Compound Heterozygous Complete Loss-of-Function SPINK1 Variants as a Novel Cause of Severe Infantile Isolated Exocrine Pancreatic Insufficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Reference Sequences, Variant Nomenclature, and Novel Variant Deposition
2.3. Variant Detection
2.4. Public Databases and Online Tools
3. Results
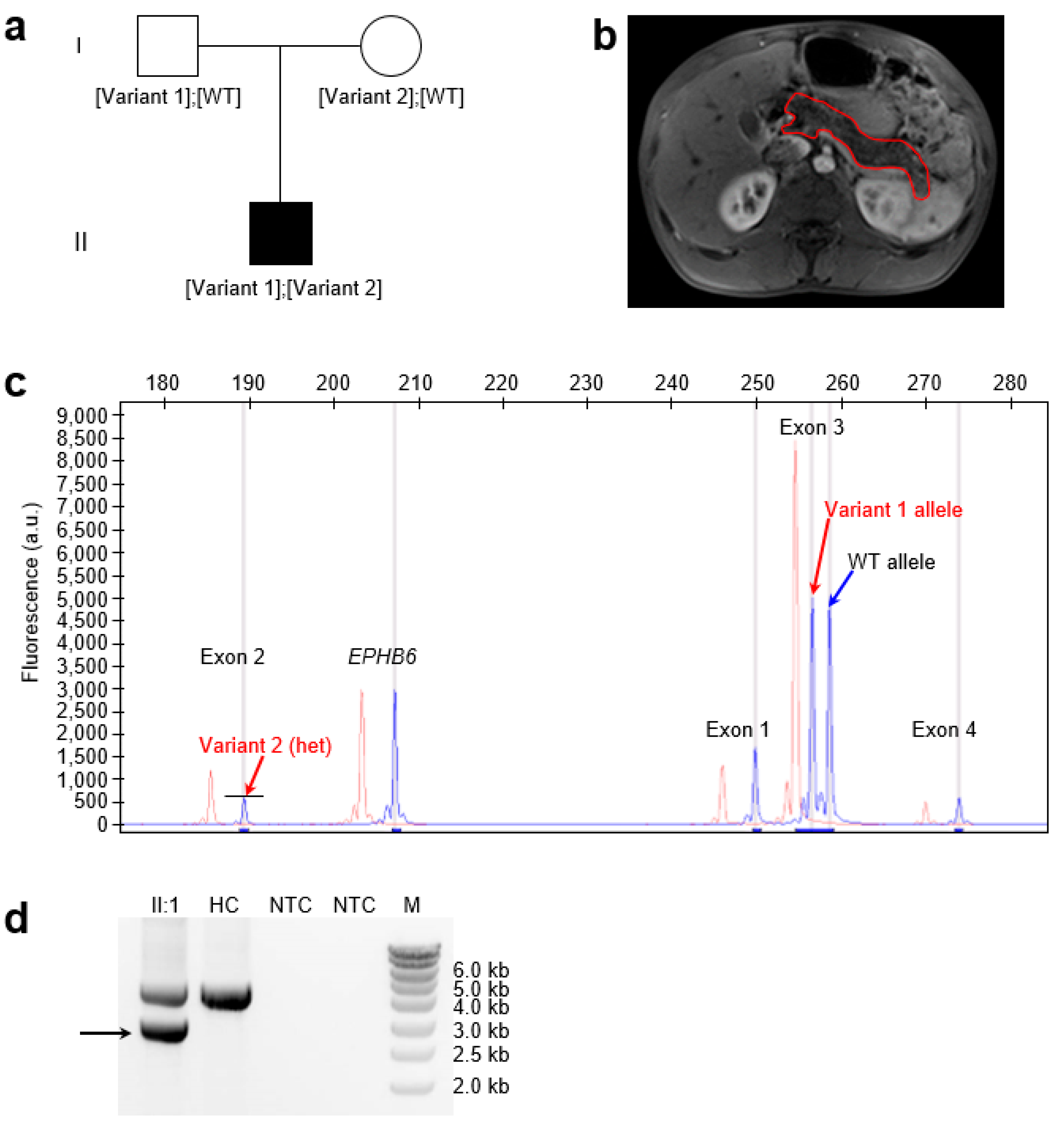
3.1. The Proband Exhibiting a Phenotype Consistent with SIIEPI
3.2. Identification of Compound Heterozygous Complete LoF SPINK1 Variants in the Proband
3.3. Generative Mechanisms and Nomenclature of the Novel Complex Rearrangement Variant
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LoF | Loss-of-function |
| SIIEPI | Severe infantile isolated exocrine pancreatic insufficiency |
| NGS | Next-generation sequencing |
| QFM-PCR | Quantitative fluorescent multiplex PCR |
| CP | Chronic pancreatitis |
| CNV | Copy number variant |
| WT | Wild-type |
| HGVS | The Human Genome Variation Society |
| gnomAD | The Genome Aggregation Database |
| nBMST | The non-B DNA Motif Search Tool |
| SRS | Serial replication slippage |
| TLS | Translesion synthesis |
References
- Yamamoto, T.; Nakamura, Y.; Nishide, J.; Emi, M.; Ogawa, M.; Mori, T.; Matsubara, K. Molecular cloning and nucleotide sequence of human pancreatic secretory trypsin inhibitor (PSTI) cDNA. Biochem. Biophys. Res. Commun. 1985, 132, 605–612. [Google Scholar] [CrossRef]
- Horii, A.; Kobayashi, T.; Tomita, N.; Yamamoto, T.; Fukushige, S.; Murotsu, T.; Ogawa, M.; Mori, T.; Matsubara, K. Primary structure of human pancreatic secretory trypsin inhibitor (PSTI) gene. Biochem. Biophys. Res. Commun. 1987, 149, 635–641. [Google Scholar] [CrossRef]
- Rinderknecht, H.; Stace, N.H.; Renner, I.G. Effects of chronic alcohol abuse on exocrine pancreatic secretion in man. Dig. Dis. Sci. 1985, 30, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Rinderknecht, H. Go, V.L.W., Dimagno, E.P., Gardner, J.D., Lebenthal, E., Scheele, G.A., Eds.; Pancreatic secretory enzymes. In The Pancreas: Biology, Pathobiology, and Disease; Raven Press: New York, NY, USA, 1993; pp. 219–251. [Google Scholar]
- Witt, H.; Luck, W.; Hennies, H.C.; Classen, M.; Kage, A.; Lass, U.; Landt, O.; Becker, M. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat. Genet. 2000, 25, 213–216. [Google Scholar] [CrossRef]
- Romac, J.M.; Ohmuraya, M.; Bittner, C.; Majeed, M.F.; Vigna, S.R.; Que, J.; Fee, B.E.; Wartmann, T.; Yamamura, K.; Liddle, R.A. Transgenic expression of pancreatic secretory trypsin inhibitor-1 rescues SPINK3-deficient mice and restores a normal pancreatic phenotype. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G518–G524. [Google Scholar] [CrossRef]
- Nathan, J.D.; Romac, J.; Peng, R.Y.; Peyton, M.; Macdonald, R.J.; Liddle, R.A. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology 2005, 128, 717–727. [Google Scholar] [CrossRef]
