Cystic Fibrosis and Male Infertility: From Genetics to Future Perspectives in Assisted Reproductive Technologies
Abstract
1. Introduction
2. Genetic Basis of Cystic Fibrosis and Its Inheritance
2.1. CFTR Gene and Mutation Classes
2.2. Autosomal Recessive Inheritance and Carrier Frequency
2.3. Genotype-Phenotype Correlation
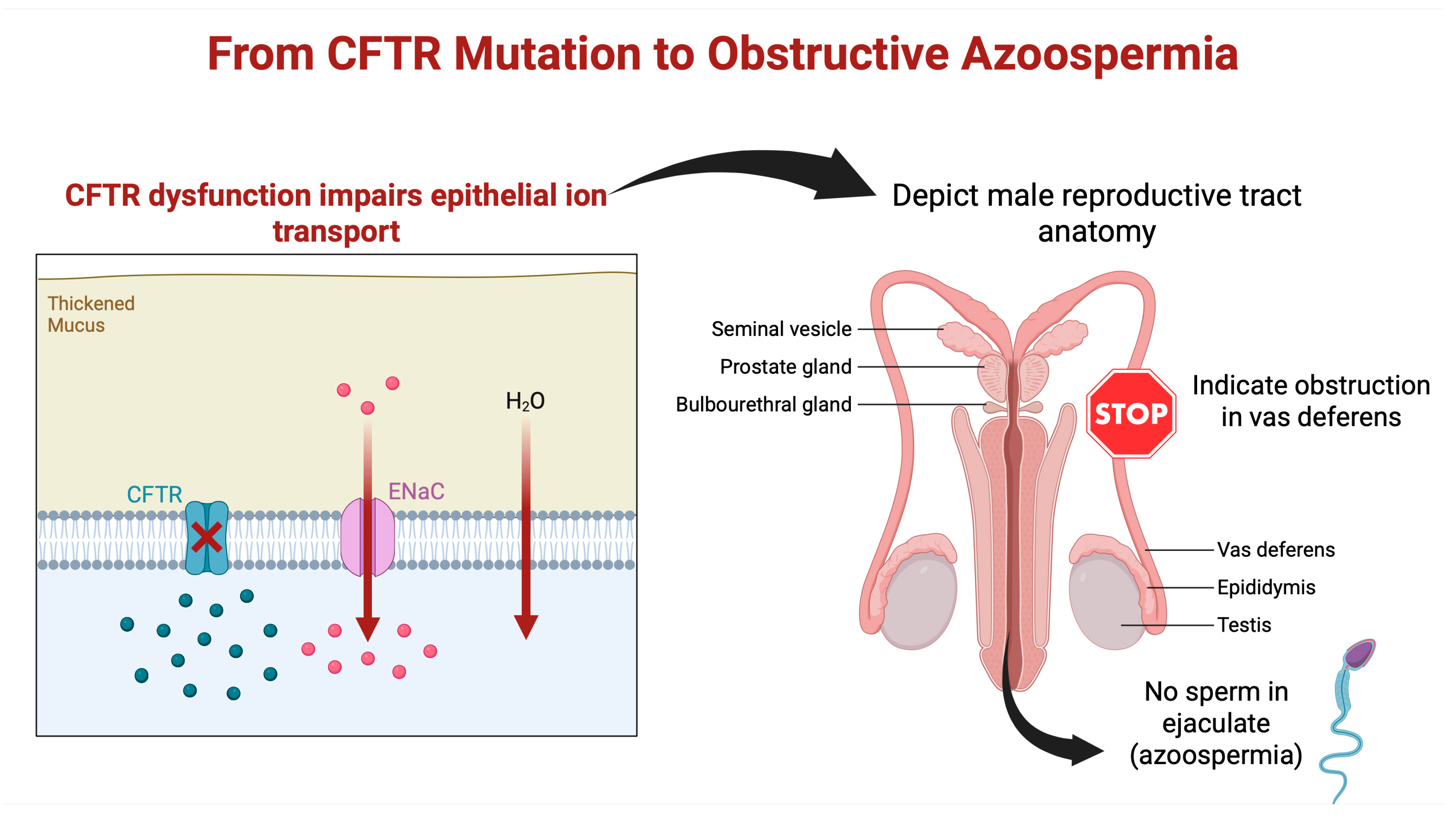
3. Role of CFTR Mutations in Male Reproductive Tract Anomalies
3.1. Congenital Bilateral Absence of the Vas Deferens
3.2. Other CFTR-Related Anomalies
3.3. Developmental Pathogenesis
4. Pathophysiology of Male Infertility in CF Patients
4.1. Obstructive Azoospermia
4.2. Spermatogenesis and Endocrine Function
5. Diagnostic Evaluation for CF-Related Infertility
5.1. History and Physical Examination
5.2. Semen Analysis
5.3. Genetic Testing for CFTR Mutations
5.4. CFTR Functional Tests
5.5. Imaging and Other Evaluations
6. Reproductive Options and Assisted Reproductive Technologies (ART)
6.1. Sperm Retrieval Techniques
6.2. IVF with ICSI
6.3. Female Partner’s Health
6.4. Sperm Cryopreservation and Timing
7. Clinical Management and Counseling for CF Patients with Infertility
7.1. Genetic Counseling
7.2. CF Care Team Involvement
7.3. Addressing Health Risks and Lifestyle
7.4. Psychosocial Support
8. Emerging Research and Future Directions
8.1. CFTR Modulator Therapies and Early Intervention
8.2. Gene Therapy and Gene Editing
8.3. Microfluidics and Sperm Selection
8.4. Molecular and Personalized Medicine
8.5. Gene Modifiers and Male Fertility
9. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef]
- Welsh, M.J.; Smith, A.E. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 1993, 73, 1251–1254. [Google Scholar] [CrossRef]
- Pilewski, J.M.; Frizzell, R.A. Role of CFTR in airway disease. Physiol. Rev. 1999, 79, S215–S255. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K.; Weren, M.; Proesmans, M.; Kerem, E. Pancreatitis among patients with cystic fibrosis: Correlation with pancreatic status and genotype. Pediatrics 2005, 115, e463–e469. [Google Scholar] [CrossRef] [PubMed]
- Ticona, J.H.; Lapinel, N.; Wang, J. Future Comorbidities in an Aging Cystic Fibrosis Population. Life 2023, 13, 1305. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, J.; DeCelie-Germana, J.K.; Kier, C.; Langfelder-Schwind, E. Reproductive Counseling and Care in Cystic Fibrosis: A Multidisciplinary Approach for a New Therapeutic Era. Life 2023, 13, 1545. [Google Scholar] [CrossRef] [PubMed]
- Jarzabek, K.; Zbucka, M.; Pepinski, W.; Szamatowicz, J.; Domitrz, J.; Janica, J.; Wolczynski, S.; Szamatowicz, M. Cystic fibrosis as a cause of infertility. Reprod. Biol. 2004, 4, 119–129. [Google Scholar]
- Jain, R.; Taylor-Cousar, J.L. Fertility, Pregnancy and Lactation Considerations for Women with CF in the CFTR Modulator Era. J. Pers. Med. 2021, 11, 418. [Google Scholar] [CrossRef]
- Diab Caceres, L.; Zamarron de Lucas, E. Cystic fibrosis: Epidemiology, clinical manifestations, diagnosis and treatment. Med. Clin. 2023, 161, 389–396. [Google Scholar] [CrossRef]
- Rommens, J.M.; Iannuzzi, M.C.; Kerem, B.; Drumm, M.L.; Melmer, G.; Dean, M.; Rozmahel, R.; Cole, J.L.; Kennedy, D.; Hidaka, N.; et al. Identification of the cystic fibrosis gene: Chromosome walking and jumping. Science 1989, 245, 1059–1065. [Google Scholar] [CrossRef]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.L.; et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef]
- Welsh, M.J. The arc of discovery, from the description of cystic fibrosis to effective treatments. J. Clin. Investig. 2024, 134, e186231. [Google Scholar] [CrossRef]
- Rogan, M.P.; Stoltz, D.A.; Hornick, D.B. Cystic fibrosis transmembrane conductance regulator intracellular processing, trafficking, and opportunities for mutation-specific treatment. Chest 2011, 139, 1480–1490. [Google Scholar] [CrossRef]
- Veit, G.; Avramescu, R.G.; Chiang, A.N.; Houck, S.A.; Cai, Z.; Peters, K.W.; Hong, J.S.; Pollard, H.B.; Guggino, W.B.; Balch, W.E.; et al. From CFTR biology toward combinatorial pharmacotherapy: Expanded classification of cystic fibrosis mutations. Mol. Biol. Cell 2016, 27, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castanos, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef]
- Moskowitz, S.M.; Chmiel, J.F.; Sternen, D.L.; Cheng, E.; Gibson, R.L.; Marshall, S.G.; Cutting, G.R. Clinical practice and genetic counseling for cystic fibrosis and CFTR-related disorders. Genet. Med. 2008, 10, 851–868. [Google Scholar] [CrossRef]
