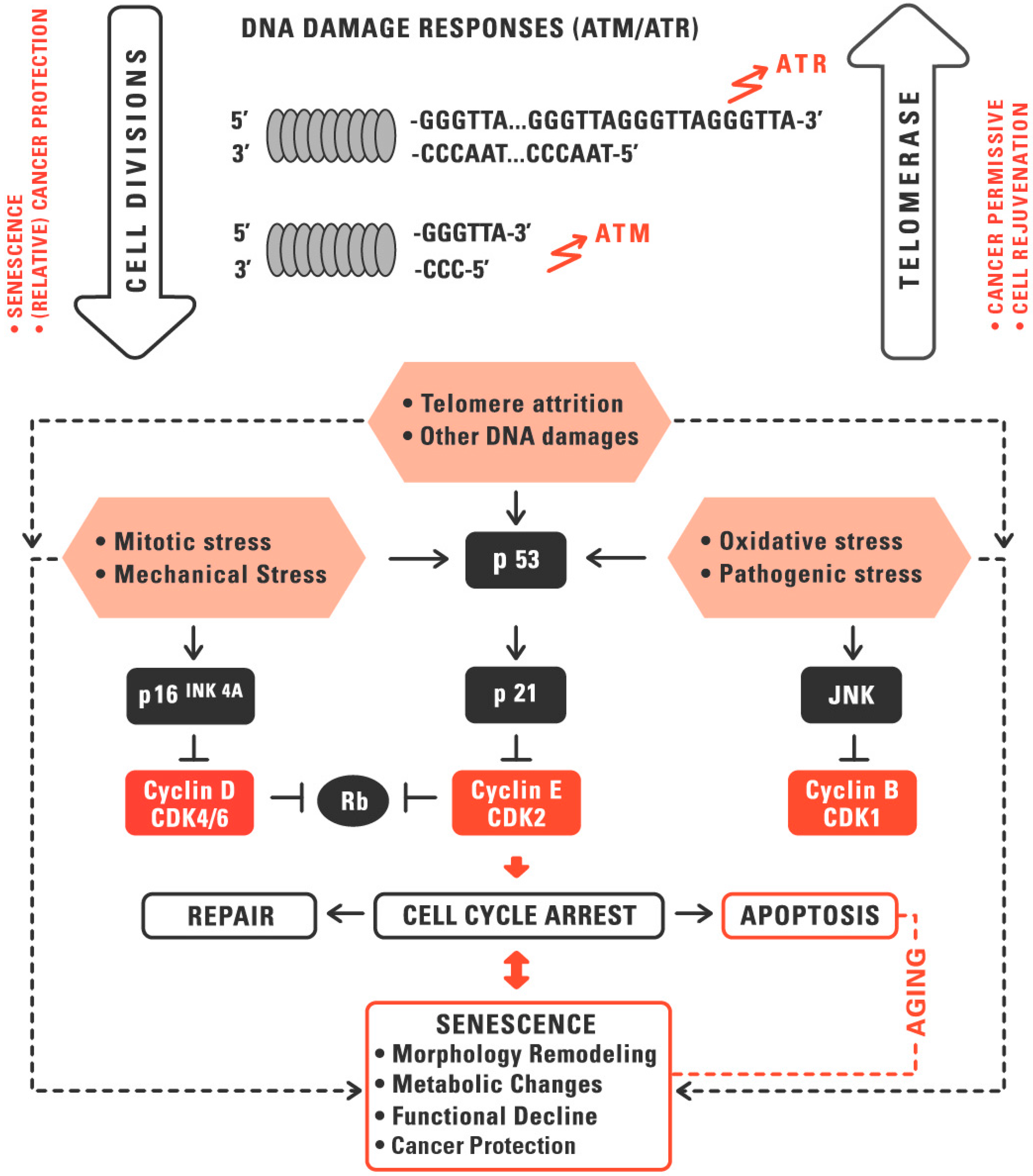
:1. Telomeres and Telomerase in Aging and Cancer
2. Telomerase as a Target for Regenerative Medicine
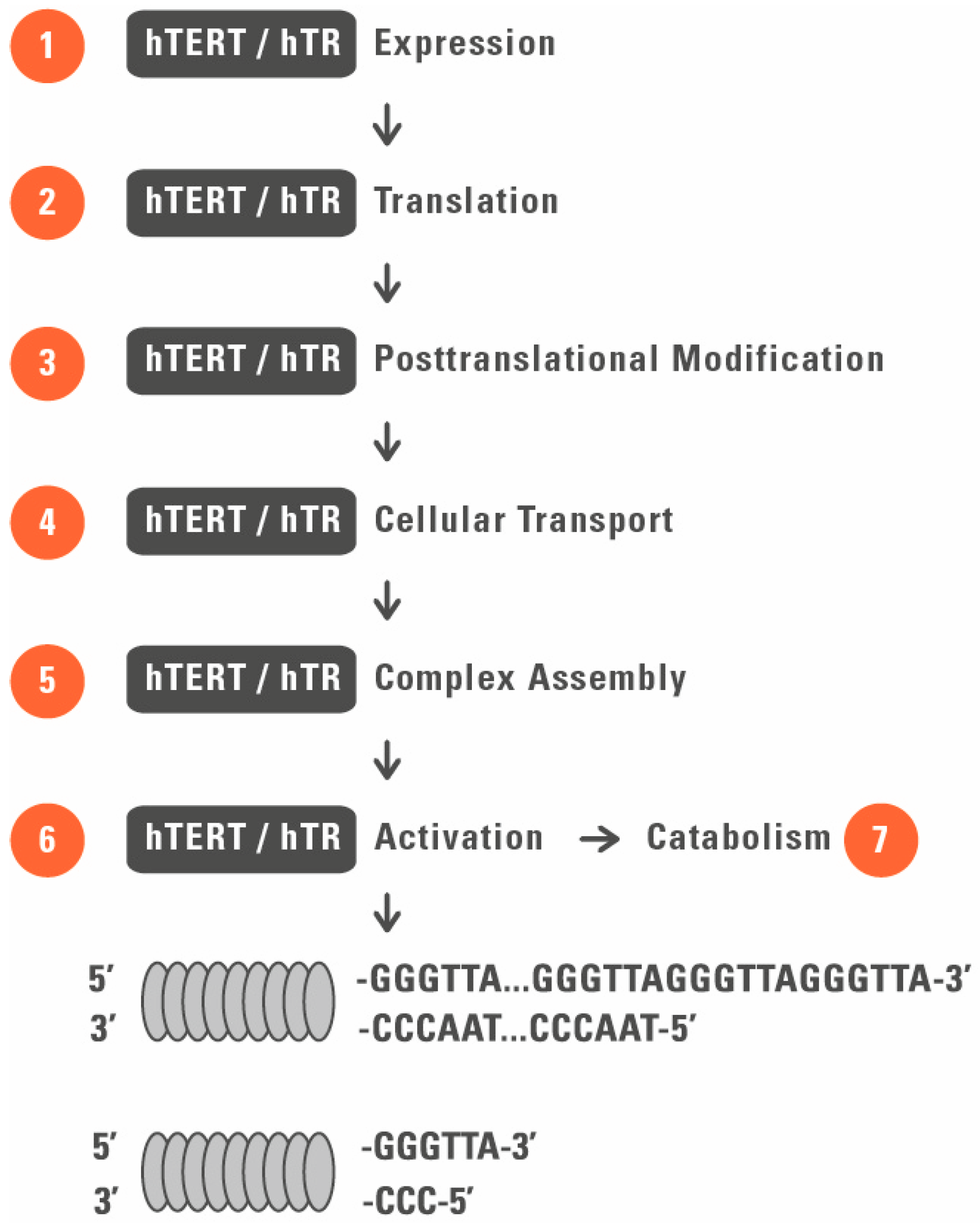
3. Telomerase as a Target for Cancer Treatment
4. Advantages, Pitfalls, and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALT | alternative lengthening of telomeres |
| CRP | C-reactive protein |
| hTERT | human telomerase reverse transcriptase |
| hTR | human telomerase RNA |
| IL-6 | interleukine-6 |
| LTL | leucocyte telomere length |
| mTERT | mouse telomerase reverse transcriptase |
| rAAV | recombinant adeno-associated virus |
| ROS | reactive oxygen species |
| TERC | telomerase RNA component |
| TERT | telomerase reverse transcriptase |
| VEGF | vascular endothelial growth |
References
- Walter, M. Interrelationships among HDL metabolism, aging, and atherosclerosis. Arterioscler Thromb. Vasc. Biol. 2009, 29, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, F.; Saijonmaa, O.; Strandberg, T. The roles of senescence and telomere shortening in cardiovascular disease. Nat. Rev. Cardiol. 2013, 10, 274–283. [Google Scholar] [CrossRef] [PubMed]
- To-Miles, F.Y.L.; Backman, C.L. What telomeres say about activity and health: A rapid review. Can. J. Occup. Ther. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Blecher, M.; van der Harst, P. Healthy aging and disease: Role for telomere biology? Clin. Sci. 2011, 120, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Herbig, U. Telomerase gene therapy: A novel approach to combat aging. EMBO Mol. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Liu, D.; Songyang, Z. The telosome/shelterin complex and its functions. Genome Biol. 2008. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Artandi, S.E.; DePinho, R.A. Telomeres and telomerase in cancer. Carcinogenesis 2010, 31, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sarin, K.Y.; Cheung, P.; Gilison, D.; Lee, E.; Tennen, R.I.; Wang, E.; Artandi, M.K.; Oro, A.E.; Artandi, S.E. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 2005, 436, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4apositive senescent cells delays ageing associated disorders. Nature 2001, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.E.; Armanios, M. The short and long telomere syndromes: Paired paradigms for molecular medicine. Curr. Opin. Gen. Dev. 2015, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, J.I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H.; et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 2009, 460, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.P.; Kuhl, M. An updated overview on Wnt signaling pathways: A prelude for more. Circ. Res. 2010, 106, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2001, 27, 339–344. [Google Scholar] [CrossRef]
- Nazari-Shafti, T.Z.; Cooke, J.P. Telomerase therapy to reserve cardiovascular senescence. Methodist Debakey Cardiovasc. J. 2015, 11, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Yegorov, Y.E. Tissue formation and tissue engineering through host cell recruitment or a potential injectable cell-based biocomposite with replicative potential: Molecular mechanisms controlling cellular senescence and the involvement of controlled transient telomerase activation therapies. J. Biomed. Mater. Res. Part A 2015, 103A, 3993–4023. [Google Scholar]
- Xu, D.; Erickson, S.; Szeps, M.; Gruber, A.; Sangfelt, O.; Einhorn, S.; Pisa, P.; Grandér, D. Interferon α down-regulates telomerase reverse transcriptase and telomerase activity in human malignant and nonmalignant hematopoietic cells. Blood 2000, 96, 4313–4318. [Google Scholar] [PubMed]
- Aviv, A.; Valdes, A.; Gardner, J.P.; Swaminathan, R.; Kimura, M.; Spector, T.D. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J. Clin. Endocrinol. Metab. 2006, 91, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Fellstrom, B.; Qureshi, A.R.; Lamb, K.; Heimbürger, O.; Bárány, P.; Radhakrishnan, K.; Lindholm, B.; Soveri, I.; et al. Telomere attrition is associated with inflammation, low fetuin—a levels and high mortality in prevalent haemodialysis patients. J. Intern. Med. 2008, 263, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Cheung, S.T.; Tsao, S.W.; Wang, X.M.; Tiwari, A.F. Telomerase activity and its association with psychological stress, mental disorders, lifestyle factors and interventions: A systematic review. Psychoneuroendocrinology 2016, 64, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.S.; Zunzunegui, M.V.; Quinlan, J.; Fahmi, H.; Tu, M.T.; Guerra, R.O. Systematic review of the association between chronic social stress and telomere length: A life course perspective. Ageing Res. Rev. 2016, 26, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Staessen, J.A.; Holvoet, P.; Struijker-Boudier, H.A.; Schiffers, P.; van Bortel, L.M.; Fagard, R.H.; Gardner, J.P.; Kimura, M.; Aviv, A. Telomere length and its associations with oxidized-LDL, carotid artery distensibility and smoking. Front. Biosci. 2010, 2, 1164–1168. [Google Scholar]
- Yaswen, P.; MacKenzieb, K.L.; Keithc, W.N.; Hentosh, P.; Rodier, F.; Zhu, J.; Firestone, G.L.; Matheu, A.; Carnero, A.; Bilsland, A.; et al. Therapeutic targeting of replicative immortality. Sem. Cancer Biol. 2015, 35, S104–S128. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.L.; de Lange, T. Significant Role for p16INK4a in p53-Independent Telomere-Directed Senescence. Curr. Biol. 2004, 14, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.L. Loss of Telomere Protection: Consequences and Opportunities. Front. Oncol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Khoo, C.M.; Naylor, M.L.; Maser, R.S.; DePinho, R.A. Telomere-based crisis: Functional differences between telomerase activation and ALT in tumor progression. Genes Dev. 2003, 17, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Hwang, S.S.; Liesa, M.; Gan, B.; Sahin, E.; Jaskelioff, M.; Ding, Z.; Ying, H.; Boutin, A.T.; Zhang, H.; et al. Antitelomerase Therapy Provokes ALT and Mitochondrial Adaptive Mechanisms in Cancer. Cell 2012, 148, 651–663. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, R.; Almouzni, G. Assembly of telomeric chromatin to create ALTernative endings. Trends Cell Biol. 2014, 24, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zheng, C.; Lindvall, C.; Hou, M.; Ekedahl, J.; Lewensohn, R.; Yan, Z.; Yang, X.; Henriksson, M.; Blennow, E.; et al. Frequent Amplification of the Telomerase Reverse Transcriptase Gene in Human Tumors. Cancer Res. 2000, 60, 6230–6235. [Google Scholar] [PubMed]
- Zhang, A.; Zheng, C.; Hou, M.; Lindvall, C.; Wallin, K.L.; Angström, T.; Yang, X.; Hellström, A.C.; Blennow, E.; Björkholm, M.; et al. Amplification of the Telomerase Reverse Transcriptase (hTERT) Gene in Cervical Carcinomas. Genes Chromos. Cancer 2002, 34, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Takuma, Y.; Nouso, K.; Kobayashi, Y.; Nakamura, S.; Tanaka, H.; Matsumoto, E.; Fujikawa, T.; Suzuki, M.; Hanafusa, T.; Shiratori, Y. Telomerase reverse transcriptase gene amplification in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2004, 19, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, P.; Autexier, C. Telomere biology: Rationale for diagnostics and therapeutics in cancer. RNA Biol. 2016, 12, 1078–1082. [Google Scholar]