- Sakata, K.; Araki, K.; Nakano, H.; Nishina, T.; Komazawa-Sakon, S.; Murai, S.; Lee, G.E.; Hashimoto, D.; Suzuki, C.; Uchiyama, Y.; et al. Novel method to rescue a lethal phenotype through integration of target gene onto the X-chromosome. Sci. Rep. 2016, 6, 37200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Mao, X.T.; Sun, C.; Wang, Y.H.; Zheng, Y.Z.; Xiong, S.H.; Liu, M.Y.; Mao, S.H.; Wang, Q.W.; Ma, G.X.; et al. Pancreas-directed AAV8-hSPINK1 gene therapy safely and effectively protects against pancreatitis in mice. Gut 2024, 73, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; Gorry, M.C.; Preston, R.A.; Furey, W.; Sossenheimer, M.J.; Ulrich, C.D.; Martin, S.P.; Gates, L.K., Jr.; Amann, S.T.; Toskes, P.P.; et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat. Genet. 1996, 14, 141–145. [Google Scholar] [CrossRef]
- Le Maréchal, C.; Masson, E.; Chen, J.M.; Morel, F.; Ruszniewski, P.; Levy, P.; Férec, C. Hereditary pancreatitis caused by triplication of the trypsinogen locus. Nat. Genet. 2006, 38, 1372–1374. [Google Scholar] [CrossRef]
- Szmola, R.; Sahin-Tóth, M. Chymotrypsin C (caldecrin) promotes degradation of human cationic trypsin: Identity with Rinderknecht’s enzyme Y. Proc. Natl. Acad. Sci. USA 2007, 104, 11227–11232. [Google Scholar] [CrossRef]
- Rosendahl, J.; Witt, H.; Szmola, R.; Bhatia, E.; Ozsvari, B.; Landt, O.; Schulz, H.U.; Gress, T.M.; Pfutzer, R.; Lohr, M.; et al. Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat. Genet. 2008, 40, 78–82. [Google Scholar] [CrossRef]
- Masson, E.; Chen, J.M.; Scotet, V.; Le Maréchal, C.; Férec, C. Association of rare chymotrypsinogen C (CTRC) gene variations in patients with idiopathic chronic pancreatitis. Hum. Genet. 2008, 123, 83–91. [Google Scholar] [CrossRef]
- Hegyi, E.; Sahin-Tóth, M. Genetic risk in chronic pancreatitis: The trypsin-dependent pathway. Dig. Dis. Sci. 2017, 62, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Masson, E.; Zou, W.B.; Génin, E.; Cooper, D.N.; Le Gac, G.; Fichou, Y.; Pu, N.; Rebours, V.; Férec, C.; Liao, Z.; et al. Expanding ACMG variant classification guidelines into a general framework. Hum. Genomics 2022, 16, 31. [Google Scholar] [CrossRef]
- Wang, Q.W.; Zou, W.B.; Masson, E.; Férec, C.; Liao, Z.; Chen, J.M. Genetics and clinical implications of SPINK1 in the pancreatitis continuum and pancreatic cancer. Hum. Genomics 2025, 19, 32. [Google Scholar] [CrossRef]
- Wang, Y.C.; Masson, E.; Wang, Q.W.; Génin, E.; Le Gac, G.; Fichou, Y.; Cooper, D.N.; Liao, Z.; Férec, C.; Zou, W.B.; et al. SPINK1-related chronic pancreatitis: A model that encapsulates the spectrum of variant effects, genetic complexity and classificatory challenges. Am. J. Hum. Genet. 2025. [Google Scholar] [CrossRef]
