Single-Cell Transcriptomics in Inherited Retinal Dystrophies: Current Findings and Emerging Perspectives
Abstract
1. Introduction
2. From Bulk to Single-Cell Transcriptomics in IRDs
3. Disease-Specific Insights from Single-Cell Transcriptomics
3.1. Retinitis Pigmentosa
3.1.1. RHO Mutations
3.1.2. PDE6B Mutations
3.1.3. RPGR Mutations
3.1.4. USH Mutations
3.1.5. Other RP Gene Mutations
3.2. Leber Congenital Amaurosis
3.3. Enhanced S-Cone Syndrome
3.4. Stargardt Disease
3.5. Achromatopsia
4. Common Themes and Therapeutic Implications
5. Challenges and Future Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated virus |
| ACHM | Achromatopsia |
| adRP | Autosomal dominant retinitis pigmentosa |
| Ca2+ | Calcium ion |
| CD | Cone dystrophy |
| CNG channels | Cyclic nucleotide-gated channels |
| CRD | Cone–rod dystrophy |
| CTRL | Control |
| CSNB | Congenital stationary night blindness |
| D | Day |
| EGR1 | Early growth response 1 |
| ER | Endoplasmic reticulum |
| ERG | Electroretinography |
| ERK | Extracellular signal-regulated kinase |
| ESCS | Enhanced S-cone syndrome |
| FGF21 | Fibroblast growth factor 21 |
| GSE ID | Gene expression omnibus series identifier |
| H3K27 | Histone H3 lysine 27 |
| HDAC | Histone deacetylase |
| HET | Heterozygous |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| iPSCs | Induced pluripotent stem cells |
| IRD | Inherited retinal dystrophy |
| JNK | c-Jun N-terminal kinase |
| LCA | Leber congenital amaurosis |
| MAPK | Mitogen-activated protein kinase |
| MD | Macular dystrophy |
| MeSH | Medical Subject Headings |
| MUT | Mutant |
| NCX | Sodium–calcium exchanger |
| P | Postnatal day |
| PARP | Poly (ADP-ribose) polymerase |
| PCR | Polymerase chain reaction |
| PKG | Protein kinase G |
| PW | Postnatal week |
| rd | Retinal degeneration |
| RNA-seq | RNA sequencing |
| rOrg | Retinal organoid |
| RP | Retinitis pigmentosa |
| RPE | Retinal pigment epithelium |
| S-cone | Short-wavelength cone |
| scRNA-seq | Single-cell RNA sequencing |
| STGD | Stargardt disease |
| USH | Usher syndrome |
| V | Version |
| VGCC | Voltage-gated calcium channel |
| vs. | Versus |
| WT | Wild-type |
| XLRP | X-linked retinitis pigmentosa |
References
- Sahel, J.A.; Marazova, K.; Audo, I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb. Perspect. Med. 2014, 5, a017111. [Google Scholar] [CrossRef]
- Wright, A.F.; Chakarova, C.F.; Abd El-Aziz, M.M.; Bhattacharya, S.S. Photoreceptor degeneration: Genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet. 2010, 11, 273–284. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- RetNet, the Retinal Information Network. Available online: https://RetNet.org/ (accessed on 11 July 2025).