- Teralı, K.; Yilmazer, A. New surprises from an old favourite: The emergence of telomerase as a key player in the regulation of cancer stemness. Biochimie 2016, 121, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Soder, A.I.; Hoare, S.F.; Muir, S.; Going, J.J.; Parkinson, E.K.; Keith, W.N. Amplification, increased dosage and in situ expression of the telomerase RNA gene in human cancer. Oncogene 1997, 14, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Hallmarks of telomeres in ageing research. J. Pathol. 2007, 211, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Depinho, R.A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 2010, 464, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Use of telomerase to create bioengineered tissues. Ann. N. Y. Acad. Sci. 2005, 1057, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Jaskelioff, M.; Muller, F.L.; Paik, J.H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadiñanos, J.; et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011, 469, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Weissman, I.L. Replicative senescence of hematiopoietic stem cells during serial transplantation: Does telomere shortening play a role? Oncogene 2002, 21, 3270–3273. [Google Scholar] [CrossRef] [PubMed]
- Pendino, F.; Tarkanyi, I.; Dudognon, C.; Hillion, J.; Lanotte, M.; Aradi, J.; Segal-Bendirdjian, S. Telomeres and telomerase: Pharmacological targets for new anticancer strategies? Curr. Cancer Drug Targets 2006, 6, 147–180. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.P.; Hoare, S.F.; Glasspool, R.M.; Keith, W.N. Lack of telomerase gene expression in alternative lengthening of telomere cells is associated with chromatin remodeling of the hTR and hTERT gene promoters. Cancer Res. 2005, 65, 7585–7590. [Google Scholar] [PubMed]
- Serenicki, N.; Hoare, S.F.; Kassem, M.; Atkinson, S.P.; Keith, W.N. Telomerase promoter reprogramming and interaction with general transcription factors in the human mesenchymal stem cell. Regen. Med. 2006, 1, 125–131. [Google Scholar]
- Doshida, M.; Ohmichi, M.; Tsutsumi, S.; Kawagoe, J.; Takahashi, T.; Du, B.; Mori-Abe, A.; Ohte, T.; Saitoh-Sekiguchi, M.; Takahashi, K.; et al. Raloxifene increases proliferation and up-regulates telomerase activity in human umbilical vein endothelial cells. J. Biol. Chem. 2006, 281, 24270–24278. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hodes, R.J.; Weng, N. Cutting edge: Telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J. Immunol. 2001, 166, 4826–4830. [Google Scholar] [CrossRef] [PubMed]
- Tarkanyi, I.; Aradi, J. Pharmacological intervention strategies for affecting telomerase activity: Future prospects to treat cancer and degenerative disease. Biochemie 2008, 90, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A. Multiple levels of telomerase regulation. Mol. Interv. 2002, 2, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Reyes, A.; Green, P.; Caron, M.J.; Bonini, M.G.; Gordon, D.M.; Holt, I.J.; Santos, J.H. Human telomerase acts as a hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res. 2012, 40, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Counter, C.M.; Meyerson, M.; Eaton, E.N.; Ellisen, L.W.; Caddle, S.D.; Haber, D.A.; Weinberg, R.A. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene 1998, 16, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, H.; Benchimol, S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 1998, 8, 279–282. [Google Scholar] [CrossRef]
- Jiang, X.R.; Jimenez, G.; Chang, E.; Frolkis, M.; Kusler, B.; Sage, M.; Beeche, M.; Bodnar, A.G.; Wahl, G.M.; Tlsty, T.D.; et al. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat. Genet. 1999, 21, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.P.; Holt, S.E.; Ouellette, M.; Kaur, K.J.; Yan, Y.; Wilson, K.S.; White, M.A.; Wright, W.E.; Shay, J.W. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat. Genet. 1999, 21, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Funk, W.D.; Wang, C.K.; Shelton, D.N.; Harley, C.B.; Pagon, G.D.; Hoeffler, W.K. Telomerase expression restores dermal integrity to in vitro-aged fibroblasts in a reconstituted skin model. Exp. Cell Res. 2000, 258, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, F.S.; Jones, C.J.; Skinner, J.W.; Haughton, M.F.; Wallis, C.; Wynford-Thomas, D.; Faragher, R.G.; Kipling, D. Telomerase prevents the accelerated cell ageing of Werner syndrome fibroblasts. Nat. Genet. 2000, 24, 16–17. [Google Scholar] [PubMed]
- Harada, H.; Nakagawa, H.; Takaoka, M.; Lee, J.; Herlyn, M.; Diehl, J.A.; Rustgi, A.K. Cleavage of MCM2 licensing protein fosters senescence in human keratinocytes. Cell Cycle 2008, 7, 3534–3538. [Google Scholar] [CrossRef] [PubMed]
- McKee, J.A.; Banik, S.S.; Boyer, M.J.; Hamad, N.M.; Lawson, J.H.; Niklason, L.E.; Counter, C.M. Human arteries engineered in vitro. EMBO Rep. 2003, 4, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Taffet, G.E.; Youker, K.A.; Entman, M.L.; Overbeek, P.A.; Michael, L.H.; Schneider, M.D. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc. Natl. Acad. Sci. USA 2001, 98, 10308–10313. [Google Scholar] [CrossRef] [PubMed]
- Wootton, M.; Steeghs, K.; Watt, D.; Munro, J.; Gordon, K.; Ireland, H.; Morrison, V.; Behan, W.; Parkinson, E.K. Telomerase alone extends the replicative life span of human skeletal muscle cells without compromising genomic stability. Hum. Gene Ther. 2003, 14, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chang, E.; Cherry, A.M.; Bangs, C.D.; Oei, Y.; Bodnar, A.; Bronstein, A.; Chiu, C.P.; Herron, G.S. Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 1999, 274, 26141–26148. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhang, J.; Brann, D.W.; Yu, F.S. Brain and retinal vascular endothelial cells with extended life span established by ectopic expression of telomerase. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3219–3225. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nagavarapu, U.; Relloma, K.; Sjaastad, M.D.; Moss, W.C.; Passaniti, A.; Herron, G.S. Telomerized human microvasculature is functional in vivo. Nat. Biotechnol. 2001, 19, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Condon, J.; Yin, S.; Mayhew, B.; Word, R.A.; Wright, W.E.; Shay, J.W.; Rainey, W.E. Telomerase immortalization of human myometrial cells. Biol. Reprod. 2002, 67, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gronthos, S.; Chen, S.; Reddi, A.; Counter, C.M.; Robey, P.G.; Wang, C.Y. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat. Biotechnol. 2002, 20, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.; Jensen, T.G.; Kassem, M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002, 20, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Chen, S.; Wang, C.Y.; Robey, P.G.; Shi, S. Telomerase accelerates osteogenesis of bone marrow stromal stem cells by upregulation of CBFA1, osterix, and osteocalcin. J. Bone Miner Res. 2003, 18, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Kobune, M.; Yamaguchi, M.; Nakamura, K.; Ito, Y.; Sasaki, K.; Takahashi, S.; Nakamura, T.; Chiba, H.; Sato, T.; et al. Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a coculture system with human telomerase catalytic subunit (hTERT)-transfected human stromal cells. Blood 2003, 101, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Darimont, C.; Avanti, O.; Tromvoukis, Y.; Vautravers-Leone, P.; Kurihara, N.; Roodman, G.D.; Colgin, L.M.; Tullberg-Reinert, H.; Pfeifer, A.M.; Offord, E.A.; et al. SV40 T antigen and telomerase are required to obtain immortalized human adult bone cells without loss of the differentiated phenotype. Cell Growth Differ. 2002, 13, 59–67. [Google Scholar] [PubMed]
