Precision Medicine for Diabetic Retinopathy: Integrating Genetics, Biomarkers, Lifestyle, and AI
Abstract
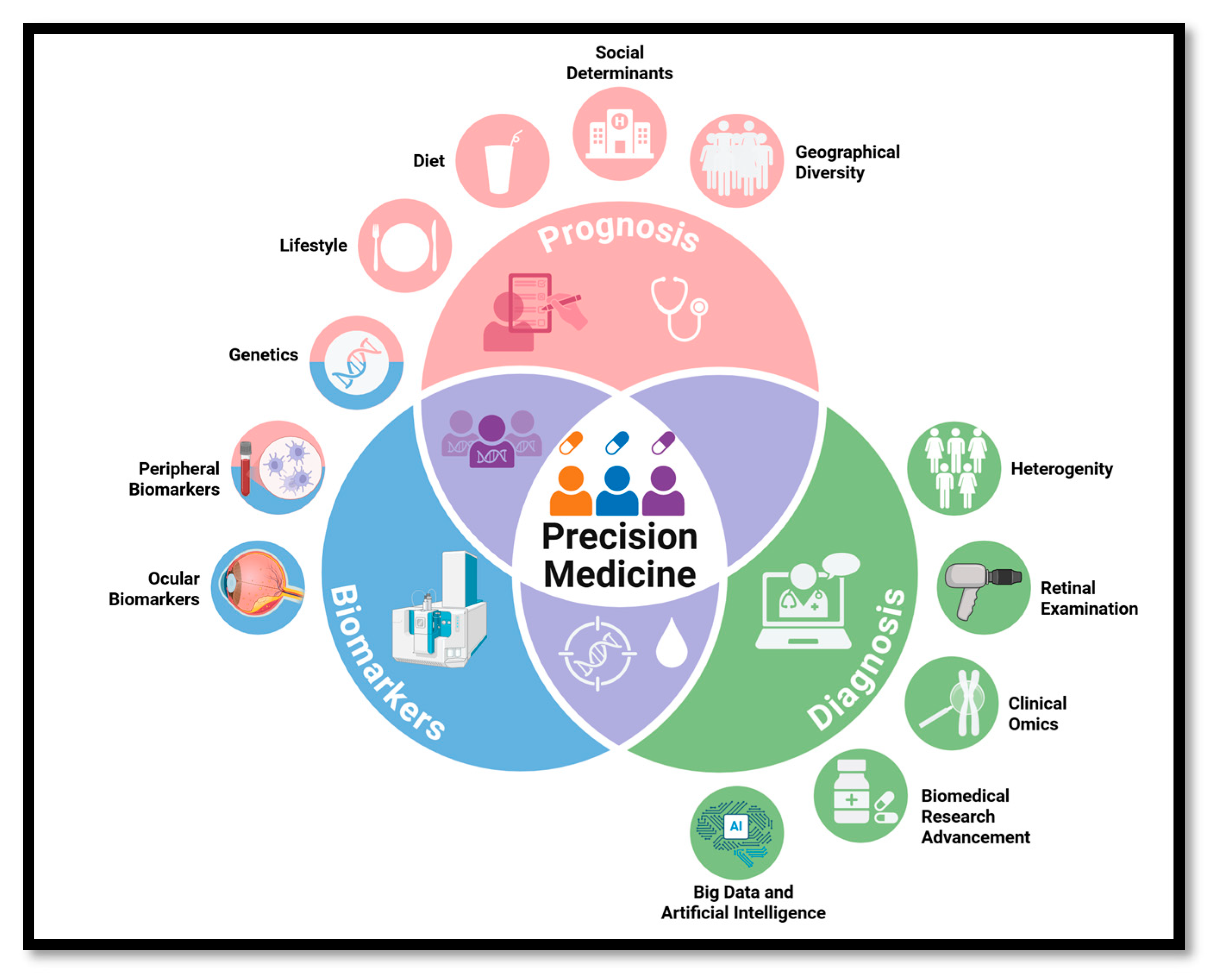
1. Introduction
2. Heterogeneity of DR
2.1. Neurodegenerative Phenotype of DR
2.2. Microvascular Phenotype of DR: Historical Perspective and Natural History of Disease
2.3. Edematous Phenotype of DR
2.4. Contribution of Assigned Sex at Birth on DR Heterogeneity
2.5. Influence of Race on DR Heterogeneity
3. Genetics
3.1. Genes and DR
3.1.1. SELP
3.1.2. MTHFR
3.1.3. NVL and CRP
3.1.4. VEGF
3.1.5. IL-6
3.1.6. eNOS
3.1.7. AR and PAI-1
3.1.8. ACE
3.1.9. ApoE and ICAM-1
4. Lifestyle and Environmental Factors
4.1. Impact of Lifestyle Factors on DR Risk
4.1.1. Physical Activity
4.1.2. Diet
4.2. Impact of Environment on DR Risk
5. Biomarkers for DR
5.1. Vitreous Humor
5.2. Aqueous Humor
5.3. Serum
5.4. Circulating Angiogenic Cells
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DR | Diabetic retinopathy |
| NPDR | Nonproliferative diabetic retinopathy |
| PDR | Proliferative diabetic retinopathy |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| VTDR | Vision-threatening diabetic retinopathy |
| DME | Diabetic macular edema |
| ETDRS | Early treatment of diabetic retinopathy study (ETDRS) |
| ICDR | International clinical diabetic retinopathy |
| OCT | Optical coherence tomography |
| OCT-A | OCT-angiography |
| NHANES | National Health and Nutrition Exam Surveys |
References
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Lundeen, E.A.; Burke-Conte, Z.; Rein, D.B.; Wittenborn, J.S.; Saaddine, J.; Lee, A.Y.; Flaxman, A.D. Prevalence of Diabetic Retinopathy in the US in 2021. JAMA Ophthalmol. 2023, 141, 747–754. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Sabanayagam, C.; Banu, R.; Chee, M.L.; Lee, R.; Wang, Y.X.; Tan, G.; Jonas, J.B.; Lamoureux, E.L.; Cheng, C.Y.; Klein, B.E.K.; et al. Incidence and progression of diabetic retinopathy: A systematic review. Lancet Diabetes Endocrinol. 2019, 7, 140–149. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, T.E.; Shao, Y.; Wong, T.Y.; Li, X. Classification of diabetic retinopathy: Past, present and future. Front. Endocrinol. 2022, 13, 1079217. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.P.; Ferris, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Jin, K.; Yu, H.; Wei, W.; Grzybowski, A. Editorial: Personalized medicine of diabetes retinopathy: From bench to bedside. Front. Endocrinol. 2025, 16, 1597332. [Google Scholar] [CrossRef]
- Sun, J.K.; Aiello, L.P.; Abràmoff, M.D.; Antonetti, D.A.; Dutta, S.; Pragnell, M.; Levine, S.R.; Gardner, T.W. Updating the Staging System for Diabetic Retinal Disease. Ophthalmology 2021, 128, 490–493. [Google Scholar] [CrossRef]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed]
- USFDA. Precision Medicine. Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/precision-medicine (accessed on 21 July 2025).
- Wang, R.C.; Wang, Z. Precision Medicine: Disease Subtyping and Tailored Treatment. Cancers 2023, 15, 3837. [Google Scholar] [CrossRef]
- Kohner, E.M.; Stratton, I.M.; Aldington, S.J.; Turner, R.C.; Matthews, D.R. Microaneurysms in the development of diabetic retinopathy (UKPDS 42). UK Prospective Diabetes Study Group. Diabetologia 1999, 42, 1107–1112. [Google Scholar] [CrossRef][Green Version]
- Channa, R.; Wolf, R.M.; Simo, R.; Brigell, M.; Fort, P.; Curcio, C.; Lynch, S.; Verbraak, F.; Abramoff, M.D.; Diabetic Retinal Neurodegeneration and Macular Edema Working Group of the Mary Tyler Moore Vision Initiative’s Diabetic Retinal Disease Staging Update Project. A New Approach to Staging Diabetic Eye Disease: Staging of Diabetic Retinal Neurodegeneration and Diabetic Macular Edema. Ophthalmol. Sci. 2024, 4, 100420. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef]
- Gardner, T.W.; Davila, J.R. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1–6. [Google Scholar] [CrossRef]
- Bearse, M.A.; Adams, A.J.; Han, Y.; Schneck, M.E.; Ng, J.; Bronson-Castain, K.; Barez, S. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog. Retin. Eye Res. 2006, 25, 425–448. [Google Scholar] [CrossRef]
- Harrison, W.W.; Bearse, M.A.; Ng, J.S.; Jewell, N.P.; Barez, S.; Burger, D.; Schneck, M.E.; Adams, A.J. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investig. Ophthalmol. Vis. Sci. 2011, 52, 772–777. [Google Scholar] [CrossRef]
- Santos, A.R.; Ribeiro, L.; Bandello, F.; Lattanzio, R.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; Gibson, J.; Grauslund, J.; Harding, S.P.; et al. Functional and Structural Findings of Neurodegeneration in Early Stages of Diabetic Retinopathy: Cross-sectional Analyses of Baseline Data of the EUROCONDOR Project. Diabetes 2017, 66, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef]
- Lakhani, E.; Wright, T.; Abdolell, M.; Westall, C. Multifocal ERG defects associated with insufficient long-term glycemic control in adolescents with type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5297–5303. [Google Scholar] [CrossRef] [PubMed]
- Laron, M.; Bearse, M.A.; Bronson-Castain, K.; Jonasdottir, S.; King-Hooper, B.; Barez, S.; Schneck, M.E.; Adams, A.J. Association between local neuroretinal function and control of adolescent type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7071–7076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reis, A.; Mateus, C.; Melo, P.; Figueira, J.; Cunha-Vaz, J.; Castelo-Branco, M. Neuroretinal dysfunction with intact blood-retinal barrier and absent vasculopathy in type 1 diabetes. Diabetes 2014, 63, 3926–3937. [Google Scholar] [CrossRef]
- Wolfensberger, T.J.; Hamilton, A.M. Diabetic retinopathy--an historical review. Semin. Ophthalmol. 2001, 16, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; McMeel, J.W.; Schepens, C.L.; Field, R.A. A new classification of diabetic retinopathy. Am. J. Ophthalmol. 1966, 62, 207–219. [Google Scholar] [CrossRef]
- Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991, 98, 786–806.
- Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991, 98, 823–833.
- Ciulla, T.A.; Amador, A.G.; Zinman, B. Diabetic retinopathy and diabetic macular edema: Pathophysiology, screening, and novel therapies. Diabetes Care 2003, 26, 2653–2664. [Google Scholar] [CrossRef]
- Otani, T.; Kishi, S.; Maruyama, Y. Patterns of diabetic macular edema with optical coherence tomography. Am. J. Ophthalmol. 1999, 127, 688–693. [Google Scholar] [CrossRef]
- Kim, M.; Lee, P.; Kim, Y.; Yu, S.Y.; Kwak, H.W. Effect of intravitreal bevacizumab based on optical coherence tomography patterns of diabetic macular edema. Ophthalmologica 2011, 226, 138–144. [Google Scholar] [CrossRef]
- Kim, J.T.; Lee, D.H.; Joe, S.G.; Kim, J.G.; Yoon, Y.H. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.R.; Lee, S.Y.; Kim, Y.H.; Byeon, H.E.; Kim, J.H.; Lee, K. Hyperreflective foci in diabetic macular edema with serous retinal detachment: Association with dyslipidemia. Acta Diabetol. 2020, 57, 861–866. [Google Scholar] [CrossRef]
- Panozzo, G.; Cicinelli, M.V.; Augustin, A.J.; Battaglia Parodi, M.; Cunha-Vaz, J.; Guarnaccia, G.; Kodjikian, L.; Jampol, L.M.; Jünemann, A.; Lanzetta, P.; et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: The European School for Advanced Studies in Ophthalmology classification. Eur. J. Ophthalmol. 2020, 30, 8–18. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Cioana, M.; Deng, J.; Nadarajah, A.; Hou, M.; Qiu, Y.; Chen, S.S.J.; Rivas, A.; Toor, P.P.; Banfield, L.; Thabane, L.; et al. Global Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw Open 2023, 6, e231887. [Google Scholar] [CrossRef] [PubMed]
- Bureau, U.S.C. Improvements to the 2020 Census Race and Hispanic Origin Question Designs, Data Processing, and Coding Procedures. Available online: https://www.census.gov/newsroom/blogs/random-samplings/2021/08/improvements-to-2020-census-race-hispanic-origin-question-designs.html (accessed on 2 September 2025).
- Cheng, Y.J.; Kanaya, A.M.; Araneta, M.R.G.; Saydah, S.H.; Kahn, H.S.; Gregg, E.W.; Fujimoto, W.Y.; Imperatore, G. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011–2016. JAMA 2019, 322, 2389–2398. [Google Scholar] [CrossRef]
- Zhang, X.; Saaddine, J.B.; Chou, C.F.; Cotch, M.F.; Cheng, Y.J.; Geiss, L.S.; Gregg, E.W.; Albright, A.L.; Klein, B.E.; Klein, R. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010, 304, 649–656. [Google Scholar] [CrossRef]
- Varma, R.; Choudhury, F.; Klein, R.; Chung, J.; Torres, M.; Azen, S.P.; Los Angeles Latino Eye Study Group. Four-year incidence and progression of diabetic retinopathy and macular edema: The Los Angeles Latino Eye Study. Am. J. Ophthalmol. 2010, 149, 752–761.e3. [Google Scholar] [CrossRef]
- Sienkiewicz-Szłapka, E.; Fiedorowicz, E.; Król-Grzymała, A.; Kordulewska, N.; Rozmus, D.; Cieślińska, A.; Grzybowski, A. The Role of Genetic Polymorphisms in Diabetic Retinopathy: Narrative Review. Int. J. Mol. Sci. 2023, 24, 15865. [Google Scholar] [CrossRef]
- Nagi, D.K.; Mansfield, M.W.; Stickland, M.H.; Grant, P.J. Angiotensin Converting Enzyme (ACE) Insertion/Deletion (I/D) Polymorphism, and Diabetic Retinopathy in Subjects with IDDM and NIDDM. Diabet. Med. 1995, 12, 997–1001. [Google Scholar] [CrossRef]
- Rabensteiner, D.; Abrahamian, H.; Irsigler, K.; Hermann, K.M.; Kiener, H.P.; Mayer, G.; Kaider, A.; Prager, R. ACE gene polymorphism and proliferative retinopathy in type 1 diabetes: Results of a case-control study. Diabetes Care 1999, 22, 1530–1535. [Google Scholar] [CrossRef]
- Fujisawa, T.; Ikegami, H.; Kawaguchi, Y.; Hamada, Y.; Ueda, H.; Shintani, M.; Fukuda, M.; Ogihara, T. Meta-analysis of association of insertion/deletion polymorphism of angiotensin I-converting enzyme gene with diabetic nephropathy and retinopathy. Diabetologia 1998, 41, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-B.; Yang, J.-K. Angiotensin-converting enzyme gene polymorphism is associated with proliferative diabetic retinopathy: A meta-analysis. Acta Diabetol. 2010, 47, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Nikzamir, A.; Rashidi, A.; Esteghamati, A.; Nakhjavani, M.; Golmohammadi, T.; Khalilzadeh, O. The relationship between ACE gene insertion/deletion polymorphism and diabetic retinopathy in Iranian patients with type 2 diabetes. Ophthalmic Genet. 2010, 31, 108–113. [Google Scholar] [CrossRef]
- Lu, Y.; Ge, Y.; Hu, Q.; Shi, Y.; Xue, C.; Shi, Y.; Chen, S.; Huang, Z. Association between angiotensin-converting enzyme gene polymorphism and diabetic retinopathy in the Chinese population. J. Renin-Angiotensin-Aldosterone Syst. 2012, 13, 289–295. [Google Scholar] [CrossRef]
- Saleem, S.; Azam, A.; Maqsood, S.I.; Muslim, I.; Bashir, S.; Fazal, N.; Riaz, M.; Ali, S.H.B.; Niazi, M.K.; Ishaq, M.; et al. Role of ACE and PAI-1 Polymorphisms in the Development and Progression of Diabetic Retinopathy. PLoS ONE 2015, 10, e0144557. [Google Scholar] [CrossRef]
- Luo, S.; Shi, C.; Wang, F.; Wu, Z. Association between the Angiotensin-Converting Enzyme (ACE) Genetic Polymorphism and Diabetic Retinopathy—A Meta-Analysis Comprising 10,168 Subjects. Int. J. Environ. Res. Public Health 2016, 13, 1142. [Google Scholar] [CrossRef]
- Qiao, Y.C.; Wang, M.; Pan, Y.H.; Zhang, X.X.; Tian, F.; Chen, Y.L.; Zhao, H.L. The relationship between ACE/AGT gene polymorphisms and the risk of diabetic retinopathy in Chinese patients with type 2 diabetes. J. Renin-Angiotensin-Aldosterone Syst. 2018, 19, 1470320317752955. [Google Scholar] [CrossRef]
- Coelho, A.R.d.P.; Silveira, L.C.; Santos, K.d.F.; Santos, R.d.S.; Reis, A.A.d.S. No Association of Angiotensin-Converting Enzyme Insertion/Deletion (ACE I/D) Gene Polymorphism in the Susceptibility to Diabetic Retinopathy in Type 2 Diabetes Mellitus Patients: An Updated Meta-Analysis. J. Pers. Med. 2023, 13, 1308. [Google Scholar] [CrossRef]
- Barrera-Perales, K.; Pérez-Cano, H.; Rojas-Juárez, S.; Morales-López, O.; Somilleda-Ventura, A.; Cuevas-Budhart, M.A.; Rueda, R.I.S. Association Between ACE Gene I/D Polymorphism and Diabetic Retinopathy in Mexican Population. Arch. Med. Res. 2025, 56, 103252. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Malik, I.R.; Bushra, H.; Alam, K.; Shakeel, M.; Ahmed, I.; Gul, H.; Elsadek, M.F.; Al-Numair, K.S.; Ahmad, N. The relationship between ACE gene insertion/deletion polymorphism and diabetes retinopathy patients with diabetes type 1. Int. J. Diabetes Dev. Ctries. 2024, 45, 764–770. [Google Scholar] [CrossRef]
- Dutta, P.; Ghosh, S.; Dasgupta, A.; Majumder, S. Association of angiotensin converting enzyme gene insertion/deletion polymorphism with diabetic retinopathy in middle-aged Indians with type 2 diabetes mellitus. Horm. Mol. Biol. Clin. Investig. 2024, 45, 111–117. [Google Scholar] [CrossRef]
- Shcherbak, N.S. Apolipoprotein E gene polymorphism is not a strong risk factor for diabetic nephropathy and retinopathy in Type I diabetes: Case-control study. BMC Med. Genet. 2001, 2, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Errera, F.; Silva, M.; Yeh, E.; Maranduba, C.; Folco, B.; Takahashi, W.; Pereira, A.; Krieger, J.E.; Passos-Bueno, M.R. Effect of polymorphisms of the MTHFR and APOE genes on susceptibility to diabetes and severity of diabetic retinopathy in Brazilian patients. Braz. J. Med. Biol. Res. 2006, 39, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Shankar, A.; Wang, J.J.; Klein, R.; Bray, M.S.; Couper, D.J.; Wong, T.Y. Apolipoprotein E gene polymorphisms are not associated with diabetic retinopathy: The atherosclerosis risk in communities study. Am. J. Ophthalmol. 2006, 142, 105–111. [Google Scholar] [CrossRef]
- Dlouha, L.; Pelikanova, T.; Veleba, J.; Adamkova, V.; Lanska, V.; Sosna, T.; Pacal, L.; Kankova, K.; Hubacek, J.A. The APOE4 allele is associated with a decreased risk of retinopathy in type 2 diabetics. Mol. Biol. Rep. 2021, 48, 5873–5879. [Google Scholar] [CrossRef]