- Venet, T.; Masson, E.; Talbotec, C.; Billiemaz, K.; Touraine, R.; Gay, C.; Destombe, S.; Cooper, D.N.; Patural, H.; Chen, J.M.; et al. Severe infantile isolated exocrine pancreatic insufficiency caused by the complete functional loss of the SPINK1 gene. Hum. Mutat. 2017, 38, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Masson, E.; Maestri, S.; Bordeau, V.; Cooper, D.N.; Férec, C.; Chen, J.M. Alu insertion-mediated dsRNA structure formation with pre-existing Alu elements as a disease-causing mechanism. Am. J. Hum. Genet. 2024, 111, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Ohmuraya, M.; Hirota, M.; Araki, M.; Mizushima, N.; Matsui, M.; Mizumoto, T.; Haruna, K.; Kume, S.; Takeya, M.; Ogawa, M.; et al. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology 2005, 129, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Demcsák, A.; Sahin-Tóth, M. Heterozygous Spink1 deficiency promotes trypsin-dependent chronic pancreatitis in mice. Cell Mol. Gastroenterol. Hepatol. 2024, 18, 101361. [Google Scholar] [CrossRef]
- Muller, N.; Sarantitis, I.; Rouanet, M.; de Mestier, L.; Halloran, C.; Greenhalf, W.; Férec, C.; Masson, E.; Ruszniewski, P.; Levy, P.; et al. Natural history of SPINK1 germline mutation related-pancreatitis. EBioMedicine 2019, 48, 581–591. [Google Scholar] [CrossRef]
- Wu, H.; Lin, J.H.; Tang, X.Y.; Marenne, G.; Zou, W.B.; Schutz, S.; Masson, E.; Génin, E.; Fichou, Y.; Le Gac, G.; et al. Combining full-length gene assay and SpliceAI to interpret the splicing impact of all possible SPINK1 coding variants. Hum. Genomics 2024, 18, 21. [Google Scholar] [CrossRef]
- Hart, R.K.; Fokkema, I.; DiStefano, M.; Hastings, R.; Laros, J.F.J.; Taylor, R.; Wagner, A.H.; den Dunnen, J.T. HGVS Nomenclature 2024: Improvements to community engagement, usability, and computability. Genome Med. 2024, 16, 149. [Google Scholar] [CrossRef]
- Masson, E.; Le Marechal, C.; Levy, P.; Chuzhanova, N.; Ruszniewski, P.; Cooper, D.N.; Chen, J.M.; Férec, C. Co-inheritance of a novel deletion of the entire SPINK1 gene with a CFTR missense mutation (L997F) in a family with chronic pancreatitis. Mol. Genet. Metab. 2007, 92, 168–175. [Google Scholar] [CrossRef]
- Chen, S.; Francioli, L.C.; Goodrich, J.K.; Collins, R.L.; Kanai, M.; Wang, Q.; Alfoldi, J.; Watts, N.A.; Vittal, C.; Gauthier, L.D.; et al. A genomic mutational constraint map using variation in 76,156 human genomes. Nature 2024, 625, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Chitipiralla, S.; Kaur, K.; Brown, G.; Chen, C.; Hart, J.; Hoffman, D.; Jang, W.; Liu, C.; Maddipatla, Z.; et al. ClinVar: Updates to support classifications of both germline and somatic variants. Nucleic Acids Res. 2025, 53, D1313–D1321. [Google Scholar] [CrossRef] [PubMed]
- Cer, R.Z.; Bruce, K.H.; Mudunuri, U.S.; Yi, M.; Volfovsky, N.; Luke, B.T.; Bacolla, A.; Collins, J.R.; Stephens, R.M. Non-B DB: A database of predicted non-B DNA-forming motifs in mammalian genomes. Nucleic Acids Res. 2011, 39, D383–D391. [Google Scholar] [CrossRef]
- Chen, J.M.; Cooper, D.N.; Férec, C.; Kehrer-Sawatzki, H.; Patrinos, G.P. Genomic rearrangements in inherited disease and cancer. Semin. Cancer Biol. 2010, 20, 222–233. [Google Scholar] [CrossRef]