- Yudoh, K.; Matsuno, H.; Nakazawa, F.; Katayama, R.; Kimura, T. Reconstituting telomerase activity using the telomerase catalytic subunit prevents the telomere shorting and replicative senescence in human osteoblasts. J. Bone Miner Res. 2001, 16, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Yudoh, K.; Nishioka, K. Telomerized presenescent osteoblasts prevent bone mass loss in vivo. Gene Ther. 2004, 11, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Narayanan, K.; Ramachandran, A.; He, G.; Almushayt, A.; Evans, C.; George, A. Odontoblast cells immortalized by telomerase produce mineralized dentin-like tissue both in vitro and in vivo. J. Biol. Chem. 2002, 277, 19976–19981. [Google Scholar] [CrossRef] [PubMed]
- Luiten, R.M.; Pene, J.; Yssel, H.; Spits, H. Ectopic hTERT expression extends the life span of human CD4 helper and regulatory T-cell clones and confers resistance to oxidative stress-induced apoptosis. Blood 2003, 101, 4512–4519. [Google Scholar] [CrossRef] [PubMed]
- Kobune, M.; Kawano, Y.; Ito, Y.; Chiba, H.; Nakamura, K.; Tsuda, H.; Sasaki, K.; Dehari, H.; Uchida, H.; Honmou, O. Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobblestone area-supporting cells. Exp. Hematol. 2003, 31, 715–722. [Google Scholar] [CrossRef]
- Di Donna, S.; Mamchaoui, K.; Cooper, R.N.; Seigneurin-Venin, S.; Tremblay, J.; Butler-Browne, G.S.; Mouly, V. Telomerase can extend the proliferative capacity of human myoblasts, but does not lead to their immortalization. Mol. Cancer Res. 2003, 1, 643–653. [Google Scholar] [PubMed]
- Schnabl, B.; Choi, Y.H.; Olsen, J.C.; Hagedorn, C.H.; Brenner, D.A. Immortal activated human hepatic stellate cells generated by ectopic telomerase expression. Lab. Invest. 2002, 82, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Shibata, N.; Westerman, K.A.; Okitsu, T.; Allain, J.E.; Sakaguchi, M.; Totsugawa, T.; Maruyama, M.; Matsumura, T.; Noguchi, H. Establishment of immortalized human hepatic stellate scavenger cells to develop bioartificial livers. Transplantation 2003, 75, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.S.; Nakano, T.; Keyoung, H.M.; Windrem, M.; Rashbaum, W.K.; Alonso, M.L.; Kang, J.; Peng, W.; Carpenter, M.K.; Lin, J.; et al. Telomerase immortalization of neuronally restricted progenitor cells derived from the human fetal spinal cord. Nat. Biotechnol. 2004, 22, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Townsley, D.M.; Dumitriu, B.; Young, N.S. Bone marrow failure and the telomeropathies. Blood 2014, 124, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. The use of telomerized cells for tissue engineering. Nat. Biotechnol. 2000, 18, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A. Telomere Maintenance in Clinical Medicine. Am. J. Med. 2004, 117, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Bär, C.; Povedano, J.M.; Serrano, R.; Benitez-Buelga, C.; Popkes, M.; Formentini, I.; Bobadilla, M.; Bosch, F.; Blasco, M.A. Telomerase gene therapy rescues telomere length, bone marrow aplasia, and survival in mice with aplastic anemia. Blood 2016, 127, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Donati, B.; Valenti, L. Telomeres, NAFLD and Chronic Liver Disease. Int. J. Mol. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T. Telomeres in lung diseases. Prog. Mol. Biol. Transl. Sci. 2014, 125, 173–183. [Google Scholar] [PubMed]
- Li, J.; Pei, M. Cell senescence: A challenge in cartilage engineering and regeneration. Tissue Eng. Part B Rev. 2012, 18, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Yegorov, Y.E. Telomere attrition in lens epithelial cells—A target for N-acetylcarnosine therapy. Front. Biosci. 2010, 15, 934–956. [Google Scholar] [CrossRef]
- Weyand, C.M.; Fujii, H.; Shao, L.; Goronzy, J.J. Rejuvenating the immune system in rheumatoid arthritis. Nat. Rev. Rheumatol. 2009, 5, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Yang, L.; Hornsby, P.J. Formation of functional tissue from transplanted adrenocortical cells expressing telomerase reverse transcriptase. Nat. Biotechnol. 2000, 18, 39–42. [Google Scholar] [PubMed]
- Fauce, S.R.; Jamieson, B.D.; Chin, A.C.; Mitsuyasu, R.T.; Parish, S.T.; Ng, H.L.; Kitchen, C.M.; Yang, O.O.; Harley, C.B.; Effros, R.B. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J. Immunol. 2008, 181, 7400–7406. [Google Scholar] [CrossRef] [PubMed]
- Dock, J.N.; Effros, R.B. Role of CD8 T Cell Replicative Senescence in Human Aging and in HIV-mediated Immunosenescence. Aging Dis. 2011, 2, 382–397. [Google Scholar] [PubMed]
- Harley, C.B.; Liu, W.; Blasco, M.; Vera, E.; Andrews, W.H.; Briggs, L.A.; Raffaele, J.M. A natural product telomerase activator as part of a health maintenance program. Rejuven. Res. 2011, 14, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Bernardes de Jesus, B.; Schneeberger, K.; Vera, E.; Tejera, A.; Harley, C.B.; Blasco, M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 2011, 10, 604–621. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.T.; Harley, C.B. Evaluation of an oral telomerase activator for early age-related macular degeneration—A pilot study. Clin. Ophthalmol. 2016, 10, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.B.; Liu, W.; Flom, P.L.; Raffaele, J.M. A natural product telomerase activator as part of a health maintenance program: Metabolic and cardiovascular response. Rejuven. Res. 2013, 16, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.; Singaravelu, G.; Harley, C.B.; Flom, P.; Suram, A.; Raffaele, J.M. A Natural Product Telomerase Activator Lengthens Telomeres in Humans: A Randomized, Double Blind, and Placebo Controlled Study. Rejuven. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pearce, V.P.; Sherrell, J.; Lou, Z.; Kopelovich, L.; Wright, W.E.; Shay, J.W. Immortalization of epithelial progenitor cells mediated by resveratrol. Oncogene 2008, 27, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wang, X.X.; Hu, X.S.; Guo, X.G.; Shang, Y.P.; Chen, H.J.; Zeng, C.L.; Zhang, F.R.; Chen, J.Z. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br. J. Pharmacol. 2008, 155, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, A.A.; Steding, C.E.; Herbert, B.-S. Pharmaceutical regulation of telomerase and its clinical potential. J. Cell. Mol. Med. 2012, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Tichon, A.; Gazit, A.; Gitler, D.; Slavin, S.; Priel, E. Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol. Med. 2012, 4, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chang, G.; Geng, X. NGF and TERT co-transfected BMSCs improve the restoration of cognitive impairment in vascular dementia rats. PLoS ONE 2014, 9, e98774. [Google Scholar] [CrossRef] [PubMed]