- Shawki, H.A.; Elzehery, R.; Abo-hashem, E.M.; Shahin, M.; Youssef, M.M. Gene polymorphism of C106T “rs759853” is not associated with diabetic retinopathy in Egyptian patients with type 2 diabetes mellitus. Gene Rep. 2020, 21, 100865. [Google Scholar] [CrossRef]
- Lin, S.; Peng, Y.; Cao, M.; Chen, R.; Hu, J.; Pu, Z.; Cai, Z.; Mou, L. Association between Aldose Reductase Gene C(-106)T Polymorphism and Diabetic Retinopathy: A Systematic Review and Meta-Analysis. Ophthalmic Res. 2020, 63, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Buraczynska, M.; Wacinski, P.; Stec, A.; Kuczmaszewska, A. Calpain-10 gene polymorphisms in type 2 diabetes and its micro- and macrovascular complications. J. Diabetes Its Complicat. 2013, 27, 54–58. [Google Scholar] [CrossRef]
- Malecki, M.T.; Cyganek, K.; Mirkiewicz-Sieradzka, B.; Wolkow, P.P.; Wanic, K.; Skupien, J.; Solnica, B.; Sieradzki, J. Alanine variant of the Pro12Ala polymorphism of the PPARγ gene might be associated with decreased risk of diabetic retinopathy in type 2 diabetes. Diabetes Res. Clin. Pract. 2008, 80, 139–145. [Google Scholar] [CrossRef]
- Derkac, I.; Januleviciene, I.; Sepetiene, R.; Valiauga, R.; Velickiene, D. The Association of CEP135 rs4865047 and NPY2R rs1902491 Single Nucleotide Polymorphisms (SNPs) with Rapid Progression of Proliferative Diabetic Retinopathy in Patients with Type 1 Diabetes Mellitus. Med. Sci. Monit. 2018, 24, 8891–8898. [Google Scholar] [CrossRef]
- Peng, D.; Wang, J.; Zhang, R.; Tang, S.; Jiang, F.; Chen, M.; Yan, J.; Sun, X.; Wang, T.; Wang, S.; et al. C-reactive protein genetic variant is associated with diabetic retinopathy in Chinese patients with type 2 diabetes. BMC Endocr. Disord. 2015, 15, 8. [Google Scholar] [CrossRef]
- Słomiński, B.; Jankowiak, M.; Maciejewska, A.; Studziński, M.; Mączyńska, A.; Skrzypkowska, M.; Gabig-Cimińska, M.; Myśliwiec, M. Genetic variation in C-reactive protein (CRP) gene is associated with retinopathy and hypertension in adolescents with type 1 diabetes. Cytokine 2022, 160, 156025. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Takahashi, A.; Matsunami, M.; Horikoshi, M.; Iwata, M.; Araki, S.-i.; Toyoda, M.; Susarla, G.; Ahn, J.; Park, K.H.; et al. Genome-wide association studies identify two novel loci conferring susceptibility to diabetic retinopathy in Japanese patients with type 2 diabetes. Hum. Mol. Genet. 2021, 30, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Taverna, M.; Sola, A.; Guyot-Argenton, C.; Pacher, N.; Bruzzo, F.; Chevalier, A.; Slama, G.; Reach, G.; Selam, J.L. eNOS4 polymorphism of the endothelial nitric oxide synthase predicts risk for severe diabetic retinopathy. Diabet. Med. 2002, 19, 240–245. [Google Scholar] [CrossRef]
- Bazzaz, J.T.; Amoli, M.M.; Pravica, V.; Chandrasecaran, R.; Boulton, A.J.; Larijani, B.; Hutchinson, I.V. eNOS gene polymorphism association with retinopathy in type 1 diabetes. Ophthalmic Genet. 2010, 31, 103–107. [Google Scholar] [CrossRef]
- Suganthalakshmi, B.; Anand, R.; Kim, R.; Mahalakshmi, R.; Karthikprakash, S.; Namperumalsamy, P.; Sundaresan, P. Association of VEGF and eNOS gene polymorphisms in type 2 diabetic retinopathy. Mol. Vis. 2006, 12, 336–341. [Google Scholar]
- Santos, K.G.; Crispim, D.; Canani, L.H.; Ferrugem, P.T.; Gross, J.L.; Roisenberg, I. Relationship of endothelial nitric oxide synthase (eNOS) gene polymorphisms with diabetic retinopathy in Caucasians with type 2 diabetes. Ophthalmic Genet. 2012, 33, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Cheema, B.S.; Kohli, H.S.; Sharma, R.; Bhansali, A.; Khullar, M. Endothelial nitric oxide synthase gene polymorphism and type 2 diabetic retinopathy among Asian Indians. Acta Diabetol. 2012, 49, 481–488. [Google Scholar] [CrossRef]
- Ma, Z.J.; Chen, R.; Ren, H.Z.; Guo, X.; Guo, J.; Chen, L.M. Association between eNOS 4b/a polymorphism and the risk of diabetic retinopathy in type 2 diabetes mellitus: A meta-analysis. J. Diabetes Res. 2014, 2014, 549747. [Google Scholar] [CrossRef]
- Gouliopoulos, N.; Siasos, G.; Oikonomou, D.; Oikonomou, E.; Konsola, T.; Kollia, C.; Athanasiou, D.; Dimitropoulos, S.; Rouvas, A.; Kassi, E.; et al. The association of T786C and G894T polymorphisms of eNOS gene with diabetic retinopathy in Greece. Eur. J. Ophthalmol. 2022, 32, 2582–2588. [Google Scholar] [CrossRef]
- Shi, Y.; Fan, X.; Zhang, K.; Ma, Y. Association of the endothelial nitric oxide synthase (eNOS) 4a/b polymorphism with the risk of incident diabetic retinopathy in patients with type 2 diabetes mellitus: A systematic review and updated meta-analysis. Ann. Med. 2023, 55, 2226908. [Google Scholar] [CrossRef]
- Abu-Hassan, D.W.; Al-Bdour, M.D.; Aolymat, I.; El-Khateeb, M. The association of endothelial nitric oxide synthase (eNOS) gene polymorphisms and diabetic retinopathy among patients with type 2 diabetes: A case-control study. Mol. Vis. 2024, 30, 390. [Google Scholar]
- Wang, A.L.; Rao, V.R.; Chen, J.J.; Lussier, Y.A.; Rehman, J.; Huang, Y.; Jager, R.D.; Grassi, M.A. Role of FAM18B in diabetic retinopathy. Mol. Vis. 2014, 20, 1146–1159. [Google Scholar] [PubMed]
- Peng, D.; Wang, J.; Zhang, R.; Jiang, F.; Tang, S.; Chen, M.; Yan, J.; Sun, X.; Wang, S.; Wang, T.; et al. Common variants in or near ZNRF1, COLEC12, SCYL1BP1 and API5 are associated with diabetic retinopathy in Chinese patients with type 2 diabetes. Diabetologia 2015, 58, 1231–1238. [Google Scholar] [CrossRef]
- Burdon, K.P.; Fogarty, R.D.; Shen, W.; Abhary, S.; Kaidonis, G.; Appukuttan, B.; Hewitt, A.W.; Sharma, S.; Daniell, M.; Essex, R.W.; et al. Genome-wide association study for sight-threatening diabetic retinopathy reveals association with genetic variation near the GRB2 gene. Diabetologia 2015, 58, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Bastos, C.M.C.; da Silva Machado, L.M.; Crispim, D.; Canani, L.H.; dos Santos, K.G. Association of the rs9896052 Polymorphism Upstream of GRB2 with Proliferative Diabetic Retinopathy in Patients with Less than 10 Years of Diabetes. Int. J. Mol. Sci. 2024, 25, 10232. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Lin, J.-M.; Lin, H.-J.; Chen, C.-C.; Chen, S.-Y.; Tsai, C.-H.; Tsai, F.-J. Genome-wide Association Study of Diabetic Retinopathy in a Taiwanese Population. Ophthalmology 2011, 118, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Kamiuchi, K.; Hasegawa, G.; Obayashi, H.; Kitamura, A.; Ishii, M.; Yano, M.; Kanatsuna, T.; Yoshikawa, T.; Nakamura, N. Intercellular adhesion molecule-1 (ICAM-1) polymorphism is associated with diabetic retinopathy in Type 2 diabetes mellitus. Diabet. Med. 2002, 19, 371–376. [Google Scholar] [CrossRef]
- Petrovič, M.G.; Osredkar, J.; Saraga-Babić, M.; Petrovič, D. K469E polymorphism of the intracellular adhesion molecule 1 gene is associated with proliferative diabetic retinopathy in Caucasians with type 2 diabetes. Clin. Exp. Ophthalmol. 2008, 36, 468–472. [Google Scholar] [CrossRef]
- Vinita, K.; Sripriya, S.; Prathiba, K.; Vaitheeswaran, K.; Sathyabaarathi, R.; Rajesh, M.; Amali, J.; Umashankar, V.; Kumaramanickavel, G.; Pal, S.S.; et al. ICAM-1 K469E polymorphism is a genetic determinant for the clinical risk factors of T2D subjects with retinopathy in Indians: A population-based case–control study. BMJ Open 2012, 2, e001036. [Google Scholar] [CrossRef]
- Simões, M.J.; Lobo, C.; Egas, C.; Nunes, S.; Carmona, S.; Costa, M.Â.; Duarte, T.; Ribeiro, L.; Faro, C.; Cunha-Vaz, J.G. Genetic Variants in ICAM1, PPARGC1A and MTHFR Are Potentially Associated with Different Phenotypes of Diabetic Retinopathy. Ophthalmologica 2014, 232, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.Y.; Liu, N.P. Meta-analysis of association between K469E polymorphism of the ICAM-1 gene and retinopathy in type 2 diabetes. Int. J. Ophthalmol. 2015, 8, 603–607. [Google Scholar] [CrossRef]