- Hamosh, A.; FitzSimmons, S.C.; Macek, M., Jr.; Knowles, M.R.; Rosenstein, B.J.; Cutting, G.R. Comparison of the clinical manifestations of cystic fibrosis in black and white patients. J. Pediatr. 1998, 132, 255–259. [Google Scholar] [CrossRef]
- Rosenstein, B.J.; Cutting, G.R. The diagnosis of cystic fibrosis: A consensus statement. Cystic Fibrosis Foundation Consensus Panel. J. Pediatr. 1998, 132, 589–595. [Google Scholar] [CrossRef]
- Bieniek, J.M.; Lapin, C.D.; Jarvi, K.A. Genetics of CFTR and male infertility. Transl. Androl. Urol. 2021, 10, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Sugarman, E.A.; Rohlfs, E.M.; Silverman, L.M.; Allitto, B.A. CFTR mutation distribution among U.S. Hispanic and African American individuals: Evaluation in cystic fibrosis patient and carrier screening populations. Genet. Med. 2004, 6, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Claustres, M. Molecular pathology of the CFTR locus in male infertility. Reprod. Biomed. Online 2005, 10, 14–41. [Google Scholar] [CrossRef]
- Grangeia, A.; Sa, R.; Carvalho, F.; Martin, J.; Girodon, E.; Silva, J.; Ferraz, L.; Barros, A.; Sousa, M. Molecular characterization of the cystic fibrosis transmembrane conductance regulator gene in congenital absence of the vas deferens. Genet. Med. 2007, 9, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Anjankar, N.; More, A.; Anjankar, A.P.; Mahajan, S.S.; Nawale, N. CFTR Gene Mutations and their Role in Male Infertility: A Case Study. J. Pharm. Bioallied Sci. 2025, 17, S1008–S1010. [Google Scholar] [CrossRef] [PubMed]
- Dumur, V.; Gervais, R.; Rigot, J.M.; Lafitte, J.J.; Manouvrier, S.; Biserte, J.; Mazeman, E.; Roussel, P. Abnormal distribution of CF delta F508 allele in azoospermic men with congenital aplasia of epididymis and vas deferens. Lancet 1990, 336, 512. [Google Scholar] [CrossRef]
- Rigot, J.M.; Lafitte, J.J.; Dumur, V.; Gervais, R.; Manouvrier, S.; Biserte, J.; Mazeman, E.; Roussel, P. Cystic fibrosis and congenital absence of the vas deferens. N. Engl. J. Med. 1991, 325, 64–65. [Google Scholar] [CrossRef]
- Anguiano, A.; Oates, R.D.; Amos, J.A.; Dean, M.; Gerrard, B.; Stewart, C.; Maher, T.A.; White, M.B.; Milunsky, A. Congenital bilateral absence of the vas deferens. A primarily genital form of cystic fibrosis. JAMA 1992, 267, 1794–1797. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, Z.; Ni, Y.; Li, Z. CFTR mutations in men with congenital bilateral absence of the vas deferens (CBAVD): A systemic review and meta-analysis. Hum. Reprod. 2012, 27, 25–35. [Google Scholar] [CrossRef]
- Friedman, K.J.; Heim, R.A.; Knowles, M.R.; Silverman, L.M. Rapid characterization of the variable length polythymidine tract in the cystic fibrosis (CFTR) gene: Association of the 5T allele with selected CFTR mutations and its incidence in atypical sinopulmonary disease. Hum. Mutat. 1997, 10, 108–115. [Google Scholar] [CrossRef]
- Morea, A.; Cameran, M.; Rebuffi, A.G.; Marzenta, D.; Marangon, O.; Picci, L.; Zacchello, F.; Scarpa, M. Gender-sensitive association of CFTR gene mutations and 5T allele emerging from a large survey on infertility. Mol. Hum. Reprod. 2005, 11, 607–614. [Google Scholar] [CrossRef]
- Liou, T.G. The Clinical Biology of Cystic Fibrosis Transmembrane Regulator Protein: Its Role and Function in Extrapulmonary Disease. Chest 2019, 155, 605–616. [Google Scholar] [CrossRef]
- de Souza, D.A.S.; Faucz, F.R.; Pereira-Ferrari, L.; Sotomaior, V.S.; Raskin, S. Congenital bilateral absence of the vas deferens as an atypical form of cystic fibrosis: Reproductive implications and genetic counseling. Andrology 2018, 6, 127–135. [Google Scholar] [CrossRef]
- Bieth, E.; Hamdi, S.M.; Mieusset, R. Genetics of the congenital absence of the vas deferens. Hum. Genet. 2021, 140, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Qing, X.; Niringiyumukiza, J.D.; Zhan, X.; Mo, D.; Zhou, Y.; Shang, X. CFTR variants and renal abnormalities in males with congenital unilateral absence of the vas deferens (CUAVD): A systematic review and meta-analysis of observational studies. Genet. Med. 2019, 21, 826–836. [Google Scholar] [CrossRef]
- Stuhrmann, M.; Dork, T. CFTR gene mutations and male infertility. Andrologia 2000, 32, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Meschede, D.; Dworniczak, B.; Behre, H.M.; Kliesch, S.; Claustres, M.; Nieschlag, E.; Horst, J. CFTR gene mutations in men with bilateral ejaculatory-duct obstruction and anomalies of the seminal vesicles. Am. J. Hum. Genet. 1997, 61, 1200–1202. [Google Scholar] [CrossRef]