- Haendeler, J.; Hoffman, J.; Diehl, J.F.; Vasa, M.; Spyridopoulos, I.; Zeiher, A.M.; Dimmeler, S. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ. Res. 2004, 94, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Moritoh, Y.; Miva, N. Age-dependent telomere-shortening is repressed by phosphorylated alpha-tocopherol together with cellular longevity and intracellular oxidative-stress reduction in human brain microvascular endotheliocytes. J. Cell Biochem. 2007, 102, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.F.; Saretzki, G.; Ahmed, S.; Nelson, G.; Richter, T.; Peters, H.; Wappler, I.; Birket, M.J.; Harold, G.; Schaeuble, K.; et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007, 5, e110. [Google Scholar] [CrossRef] [PubMed]
- Brouilette, S.W.; Moore, J.S.; McMahon, A.D.; Thompson, J.R.; Ford, I.; Shepherd, J.; Packard, C.J.; Samani, N.J. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: A nested case-control study. Lancet 2007, 369, 107–114. [Google Scholar] [CrossRef]
- Spyridopoulos, I.; Haendeler, J.; Urbich, C.; Brummendorf, T.H.; Oh, H.; Schneider, M.D.; Zeiher, A.M.; Dimmeler, S. Statins enhance migratory capacity by upregulation of the telomere repeat binding factor TRF2 in endothelial progenitor cells. Circulation 2004, 110, 3136–3142. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.X.; Hui, Y.J.; Xiang, W.X.; Rong, Z.F.; Jian, S.; Zhu, C.J. Ginkgo Biloba extract reduces endothelial progenitor-cell senescence trough augmentation of telomerase activity. J. Cardiovasc. Pharmacol. 2007, 49, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Batra, S.K. Recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells 2006, 24, 2319–2345. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Mitsuno, H.; Nonaka, I.; Sen, Y.; Kawanishi, K.; Inada, Y.; Takakura, Y.; Okuchi, K.; Nonomura, A. Wound therapy by marrow mesenchymal cell transplantation. Plast. Reconstr. Surg. 2008, 121, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Kung, E.F.; Wang, F.; Schechner, J.S. In vivo perfusion of human skin substitutes with microvessels formed by adult circulating endothelial progenitor cells. Dermatol. Surg. 2008, 34, 137–146. [Google Scholar] [PubMed]
- Zhang, C.P.; Fu, X.B. Therapeutic potential of stem cells in skin repair and regeneration. Chin. J. Traumatol. 2008, 11, 209–221. [Google Scholar] [CrossRef]
- Park, B.S.; Jang, K.A.; Sung, J.H.; Park, J.S.; Kwon, Y.H.; Kim, K.J.; Kim, W.S. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol. Surg. 2008, 34, 1323–1326. [Google Scholar] [PubMed]
- Branski, L.K.; Gauglitz, G.G.; Herndon, D.N.; Jeschke, M.G. A review of gene and stem cell therapy in cutaneous wound healing. Burns 2008, 35, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Cho, H.J.; Yang, S.K.; Shin, J.W.; Huh, C.H.; Park, K.C. IGFBP-2 Contributes to the proliferation of less proliferative cells in forming skin equivalents. Tissue Eng. Part A 2009, 15, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Siegl-Cachedenier, I.; Flores, I.; Klatt, P.; Blasco, M.A. Telomerase reverses epidermal hair follicle stem cell defects and loss of longterm survival associated with critically short telomeres. J. Cell Biol. 2007, 179, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Scott, P.G.; Tredget, E.E. Bone marrow-derived stem cells in wound healing: A review. Wound Repair Regen. 2007, 15, S18–S26. [Google Scholar] [CrossRef] [PubMed]
- Jan, H.M.; Wei, M.F.; Peng, C.L.; Lin, S.J.; Lai, P.S.; Shieh, M.J. The use of polyethylenimine-DNA to topically deliver hTERT to promote hair growth. Gene Ther. 2012, 19, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Lee, T.R.; Shin, D.W. Novel in vitro culture condition improves the stemness of human dermal stem/progenitor cells. Mol. Cells 2013, 36, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Neuner, B.; Lenfers, A.; Kelsch, R.; Jäger, K.; Brüggmann, N.; van der Harst, P.; Walter, M. Telomere length is not related to established cardiovascular risk factors but does correlate with red and white blood cell counts in a German blood donor population. PLoS ONE 2015, 10, e0139308. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Forsyth, N.R.; Wright, W.; Shay, J.W.; Roth, M.G. The establishment of telomerase-immortalized Tangier disease cell lines indicates the existence of an apolipoprotein A-I-inducible but ABCA1-independent cholesterol efflux pathway. J. Biol. Chem. 2004, 279, 20866–20873. [Google Scholar] [CrossRef] [PubMed]
- Kannenberg, F.; Gorzelniak, K.; Jäger, K.; Fobker, M.; Rust, S.; Repa, J.; Roth, M.; Björkhem, I.; Walter, M. Characterization of cholesterol homeostasis in telomerase-immortalized Tangier disease fibroblasts reveals marked phenotype variability. J. Biol. Chem. 2013, 288, 36936–36947. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Nedel, F.; Collares, T.V.; Tarquinio, S.B.; Nör, J.E.; Demarco, F.F. Telomeres and tissue engineering: The potential roles of TERT in VEGF-mediated angiogenesis. Stem Cell Rev. 2012, 8, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Chang, E.; Glassford, A.J.; Cooke, J.P.; Chiu, C.P.; Tsao, P.S. eNOS Activity Is Reduced in Senescent Human Endothelial Cells Preservation by hTERT Immortalization. Circ. Res. 2001, 89, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.G.; Hu, Y.; Wu, D.L.; Zhu, L.J.; Chen, C.; Jin, X.; Luo, C.X.; Wu, H.Y.; Zhang, J.; Zhu, D.Y. Hippocampal telomerase is involved in the modulation of depressive behaviors. J. Neurosci. 2011, 31, 12258–12269. [Google Scholar] [CrossRef] [PubMed]
- Wolkowitz, O.M.; Mellon, S.H.; Epel, E.S.; Lin, J.; Reus, V.I.; Rosser, R.; Burke, H.; Compagnone, M.; Nelson, J.C.; Dhabhar, F.S.; et al. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol. Psychiatry 2012, 17, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.M.; Walton, Z.E.; Bui, E.; Prescott, J.; Hoge, E.; Keshaviah, A.; Schwarz, N.; Dryman, T.; Ojserkis, R.A.; Kovachy, B.; et al. Telomere length and telomerase in a well-characterized sample of individuals with major depressive disorder compared to controls. Psychoneuroendocrinology 2015, 58, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.B.; Backlund, L.; Wegener, G.; Mathé, A.A.; Lavebratt, C. Telomerase dysregulation in the hippocampus of a rat model of depression. Normalization by lithium. Int. J. Neuropsychopharmacol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Martinsson, L.; Wei, Y.; Xu, D.; Melas, P.A.; Mathé, A.A.; Schalling, M.; Lavebratt, C.; Backlund, L. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl. Psychiatry 2013. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Y.; Chang, H.W.; Lin, C.H.; Cho, C.L. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J. Psychiatry Neurosci. 2008, 33, 244–247. [Google Scholar] [PubMed]
- Bersani, F.S.; Lindqvist, D.; Mellon, S.H.; Penninx, B.W.; Verhoeven, J.E.; Révész, D.; Reus, V.I.; Wolkowitz, O.M. Telomerase activation as a possible mechanism of action for psychopharmacological interventions. Drug Discov. Today 2015, 20, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Malberg, J.; Nakagawa, S. Regulation of adult neurogenesis by psychotropic drugs and stress. J. Pharmacol. Exp. Ther. 2001, 299, 401–407. [Google Scholar] [PubMed]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Podlevsky, J.D.; Chen, J.J. It all comes together at the ends: Telomerase structure, function, and biogenesis. Mutat. Res. 2012, 730, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Funk, W.D.; Wang, S.-S.; Weinrich, S.L.; Avilion, A.A.; Chiu, C.P.; Adams, R.R.; Chang, E.; Allsopp, R.C.; Yu, J.; et al. The RNA component of human telomerase. Science 1995, 269, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.C.; Piatyszek, M.A.; Wright, W.E.; Shay, J.W.; Corey, D.R. Inhibition of human telomerase activity by peptide nucleic acids. Nat. Biotechnol. 1996, 14, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Tanaka, Y.; Kondo, Y.; Hitomi, M.; Barnett, G.H.; Ishizaka, Y.; Liu, J.; Haqqi, T.; Nishiyama, A.; Villeponteau, B.; et al. Antisense telomerase treatment: Induction of two distinct pathways, apoptosis and differentiation. FASEB J. 1988, 12, 801–811. [Google Scholar]