- Chen, J.M.; Chuzhanova, N.; Stenson, P.D.; Férec, C.; Cooper, D.N. Complex gene rearrangements caused by serial replication slippage. Hum. Mutat. 2005, 26, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Carvalho, C.M.; Lupski, J.R. A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell 2007, 131, 1235–1247. [Google Scholar] [CrossRef]
- Chen, J.M.; Férec, C.; Cooper, D.N. Complex multiple-nucleotide substitution mutations causing human inherited disease reveal novel insights into the action of translesion synthesis DNA polymerases. Hum. Mutat. 2015, 36, 1034–1038. [Google Scholar] [CrossRef]
- Duardo, R.C.; Guerra, F.; Pepe, S.; Capranico, G. Non-B DNA structures as a booster of genome instability. Biochimie 2023, 214, 176–192. [Google Scholar] [CrossRef]
- Masson, E.; Le Maréchal, C.; Chen, J.M.; Frebourg, T.; Lerebours, E.; Férec, C. Detection of a large genomic deletion in the pancreatic secretory trypsin inhibitor (SPINK1) gene. Eur. J. Hum. Genet. 2006, 14, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.A.; Burgel, P.R.; Castellani, C.; Davies, J.C.; Salathe, M.; Taylor-Cousar, J.L. Cystic fibrosis. Nat. Rev. Dis. Primers 2024, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gong, S.; Li, M.; Wang, X.; Wang, F.; Cai, X.; Liu, W.; Luo, Y.; Zhang, S.; Zhang, R.; et al. Clinical and genetic characteristics of CEL-MODY (MODY8): A literature review and screening in Chinese individuals diagnosed with early-onset type 2 diabetes. Endocrine 2024, 83, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Scheers, I.; Berardis, S. Congenital etiologies of exocrine pancreatic insufficiency. Front. Pediatr. 2022, 10, 909925. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, F.; Lupski, J.R. Mechanisms for human genomic rearrangements. PathoGenetics 2008, 1, 4. [Google Scholar] [CrossRef]
- Lange, S.S.; Takata, K.; Wood, R.D. DNA polymerases and cancer. Nat. Rev. Cancer 2011, 11, 96–110. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masson, E.; Wangermez, M.; Tougeron, D.; Rebours, V.; Férec, C.; Chen, J.-M. Compound Heterozygous Complete Loss-of-Function SPINK1 Variants as a Novel Cause of Severe Infantile Isolated Exocrine Pancreatic Insufficiency. Genes 2025, 16, 998. https://doi.org/10.3390/genes16090998
Masson E, Wangermez M, Tougeron D, Rebours V, Férec C, Chen J-M. Compound Heterozygous Complete Loss-of-Function SPINK1 Variants as a Novel Cause of Severe Infantile Isolated Exocrine Pancreatic Insufficiency. Genes. 2025; 16(9):998. https://doi.org/10.3390/genes16090998
Chicago/Turabian StyleMasson, Emmanuelle, Marc Wangermez, David Tougeron, Vinciane Rebours, Claude Férec, and Jian-Min Chen. 2025. "Compound Heterozygous Complete Loss-of-Function SPINK1 Variants as a Novel Cause of Severe Infantile Isolated Exocrine Pancreatic Insufficiency" Genes 16, no. 9: 998. https://doi.org/10.3390/genes16090998
APA StyleMasson, E., Wangermez, M., Tougeron, D., Rebours, V., Férec, C., & Chen, J.-M. (2025). Compound Heterozygous Complete Loss-of-Function SPINK1 Variants as a Novel Cause of Severe Infantile Isolated Exocrine Pancreatic Insufficiency. Genes, 16(9), 998. https://doi.org/10.3390/genes16090998