- Mak, V.; Zielenski, J.; Tsui, L.C.; Durie, P.; Zini, A.; Martin, S.; Longley, T.B.; Jarvi, K.A. Cystic fibrosis gene mutations and infertile men with primary testicular failure. Hum. Reprod. 2000, 15, 436–439. [Google Scholar] [CrossRef]
- van der Ven, K.; Messer, L.; van der Ven, H.; Jeyendran, R.S.; Ober, C. Cystic fibrosis mutation screening in healthy men with reduced sperm quality. Hum. Reprod. 1996, 11, 513–517. [Google Scholar] [CrossRef]
- McCallum, T.; Milunsky, J.; Munarriz, R.; Carson, R.; Sadeghi-Nejad, H.; Oates, R. Unilateral renal agenesis associated with congenital bilateral absence of the vas deferens: Phenotypic findings and genetic considerations. Hum. Reprod. 2001, 16, 282–288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlegel, P.N.; Shin, D.; Goldstein, M. Urogenital anomalies in men with congenital absence of the vas deferens. J. Urol. 1996, 155, 1644–1648. [Google Scholar] [CrossRef]
- Gaillard, D.A.; Carre-Pigeon, F.; Lallemand, A. Normal vas deferens in fetuses with cystic fibrosis. J. Urol. 1997, 158, 1549–1552. [Google Scholar] [CrossRef]
- Shamohammadi, I.; Haghpanah, A.; Eslahi, A.; Sadighi Gilani, M.A.; Adib, A.; Kashfi, S.S. Seminal vesicle status and its association with semen parameters in congenital bilateral absence of the vas deferens (CBAVD). Basic Clin. Androl. 2025, 35, 24. [Google Scholar] [CrossRef]
- Daudin, M.; Bieth, E.; Bujan, L.; Massat, G.; Pontonnier, F.; Mieusset, R. Congenital bilateral absence of the vas deferens: Clinical characteristics, biological parameters, cystic fibrosis transmembrane conductance regulator gene mutations, and implications for genetic counseling. Fertil. Steril. 2000, 74, 1164–1174. [Google Scholar] [CrossRef]
- Yoon, J.C.; Casella, J.L.; Litvin, M.; Dobs, A.S. Male reproductive health in cystic fibrosis. J. Cyst. Fibros. 2019, 18 (Suppl. S2), S105–S110. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, H. Congenital Bilateral Absence of the Vas Deferens. Front. Genet. 2022, 13, 775123. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Zarli, M.; Schuppe, K.; Wong, R.; Rahman, F.; Ramasamy, R. Sexual and Reproductive Health Among Men With Cystic Fibrosis. Urology 2023, 179, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cocuzza, M.; Alvarenga, C.; Pagani, R. The epidemiology and etiology of azoospermia. Clinics 2013, 68 (Suppl. S1), 15–26. [Google Scholar] [CrossRef]
- Attardo, T.; Vicari, E.; Mollica, F.; Grazioso, C.; Burrello, N.; Garofalo, M.R.; Lizzio, M.N.; Garigali, G.; Cannizzaro, M.; Ruvolo, G.; et al. Genetic, andrological and clinical characteristics of patients with congenital bilateral absence of the vas deferens. Int. J. Androl. 2001, 24, 73–79. [Google Scholar] [CrossRef]
- Borowitz, D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr. Pulmonol. 2015, 50 (Suppl. S40), S24–S30. [Google Scholar] [CrossRef]
- Jarvi, K.; Zielenski, J.; Wilschanski, M.; Durie, P.; Buckspan, M.; Tullis, E.; Markiewicz, D.; Tsui, L.C. Cystic fibrosis transmembrane conductance regulator and obstructive azoospermia. Lancet 1995, 345, 1578. [Google Scholar] [CrossRef] [PubMed]
- Fedder, J.; Jorgensen, M.W.; Engvad, B. Prevalence of CBAVD in azoospermic men carrying pathogenic CFTR mutations—Evaluated in a cohort of 639 non-vasectomized azoospermic men. Andrology 2021, 9, 588–598. [Google Scholar] [CrossRef]
- Artusi, I.; Rubin, M.; Cozza, G. Redox Imbalance in Cystic Fibrosis: The Multifaceted Role of Oxidative Stress. Pharmaceuticals 2025, 18, 784. [Google Scholar] [CrossRef]
- Sudhakaran, G.; Kesavan, D.; Kandaswamy, K.; Guru, A.; Arockiaraj, J. Unravelling the epigenetic impact: Oxidative stress and its role in male infertility-associated sperm dysfunction. Reprod. Toxicol. 2024, 124, 108531. [Google Scholar] [CrossRef]
- Naz Khan, F.; Mason, K.; Roe, A.H.; Tangpricha, V. CF and male health: Sexual and reproductive health, hypogonadism, and fertility. J. Clin. Transl. Endocrinol. 2022, 27, 100288. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zikopoulos, A.; Dimitriadis, F.; Sheshi, D.; Politis, M.; Moustakli, E.; Symeonidis, E.N.; Chrisofos, M.; Sofikitis, N.; Zachariou, A. Oxidative Stress and Erectile Dysfunction: Pathophysiology, Impacts, and Potential Treatments. Curr. Issues Mol. Biol. 2024, 46, 8807–8834. [Google Scholar] [CrossRef] [PubMed]