- Pitts, A.E.; Corey, D.R. Inhibition of human telomerase by 2’-O-methyl-RNA. Proc. Natl. Acad. Sci. USA 1988, 95, 11549–11554. [Google Scholar] [CrossRef]
- Herbert, B.; Pitts, A.E.; Baker, S.I.; Hamilton, S.E.; Wright, W.E.; Shay, J.W.; Corey, D.R. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc. Natl. Acad. Sci. USA 1999, 96, 14276–14281. [Google Scholar] [CrossRef] [PubMed]
- Corey, D.R. Telomerase inhibition, oligonucleotides, and clinical trials. Oncogene 2002, 21, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Oshima, Y.; Yamamoto, Y.; Uochi, T.A.; Kusaka, H.; Akinaga, S.; Yamashita, Y.; Pongracz, K.; Pruzan, R.; Wunder, E.; et al. A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res. 2003, 63, 3931–3939. [Google Scholar] [PubMed]
- Harley, C.B. Telomerase and cancer therapeutics. Nat. Rev. Cancer 2008, 8, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Marian, C.O.; Cho, S.K.; McEllin, B.M.; Maher, E.A.; Hatanpaa, K.J.; Madden, C.J.; Mickey, B.E.; Wright, W.E.; Shay, J.W.; Bachoo, R.M. The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumor-initiating cells leading to decreased proliferation and tumor growth. Clin. Cancer Res. 2010, 16, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Naasani, I.; Seimiya, H.; Yamori, T.; Tsuruo, T. FJ5002: A potent telomerase inhibitor identified by exploiting the disease-oriented screening program with COMPARE analysis. Cancer Res. 1999, 59, 4004–4011. [Google Scholar] [PubMed]
- Hayakawa, N.; Nozawa, K.; Ogawa, A.; Kato, N.; Yoshida, K.; Akamatsu, K.I.; Tsuchiya, M.; Nagasaka, A.; Yoshida, S. Isothiazolone derivatives selectively inhibit telomerase from human and rat cancer cells in vitro. Biochemistry 1999, 38, 11501–11507. [Google Scholar] [CrossRef] [PubMed]
- Damm, K.; Hemmann, U.; Garin-Chesa, P.; Hauel, N.; Kauffmann, I.; Priepke, H.; Niestroj, C.; Daiber, C.; Enenkel, B.; Guilliard, B.; et al. A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J. 2001, 20, 6958–6968. [Google Scholar] [CrossRef] [PubMed]
- Kleideiter, E.; Piotrowska, K.; Klotz, U. Screening of telomerase inhibitors. Methods Mol. Biol. 2007, 405, 167–180. [Google Scholar] [PubMed]
- Wong, L.H.; Unciti-Broceta, A.; Spitzer, M.; White, R.; Tyers, M.; Harrington, L. A yeast chemical genetic screen identifies inhibitors of human telomerase. Chem. Biol. 2013, 20, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Peterson, T.R.; Sabatini, D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 2010, 40, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Jia, F.; Wang, W.; Guo, X.; Wu, M.; Wei, L. Coupled down-regulation of mTOR and telomerase activity during fluorouracil-induced apoptosis of hepatocarcinoma cells. BMC Cancer 2007. [Google Scholar] [CrossRef] [PubMed]
- Sundin, T.; Peffley, D.M.; Gauthier, D.; Hentosh, P. The isoprenoid perillyl alcohol inhibits telomerase activity in prostate cancer cells. Biochimie 2012, 94, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Sundin, T.; Peffley, D.M.; Hentosh, P. Disruption of an hTERT-mTOR-RAPTOR protein complex by a phytochemical perillyl alcohol and rapamycin. Mol. Cell Biochem. 2013, 375, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Chen, W.; Schwarer, A.P.; Li, H. Telomerase in cancer immunotherapy. Biochim. Biophys. Acta 2010, 1805, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ugel, S.; Scarselli, E.; Iezzi, M.; Mennuni, C.; Pannellini, T.; Calvaruso, F.; Cipriani, B.; De Palma, R.; Ricci-Vitiani, L.; Peranzoni, E. Autoimmune B-cell lymphopenia after successful adoptive therapy with telomerase-specific T lymphocytes. Blood 2010, 115, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M. A second chance for telomerase reverse transcriptase in anticancer immunotherapy. Nat. Rev. Clin. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Dannull, J.; Heiser, A.; Yancey, D.; Pruitt, S.; Madden, J.; Coleman, D.; Niedzwiecki, D.; Gilboa, E.; Vieweg, J. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003, 63, 2127–2133. [Google Scholar] [PubMed]
- Parkhurst, M.R.; Riley, J.P.; Igarashi, T.; Li, Y.; Robbins, P.F.; Rosenberg, S.A. Immunization of patients with the hTERT: 540–548 peptide induces peptide-reactive T lymphocytes that do not recognize tumors endogenously expressing telomerase. Clin. Cancer Res. 2004, 10, 4688–4698. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H.; Domchek, S.M.; Schultze, J.L.; George, D.J.; Hoar, K.M.; Chen, D.Y.; Stephans, K.F.; Masutomi, K.; Loda, M.; Xia, Z.; et al. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin. Cancer Res. 2004, 10, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Dannull, J.; Yang, B.K.; Dahm, P.; Coleman, D.; Yancey, D.; Sichi, S.; Niedzwiecki, D.; Boczkowski, D.; Gilboa, E.; et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J. Immunol. 2005, 174, 3798–3807. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Gonzalez, X.; Zanetti, M. Telomerase immunity from bench to bedside: Round one. J. Transl. Med. 2007. [Google Scholar] [CrossRef] [PubMed]
- Brunsvig, P.F.; Aamdal, S.; Gjertsen, M.K.; Kvalheim, G.; Markowski-Grimsrud, C.J.; Sve, I.; Dyrhaug, M.; Trachsel, S.; Møller, M.; Eriksen, J.A.; et al. Telomerase peptide vaccination: A phase I/II study in patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2006, 55, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, S.L.; Gjertsen, M.K.; Trachsel, S.; Møller, M.; Eriksen, J.A.; Meo, M.; Buanes, T.; Gaudernack, G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br. J. Cancer 2006, 95, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, D.; Bolonakis, I.; Cornet, S.; Myllaki, G.; Kanellou, P.; Kotsakis, A.; Galanis, A.; Nikoloudi, I.; Spyropoulou, M.; Menez, J.; et al. A phase I study of the optimized cryptic peptide TERT572y in patients with advanced malignancies. Oncology 2006, 70, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Bolonaki, I.; Kotsakis, A.; Papadimitraki, E.; Aggouraki, D.; Konsolakis, G.; Vagia, A.; Christophylakis, C.; Nikoloudi, I.; Magganas, E.; Galanis, A.; et al. Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J. Clin. Oncol. 2007, 25, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, A.; Trepiakas, R.; Wenandy, L.; Geertsen, P.F.; thor Straten, P.; Andersen, M.H.; Pedersen, A.E.; Claesson, M.H.; Lorentzen, T.; Johansen, J.S.; et al. Therapeutic dendritic cell vaccination of patients with metastatic renal cell carcinoma: A clinical phase 1/2 trial. J. Immunother. 2008, 31, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Hunger, R.E.; Kernland Lang, K.; Markowski, C.J.; Trachsel, S.; Møller, M.; Eriksen, J.A.; Rasmussen, A.M.; Braathen, L.R.; Gaudernack, G. Vaccination of patients with cutaneous melanoma with telomerase-specific peptides. Cancer Immunol. Immunother. 