- Lv, Z.; Li, Y.; Wu, Y.; Qu, Y. Association of ICAM-1 and HMGA1 Gene Variants with Retinopathy in Type 2 Diabetes Mellitus Among Chinese Individuals. Curr. Eye Res. 2016, 41, 1118–1122. [Google Scholar] [CrossRef]
- Xie, Z.; Liang, H. Association between diabetic retinopathy in type 2 diabetes and the ICAM-1 rs5498 polymorphism: A meta-analysis of case-control studies. BMC Ophthalmol. 2018, 18, 297. [Google Scholar] [CrossRef]
- Lu, Q.K.; Zhang, J.T.; Zhao, N.; Wang, H.Y.; Tong, Q.H.; Wang, S.L. Association of IL-6 gene (-174 and-572 G/C) polymorphisms with proliferative diabetic retinopathy of type 2 diabetes in a Chinese population. Ophthalmic Res. 2017, 58, 162–167. [Google Scholar] [CrossRef]
- Sun, X.; Guo, S. Association between diabetic retinopathy and interleukin-related gene polymorphisms: A machine learning aided meta-analysis. Ophthalmic Genet. 2020, 41, 216–222. [Google Scholar] [CrossRef]
- Chen, B.; Wu, M.; Zang, C.; Li, Y.; Xu, Z. Association between IL-6 polymorphisms and diabetic nephropathy risk: A meta-analysis. Am. J. Med. Sci. 2019, 358, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.Y.; Hui, E.Y.L.; Lee, C.-H.; Kwok, K.H.M.; Gangwani, R.A.; Li, K.K.W.; Chan, J.C.W.; Woo, Y.-C.; Chow, W.-S.; Yuen, M.M.A.; et al. Impact of Genetic Loci Identified in Genome-Wide Association Studies on Diabetic Retinopathy in Chinese Patients With Type 2 Diabetes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5518–5524. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Lin, H.-Y.; Lin, H.-J.; Chen, S.-Y.; Liu, S.-P.; Liao, W.-L.; Lin, J.-M.; Chen, Y.-H.; Tsai, F.-J. JPH2 is a novel susceptibility gene on chromosome 20q associated with diabetic retinopathy in a Taiwanese population. ScienceAsia 2013, 39, 167. [Google Scholar] [CrossRef]
- Morgante, C.; Latini, A.; De Benedittis, G.; Novelli, G.; Spallone, V.; Ciccacci, C.; Borgiani, P. A genetic variant in the potassium channel (KCNJ11) gene is associated with diabetic retinopathy in type 2 diabetes patients. Gene 2025, 945, 149289. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.-J.; Wu, H.-H.; Li, Y.-L.; Yang, Z.; Tao, X.-M.; Du, Y.-P.; Wang, X.-C.; Lu, B.; Zhang, Z.-Y.; Hu, R.-M.; et al. An analysis of the association between a polymorphism of KCNJ11 and diabetic retinopathy in a Chinese Han population. Eur. J. Med. Res. 2015, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Yamamoto, I.; Fukuda, M.; Motomura, T.; Nishida, M.; Nonen, S.; Fujio, Y.; Kasayama, S.; Azuma, J. MTHFR gene polymorphism is susceptible to diabetic retinopathy but not to diabetic nephropathy in Japanese type 2 diabetic patients. J. Diabetes Complicat. 2008, 22, 119–125. [Google Scholar] [CrossRef]
- Luo, S.; Wang, F.; Shi, C.; Wu, Z. A meta-analysis of association between methylenetetrahydrofolate reductase gene (MTHFR) 677C/T polymorphism and diabetic retinopathy. Int. J. Environ. Res. Public Health 2016, 13, 806. [Google Scholar] [CrossRef]
- Garcia-Hernandez, S.C.; Porchia, L.M.; López-Bayghen, E.; Gonzalez-Mejia, M.E. The A1298C methylenetetrahydrofolate reductase polymorphism augments the risk of developing of diabetic retinopathy: A meta-analysis. Meta Gene 2019, 20, 100560. [Google Scholar] [CrossRef]
- Khan, N.; Paterson, A.D.; Roshandel, D.; Maqbool, S.; Fazal, N.; Ali, L.; Khurram, R.; Maqsood, S.I.; Ali, S.H.B.; Khan, H. Role of 19 SNPs in 10 genes with type 2 diabetes in the Pakistani population. Gene 2023, 848, 146899. [Google Scholar] [CrossRef]
- Hsieh, Y.-Y.; Huang, Y.-C.; Chang, C.-C.; Wang, Y.-K.; Lin, W.-H.; Tsai, F.-J. Chromosome 15q21-22–Related Polymorphisms and Haplotypes Are Associated with Susceptibility to Type-2 Diabetic Nonproliferative Retinopathy. Genet. Test. Mol. Biomark. 2012, 16, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Pollack, S.; Igo Jr, R.P.; Jensen, R.A.; Christiansen, M.; Li, X.; Cheng, C.-Y.; Ng, M.C.; Smith, A.V.; Rossin, E.J.; Segre, A.V. Multiethnic genome-wide association study of diabetic retinopathy using liability threshold modeling of duration of diabetes and glycemic control. Diabetes 2019, 68, 441–456. [Google Scholar] [CrossRef]
- Ezzidi, I.; Mtiraoui, N.; Chaieb, M.; Kacem, M.; Mahjoub, T.; Almawi, W.Y. Diabetic retinopathy, PAI-1 4G/5G and −844G/A polymorphisms, and changes in circulating PAI-1 levels in Tunisian type 2 diabetes patients. Diabetes Metab. 2009, 35, 214–219. [Google Scholar] [CrossRef]
- Siokas, V.; Dardiotis, E.; Sokolakis, T.; Kotoula, M.; Tachmitzi, S.V.; Chatzoulis, D.Z.; Almpanidou, P.; Stefanidis, I.; Hadjigeorgiou, G.M.; Tsironi, E.E. Plasminogen Activator Inhibitor Type-1 Tag Single-Nucleotide Polymorphisms in Patients with Diabetes Mellitus Type 2 and Diabetic Retinopathy. Curr. Eye Res. 2017, 42, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Dastgheib, S.A.; Najafi, F.; Shajari, A.; Bahrami, R.; Asadian, F.; Sadeghizadeh-Yazdi, J.; Akbarian, E.; Emarati, S.A.; Neamatzadeh, H. Association of plasminogen activator inhibitor-1 4G5G Polymorphism with risk of diabetic nephropathy and retinopathy: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2020, 19, 2005–2016. [Google Scholar] [CrossRef]
- Qi, S.; Wang, C.; Zhang, Y.; Cheng, Y.; Wang, S.; Zhao, Y. The association of peroxisome proliferator-activated receptor α with diabetic retinopathy, and additional gene–obesity interaction in Chinese type 2 diabetes mellitus patients. Obes. Res. Clin. Pract. 2016, 10, S103–S109. [Google Scholar] [CrossRef]
- Costa, V.; Casamassimi, A.; Esposito, K.; Villani, A.; Capone, M.; Iannella, R.; Schisano, B.; Ciotola, M.; Di Palo, C.; Capone Corrado, F.; et al. Characterization of a Novel Polymorphism in PPARG Regulatory Region Associated with Type 2 Diabetes and Diabetic Retinopathy in Italy. BioMed Res. Int. 2009, 2009, 126917. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.H.; Li, R.X. Interaction between peroxisome proliferator- activated receptor gamma polymorphism and overweight on diabetic retinopathy in a Chinese case-control study. Int. J. Clin. Exp. Med. 2015, 8, 21647–21652. [Google Scholar] [PubMed]
- Li, X.F.; Jiang, G.B.; Cheng, S.Y.; Song, Y.F.; Deng, C.; Niu, Y.M.; Cai, J.W. Association between PPAR-γ2 gene polymorphisms and diabetic retinopathy risk: A meta-analysis. Aging (Albany NY) 2021, 13, 5136–5149. [Google Scholar] [CrossRef] [PubMed]
- Buraczynska, M.; Gwiazda-Tyndel, K.; Drop, B.; Zaluska, W. Renalase gene Glu37Asp polymorphism affects susceptibility to diabetic retinopathy in type 2 diabetes mellitus. Acta Diabetol. 2021, 58, 1595–1602. [Google Scholar] [CrossRef]
- Sobrin, L.; Green, T.; Sim, X.; Jensen, R.A.; Tai, E.S.; Tay, W.T.; Wang, J.J.; Mitchell, P.; Sandholm, N.; Liu, Y. Candidate gene association study for diabetic retinopathy in persons with type 2 diabetes: The Candidate gene Association Resource (CARe). Investig. Ophthalmol. Vis. Sci. 2011, 52, 7593–7602. [Google Scholar] [CrossRef]
- Penman, A.; Hoadley, S.; Wilson, J.G.; Taylor, H.A.; Chen, C.J.; Sobrin, L. P-selectin plasma levels and genetic variant associated with diabetic retinopathy in African Americans. Am. J. Ophthalmol. 2015, 159, 1152–1160. e1152. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouz, P.; Farashahi Yazd, E.; Tajamolian, M.; Manaviat, M.R.; Sheikhha, M.H. The rs3917779 polymorphism of P-selectin’s significant association with proliferative diabetic retinopathy in Yazd, Iran. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1967–1972. [Google Scholar] [CrossRef]
- Beránek, M.; Kanková, K.; Tschöplová, S.; Kolár, P.; Vácha, J. Three novel polymorphisms in the promoter region of the TIMP-3 gene are not associated with proliferative diabetic retinopathy in type 2 diabetes mellitus. Curr. Eye Res. 2003, 27, 91–93. [Google Scholar] [CrossRef]
- Awata, T.; Inoue, K.; Kurihara, S.; Ohkubo, T.; Watanabe, M.; Inukai, K.; Inoue, I.; Katayama, S. A Common Polymorphism in the 5′-Untranslated Region of the VEGF Gene Is Associated With Diabetic Retinopathy in Type 2 Diabetes. Diabetes 2002, 51, 1635–1639. [Google Scholar] [CrossRef]