- Sofikitis, N.; Kaltsas, A.; Dimitriadis, F.; Rassweiler, J.; Grivas, N.; Zachariou, A.; Kaponis, A.; Tsounapi, P.; Paterakis, N.; Karagiannis, A.; et al. The Effect of PDE5 Inhibitors on the Male Reproductive Tract. Curr. Pharm. Des. 2021, 27, 2697–2713. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Zachariou, A.; Dimitriadis, F.; Chrisofos, M.; Sofikitis, N. Empirical Treatments for Male Infertility: A Focus on Lifestyle Modifications and Medicines. Diseases 2024, 12, 209. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Liao, H.; Leung, X.; He, J.; Wang, X.; Li, F.; Yue, H.; Xu, W. CFTR mutation compromises spermatogenesis by enhancing miR-15b maturation and suppressing its regulatory target CDC25Adagger. Biol. Reprod. 2019, 101, 50–62. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Bernardino, R.L.; Carrageta, D.F.; Soveral, G.; Calamita, G.; Alves, M.G.; Oliveira, P.F. CFTR modulates aquaporin-mediated glycerol permeability in mouse Sertoli cells. Cell Mol. Life Sci. 2022, 79, 592. [Google Scholar] [CrossRef]
- Kaltsas, A.; Markou, E.; Kyrgiafini, M.A.; Zikopoulos, A.; Symeonidis, E.N.; Dimitriadis, F.; Zachariou, A.; Sofikitis, N.; Chrisofos, M. Oxidative-Stress-Mediated Epigenetic Dysregulation in Spermatogenesis: Implications for Male Infertility and Offspring Health. Genes 2025, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiafini, M.A.; Kaltsas, A.; Chatziparasidou, A.; Mamuris, Z. The Small RNA Landscape in Azoospermia: Implications for Male Infertility and Sperm Retrieval-A Preliminary Study. Int. J. Mol. Sci. 2025, 26, 3537. [Google Scholar] [CrossRef] [PubMed]
- Anton-Paduraru, D.T.; Azoicai, A.N.; Trofin, F.; Mindru, D.E.; Murgu, A.M.; Bocec, A.S.; Iliescu Halitchi, C.O.; Ciongradi, C.I.; Sarbu, I.; Iliescu, M.L. Diagnosing Cystic Fibrosis in the 21st Century-A Complex and Challenging Task. Diagnostics 2024, 14, 763. [Google Scholar] [CrossRef]
- Andrade, D.L.; Viana, M.C.; Esteves, S.C. Differential Diagnosis of Azoospermia in Men with Infertility. J. Clin. Med. 2021, 10, 3144. [Google Scholar] [CrossRef]
- Lissens, W.; Mercier, B.; Tournaye, H.; Bonduelle, M.; Ferec, C.; Seneca, S.; Devroey, P.; Silber, S.; Van Steirteghem, A.; Liebaers, I. Cystic fibrosis and infertility caused by congenital bilateral absence of the vas deferens and related clinical entities. Hum. Reprod. 1996, 11 (Suppl. S4), 55–78, discussion 79–80. [Google Scholar] [CrossRef]
- Barratt, C.L.R.; Bjorndahl, L.; De Jonge, C.J.; Lamb, D.J.; Osorio Martini, F.; McLachlan, R.; Oates, R.D.; van der Poel, S.; St John, B.; Sigman, M.; et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Male Reproduction and Urology. Evaluation of the azoospermic male: A committee opinion. Fertil. Steril. 2018, 109, 777–782. [Google Scholar] [CrossRef]
- Minhas, S.; Boeri, L.; Capogrosso, P.; Cocci, A.; Corona, G.; Dinkelman-Smit, M.; Falcone, M.; Jensen, C.F.; Gul, M.; Kalkanli, A.; et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2025 Update on Male Infertility. Eur. Urol. 2025, 87, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Mak, V.; Zielenski, J.; Tsui, L.C.; Durie, P.; Zini, A.; Martin, S.; Longley, T.B.; Jarvi, K.A. Proportion of cystic fibrosis gene mutations not detected by routine testing in men with obstructive azoospermia. JAMA 1999, 281, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Goeminne, P.C.; Dupont, L.J. The sinusitis-infertility syndrome: Young’s saint, old devil. Eur. Respir. J. 2010, 35, 698. [Google Scholar] [CrossRef]
- Friedman, K.J.; Teichtahl, H.; De Kretser, D.M.; Temple-Smith, P.; Southwick, G.J.; Silverman, L.M.; Highsmith, W.E., Jr.; Boucher, R.C.; Knowles, M.R. Screening Young syndrome patients for CFTR mutations. Am. J. Respir. Crit. Care Med. 1995, 152, 1353–1357. [Google Scholar] [CrossRef]
- Mohammed, S.K.; Jan, A. Young Syndrome; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bombieri, C.; Claustres, M.; De Boeck, K.; Derichs, N.; Dodge, J.; Girodon, E.; Sermet, I.; Schwarz, M.; Tzetis, M.; Wilschanski, M.; et al. Recommendations for the classification of diseases as CFTR-related disorders. J. Cyst. Fibros. 2011, 10 (Suppl. S2), S86–S102. [Google Scholar] [CrossRef]
- Donkol, R.H. Imaging in male-factor obstructive infertility. World J. Radiol. 2010, 2, 172–179. [Google Scholar] [CrossRef]