2011, 60, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.A.; Gaudernack, G.; Dueland, S.; Trachsel, S.; Julsrud, L.; Aamdal, S. Telomerase peptide vaccination combined with temozolomide: A clinical trial in stage IV melanoma patients. Clin. Cancer Res. 2011, 17, 4568–4580. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.P.; Aqui, N.A.; Stadtmauer, E.A.; Vogl, D.T.; Fang, H.B.; Cai, L.; Janofsky, S.; Chew, A.; Storek, J.; Akpek, G.; et al. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood 2011, 117, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Vik-Mo, E.O.; Nyakas, M.; Mikkelsen, B.V.; Moe, M.C.; Due-Tønnesen, P.; Suso, E.M.; Sæbøe-Larssen, S.; Sandberg, C.; Brinchmann, J.E.; Helseth, E.; et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol. Immunother. 2013, 62, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, D.; Traverso, P.; Parodi, A.; Tomasello, L.; Negrini, S.; Kalli, F.; Battaglia, F.; Ferrera, F.; Sciallero, S.; Murdaca, G.; et al. A multi-peptide, dual-adjuvant telomerase vaccine (GX301) is highly immunogenic in patients with prostate and renal cancer. Cancer Immunol. Immunother. 2013, 62, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Staff, C.; Mozaffari, F.; Frodin, J.E.; Mellstedt, H.; Liljefors, M. Telomerase (GV1001) vaccination together with gemcitabine in advanced pancreatic cancer patients. Int. J. Oncol. 2014, 45, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Davoli, T.; de Lange, T. Telomere-driven tetraploidization occurs in human cells undergoing crisis and promotes transformation of mouse cells. Cancer Cell 2012, 21, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Forner, A.; Korangy, F.; N'Kontchou, G.; Barget, N.; Ayuso, C.; Ormandy, L.A.; Manns, M.P.; Beaugrand, M.; Bruix, J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer 2010. [Google Scholar] [CrossRef] [PubMed]
- Brunsvig, P.F.; Kyte, J.A.; Kersten, C.; Sundstrøm, S.; Møller, M.; Nyakas, M.; Hansen, G.L.; Gaudernack, G.; Aamdal, S. Telomerase peptide vaccination in NSCLC: A phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin. Cancer Res. 2001, 17, 6847–6857. [Google Scholar] [CrossRef] [PubMed]
- Ellebaek, E.; Engell-Noerregaard, L.; Iversen, T.Z.; Froesig, T.M.; Munir, S.; Hadrup, S.R.; Andersen, M.H.; Svane, I.M. Metastatic melanoma patients treated with dendritic cell vaccination, interleukin-2 and metronomic cyclophosphamide: Results from a phase II trial. Cancer Immunol. Immunother. 2012, 61, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, A.; Vetsika, E.K.; Christou, S.; Hatzidaki, D.; Vardakis, N.; Aggouraki, D.; Konsolakis, G.; Georgoulias, V.; Christophyllakis, C.; Cordopatis, P.; et al. Clinical outcome of patients with various advanced cancer types vaccinated with an optimized cryptic human telomerase reverse transcriptase (TERT) peptide: Results of an expanded phase II study. Ann. Oncol. 2012, 23, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Middleton, G.; Silcocks, P.; Cox, T.; Valle, J.; Wadsley, J.; Propper, D.; Coxon, F.; Ross, P.; Madhusudan, S.; Roques, T.; et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): An open-label, randomised, phase 3 trial. Lancet Oncol. 2014, 15, 829–840. [Google Scholar] [CrossRef]
- Mizukoshi, E.; Nakagawa, H.; Kitahara, M.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Iida, N.; Fushimi, K.; Kaneko, S. Phase I trial of multidrug resistance-associated protein 3-derived peptide in patients with hepatocellular carcinoma. Cancer Lett. 2015, 369, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.; Tong, A.W.; Nemunaitis, M.; Senzer, N.; Phadke, A.P.; Bedell, C.; Adams, N.; Zhang, Y.A.; Maples, P.B.; Chen, S.; et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumours. Mol. Ther. 2010, 18, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Keith, W.N.; Bilsland, A.; Hardie, M.; Evans, T.R. Drug insight: Cancer cell immortality-telomerase as a target for novel cancer gene therapies. Nat. Clin. Pract. Oncol. 2004, 1, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y. Functional and mechanistic analysis of telomerase: An antitumor drug target. Pharmacol. Ther. 2016, 163, 24–47. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, X. Effects of allicin on both telomerase activity and apoptosis in gastric cancer SGC-7901 cells. World J. Gastroenterol. 2003, 9, 1930–1934. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ghosh, U.; Bhattacharyya, N.P.; Bhattacharya, R.K.; Roy, M. Inhibition of telomerase activity and induction of apoptosis by curcumin in K-562 cells. Mutat. Res. 2006, 596, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Noguchi, M.; Nakao, Y.; Ysunaga, M.; Yamasaki, F.; Iwasaka, T. Antiproliferative effects of the major tea polyphenol, (−)-epigallocatechin gallate and retinoic acid in cervical adenocarcinoma. Gynecol Oncol. 2008, 108, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Fonseca, H.B.; Jhabvala, P.; Escalon, E.A.; Melnick, S.J. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Lett. 2002, 184, 1–6. [Google Scholar] [CrossRef]
- Hsin, I.L.; Sheu, G.T.; Chen, H.H.; Chiu, L.Y.; Wang, H.D.; Chan, H.W.; Hsu, C.P.; Ko, J.L. N-acetyl cysteine mitigates curcumin-mediated telomerase inhibition through rescuing of Sp1 reduction in A549 cells. Mutat. Res. 2010, 688, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chung, I.K. Curcumin inhibits nuclear localization of telomerase by dissociating the Hsp90 co-chaperone p23 from hTERT. Cancer Lett. 2010, 290, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol. Cell Biochem. 2009, 325, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee Nee Chakraborty, S.; Ghosh, U.; Bhattacharyya, N.P.; Bhattacharya, R.K.; Dey, S.; Roy, M. Curcumin-induced apoptosis in human leukemia cell HL-60 is associated with inhibition of telomerase activity. Mol. Cell Biochem. 2007, 297, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Berletch, J.B.; Liu, C.; Love, W.K.; Andrews, L.G.; Katiyar, S.K.; Tollefsbol, T.O. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J. Cell Biochem. 2008, 103, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Andrews, L.G.; Tollefsbol, T.O. Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. Int. J. Cancer 2009, 125, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Patel, S.N.; Chan, T.H.; Tollefsbol, T.O. A novel prodrug of epigallocatechin-3-gallate: Differential epigenetic hTERT repression in human breast cancer cells. Cancer Prev. Res. (Phila) 2011, 4, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kang, S.H.; Kim, K.C.; Kim, M.O.; Choi, Y.H.; Kim, G.Y. Sulforaphane decreases viability and telomerase activity in hepatocellular carcinoma Hep3B cells through the reactive oxygen species-dependent pathway. Cancer Lett. 2010, 295, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Pate, M.S.; Wylie, R.C.; Tollefsbol, T.O.; Katiyar, S.K. EGCG down-regulates telomerase in human breast carcinoma MCF-7 cells, leading to suppression of cell viability and induction of apoptosis. Int. J. Oncol. 