- Buraczynska, M.; Ksiazek, P.; Baranowicz-Gaszczyk, I.; Jozwiak, L. Association of the VEGF gene polymorphism with diabetic retinopathy in type 2 diabetes patients. Nephrol. Dial. Transplant. 2007, 22, 827–832. [Google Scholar] [CrossRef][Green Version]
- Szaflik, J.P.; Wysocki, T.; Kowalski, M.; Majsterek, I.; Borucka, A.I.; Blasiak, J.; Szaflik, J. An association between vascular endothelial growth factor gene promoter polymorphisms and diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Uthra, S.; Raman, R.; Mukesh, B.N.; Rajkumar, S.A.; Kumari R, P.; Paul, P.G.; Lakshmipathy, P.; Gnanamoorthy, P.; Sharma, T.; McCarty, C.A. Association of VEGF gene polymorphisms with diabetic retinopathy in a south Indian cohort. Ophthalmic Genet. 2008, 29, 11–15. [Google Scholar] [CrossRef]
- Churchill, A.J.; Carter, J.G.; Ramsden, C.; Turner, S.J.; Yeung, A.; Brenchley, P.E.; Ray, D.W. VEGF polymorphisms are associated with severity of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3611–3616. [Google Scholar] [CrossRef]
- Yang, X.; Deng, Y.; Gu, H.; Lim, A.; Altankhuyag, A.; Jia, W.; Ma, K.; Xu, J.; Zou, Y.; Snellingen, T.; et al. Polymorphisms in the vascular endothelial growth factor gene and the risk of diabetic retinopathy in Chinese patients with type 2 diabetes. Mol. Vis. 2011, 17, 3088–3096. [Google Scholar] [PubMed]
- Gong, J.-Y.; Sun, Y.-H. Association of VEGF gene polymorphisms with diabetic retinopathy: A meta-analysis. PLoS ONE 2013, 8, e84069. [Google Scholar] [CrossRef][Green Version]
- Hu, L.; Gong, C.; Chen, X.; Zhou, H.; Yan, J.; Hong, W. Associations between Vascular Endothelial Growth Factor Gene Polymorphisms and Different Types of Diabetic Retinopathy Susceptibility: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2021, 2021, 7059139. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, A.R.; Surudarma, I.W.; Wihandani, D.M.; Putra, I. Polymorphisms of vascular endothelial growth factor-2578C/A rs699947 are risk factors for diabetic retinopathy in type-2 diabetes mellitus patients in Bali, Indonesia. Biomedicine 2021, 11, 11–17. [Google Scholar] [CrossRef]
- Hillmy, E.; Abdelazim, A.; Elborgy, E.; El-Habit, O.H.; Elbeltagy, R.S. Gene polymorphisms of the Vascular Endothelial Growth Factor (VEGF) in Promotor Region -2578 C/A and -460 T/C as a Risk Factor of Diabetic Retinopathy in Egyptian Patients with Diabetes Mellitus. Adv. Basic Appl. Sci. 2024, 3, 33–46. [Google Scholar] [CrossRef]
- Rabbind Singh, A.; Gupta, R.; Shukla, M.; Jain, A.; Shukla, D. Association of VEGFA promoter polymorphisms rs699947 and rs35569394 with diabetic retinopathy among North-Central Indian subjects: A case-control study. Ophthalmic Genet. 2022, 43, 80–87. [Google Scholar] [CrossRef]
- Panicker, S.R.; Mehta-D’souza, P.; Zhang, N.; Klopocki, A.G.; Shao, B.; McEver, R.P. Circulating soluble P-selectin must dimerize to promote inflammation and coagulation in mice. Blood J. Am. Soc. Hematol. 2017, 130, 181–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, G.; Wang, W.; Jing, Y. C-reactive protein to high-density lipoprotein cholesterol ratio: An independent risk factor for diabetic retinopathy in type 2 diabetes patients. Front. Nutr. 2025, 12, 1537707. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Adamis, A.P.; Miller, J.W.; Bernal, M.T.; D’Amico, D.J.; Folkman, J.; Yeo, T.K.; Yeo, K.T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 1994, 118, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.H.; Minaker, S.A.; Lahaie Luna, G.; Bapat, P.; Farahvash, A.; Garg, A.; Bhambra, N.; Muni, R.H. Changes in aqueous and vitreous inflammatory cytokine levels in proliferative diabetic retinopathy: A systematic review and meta-analysis. Eye 2022, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Wirostko, B.; Wong, T.Y.; Simó, R. Vascular endothelial growth factor and diabetic complications. Prog. Retin. Eye Res. 2008, 27, 608–621. [Google Scholar] [CrossRef]
- Abhary, S.; Burdon, K.P.; Gupta, A.; Lake, S.; Selva, D.; Petrovsky, N.; Craig, J.E. Common sequence variation in the VEGFA gene predicts risk of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5552–5558. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Y.; Zhang, X.; Li, X.; Liu, J. Association of VEGF gene polymorphisms with susceptibility to diabetic retinopathy: A systematic review and meta-analysis. Horm. Metab. Res. 2020, 52, 264–279. [Google Scholar] [CrossRef]
- Yuan, Y.; Wen, Z.; Guan, Y.; Sun, Y.; Yang, J.; Fan, X.; Yang, X.; Liu, R. The relationships between type 2 diabetic retinopathy and VEGF-634G/C and VEGF-460C/T polymorphisms in Han Chinese subjects. J. Diabetes Its Complicat. 2014, 28, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, L.; Xing, W.; Zhuo, R.; Lin, X.; Hao, Y.; Wu, Q.; Zhao, J. The Associations between VEGF Gene Polymorphisms and Diabetic Retinopathy Susceptibility: A Meta-Analysis of 11 Case-Control Studies. J. Diabetes Res. 2014, 2014, 805801. [Google Scholar] [CrossRef]
- Sallami, N.; AmiraTurki; Ben Lamine, L.; Ghorbel, M.; Khairallah, M.; Mahjoub, T.; Almawi, W.Y. Vascular endothelial growth factor gene variants and haplotypes associated with susceptibility to diabetic retinopathy in type 2 diabetes mellitus. Gene Rep. 2025, 40, 102258. [Google Scholar] [CrossRef]
- Sepah, Y.J.; Do, D.V.; Mesquida, M.; Day, B.-M.; Blotner, S.; Afridi, R.; Halim, M.S.; Hong, K.; Wakshull, E.; Fauser, S.; et al. Aqueous humour interleukin-6 and vision outcomes with anti-vascular endothelial growth factor therapy. Eye 2024, 38, 1755–1761. [Google Scholar] [CrossRef]
- Yao, Y.; Li, R.; Du, J.; Long, L.; Li, X.; Luo, N. Interleukin-6 and Diabetic Retinopathy: A Systematic Review and Meta-Analysis. Curr. Eye Res. 2019, 44, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Lei, C.; Zhang, Y.; Zhang, M. Interleukin-6 in retinal diseases: From pathogenesis to therapy. Exp. Eye Res. 2023, 233, 109556. [Google Scholar] [CrossRef]
- Khaloo, P.; Qahremani, R.; Rabizadeh, S.; Omidi, M.; Rajab, A.; Heidari, F.; Farahmand, G.; Bitaraf, M.; Mirmiranpour, H.; Esteghamati, A. Nitric oxide and TNF-α are correlates of diabetic retinopathy independent of hs-CRP and HbA1c. Endocrine 2020, 69, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fan, L.; Luo, H.; Ju, X.; Li, H.; Rong, S.; Yuan, Y.; Xiao, J.; Zhang, R.; Wang, K.; et al. Genetic association of MIR-449B, GCLC, eNOS, SORD, and ENPP1 with diabetic retinopathy. Exp. Eye Res. 2025, 253, 110287. [Google Scholar] [CrossRef]
- Rahimi, Z.; Moradi, M.; Nasri, H. A systematic review of the role of renin angiotensin aldosterone system genes in diabetes mellitus, diabetic retinopathy and diabetic neuropathy. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 1090. [Google Scholar]
- Bhatwadekar, A.D.; Shughoury, A.; Belamkar, A.; Ciulla, T.A. Genetics of Diabetic Retinopathy, a Leading Cause of Irreversible Blindness in the Industrialized World. Genes 2021, 12, 1200. [Google Scholar] [CrossRef]
- Sayed-Tabatabaei, F.; Oostra, B.; Isaacs, A.; Van Duijn, C.; Witteman, J. ACE polymorphisms. Circ. Res. 2006, 98, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Globočnik-Petrovič, M.; Hawlina, M.; Peterlin, B.; Petrovič, D. Insertion/deletion plasminogen activator inhibitor 1 and insertion/deletion angiotensin-converting enzyme gene polymorphisms in diabetic retinopathy in type 2 diabetes. Ophthalmologica 2003, 217, 219–224. [Google Scholar] [CrossRef]
- Anthopoulos, P.; Hamodrakas, S.; Bagos, P. Apolipoprotein E polymorphisms and type 2 diabetes: A meta-analysis of 30 studies including 5423 cases and 8197 controls. Mol. Genet. Metab. 2010, 100, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Moss, S.E.; Cruickshanks, K.J. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology 1995, 102, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 854–865.