- Kaltsas, A.; Dimitriadis, F.; Zachariou, D.; Zikopoulos, A.; Symeonidis, E.N.; Markou, E.; Tien, D.M.B.; Takenaka, A.; Sofikitis, N.; Zachariou, A. From Diagnosis to Treatment: Comprehensive Care by Reproductive Urologists in Assisted Reproductive Technology. Medicina 2023, 59, 1835. [Google Scholar] [CrossRef]
- Esteves, S.C.; Miyaoka, R.; Orosz, J.E.; Agarwal, A. An update on sperm retrieval techniques for azoospermic males. Clinics 2013, 68 (Suppl. S1), 99–110. [Google Scholar] [CrossRef]
- Hibi, H.; Sumitomo, M.; Fukunaga, N.; Sonohara, M.; Asada, Y. Superior clinical pregnancy rates after microsurgical epididymal sperm aspiration. Reprod. Med. Biol. 2018, 17, 59–63. [Google Scholar] [CrossRef]
- Anger, J.T.; Wang, G.J.; Boorjian, S.A.; Goldstein, M. Sperm cryopreservation and in vitro fertilization/intracytoplasmic sperm injection in men with congenital bilateral absence of the vas deferens: A success story. Fertil. Steril. 2004, 82, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Markou, E.; Zachariou, A.; Dimitriadis, F.; Symeonidis, E.N.; Zikopoulos, A.; Mamoulakis, C.; Tien, D.M.B.; Takenaka, A.; Sofikitis, N. Evaluating the Predictive Value of Diagnostic Testicular Biopsy for Sperm Retrieval Outcomes in Men with Non-Obstructive Azoospermia. J. Pers. Med. 2023, 13, 1362. [Google Scholar] [CrossRef]
- Esteves, S.C. Percutaneous epididymal sperm aspiration as a method for sperm retrieval in men with obstructive azoospermia seeking fertility: Operative and laboratory aspects. Int. Braz. J. Urol. 2015, 41, 817, discussion 818. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sun, Z.; Wang, J.; Wang, B.; Hongjun, L. Fertility outcome of patients with CBAVD: A single-institution experience. J. Men’s Health 2022, 18, 80. [Google Scholar] [CrossRef]
- Kaltsas, A.; Stavros, S.; Kratiras, Z.; Zikopoulos, A.; Machairiotis, N.; Potiris, A.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M.; Zachariou, A. Predictors of Successful Testicular Sperm Extraction: A New Era for Men with Non-Obstructive Azoospermia. Biomedicines 2024, 12, 2679. [Google Scholar] [CrossRef] [PubMed]
- Gangrade, B.K. Cryopreservation of testicular and epididymal sperm: Techniques and clinical outcomes of assisted conception. Clinics 2013, 68 (Suppl. S1), 131–140. [Google Scholar] [CrossRef]
- Palermo, G.D.; O’Neill, C.L.; Chow, S.; Cheung, S.; Parrella, A.; Pereira, N.; Rosenwaks, Z. Intracytoplasmic sperm injection: State of the art in humans. Reproduction 2017, 154, F93–F110. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.N.; Cohen, J.; Goldstein, M.; Alikani, M.; Adler, A.; Gilbert, B.R.; Palermo, G.D.; Rosenwaks, Z. Cystic fibrosis gene mutations do not affect sperm function during in vitro fertilization with micromanipulation for men with bilateral congenital absence of vas deferens. Fertil. Steril. 1995, 64, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Hughan, K.S.; Daley, T.; Rayas, M.S.; Kelly, A.; Roe, A. Female reproductive health in cystic fibrosis. J. Cyst. Fibros. 2019, 18 (Suppl. S2), S95–S104. [Google Scholar] [CrossRef]
- Kushary, S.; Ali, N.; Spencer, J.B.; Dokson, J.; Hunt, W.R. Assessment of a novel genetic counselling intervention to inform assisted reproductive technology treatments and other family-building options in adults with cystic fibrosis. Reprod. Biomed. Soc. Online 2021, 13, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Stransky, O.M.; Bernard, M.; Hughan, K.S.; Ladores, S.; Sawicki, G.S.; Stalvey, M.S.; Kazmerski, T.M. Men’s sexual and reproductive health in cystic fibrosis in the era of highly effective modulator therapies-A qualitative study. J. Cyst. Fibros. 2022, 21, 657–661. [Google Scholar] [CrossRef]
- Lotti, F.; Studniarek, M.; Balasa, C.; Belfield, J.; De Visschere, P.; Freeman, S.; Kozak, O.; Markiet, K.; Ramanathan, S.; Richenberg, J.; et al. The role of the radiologist in the evaluation of male infertility: Recommendations of the European Society of Urogenital Radiology-Scrotal and Penile Imaging Working Group (ESUR-SPIWG) for scrotal imaging. Eur. Radiol. 2025, 35, 752–766. [Google Scholar] [CrossRef]
- Li, L.; Lin, W.; Wang, Z.; Huang, R.; Xia, H.; Li, Z.; Deng, J.; Ye, T.; Huang, Y.; Yang, Y. Hormone Regulation in Testicular Development and Function. Int. J. Mol. Sci. 2024, 25, 5805. [Google Scholar] [CrossRef]
- Hussein, A.A.; Tran, N.D.; Smith, J.F. Fertility preservation for boys and adolescents facing sterilizing medical therapy. Transl. Androl. Urol. 2014, 3, 382–390. [Google Scholar] [CrossRef]