2004, 24, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.; Granot, G.; Mor-Tzuntz, R.; Raanani, P.; Uziel, O.; Lahav, M.; Shpilberg, O. Second-generation tyrosine kinase inhibitors reduce telomerase activity in K562 cells. Cancer Lett. 2012, 323, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Mor-Tzuntz, R.; Uziel, O.; Shpilberg, O.; Lahav, J.; Raanani, P.; Bakhanashvili, M.; Rabizadeh, E.; Zimra, Y.; Lahav, M.; Granot, G. Effect of imatinib on the signal transduction cascade regulating telomerase activity in K562 (BCR-ABL-positive) cells sensitive and resistant to imatinib. Exp. Hematol. 2010, 8, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, M.O.; Heo, M.S.; Lee, J.D.; Choi, Y.H.; Kim, G.Y. Gefitinib induces apoptosis and decreases telomerase activity in MDA-MB-231 human breast cancer cells. Arch. Pharm. Res. 2009, 32, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, M.; Shi, W.; Yang, Q.; Chen, C.; Wang, Z.; Zhou, X. Arsenic trioxide suppresses transcription of hTERT through down-regulation ofmultiple transcription factors in HL-60 leukemia cells. Toxicol. Lett. 2015, 232, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; de Jonge, N.; Bjorkholm, M.; Xu, D. The DNA methylation inhibitor induces telomere dysfunction and apoptosis of leukemia cells that is attenuated by telomerase over-expression. Oncotarget 2015, 6, 4888–4900. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa, T.; Germano, I.M.; Kondo, Y.; Ito, H.; Kyo, S.; Kondo, S. Inhibition of telomerase activity in malignant glioma cells correlates with their sensitivity to temozolomide. Br. J. Cancer 2003, 89, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Lu, J.; Yeung, B.Z.; Cottage, C.T.; Wientjes, M.G.; Au, J.L. Pharmacodynamics of telomerase inhibition and telomere shortening by noncytotoxic suramin. AAPS J. 2015, 17, 268–276. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xia, H.H.; Wang, J.D.; Gu, Q.; Lin, M.C.; Zou, B.; Lam, S.K.; Chan, A.O.; Yuen, M.F.; Kung, H.F.; et al. Inhibition of human telomerase reverse transcriptase by nonsteroidal antiinflammatory drugs in colon carcinoma. Cancer 2006, 106, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Q.; Feng, H.-W.; Jia, T.; Chen, X.-M.; Zhang, H.; Xu, A.-T.; Zhang, H.L.; Fan, X.-L. Antiproliferative effects of celecoxib in Hep-2 cells through telomerase inhibition and induction of apoptosis. Asian Pac. J. Cancer Prev. 2014, 15, 4919–4923. [Google Scholar] [CrossRef] [PubMed]
- Rashid-Kolvear, F.; Taboski, M.A.; Nguyen, J.; Wang, D.Y.; Harrington, L.A.; Done, S.J. Troglitazone suppresses telomerase activity independently of PPARgamma in estrogen-receptor negative breast cancer cells. BMC Cancer 2011. [Google Scholar] [CrossRef]
- Kiran, K.G.; Palaniswamy, M.; Angayarkanni, J. Human telomerase inhibitors from microbial source. World J. Microbiol. Biotechnol. 2015, 31, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Li, C.T.; Hsiao, Y.M.; Wu, T.C.; Lin, Y.W.; Yeh, K.T.; Ko, J.L. Vorinostat, SAHA, represses telomerase activity via epigenetic regulation of telomerase reverse transcriptase in non–small cell lung cancer cells. J. Cell Biochem. 2011, 112, 3044–3053. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Zhou, Q.; Xu, Y.; Lai, X.Y.; Huang, H. Antiproliferative effect of rapamycin on human T-cell leukemia cell line Jurkat by cell cycle arrest and telomerase inhibition. Acta Pharmacol. Sin. 2008, 29, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.J.; Choi, Y.H. Growth inhibition of A549 human lung carcinoma cells by beta-lapachone through induction of apoptosis and inhibition of telomerase activity. Int. J. Oncol. 2005, 26, 1017–1023. [Google Scholar] [PubMed]
- Burger, A.M.; Double, J.A.; Newell, D.R. Inhibition of telomerase activity by cisplatin in human testicular cancer cells. Eur. J. Cancer 1997, 33, 638–644. [Google Scholar] [CrossRef]
- Leon-Blanco, M.M.; Guerrero, J.M.; Reiter, R.J.; Calvo, J.R.; Pozo, D. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. J. Pineal. Res. 2003, 35, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Holohan, B.; Hagiopian, M.M.; Lai, T.P.; Huang, E.; Friedman, D.R.; Wright, W.E.; Shay, J.W. Perifosine as a potential novel anti-telomerase therapy. Oncotarget 2015, 6, 21816–21826. [Google Scholar] [CrossRef] [PubMed]
- Baoping, Y.; Guoyong, H.; Jieping, Y.; Zongxue, R.; Hesheng, L. Cyclooxygenase-2 inhibitor nimesulide suppresses telomerase activity by blocking Akt/PKB activation in gastric cancer cell line. Dig. Dis. Sci. 2004, 49, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-H.; Park, H.J.; Oh, M.-K.; Kim, I.-S. Antiproliferative effect of gold (I) compound auranofin through inhibition of STAT3 and telomerase activity in MDA-MB 231 human breast cancer cells. BMB Rep. 2013, 46, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Khorramizadeh, M.R.; Saadat, F.; Vaezzadeh, F.; Safavifar, F.; Bashiri, H.; Jahanshiri, Z. Suppression of telomerase activity by pyrimethamine: Implication to cancer. Iran Biomed. J. 2007, 11, 223–228. [Google Scholar] [PubMed]
- Brown, T.; Sigurdson, E.; Rogatko, A.; Broccoli, D. Telomerase inhibition using azidothymidine in the HT-29 colon cancer cell line. Ann. Surg. Oncol. 2003, 10, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yu, B.P.; Li, Y.; Dong, W.G.; Luo, H.S. Antiproliferative effect of octreotide on gastric cancer cells mediated by inhibition of Akt/PKB and telomerase. World J. Gastroenterol. 2003, 9, 2362–2365. [Google Scholar] [CrossRef] [PubMed]
- Yamakuchi, M.; Nakata, M.; Kawahara, K.; Kitajima, I.; Maruyama, I. New quinolones, ofloxacin and levofloxacin, inhibit telomerase activity in transitional cell carcinoma cell lines. Cancer Lett. 1997, 119, 213–219. [Google Scholar] [CrossRef]
- Sun, H.; Xiang, J.; Li, Q.; Liu, Y.; Li, L.; Shang, Q.; Xu, G.; Tang, Y. Recognize three different human telomeric G-quadruplex conformations by quinacrine. Analyst 2012, 137, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Ci, X.; Li, B.; Ma, X.; Kong, F.; Zheng, C.; Björkholm, M.; Jia, J.; Xu, D. Bortezomibmediated down-regulation of telomerase and disruption of telomere homeostasis contributes to apoptosis of malignant cells. Oncotarget 2015, 6, 38079–38092. [Google Scholar] [PubMed]
- Kato, M.; Nakayama, M.; Agata, M.; Yoshida, K. Gene expression levels of human shelterin complex and shelterin-associated factors regulated by the topoisomerase II inhibitors doxorubicin and etoposide in human cultured cells. Tumour Biol. 2013, 34, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qian, D.; Ma, H.H.; Jin, R.; Yang, P.X.; Cai, M.Y.; Liu, Y.H.; Liao, Y.J.; Deng, H.X.; Mai, S.J.; et al. Anthracyclines disrupt telomere maintenance by telomerase through inducing PinX1 ubiquitination and degradation. Oncogene 2012, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Hauke, R.; Mehta, P.P.; Batra, S.K. Recent advances on cancer stem/progenitor cell research: Therapeutic implications for overcoming resistance to the most aggressive cancers. J. Mol. Cell Med. 2007, 11, 981–1011. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Batra, S.K. Recent advances on the development of novel anti-cancer drugs targeting cancer stem/progenitor cells. Drug Dev. Res. 2008, 69, 415–430. [Google Scholar] [CrossRef]