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Yang, B.Y.; Fan, S.; Thiering, E.; Seissler, J.; Nowak, D.; Dong, G.H.; Heinrich, J. Ambient air pollution and diabetes: A systematic review and meta-analysis. Environ. Res. 2020, 180, 108817. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, L.; Guo, J.; Li, J.; Li, X.; Li, L.; Zhang, J.; Suo, X. Volatile organic compounds exposure in relation to glucose homeostasis and type 2 diabetes in older adults from the NHANES. Sci. Rep. 2024, 14, 30075. [Google Scholar] [CrossRef]
- Mercado, C.I.; McKeever Bullard, K.; Gregg, E.W.; Ali, M.K.; Saydah, S.H.; Imperatore, G. Differences in U.S. Rural-Urban Trends in Diabetes ABCS, 1999-2018. Diabetes Care 2021, 44, 1766–1773. [Google Scholar] [CrossRef]
- Stratton, I.M.; Kohner, E.M.; Aldington, S.J.; Turner, R.C.; Holman, R.R.; Manley, S.E.; Matthews, D.R. UKPDS 50: Risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001, 44, 156–163. [Google Scholar] [CrossRef]
- Atchison, E.; Barkmeier, A. The Role of Systemic Risk Factors in Diabetic Retinopathy. Curr. Ophthalmol. Rep. 2016, 4, 84–89. [Google Scholar] [CrossRef]
- Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; Lovato, J.F.; Perdue, L.H.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef]
- Yardley, J.E.; Hay, J.; Abou-Setta, A.M.; Marks, S.D.; McGavock, J. A systematic review and meta-analysis of exercise interventions in adults with type 1 diabetes. Diabetes Res. Clin. Pract. 2014, 106, 393–400. [Google Scholar] [CrossRef]
- Ren, C.; Liu, W.; Li, J.; Cao, Y.; Xu, J.; Lu, P. Physical activity and risk of diabetic retinopathy: A systematic review and meta-analysis. Acta Diabetol. 2019, 56, 823–837. [Google Scholar] [CrossRef]
- Li, B.; Zhou, C.; Gu, C.; Cheng, X.; Wang, Y.; Li, C.; Ma, M.; Fan, Y.; Xu, X.; Chen, H.; et al. Modifiable lifestyle, mental health status and diabetic retinopathy in U.S. adults aged 18-64 years with diabetes: A population-based cross-sectional study from NHANES 1999-2018. BMC Public Health 2024, 24, 11. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Pan, A.; Hu, Y.; Chen, S.; Qian, F.; Rimm, E.B.; Manson, J.E.; Stampfer, M.J.; Giatsidis, G.; et al. Adherence to a Healthy Lifestyle in Association With Microvascular Complications Among Adults With Type 2 Diabetes. JAMA Netw Open 2023, 6, e2252239. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef]
- Uusitupa, M.; Khan, T.A.; Viguiliouk, E.; Kahleova, H.; Rivellese, A.A.; Hermansen, K.; Pfeiffer, A.; Thanopoulou, A.; Salas-Salvadó, J.; Schwab, U.; et al. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2611. [Google Scholar] [CrossRef] [PubMed]
- Bryl, A.; Mrugacz, M.; Falkowski, M.; Zorena, K. A Mediterranean Diet May Be Protective in the Development of Diabetic Retinopathy. Int. J. Mol. Sci. 2023, 24, 11145. [Google Scholar] [CrossRef]
- Díaz-López, A.; Babio, N.; Martínez-González, M.A.; Corella, D.; Amor, A.J.; Fitó, M.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Mediterranean Diet, Retinopathy, Nephropathy, and Microvascular Diabetes Complications: A Post Hoc Analysis of a Randomized Trial. Diabetes Care 2015, 38, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.; Mancini, F.; Rajaobelina, K.; Boutron-Ruault, M.C.; Balkau, B.; Bonnet, F.; Fagherazzi, G. Diet and risk of diabetic retinopathy: A systematic review. Eur. J. Epidemiol. 2018, 33, 141–156. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Ros, E.; Estruch, R. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 379, 1388–1389. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; a Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Tanaka, S.; Yoshimura, Y.; Kawasaki, R.; Kamada, C.; Horikawa, C.; Ohashi, Y.; Araki, A.; Ito, H.; Akanuma, Y.; Yamada, N.; et al. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology 2013, 24, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Granado-Casas, M.; Ramírez-Morros, A.; Martín, M.; Real, J.; Alonso, N.; Valldeperas, X.; Traveset, A.; Rubinat, E.; Alcubierre, N.; Hernández, M.; et al. Type 1 Diabetic Subjects with Diabetic Retinopathy Show an Unfavorable Pattern of Fat Intake. Nutrients 2018, 10, 1184. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, J.; Gu, W.; Zhao, X.; Xiao, L.; Yang, C. Dietary Inflammatory Index and diabetic retinopathy risk in US adults: Findings from NHANES (2005–2008). BMC Ophthalmol. 2024, 24, 46. [Google Scholar] [CrossRef]
- Shan, A.; Chen, X.; Yang, X.; Yao, B.; Liang, F.; Yang, Z.; Liu, F.; Chen, S.; Yan, X.; Huang, J.; et al. Association between long-term exposure to fine particulate matter and diabetic retinopathy among diabetic patients: A national cross-sectional study in China. Environ. Int. 2021, 154, 106568. [Google Scholar] [CrossRef]
- Pan, S.C.; Huang, C.C.; Chin, W.S.; Chen, B.Y.; Chan, C.C.; Guo, Y.L. Association between air pollution exposure and diabetic retinopathy among diabetics. Environ. Res. 2020, 181, 108960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Y.; Ye, Y.; Wang, L.; Bao, Y.; Wang, X.; Zhou, X.; Zhao, J. Associations Between Ambient Air Pollution and Five Common Vision-Threatening Ocular Diseases in Middle-Aged and Older Adults: A Large Prospective Cohort Study. Am. J. Ophthalmol. 2025, 274, 276–285. [Google Scholar] [CrossRef]
- Zuo, B.; Hu, Q.; Wu, Y.; Li, X.; Wang, B.; Yan, M.; Li, Y. Association between long-term exposure to air pollution and diabetic retinopathy: Evidence from the Fujian Eye Study. Ecotoxicol. Environ. Saf. 2024, 279, 116459. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, D.; Peng, L.; Wang, X.; Ding, Q.; Li, L.; Xu, T. Exposure to volatile organic compounds is a risk factor for diabetes retinopathy: A cross-sectional study. Front. Public Health 2024, 12, 1347671. [Google Scholar] [CrossRef]
- Moshfeghi, A.; Garmo, V.; Sheinson, D.; Ghanekar, A.; Abbass, I. Five-Year Patterns of Diabetic Retinopathy Progression in US Clinical Practice. Clin. Ophthalmol. 2020, 14, 3651–3659. [Google Scholar] [CrossRef]
- Blinder, K.J.; Dugel, P.U.; Chen, S.; Jumper, J.M.; Walt, J.G.; Hollander, D.A.; Scott, L.C. Anti-VEGF treatment of diabetic macular edema in clinical practice: Effectiveness and patterns of use (ECHO Study Report 1). Clin. Ophthalmol. 2017, 11, 393–401. [Google Scholar] [CrossRef]
- Ferrara, M.; Loda, A.; Coco, G.; Grassi, P.; Cestaro, S.; Rezzola, S.; Romano, V.; Semeraro, F. Diabetic Retinopathy: Soluble and Imaging Ocular Biomarkers. J. Clin. Med. 2023, 12, 912. [Google Scholar] [CrossRef]
- Katagiri, M.; Shoji, J.; Inada, N.; Kato, S.; Kitano, S.; Uchigata, Y. Evaluation of vitreous levels of advanced glycation end products and angiogenic factors as biomarkers for severity of diabetic retinopathy. Int. Ophthalmol. 2018, 38, 607–615. [Google Scholar] [CrossRef]
- Funatsu, H.; Yamashita, H.; Sakata, K.; Noma, H.; Mimura, T.; Suzuki, M.; Eguchi, S.; Hori, S. Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology 2005, 112, 806–816. [Google Scholar] [CrossRef]
- Funatsu, H.; Noma, H.; Mimura, T.; Eguchi, S.; Hori, S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009, 116, 73–79. [Google Scholar] [CrossRef]
- Yoshimura, T.; Sonoda, K.H.; Sugahara, M.; Mochizuki, Y.; Enaida, H.; Oshima, Y.; Ueno, A.; Hata, Y.; Yoshida, H.; Ishibashi, T. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS ONE 2009, 4, e8158. [Google Scholar] [CrossRef]
- Brooks, H.L.; Caballero, S.; Newell, C.K.; Steinmetz, R.L.; Watson, D.; Segal, M.S.; Harrison, J.K.; Scott, E.W.; Grant, M.B. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch. Ophthalmol. 2004, 122, 1801–1807. [Google Scholar] [CrossRef]
- McAuley, A.K.; Sanfilippo, P.G.; Hewitt, A.W.; Liang, H.; Lamoureux, E.; Wang, J.J.; Connell, P.P. Vitreous biomarkers in diabetic retinopathy: A systematic review and meta-analysis. J. Diabetes Complicat. 2014, 28, 419–425. [Google Scholar] [CrossRef]
- Newman, D.K. Surgical management of the late complications of proliferative diabetic retinopathy. Eye 2010, 24, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Jiang, F.; You, C.; Mao, C.; Yu, J.; Han, J.; Zhang, Z.; Yan, H. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS ONE 2014, 9, e110531. [Google Scholar] [CrossRef]