- Goossens, E.; Jahnukainen, K.; Mitchell, R.T.; van Pelt, A.; Pennings, G.; Rives, N.; Poels, J.; Wyns, C.; Lane, S.; Rodriguez-Wallberg, K.A.; et al. Fertility preservation in boys: Recent developments and new insights (dagger). Hum. Reprod. Open 2020, 2020, hoaa016. [Google Scholar] [CrossRef]
- Wyns, C.; Kanbar, M.; Giudice, M.G.; Poels, J. Fertility preservation for prepubertal boys: Lessons learned from the past and update on remaining challenges towards clinical translation. Hum. Reprod. Update 2021, 27, 433–459. [Google Scholar] [CrossRef]
- Hardy, T. The role of prenatal diagnosis following preimplantation genetic testing for single-gene conditions: A historical overview of evolving technologies and clinical practice. Prenat. Diagn. 2020, 40, 647–651. [Google Scholar] [CrossRef]
- Handyside, A.H.; Lesko, J.G.; Tarin, J.J.; Winston, R.M.; Hughes, M.R. Birth of a normal girl after in vitro fertilization and preimplantation diagnostic testing for cystic fibrosis. N. Engl. J. Med. 1992, 327, 905–909. [Google Scholar] [CrossRef]
- Girardet, A.; Viart, V.; Plaza, S.; Daina, G.; De Rycke, M.; Des Georges, M.; Fiorentino, F.; Harton, G.; Ishmukhametova, A.; Navarro, J.; et al. The improvement of the best practice guidelines for preimplantation genetic diagnosis of cystic fibrosis: Toward an international consensus. Eur. J. Hum. Genet. 2016, 24, 469–478. [Google Scholar] [CrossRef]
- Sawyer, S.M.; Tully, M.A.; Dovey, M.E.; Colin, A.A. Reproductive health in males with cystic fibrosis: Knowledge, attitudes, and experiences of patients and parents. Pediatr. Pulmonol. 1998, 25, 226–230. [Google Scholar] [CrossRef]
- Bourke, S.J.; Anderson, A.; Briggs, J.; Doe, S.; Echevarria, C.; Choudhary, M.; McEleny, K.; Stewart, J. Current status of fertility and family formation in men with cystic fibrosis. Hum. Fertil. 2021, 24, 298–303. [Google Scholar] [CrossRef]
- Taussig, L.M.; Lobeck, C.C.; di Sant’Agnese, P.A.; Ackerman, D.R.; Kattwinkel, J. Fertility in males with cystic fibrosis. N. Engl. J. Med. 1972, 287, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, J.; Campbell, C.; Ladores, S. Provider Perspectives on Fertility and Fertility Preservation Discussions Among Women With Cystic Fibrosis. Inquiry 2023, 60, 469580231159488. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Campbell, K.; Deebel, N.; Ghomeshi, A.; Zarli, M.; Domaradzki, L.; Tupayachi Ortiz, M.G.; Ramasamy, R. Assessing Infertility Literacy and Knowledge Gaps Among Patients with Cystic Fibrosis. Urol. Res. Pract. 2023, 49, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Kazmerski, T.M.; West, N.E.; Jain, R.; Uluer, A.; Georgiopoulos, A.M.; Aitken, M.L.; Taylor-Cousar, J.L. Family-building and parenting considerations for people with cystic fibrosis. Pediatr. Pulmonol. 2022, 57 (Suppl. S1), S75–S88. [Google Scholar] [CrossRef]
- Jacob, A.; Journiac, J.; Fischer, L.; Astrologo, L.; Flahault, C. How do cystic fibrosis patients experience parenthood? A systematic review. J. Health Psychol. 2021, 26, 60–81. [Google Scholar] [CrossRef]
- Frost, F.; Dyce, P.; Ochota, A.; Pandya, S.; Clarke, T.; Walshaw, M.J.; Nazareth, D.S. Cystic fibrosis-related diabetes: Optimizing care with a multidisciplinary approach. Diabetes Metab. Syndr. Obes. 2019, 12, 545–552. [Google Scholar] [CrossRef]
- Huang, R.; Chen, J.; Guo, B.; Jiang, C.; Sun, W. Diabetes-induced male infertility: Potential mechanisms and treatment options. Mol. Med. 2024, 30, 11. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Miller, S.; Sorscher, E.J. Cystic fibrosis. N. Engl. J. Med. 2005, 352, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Koumenis, A.; Stavropoulos, M.; Kratiras, Z.; Deligiannis, D.; Adamos, K.; Chrisofos, M. Male Infertility and Reduced Life Expectancy: Epidemiology, Mechanisms, and Clinical Implications. J. Clin. Med. 2025, 14, 3930. [Google Scholar] [CrossRef]
- Sharma, A.; Shrivastava, D. Psychological Problems Related to Infertility. Cureus 2022, 14, e30320. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Neto, B.; Barreiro, M.; Tome, A.; Vale-Fernandes, E. Psychosocial aspects of infertility and the impact of assisted reproductive techniques—A comprehensive review. JBRA Assist. Reprod. 2025, 29, 378–393. [Google Scholar] [CrossRef]