- Tang, J.Y.; So, P.L.; Epstein, E.H., Jr. Novel Hedgehog pathway targets against basal cell carcinoma. Toxicol. Appl. Pharmacol. 2006, 224, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Schneider, M.; Knyazev, P.; Ullrich, A. UV-induced EGFR signal transactivation is dependent on proligand shedding by activated metalloproteases in skin cancer cell lines. Int. J. Cancer 2009, 124, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Stecca, B.; Mas, C.; Clement, V.; Zbinden, M.; Correa, R.; Piguet, V.; Beermann, F.; Ruiz, I.; Altaba, A. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc. Natl. Acad. Sci. USA 2007, 104, 5895–5900. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeyer, K.; Raggioli, A.; Rudloff, S.; Anton, R.; Hierholzer, A.; Del Valle, I.; Hein, K.; Vogt, R.; Kemler, R. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science 2012, 336, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Young, N.S. Telomere maintenance and human bone marrow failure. Blood 2008, 111, 4446–4455. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Nihrane, A.; Aglipay, J.; Sironi, J.; Arkin, S.; Lipton, J.M.; Ouchi, T.; Liu, J.M. Upregulated ATM gene expression and activated DNA crosslink-induced damage response checkpoint in Fanconi anemia: Implications for carcinogenesis. Mol. Med. 2008, 14, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Meshorer, E.; Gruenbaum, Y. Gone with the Wnt/Notch: Stem cells in laminopathies, progeria, and aging. J. Cell Biol. 2008, 181, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat. Cell Biol. 2008, 10, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Bergoglio, V.; Magnaldo, T. Nucleotide excision repair and related human diseases. Genome Dyn. 2006, 1, 35–52. [Google Scholar] [PubMed]
- Stout, G.J.; Blasco, M.A. Genetic dissection of the mechanisms underlying telomereassociated diseases: Impact of the TRF2 telomeric protein on mouse epidermal stem cells. Dis. Model Mech. 2009, 2, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Lansdorp, P.M. Telomeres and aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.J.; Wilson, D.B.; Bessler, M. Dyskeratosis congenital—A disease of dysfunctional telomere maintenance. Curr. Mol. Med. 2005, 5, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.; Gerson, S.L. The role of DNA damage repair in aging of adult stem cells. Nucleic Acids Res. 2007, 35, 7557–7565. [Google Scholar] [CrossRef] [PubMed]
- Nijnik, A.; Woodbine, L.; Marchetti, C.; Dawson, S.; Lambe, T.; Liu, C.; Rodrigues, N.P.; Crockford, T.L.; Cabuy, E.; Vindigni, A.; et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature 2007, 447, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Chigancas, V.; Lima-Bessa, K.M.; Stary, A.; Menck, C.F.; Sarasin, A. Defective transcription/repair factor IIH recruitment to specific UV lesions in trichothiodystrophy syndrome. Cancer Res. 2008, 68, 6074–6083. [Google Scholar] [CrossRef] [PubMed]
- Budiyanto, A.; Bito, T.; Kunisada, M.; Ashida, M.; Ichihashi, M.; Ueda, M. Inhibition of the epidermal growth factor receptor suppresses telomerase activity in HSC-1 human cutaneous squamous cell carcinoma cells. J. Invest. Dermatol. 2003, 121, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase in normal and cancer stem cells. FEBS Lett. 2010, 584, 3819–3825. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.C.; Counter, C.M.; Lundberg, A.S.; Beijersbergen, R.L.; Brooks, M.W.; Weinberg, R.A. Creation of human tumor cells with defined genetic elements. Nature 1999, 400, 464–468. [Google Scholar] [PubMed]
- Braig, M.; Lee, S.; Loddenkemper, C.; Rudolph, C.; Peters, A.H.; Schlegelberger, B.; Stein, H.; Dörken, B.; Jenuwein, T.; Schmitt, C.A. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 2005, 436, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Trotman, L.C.; Shaffer, D.; Lin, H.K.; Dotan, Z.A.; Niki, M.; Koutcher, J.A.; Scher, H.I.; Ludwig, T.; Gerald, W.; et al. Crucial role ofp53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005, 436, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Serrano, M. The power and the promise of oncogene-induced senescence markers. Nat. Rev. Cancer 2006, 6, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Zender, L.; Miething, C.; Dickins, R.A.; Hernando, E.; Krizhanovsky, V.; Cordon-Cardo, C.; Lowe, S.W. Senescence and tumour clearance is triggered by p53 restoration in murineliver carcinomas. Nature 2007, 445, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Kirsch, D.G.; McLaughlin, M.E.; Tuveson, D.A.; Grimm, J.; Lintault, L.; Newman, J.; Reczek, E.E.; Weissleder, R.; Jacks, T. Restoration of p53 function leads to tumour regression in vivo. Nature 2007, 445, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Ewald, J.A.; Desotelle, J.A.; Wilding, G.; Jarrard, D.F. Therapy-induced senescence in cancer. J. Natl. Cancer Inst. 2010, 102, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Te Poele, R.H.; Okorokov, A.L.; Jardine, L.; Cummings, J.; Joel, S.P. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002, 62, 1876–1883. [Google Scholar] [PubMed]
- Rudolph, K.L.; Millard, M.; Bosenberg, M.W.; DePinho, R.A. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat. Genet. 2001, 28, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.; Artandi, S.E.; Shen, Q.; Tam, A.; Lee, S.-L.; Gottlieb, G.J.; Greider, C.W.; DePinho, R.A. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 1999, 97, 527–538. [Google Scholar] [CrossRef]
- Artandi, S.E.; Chang, S.; Lee, S.L.; Alson, S.; Gottlieb, G.J.; Chin, L.; DePinho, R.A. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 2000, 406, 641–645. [Google Scholar] [PubMed]
- O’Sullivan, J.N.; Bronner, M.P.; Brentnall, T.A.; Finley, J.C.; Shen, W.T.; Emerson, S.; Emond, M.J.; Gollahon, K.A.; Moskovitz, A.H.; Crispin, D.A.; et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat. Genet. 2002, 32, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Romanov, S.R.; Kozakiewicz, B.K.; Holst, C.R.; Stampfer, M.R.; Haupt, L.M.; Tlsty, T.D. Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature 2001, 409, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Seger, Y.R.; García-Cao, M.; Piccinin, S.; Cunsolo, C.L.; Doglioni, C.; Blasco, M.A.; Hannon, G.J.; Maestro, R. Transformation of normal human cells in the absence of telomerase activation. Cancer Cell 2002, 2, 401–413. [Google Scholar] [CrossRef]
- Tomas-Loba, A.; Flores, I.; Fernandez-Marcos, P.J.; Cayuela, M.L.; Maraver, A.; Tejera, A.; Borras, C.; Matheu, A.; Klatt, P.; Flores, J.M.; et al. Telomerase reverse transcriptase delays aging in cancer resistant mice. Cell 2008, 135, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, E.; Geserick, C.; Flores, J.M.; Blasco, M.A. Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene 2005, 24, 2256–2270. [Google Scholar] [CrossRef] [PubMed]
- Bernardes de Jesus, B.; Vera, E.; Schneeberger, K.; Tejera, A.M.; Ayuso, E.; Bosch, F.; Blasco, M.A. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 2012, 4, 691–704. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jäger, K.; Walter, M. Therapeutic Targeting of Telomerase. Genes 2016, 7, 39. https://doi.org/10.3390/genes7070039
Jäger K, Walter M. Therapeutic Targeting of Telomerase. Genes. 2016; 7(7):39. https://doi.org/10.3390/genes7070039
Chicago/Turabian StyleJäger, Kathrin, and Michael Walter. 2016. "Therapeutic Targeting of Telomerase" Genes 7, no. 7: 39. https://doi.org/10.3390/genes7070039
APA StyleJäger, K., & Walter, M. (2016). Therapeutic Targeting of Telomerase. Genes, 7(7), 39. https://doi.org/10.3390/genes7070039