- Al-Dwairi, R.; El-Elimat, T.; Aleshawi, A.; Al Sharie, A.H.; Abu Mousa, B.M.; Al Beiruti, S.; Alkazaleh, A.; Mohidat, H. Vitreous Levels of Vascular Endothelial Growth Factor and Platelet-Derived Growth Factor in Patients with Proliferative Diabetic Retinopathy: A Clinical Correlation. Biomolecules 2023, 13, 1630. [Google Scholar] [CrossRef]
- Funatsu, H.; Yamashita, H.; Noma, H.; Mimura, T.; Nakamura, S.; Sakata, K.; Hori, S. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 243, 3–8. [Google Scholar] [CrossRef]
- Noma, H.; Funatsu, H.; Yamasaki, M.; Tsukamoto, H.; Mimura, T.; Sone, T.; Hirayama, T.; Tamura, H.; Yamashita, H.; Minamoto, A.; et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye 2008, 22, 42–48. [Google Scholar] [CrossRef]
- Trivedi, D.; Denniston, A.K.; Murray, P.I. Safety profile of anterior chamber paracentesis performed at the slit lamp. Clin. Exp. Ophthalmol. 2011, 39, 725–728. [Google Scholar] [CrossRef]
- Hillier, R.J.; Ojaimi, E.; Wong, D.T.; Mak, M.Y.K.; Berger, A.R.; Kohly, R.P.; Kertes, P.J.; Forooghian, F.; Boyd, S.R.; Eng, K.; et al. Aqueous Humor Cytokine Levels and Anatomic Response to Intravitreal Ranibizumab in Diabetic Macular Edema. JAMA Ophthalmol. 2018, 136, 382–388. [Google Scholar] [CrossRef]
- Felfeli, T.; Juncal, V.R.; Hillier, R.J.; Mak, M.Y.K.; Wong, D.T.; Berger, A.R.; Kohly, R.P.; Kertes, P.J.; Eng, K.T.; Boyd, S.R.; et al. Aqueous Humor Cytokines and Long-Term Response to Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema. Am. J. Ophthalmol. 2019, 206, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Manda, A.; Lee, L.H.; Steinkerchner, M.; Sheng, J.; Veach, L.; Gangaputra, S.; Kim, S.J. Analysis of Aqueous Interleukin-6 in Diabetic Retinopathy: A Prospective, Controlled Trial of 328 Eyes. Ophthalmol. Retin. 2025. [Google Scholar] [CrossRef]
- Haliyur, R.; Marwah, S.; Mittal, S.; Stein, J.D.; Shah, A.R.; Consortium, S. Demographic and Metabolic Risk Factors Associated with Development of Diabetic Macular Edema among Persons with Diabetes Mellitus. Ophthalmol. Sci. 2024, 4, 100557. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Saxena, S.; Akduman, L.; Meyer, C.H.; Kruzliak, P.; Khanna, V.K. Serum vascular endothelial growth factor is a biomolecular biomarker of severity of diabetic retinopathy. Int. J. Retin. Vitr. 2019, 5, 29. [Google Scholar] [CrossRef]
- Zhou, Z.; Ju, H.; Sun, M.; Chen, H. Serum Vascular Endothelial Growth Factor Levels Correlate with Severity of Retinopathy in Diabetic Patients: A Systematic Review and Meta-Analysis. Dis. Markers 2019, 2019, 9401628. [Google Scholar] [CrossRef] [PubMed]
- Frudd, K.; Sivaprasad, S.; Raman, R.; Krishnakumar, S.; Revathy, Y.R.; Turowski, P. Diagnostic circulating biomarkers to detect vision-threatening diabetic retinopathy: Potential screening tool of the future? Acta Ophthalmol. 2022, 100, e648–e668. [Google Scholar] [CrossRef]
- Kaviarasan, K.; Jithu, M.; Arif Mulla, M.; Sharma, T.; Sivasankar, S.; Das, U.N.; Angayarkanni, N. Low blood and vitreal BDNF, LXA4 and altered Th1/Th2 cytokine balance are potential risk factors for diabetic retinopathy. Metabolism 2015, 64, 958–966. [Google Scholar] [CrossRef]
- Fadini, G.P.; Avogaro, A. It is all in the blood: The multifaceted contribution of circulating progenitor cells in diabetic complications. Exp. Diabetes Res. 2012, 2012, 742976. [Google Scholar] [CrossRef]
- Caballero, S.; Hazra, S.; Bhatwadekar, A.; Li Calzi, S.; Paradiso, L.J.; Miller, L.P.; Chang, L.J.; Kern, T.S.; Grant, M.B. Circulating mononuclear progenitor cells: Differential roles for subpopulations in repair of retinal vascular injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3000–3009. [Google Scholar] [CrossRef][Green Version]
- Bhatwadekar, A.D.; Duan, Y.; Korah, M.; Thinschmidt, J.S.; Hu, P.; Leley, S.P.; Caballero, S.; Shaw, L.; Busik, J.; Grant, M.B. Hematopoietic stem/progenitor involvement in retinal microvascular repair during diabetes: Implications for bone marrow rejuvenation. Vision. Res. 2017, 139, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bhatwadekar, A.D.; Guerin, E.P.; Jarajapu, Y.P.; Caballero, S.; Sheridan, C.; Kent, D.; Kennedy, L.; Lansang, M.C.; Ruscetti, F.W.; Pepine, C.J.; et al. Transient inhibition of transforming growth factor-beta1 in human diabetic CD34+ cells enhances vascular reparative functions. Diabetes 2010, 59, 2010–2019. [Google Scholar] [CrossRef]
- Bhatwadekar, A.D.; Yan, Y.; Stepps, V.; Hazra, S.; Korah, M.; Bartelmez, S.; Chaqour, B.; Grant, M.B. miR-92a Corrects CD34+ Cell Dysfunction in Diabetes by Modulating Core Circadian Genes Involved in Progenitor Differentiation. Diabetes 2015, 64, 4226–4237. [Google Scholar] [CrossRef] [PubMed]
- Bhatwadekar, A.D.; Duan, Y.; Chakravarthy, H.; Korah, M.; Caballero, S.; Busik, J.V.; Grant, M.B. Ataxia Telangiectasia Mutated Dysregulation Results in Diabetic Retinopathy. Stem Cells 2016, 34, 405–417. [Google Scholar] [CrossRef][Green Version]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.; Zawadzki, R.J.; Werner, J.S.; Nolta, J. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: Preliminary phase 1 clinical trial findings. Investig. Ophthalmol. Vis. Sci. 2014, 56, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S. Cell Therapy Applications for Retinal Vascular Diseases: Diabetic Retinopathy and Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFj1–ORSFj10. [Google Scholar] [CrossRef]
- Jarajapu, Y.P.; Hazra, S.; Segal, M.; Li Calzi, S.; Jadhao, C.; Qian, K.; Mitter, S.K.; Raizada, M.K.; Boulton, M.E.; Grant, M.B. Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms. PLoS ONE 2014, 9, e93965. [Google Scholar] [CrossRef]
- Balaiya, S.; Grant, M.B.; Priluck, J.; Chalam, K.V. Growth factors/chemokines in diabetic vitreous and aqueous alter the function of bone marrow-derived progenitor (CD34+) cells in humans. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E695–E702. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.; Luo, Q.; Abhyankar, S.; Bhatwadekar, A.D. Transcriptomic Profile of Lin. BMC Genom. 2024, 25, 782. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Bhamidipalli, S.S.; Eckert, G.J.; Bhatwadekar, A.D. Hypermethylation of miRNA-17-92 cluster in peripheral blood mononuclear cells in diabetic retinopathy. Diabetes Metab. Syndr. 2022, 16, 102390. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Lavin, P.T.; Birch, M.; Shah, N.; Folk, J.C. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit. Med. 2018, 1, 39. [Google Scholar] [CrossRef]
- van der Heijden, A.A.; Abramoff, M.D.; Verbraak, F.; van Hecke, M.V.; Liem, A.; Nijpels, G. Validation of automated screening for referable diabetic retinopathy with the IDx-DR device in the Hoorn Diabetes Care System. Acta Ophthalmol. 2018, 96, 63–68. [Google Scholar] [CrossRef]
- Verbraak, F.D.; Abramoff, M.D.; Bausch, G.C.F.; Klaver, C.; Nijpels, G.; Schlingemann, R.O.; van der Heijden, A.A. Diagnostic Accuracy of a Device for the Automated Detection of Diabetic Retinopathy in a Primary Care Setting. Diabetes Care 2019, 42, 651–656. [Google Scholar] [CrossRef]
- Huang, X.; Wang, H.; She, C.; Feng, J.; Liu, X.; Hu, X.; Chen, L.; Tao, Y. Artificial intelligence promotes the diagnosis and screening of diabetic retinopathy. Front. Endocrinol. 2022, 13, 946915. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, K.; Zhang, X.; Wang, Y.; Chen, W.; Wang, X.; Ye, Y.; Lou, Y.; Li, L. A machine learning model for predicting anatomical response to Anti-VEGF therapy in diabetic macular edema. Front. Cell Dev. Biol. 2025, 13, 1603958. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, B.; Hu, Y.; Cao, D.; Yang, D.; Wu, Q.; Yang, J.; Peng, Q.; Huang, M.; Zhong, P.; et al. Automatic prediction of treatment outcomes in patients with diabetic macular edema using ensemble machine learning. Ann. Transl. Med. 2021, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Nandi, A.; Pramanik, S.; Mondal, L.K. Application of deep learning algorithm for judicious use of anti-VEGF in diabetic macular edema. Sci. Rep. 2025, 15, 4569. [Google Scholar] [CrossRef]