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shi, W.; Cheng, L.; Wang, Y.; Chen, D.; Hu, X.; Xu, J.; Xu, L.; Wu, Y.; Qu, J.; et al. Identification of a rhodopsin gene mutation in a large family with autosomal dominant retinitis pigmentosa. Sci. Rep. 2016, 6, 19759. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gil, N.; Maneu, V.; Kutsyr, O.; Fernández-Sánchez, L.; Sánchez-Sáez, X.; Sánchez-Castillo, C.; Campello, L.; Lax, P.; Pinilla, I.; Cuenca, N. Cellular and molecular alterations in neurons and glial cells in inherited retinal degeneration. Front. Neuroanat. 2022, 16, 984052. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.B.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Menon, M.; Mohammadi, S.; Davila-Velderrain, J.; Goods, B.A.; Cadwell, T.D.; Xing, Y.; Stemmer-Rachamimov, A.; Shalek, A.K.; Love, J.C.; Kellis, M.; et al. Single-cell transcriptomic atlas of the human retina identifies cell types associated with age-related macular degeneration. Nat. Commun. 2019, 10, 4902. [Google Scholar]
- Voigt, A.P.; Mullin, N.K.; Stone, E.M.; Tucker, B.A.; Scheetz, T.E.; Mullins, R.F. Single-cell RNA sequencing in vision research: Insights into human retinal health and disease. Prog. Retin. Eye Res. 2021, 83, 100934. [Google Scholar] [CrossRef]
- Tzec-Interián, J.A.; González-Padilla, D.; Góngora-Castillo, E.B. Bioinformatics perspectives on transcriptomics: A comprehensive review of bulk and single-cell RNA sequencing analyses. Quant. Biol. 2025, 13, e78. [Google Scholar] [CrossRef]
- Nguyen, A.; Khoo, W.H.; Moran, I.; Croucher, P.I.; Phan, T.G. Single Cell RNA Sequencing of Rare Immune Cell Populations. Front. Immunol. 2018, 9, 1553. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, K.; Whitney, I.E.; Butrus, S.; Peng, Y.-R.; Sanes, J.R. Diversification of multipotential postmitotic mouse retinal ganglion cell precursors into discrete types. eLife 2022, 11, e73809. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef]
- Tomita, Y.; Qiu, C.; Bull, E.; Allen, W.; Kotoda, Y.; Talukdar, S.; Smith, L.E.H.; Fu, Z. Müller glial responses compensate for degenerating photoreceptors in retinitis pigmentosa. Exp. Mol. Med. 2021, 53, 1748–1758. [Google Scholar] [CrossRef]
- Fu, Z.; Qiu, C.; Cagnone, G.; Tomita, Y.; Huang, S.; Cakir, B.; Kotoda, Y.; Allen, W.; Bull, E.; Akula, J.D.; et al. Retinal glial remodeling by FGF21 preserves retinal function during photoreceptor degeneration. iScience 2021, 24, 102376. [Google Scholar] [CrossRef]
- Santhanam, A.; Shihabeddin, E.; Wei, H.; Wu, J.; O’Brien, J. Molecular basis of retinal remodeling in a zebrafish model of retinitis pigmentosa. Cell. Mol. Life Sci. 2023, 80, 362. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Z.L.; Zhang, X.; Wang, W.; Huang, Z.Q.; Sun, S.N.; Jin, Z.B. Modeling autosomal dominant retinitis pigmentosa by using patient-specific retinal organoids with a class-3 RHO mutation. Exp. Eye Res. 2024, 241, 109856. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, Y.; Yan, J.; Wang, L.; Yu, S.; Jiao, K.; Paquet-Durand, F. Single-Cell Transcriptomic Profiling in Inherited Retinal Degeneration Reveals Distinct Metabolic Pathways in Rod and Cone Photoreceptors. Int. J. Mol. Sci. 2022, 23, 12170. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, W.; Li, Y.; Wei, C.; Hu, Y.; Hu, Z.; Paquet-Durand, F.; Jiao, K. Inhibition of the MAPK/c-Jun-EGR1 Pathway Decreases Photoreceptor Cell Death in the Rd1 Mouse Model Inherit. Retin. Degeneration. Int. J. Mol. Sci. 2022, 23, 14600. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, J.; Xu, W.; Paquet-Durand, F.; Hu, Z.; Jiao, K. HDAC inhibition delays photoreceptor loss in Pde6b Mutant Mice Retin. Pigment. Insights ScRNA-Seq CUT&Tag. PeerJ 2023, 11, e15659. [Google Scholar] [PubMed]
- Yan, J.; Wang, L.; Yang, Q.L.; Yang, Q.X.; He, X.; Dong, Y.; Hu, Z.; Seeliger, M.W.; Jiao, K.; Paquet-Durand, F. T-type voltage-gated channels, Na+/Ca2+-exchanger, and calpain-2 promote photoreceptor cell death in inherited retinal degeneration. Cell Commun. Signal. 2024, 22, 92. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Chen, S.; Chen, X.; Yi, W.; Fan, Y.; Chen, Y.; Ye, T.; Chen, Y. Inhibition of JNK ameliorates rod photoreceptor degeneration in a mouse model of retinitis pigmentosa. FEBS Lett. 2024, 598, 2683–2701. [Google Scholar] [CrossRef] [PubMed]
- Karademir, D.; Todorova, V.; Ebner, L.J.A.; Samardzija, M.; Grimm, C. Single-cell RNA sequencing of the retina in a model of retinitis pigmentosa reveals early responses to degeneration in rods and cones. BMC Biol. 2022, 20, 86. [Google Scholar] [CrossRef]
- Sigurdsson, D.; Grimm, C. Single-Cell Transcriptomic Profiling of Müller Glia in the rd10 Retina. Adv. Exp. Med. Biol. 2023, 1415, 377–381. [Google Scholar]
- Newton, F.; Halachev, M.; Nguyen, L.; McKie, L.; Mill, P.; Megaw, R. Autophagy disruption and mitochondrial stress precede photoreceptor necroptosis in multiple mouse models of inherited retinal disorders. Nat. Commun. 2025, 16, 4024. [Google Scholar] [CrossRef]
- Li, T.; Ma, Y.; Cheng, Y.; Zhao, Y.; Qiu, Z.; Liu, H.; Zhang, D.; Wu, J.; Li, J.; Zhang, S.; et al. Single-Cell Transcriptomic Dataset of RPGR-associated Retinitis Pigmentosa Patient-Derived Retinal Organoids. Sci. Data 2024, 11, 1285. [Google Scholar] [CrossRef]
- Leong, Y.C.; Di Foggia, V.; Pramod, H.; Bitner-Glindzicz, M.; Patel, A.; Sowden, J.C. Molecular pathology of Usher 1B patient-derived retinal organoids at single cell resolution. Stem Cell Rep. 2022, 17, 2421–2437. [Google Scholar] [CrossRef]
- Bertrand, R.E.; Wang, J.; Li, Y.; Cheng, X.; Wang, K.; Stoilov, P.; Chen, R. Cwc27, associated with retinal degeneration, functions as a splicing factor in Vivo. Hum. Mol. Genet. 2022, 31, 1278–1292. [Google Scholar] [CrossRef]
- Atkinson, R.; Georgiou, M.; Yang, C.; Szymanska, K.; Lahat, A.; Vasconcelos, E.J.R.; Ji, Y.; Moya Molina, M.; Collin, J.; Queen, R.; et al. PRPF8-mediated dysregulation of hBrr2 helicase disrupts human spliceosome kinetics and 5′-splice-site selection causing tissue-specific defects. Nat. Commun. 2024, 15, 3138. [Google Scholar] [CrossRef]
- Boon, N.; Lu, X.; Andriessen, C.A.; Moustakas, I.; Buck, T.M.; Freund, C.; Arendzen, C.H.; Böhringer, S.; Mei, H.; Wijnholds, J. AAV-mediated gene augmentation therapy of CRB1 patient-derived retinal organoids restores the histological and transcriptional retinal phenotype. Stem Cell Rep. 2023, 18, 1123–1137. [Google Scholar] [CrossRef] [PubMed]