- Kaltsas, A.; Dimitriadis, F.; Zachariou, A.; Sofikitis, N.; Chrisofos, M. Phosphodiesterase Type 5 Inhibitors in Male Reproduction: Molecular Mechanisms and Clinical Implications for Fertility Management. Cells 2025, 14, 120. [Google Scholar] [CrossRef]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Shteinberg, M.; Cohen-Cymberknoh, M.; Jain, R. The Impact of Highly Effective Cystic Fibrosis Transmembrane Conductance Regulator Modulators on the Health of Female Subjects With Cystic Fibrosis. Clin. Ther. 2023, 45, 278–289. [Google Scholar] [CrossRef]
- Fortner, C.N.; Seguin, J.M.; Kay, D.M. Normal pancreatic function and false-negative CF newborn screen in a child born to a mother taking CFTR modulator therapy during pregnancy. J. Cyst. Fibros. 2021, 20, 835–836. [Google Scholar] [CrossRef]
- Trimble, A.; McKinzie, C.; Terrell, M.; Stringer, E.; Esther, C.R., Jr. Measured fetal and neonatal exposure to Lumacaftor and Ivacaftor during pregnancy and while breastfeeding. J. Cyst. Fibros. 2018, 17, 779–782. [Google Scholar] [CrossRef]
- Jones, G.H.; Walshaw, M.J. Potential impact on fertility of new systemic therapies for cystic fibrosis. Paediatr. Respir. Rev. 2015, 16 (Suppl. S1), 25–27. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.; Nazareth, D. A successful uncomplicated CF pregnancy while remaining on Ivacaftor. J. Cyst. Fibros. 2016, 15, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Ladores, S.; Kazmerski, T.M.; Rowe, S.M. A Case Report of Pregnancy During Use of Targeted Therapeutics for Cystic Fibrosis. J. Obstet. Gynecol. Neonatal Nurs. 2017, 46, 72–77. [Google Scholar] [CrossRef]
- Heltshe, S.L.; Godfrey, E.M.; Josephy, T.; Aitken, M.L.; Taylor-Cousar, J.L. Pregnancy among cystic fibrosis women in the era of CFTR modulators. J. Cyst. Fibros. 2017, 16, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Scialli, A.R.; Bailey, G.; Beyer, B.K.; Bogh, I.B.; Breslin, W.J.; Chen, C.L.; DeLise, A.M.; Hui, J.Y.; Moffat, G.J.; Stewart, J.; et al. Potential seminal transport of pharmaceuticals to the conceptus. Reprod. Toxicol. 2015, 58, 213–221. [Google Scholar] [CrossRef]
- Nash, E.F.; Middleton, P.G.; Taylor-Cousar, J.L. Outcomes of pregnancy in women with cystic fibrosis (CF) taking CFTR modulators—An international survey. J. Cyst. Fibros. 2020, 19, 521–526. [Google Scholar] [CrossRef]
- Davies, J.C.; Polineni, D.; Boyd, A.C.; Donaldson, S.; Gill, D.R.; Griesenbach, U.; Hyde, S.C.; Jain, R.; McLachlan, G.; Mall, M.A.; et al. Lentiviral Gene Therapy for Cystic Fibrosis: A Promising Approach and First-in-Human Trial. Am. J. Respir. Crit. Care Med. 2024, 210, 1398–1408. [Google Scholar] [CrossRef]
- Rowe, S.M.; Zuckerman, J.B.; Dorgan, D.; Lascano, J.; McCoy, K.; Jain, M.; Schechter, M.S.; Lommatzsch, S.; Indihar, V.; Lechtzin, N.; et al. Inhaled mRNA therapy for treatment of cystic fibrosis: Interim results of a randomized, double-blind, placebo-controlled phase 1/2 clinical study. J. Cyst. Fibros. 2023, 22, 656–664. [Google Scholar] [CrossRef]
- Rubeis, G.; Steger, F. Risks and benefits of human germline genome editing: An ethical analysis. Asian Bioeth. Rev. 2018, 10, 133–141. [Google Scholar] [CrossRef]
- Wei, J.; Baptista-Hon, D.T.; Wang, Z.; Li, G.; Herrler, T.; Dai, C.; Liu, K.; Yu, B.; Chen, X.; Yang, M.; et al. Bioengineered human tissue regeneration and repair using endogenous stem cells. Cell Rep. Med. 2023, 4, 101156. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.R.; Ziarati, N.; Dadkhah, E.; Bucak, M.N.; Rahimizadeh, P.; Shahverdi, A.; Sadighi Gilani, M.A.; Topraggaleh, T.R. Microfluidics: The future of sperm selection in assisted reproduction. Andrology 2024, 12, 1236–1252. [Google Scholar] [CrossRef]
- De Geyter, J.; Gallati-Kraemer, S.; Zhang, H.; De Geyter, C. Identification and selection of healthy spermatozoa in heterozygous carriers of the Phe508del-variant of the CFTR-gene in assisted reproduction. Sci. Rep. 2022, 12, 1866. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Guan, S.; Yan, Z.; Zhu, X.; Kuo, Y.; Wang, N.; Zhi, X.; Lian, Y.; Huang, J.; et al. Application of the PGT-M strategy using single sperm and/or affected embryos as probands for linkage analysis in males with hereditary tumor syndromes without family history. J. Hum. Genet. 2023, 68, 813–821. [Google Scholar] [CrossRef]
- Kaltsas, A.; Giannakodimos, I.; Markou, E.; Stavropoulos, M.; Deligiannis, D.; Kratiras, Z.; Chrisofos, M. The Androbactome and the Gut Microbiota-Testis Axis: A Narrative Review of Emerging Insights into Male Fertility. Int. J. Mol. Sci. 2025, 26, 6211. [Google Scholar] [CrossRef]