- Dai, L.; Sheng, B.; Chen, T.; Wu, Q.; Liu, R.; Cai, C.; Wu, L.; Yang, D.; Hamzah, H.; Liu, Y.; et al. A deep learning system for predicting time to progression of diabetic retinopathy. Nat. Med. 2024, 30, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Limoli, C.; Nucci, P. Novel artificial intelligence for diabetic retinopathy and diabetic macular edema: What is new in 2024? Curr. Opin. Ophthalmol. 2024, 35, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Channa, R.; Wolf, R.M.; Abràmoff, M.D.; Lehmann, H.P. Effectiveness of artificial intelligence screening in preventing vision loss from diabetes: A policy model. NPJ Digit. Med. 2023, 6, 53. [Google Scholar] [CrossRef] [PubMed]

| Genes | Chromosome | SNPs | Type of Diabetes | Type of DR | Geographical Location | References |
|---|---|---|---|---|---|---|
| ACE | 17 | Insertion/deletion (I/D) polymorphism in the ACE gene | T1DM and T2DM | DR | - | [41] |
| Insertion/deletion (I/D) polymorphism | T1DM | NPDR and PDR | Vienna | [42] | ||
| Insertion/deletion (I/D) polymorphism | T1DM and T2DM | DR | - | [43] | ||
| Insertion/deletion (I/D) polymorphism | T1DM and T2DM | DR | - | [44] | ||
| Insertion/deletion (I/D) polymorphism | T2DM | DR | Iran | [45] | ||
| Insertion/deletion (I/D) polymorphism | - | PDR and BDR | China | [46] | ||
| Insertion/deletion (I/D) polymorphism | T2DM | DR | Pakistan | [47] | ||
| Insertion/deletion (I/D) polymorphism | T2DM | DR | Asia | [48] | ||
| ACE I/D and AGT M/T gene polymorphisms | T2DM | DR | China | [49] | ||
| ACE gene (I/D) polymorphism (rs1799752) | T2DM | DR | - | [50] | ||
| Insertion/deletion (I/D) polymorphism | T2DM | DR | Mexico | [51] | ||
| Insertion/deletion (I/D) polymorphism | T1DM | DR | Pakistan | [52] | ||
| Insertion/deletion (I/D) polymorphism | T2DM | DR | India | [53] | ||
| APOE | 19 | e2/e3/e4 polymorphisms | T1DM | DR | Russia | [54] |
| e2/e3/e4 polymorphisms | T1DM and T2DM | NPDR and PDR | Brazil | [55] | ||
| e2/e3/e4 polymorphisms | T2DM | DR | USA | [56] | ||
| rs429358 and rs7412 | T2DM | DR | Czech Republic | [57] | ||
| AR | 7 | rs759853 | T2DM | DR | Egypt | [58] |
| C(-106)T polymorphism | T1DM and T2DM | DR | - | [59] | ||
| CAPN10 | 2 | SNP-43 1/1 | T2DM | DR | Poland | [60] |
| SNP43 | T2DM | DR | Poland | [61] | ||
| CEP135 | 4 | rs4865047 | T1DM | PDR | Lithuania | [62] |
| CRP | 1 | rs2808629, rs3093077, rs1130864 and rs2808634 | T2DM | DR | China | [63] |
| +1846 C>T polymorphism | T1DM | DR | Poland | [64] | ||
| EHD3 | 2 | - | T2DM | DR | Japan | [65] |
| eNOS | 7 | eNOS4b/b, eNOS4b/a and eNOS4a/a | T1DM | NPDR and PDR | Parice, France | [66] |
| −786*C/T | T1DM | DR | - | [67] | ||
| eNOS4 allele | T2DM | PDR | India | [68] | ||
| −786T/C, the VNTR intron 4 a/b and the 894G/T (Glu298Asp) polymorphisms | T2DM | DR | Brazil | [69] | ||
| T-786C, G894T and 27VNTR | T2DM | DR | Asian Indian | [70] | ||
| eNOS-4b/a polymorphism | T2DM | DR | - | [71] | ||
| T786C and G894T | T2DM | DR | Greece | [72] | ||
| eNOS 4a/b polymorphism | T2DM | DR | - | [73] | ||
| intron 4ab, exon 7 Glu298Asp variant (G894T), and T-786C | T2DM | DR | Jordan | [74] | ||
| FAM18B | 17 | rs11871508 (G>A) | - | PDR | - | [75] |
| GORAB or SCYL1BP1 | 1 | rs6427247 | T2DM | DR | China | [76] |
| GRB2 | 17 | rs3805931 and rs9896052 | T2DM | STDR | Australia | [77] |
| rs9896052 | T2DM | PDR | Brazil | [78] | ||
| HS6ST3 | 13 | rs2038823 | T2DM | DR | Taiwan | [79] |
| ICAM1 | 19 | 469 (K/K, K/E and E/E allele) | T2DM | DR | Japan | [80] |
| 469E (EE) and G241A | T2DM | PDR | Slovenia | [81] | ||
| K469E (rs5498) | T2DM | DR | India | [82] | ||
| rs1801714 | T2DM | NPDR | - | [83] | ||
| rs5498 | T2DM | DR | China | [84] | ||
| rs5498 | T2DM | PDR and NPDR | China | [85] | ||
| rs5498 | T2DM | DR | - | [86] | ||
| IL-6 | 7 | rs1800795 GC and rs1800796 GG | T2DM | PDR | China | [87] |
| rs1800795 and rs1800796, | - | DR | - | [88] | ||
| rs1800795, rs1800796 and rs1800797 rs2069837 and rs2069840 | - | DR | - | [89] | ||
| INSR | 19 | rs2115386 | T2DM | STDR and PDR | China | [90] |
| JPH2 | 20 | rs761207 and rs6031415 | T2DM | DR | Taiwan | [91] |
| KCNJ11 | 11 | rs2285676 | T2DM | DR | Italy | [92] |
| rs5219 | T2DM | DR | China | [93] | ||
| MTHFR | 1 | C677T polymorphism | T2DM | DR | Japan | [94] |
| 677C/T polymorphism | T1DM and T2DM | DR | - | [95] | ||
| A1298C | DM | DR | Middle East Countries | [96] | ||
| rs1801133 | T2DM | DR | Pakistan | [97] | ||
| MYO5C | 15 | rs3751624 | T2DM | NPDR and PDR | - | [98] |
| MYSM1 | 1 | rs2811893 and rs12092121 | T2DM | DR | Taiwan | [79] |
| NPY2R | 4 | rs1902491 | T1DM | PDR | Lithuania | [62] |
| NVL | 1 | rs142293996 | T2DM | DR | - | [99] |
| PAI-1 | 7 | 4G/5G (deletion/insertion) polymorphism | T2DM | DR | Pakistan | [47] |
| 4G/5G and −844G/A | T2DM | DR | Tunisia | [100] | ||
| rs2070682 | T2DM | DR | Greece | [101] | ||
| 4G5G polymorphism | DM | DR | - | [102] | ||
| PALM2 | 9 | rs140508424 | T2DM | DR | Japan | [65] |
| PLXDC2 | 10 | rs1571942 (C/T) | T2DM | DR | Taiwan | [79] |
| PPARα | 3 | rs4253778, rs135539 and rs1800206 | T2DM | DR | China | [103] |
| PPARγ | 3 | Pro12Ala, C1431T, C-2821T, A-2819G | T2DM | PDR | Italy | [104] |
| rs1805192, rs709158, rs3856806, rs4684847 | T2DM | DR | China | [105] | ||
| rs1801282 (Pro12Ala) | T2DM | DR | Poland | [61] | ||
| rs1801282 C/G and rs3856806 C/T polymorphism | DM | DR | Europe and Asia | [106] | ||
| PTPN1 | 20 | rs3787345 and rs754118 | T2DM | DR | Poland | [61] |
| RNLS | 10 | rs2296545 | T2DM | DR | Poland | [107] |
| SELP | 1 | rs6128, rs6133, and rs3917779 | T2DM | DR | - | [108] |
| rs6128 | T2DM | DR; PDR | USA | [109] | ||
| rs6128, rs6133, and rs3917779 | T2DM | PDR | Iran | [110] | ||
| STT3B | 3 | rs12630354 | T2DM | DR | Japan | [65] |
| TIMP3 | 22 | −899T/A, −915A/G and −1296T/C | T2DM | PDR | - | [111] |
| VEGF | 6 | C(−634)G polymorphism | T2DM | DR; PDR | Japan | [112] |
| C(−7)T and C(−634)G in the 5′ UTR | T2DM | DR | India | [68] | ||
| I/D polymorphism | T2DM | DR | Lublin | [113] | ||
| −634 (the G/C polymorphism) and −460 (the C/T polymorphism) | T2DM | NPDR and PDR | Poland | [114] | ||
| rs201963 or −634C/G and 936C/T polymorphisms | T2DM | DR | India | [115] | ||
| −160C, −152A (rs13207351), and −116A (rs1570360); +4618 (rs735286) and +5092 (rs2146323) | DM | PDR | Northern European Region | [116] | ||
| rs699947, rs833061, rs13207351, rs2010963, rs833069, rs2146323, rs3025021, and rs3025039 | T2DM | DR | China | [117] | ||
| −460T/C and −2578C/A polymorphisms | - | DR | - | [118] | ||
| rs2010963, rs833061 and rs699947 | T1DM and T2DM | NPDR and PDR | - | [119] | ||
| rs699947 | T2DM | DR | Indonesia | [120] | ||
| −2578C/A (rs699947) and −460T/C (rs833061) | DM | NPDR and PDR | Egypt | [121] | ||
| rs699947 and rs35569394 | T2DM | DR | India | [122] | ||
| ZNRF1 | 16 | rs17684886 | T2DM | NPDR and PDR | China | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaurich, C.; Mahajan, N.; Bhatwadekar, A.D. Precision Medicine for Diabetic Retinopathy: Integrating Genetics, Biomarkers, Lifestyle, and AI. Genes 2025, 16, 1096. https://doi.org/10.3390/genes16091096
Kaurich C, Mahajan N, Bhatwadekar AD. Precision Medicine for Diabetic Retinopathy: Integrating Genetics, Biomarkers, Lifestyle, and AI. Genes. 2025; 16(9):1096. https://doi.org/10.3390/genes16091096
Chicago/Turabian StyleKaurich, Connor, Neha Mahajan, and Ashay D. Bhatwadekar. 2025. "Precision Medicine for Diabetic Retinopathy: Integrating Genetics, Biomarkers, Lifestyle, and AI" Genes 16, no. 9: 1096. https://doi.org/10.3390/genes16091096
APA StyleKaurich, C., Mahajan, N., & Bhatwadekar, A. D. (2025). Precision Medicine for Diabetic Retinopathy: Integrating Genetics, Biomarkers, Lifestyle, and AI. Genes, 16(9), 1096. https://doi.org/10.3390/genes16091096