- Shigesada, N.; Shikada, N.; Shirai, M.; Toriyama, M.; Higashijima, F.; Kimura, K.; Kondo, T.; Bessho, Y.; Shinozuka, T.; Sasai, N. Combination of blockade of endothelin signalling and compensation of IGF1 expression protects the retina from degeneration. Cell. Mol. Life Sci. 2024, 81, 51. [Google Scholar] [CrossRef]
- Lewandowski, D.; Foik, A.T.; Smidak, R.; Choi, E.H.; Zhang, J.; Hoang, T.; Tworak, A.; Suh, S.; Leinonen, H.; Dong, Z.; et al. Inhibition of ceramide accumulation in AdipoR1-/- mice increases photoreceptor survival and improves vision. JCI Insight 2022, 7, e156301. [Google Scholar] [CrossRef] [PubMed]
- Fogerty, J.; Song, P.; Boyd, P.; Grabinski, S.E.; Hoang, T.; Reich, A.; Cianciolo, L.T.; Blackshaw, S.; Mumm, J.S.; Hyde, D.R.; et al. Notch Inhibition Promotes Regeneration and Immunosuppression Supports Cone Survival in a Zebrafish Model of Inherited Retinal Dystrophy. J. Neurosci. 2022, 42, 5144–5158. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Suh, S.; Foik, A.T.; Leinonen, H.; Newby, G.A.; Gao, X.D.; Banskota, S.; Hoang, T.; Du, S.W.; Dong, Z.; et al. In Vivo Base Editing Rescues Cone Photoreceptors A Mouse Model of Early-Onset Inherited Retinal Degeneration. Nat. Commun. 2022, 13, 1830. [Google Scholar] [CrossRef]
- Kruczek, K.; Qu, Z.; Gentry, J.; Fadl, B.R.; Gieser, L.; Hiriyanna, S.; Batz, Z.; Samant, M.; Samanta, A.; Chu, C.J.; et al. Gene Therapy of Dominant CRX-Leber Congenital Amaurosis using Patient Stem Cell-Derived Retinal Organoids. Stem Cell Rep. 2021, 16, 252–263. [Google Scholar] [CrossRef]
- Mullin, N.K.; Bohrer, L.R.; Voigt, A.P.; Lozano, L.P.; Wright, A.T.; Bonilha, V.L.; Mullins, R.F.; Stone, E.M.; Tucker, B.A. NR2E3 loss disrupts photoreceptor cell maturation and fate in human organoid models of retinal development. J. Clin. Investig. 2024, 134, e173892. [Google Scholar] [CrossRef]
- Aísa-Marín, I.; Rovira, Q.; Díaz, N.; Calvo-López, L.; Vaquerizas, J.M.; Marfany, G. Specific photoreceptor cell fate pathways are differentially altered in NR2E3-associated diseases. Neurobiol. Dis. 2024, 194, 106463. [Google Scholar] [CrossRef]
- Watson, A.; Queen, R.; Ferrández-Peral, L.; Dorgau, B.; Collin, J.; Nelson, A.; Hussain, R.; Coxhead, J.; McCorkindale, M.; Atkinson, R.; et al. Unravelling genotype-phenotype correlations in Stargardt disease using patient-derived retinal organoids. Cell Death Dis. 2025, 16, 108. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, Y.; Li, T.; Wu, J.; Li, C.; Zhang, S.; Wu, J. Longitudinal scRNA-seq of retinal organoids derived from Stargardt disease patient with ABCA4 mutation. Sci. Data 2025, 12, 878. [Google Scholar] [CrossRef]
- Miller, A.L.; Fuller-Carter, P.I.; Masarini, K.; Samardzija, M.; Carter, K.W.; Rashwan, R.; Lim, X.R.; Brunet, A.A.; Chopra, A.; Ram, R.; et al. Increased H3K27 trimethylation contributes to cone survival in a mouse model of cone dystrophy. Cell. Mol. Life Sci. 2022, 79, 409. [Google Scholar] [CrossRef]
- Cross, N.; van Steen, C.; Zegaoui, Y.; Satherley, A.; Angelillo, L. Retinitis Pigmentosa: Burden of Disease and Current Unmet Needs. Clin. Ophthalmol. 2022, 16, 1993–2010. [Google Scholar] [CrossRef]