- Kaltsas, A. Multi-Omics Perspectives on Testicular Aging: Unraveling Germline Dysregulation, Niche Dysfunction, and Epigenetic Remodeling. Cells 2025, 14, 899. [Google Scholar] [CrossRef]
- Cui, X.; Wu, X.; Li, Q.; Jing, X. Mutations of the cystic fibrosis transmembrane conductance regulator gene in males with congenital bilateral absence of the vas deferens: Reproductive implications and genetic counseling (Review). Mol. Med. Rep. 2020, 22, 3587–3596. [Google Scholar] [CrossRef]
- Desai, M.; Hine, C.; Whitehouse, J.L.; Brownlee, K.; Charman, S.C.; Nagakumar, P. Who are the 10%?—Non eligibility of cystic fibrosis (CF) patients for highly effective modulator therapies. Respir. Med. 2022, 199, 106878. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Schimenti, J.C. Strategies to Identify Genetic Variants Causing Infertility. Trends Mol. Med. 2021, 27, 792–806. [Google Scholar] [CrossRef]

| CFTR Variant (Class) | Functional Effect | Clinical Severity | Male Fertility Impact |
|---|---|---|---|
| F508del (Class II) | Misfolding → little/no protein at surface | Classic severe CF (pancreatic insufficiency, lung disease) | ~98% of male CF patients with F508del have CBAVD (infertility). |
| G542X (Class I) | Nonsense mutation → no functional protein | Classic severe CF (common in some populations) | Causes azoospermia via CBAVD in virtually all male patients (infertile). |
| W1282X (Class I) | Nonsense mutation → truncated protein | Severe CF (prevalent in Ashkenazi Jewish CF patients) | Associated with CBAVD in male CF (infertility expected). |
| N1303K (Class II) | Misprocessing of protein (trafficking defect) | Severe CF (pancreatic insufficiency) | Associated with obstructive azoospermia (CBAVD) when biallelic. |
| G551D (Class III) | Gating defect (channel does not open properly) | Severe CF, but responsive to ivacaftor therapy | Male patients have CBAVD and require ART; CFTR modulator therapy improves health but does not restore ducts |
| R117H (Class IV) | Reduced channel conductance | Variable: Often mild or atypical CF; phenotype depends on poly-T tract (see 5T) | Common in CBAVD especially when in cis with 5T variant. With 5T, can cause azoospermia even if lung/pancreas are minimally affected. |
| Poly-T 5T variant (Class V) | Intron 8 polymorphism reducing CFTR mRNA splicing (exon 9 skipping) | Not CF by itself; a polymorphic variant with variable penetrance | Key modifier: When one allele is 5T and the other a mild/severe mutation, commonly causes CBAVD (particularly when 5T is in cis with a TG12 or TG13 repeat). 5T alone usually does not cause infertility (seen in fertile men). |
| 3849+10kb C>T (Class V) | Aberrant splicing with residual functional transcript | Mild CF phenotype (pancreatic sufficient; late diagnosis) | Often preserves some vas deferens function. Men with this mild mutation have a higher chance of natural fertility (reported in 2–3% of CF males), though many still have infertility. |
| CFTRdele2,3(21kb del) (Class I) | Deletion of exons 2–3 → no functional protein | Severe CF (second most common mutation in some regions) | Causes CBAVD in essentially all affected males (complete vas agenesis). |
| L138ins (p.Leu138dup, Class V) | Small in-frame insertion → partially functional protein | Mild CFTR-related disorder (common Slavic variant; often CFTR-RD) | Can contribute to CBAVD when combined with another mutation. Some men with L138ins (especially heterozygous) may be fertile or have only unilateral vas absence. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, A. Cystic Fibrosis and Male Infertility: From Genetics to Future Perspectives in Assisted Reproductive Technologies. Genes 2025, 16, 994. https://doi.org/10.3390/genes16090994
Kaltsas A. Cystic Fibrosis and Male Infertility: From Genetics to Future Perspectives in Assisted Reproductive Technologies. Genes. 2025; 16(9):994. https://doi.org/10.3390/genes16090994
Chicago/Turabian StyleKaltsas, Aris. 2025. "Cystic Fibrosis and Male Infertility: From Genetics to Future Perspectives in Assisted Reproductive Technologies" Genes 16, no. 9: 994. https://doi.org/10.3390/genes16090994
APA StyleKaltsas, A. (2025). Cystic Fibrosis and Male Infertility: From Genetics to Future Perspectives in Assisted Reproductive Technologies. Genes, 16(9), 994. https://doi.org/10.3390/genes16090994