- Dryja, T.P.; McGee, T.L.; Hahn, L.B.; Cowley, G.S.; Olsson, J.E.; Reichel, E.; Sandberg, M.A.; Berson, E.L. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N. Engl. J. Med. 1990, 323, 1302–1307. [Google Scholar] [CrossRef]
- Zhen, F.; Zou, T.; Wang, T.; Zhou, Y.; Dong, S.; Zhang, H. Rhodopsin-associated retinal dystrophy: Disease mechanisms and therapeutic strategies. Front. Neurosci. 2023, 17, 1132179. [Google Scholar] [CrossRef]
- Yang, C.; Georgiou, M.; Atkinson, R.; Collin, J.; Al-Aama, J.; Nagaraja-Grellscheid, S.; Johnson, C.; Ali, R.; Armstrong, L.; Mozaffari-Jovin, S.; et al. Pre-mRNA Processing Factors and Retinitis Pigmentosa: RNA Splicing and Beyond. Front. Cell Dev. Biol. 2021, 9, 700276. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, Y.; Yi, J.; Xu, H.; Yuan, L.; Yang, Z.; Deng, H. Heterozygous RHO P.R135W Missense Mutat. A Large Han-Chin. Fam. Retin. Pigment. Differ. Refract. Errors. Biosci. Rep. 2019, 39, BSR20182198. [Google Scholar] [CrossRef] [PubMed]
- Mendes, H.F.; van der Spuy, J.; Chapple, J.P.; Cheetham, M.E. Mechanisms of cell death in rhodopsin retinitis pigmentosa: Implications for therapy. Trends Mol. Med. 2005, 11, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xia, X.; Li, H.; Zhang, Y.; Zhou, X.; Jiang, H. A new rhodopsin R135W mutation induces endoplasmic reticulum stress and apoptosis in retinal pigment epithelial cells. J. Cell. Physiol. 2019, 234, 14100–14108. [Google Scholar] [CrossRef]
- Khramtsov, N.V.; Feshchenko, E.A.; Suslova, V.A.; Shmukler, B.E.; Terpugov, B.E.; Rakitina, T.V.; Atabekova, N.V.; Lipkin, V.M. The human rod photoreceptor cGMP phosphodiesterase beta-subunit. Structural studies of its cDNA and gene. FEBS Lett. 1993, 327, 275–278. [Google Scholar] [CrossRef]
- Huang, S.H.; Pittler, S.J.; Huang, X.; Oliveira, L.; Berson, E.L.; Dryja, T.P. Autosomal recessive retinitis pigmentosa caused by mutations in the alpha subunit of rod cGMP phosphodiesterase. Nat. Genet. 1995, 11, 468–471. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.; Dai, H.; Li, G. Novel variants in PDE6A and PDE6B genes and its phenotypes in patients with retinitis pigmentosa in Chinese families. BMC Ophthalmol. 2022, 22, 27. [Google Scholar] [CrossRef]
- Mittal, R.; Bencie, N.; Parrish, J.M.; Liu, G.; Mittal, J.; Yan, D.; Liu, X.Z. An Update on Phosphodiesterase Mutations Underlying Genetic Etiology of Hearing Loss and Retinitis Pigmentosa. Front. Genet. 2018, 9, 9. [Google Scholar] [CrossRef]
- Kuehlewein, L.; Zobor, D.; Stingl, K.; Kempf, M.; Nasser, F.; Bernd, A.; Biskup, S.; Cremers, F.P.M.; Khan, M.I.; Mazzola, P.; et al. Clinical Phenotype of PDE6B-Assoc. Retin. Pigmentosa. Int. J. Mol. Sci. 2021, 22, 2374. [Google Scholar] [CrossRef]
- Caley, D.W.; Johnson, C.; Liebelt, R.A. The postnatal development of the retina in the normal and rodless CBA mouse: A light and electron microscopic study. Am. J. Anat. 1972, 133, 179–212. [Google Scholar] [CrossRef] [PubMed]
- LaVail, M.M.; Sidman, R.L. C57BL-6J mice with inherited retinal degeneration. Arch. Ophthalmol. 1974, 91, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Carter-Dawson, L.D.; LaVail, M.M.; Sidman, R.L. Differential effect of the rd mutation on rods and cones in the mouse retina. Investig. Ophthalmol. Vis. Sci. 1978, 17, 489–498. [Google Scholar]
- Samardzija, M.; Wariwoda, H.; Imsand, C.; Huber, P.; Heynen, S.R.; Gubler, A.; Grimm, C. Activation of survival pathways in the degenerating retina of rd10 mice. Exp. Eye Res. 2012, 99, 17–26. [Google Scholar] [CrossRef]
- Gargini, C.; Terzibasi, E.; Mazzoni, F.; Strettoi, E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: A morphological and ERG study. J. Comp. Neurol. 2007, 500, 222–238. [Google Scholar] [CrossRef]
- Barhoum, R.; Martínez-Navarrete, G.; Corrochano, S.; Germain, F.; Fernandez-Sanchez, L.; de la Rosa, E.J.; de la Villa, P.; Cuenca, N. Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience 2008, 155, 698–713. [Google Scholar] [CrossRef]
- Scimeca, J.C.; Servant, M.J.; Dyer, J.O.; Meloche, S. Essential role of calcium in the regulation of MAP kinase phosphatase-1 expression. Oncogene 1997, 15, 717–725. [Google Scholar] [CrossRef]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef]
- Hoffmann, E.; Ashouri, J.; Wolter, S.; Doerrie, A.; Dittrich-Breiholz, O.; Schneider, H.; Wagner, E.F.; Troppmair, J.; Mackman, N.; Kracht, M. Transcriptional regulation of EGR-1 by the interleukin-1-JNK-MKK7-c-Jun pathway. J. Biol. Chem. 2008, 283, 12120–12128. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Black, G.C.; Rice, J.M.; Hart-Holden, N.; Jones, A.; O’Grady, A.; Ramsden, S.; Wright, A.F. RPGR mutation analysis and disease: An update. Hum. Mutat. 2007, 28, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Megaw, R.D.; Soares, D.C.; Wright, A.F. RPGR: Its role in photoreceptor physiology, human disease, and future therapies. Exp. Eye Res. 2015, 138, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Cehajic Kapetanovic, J.; McClements, M.E.; Martinez-Fernandez de la Camara, C.; MacLaren, R.E. Molecular Strategies for RPGR Gene Therapy. Genes 2019, 10, 674. [Google Scholar] [CrossRef]
- Megaw, R.; Abu-Arafeh, H.; Jungnickel, M.; Mellough, C.; Gurniak, C.; Witke, W.; Zhang, W.; Khanna, H.; Mill, P.; Dhillon, B.; et al. Gelsolin dysfunction causes photoreceptor loss in induced pluripotent cell and animal retinitis pigmentosa models. Nat. Commun. 2017, 8, 271. [Google Scholar] [CrossRef]
- Megaw, R.; Moye, A.; Zhang, Z.; Newton, F.; McPhie, F.; Murphy, L.C.; McKie, L.; He, F.; Jungnickel, M.K.; von Kriegsheim, A.; et al. Ciliary tip actin dynamics regulate photoreceptor outer segment integrity. Nat. Commun. 2024, 15, 4316. [Google Scholar] [CrossRef]
- Birch, D.G.; Cheetham, J.K.; Daiger, S.P.; Hoyng, C.; Kay, C.; MacDonald, I.M.; Pennesi, M.E.; Sullivan, L.S. Overcoming the Challenges to Clinical Development of X-Linked Retinitis Pigmentosa Therapies: Proceedings of an Expert Panel. Transl. Vis. Sci. Technol. 2023, 12, 5. [Google Scholar] [CrossRef]
- Sandberg, M.A.; Rosner, B.; Weigel-DiFranco, C.; Dryja, T.P.; Berson, E.L. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1298–1304. [Google Scholar] [CrossRef]
- Zito, I.; Downes, S.M.; Patel, R.J.; Cheetham, M.E.; Ebenezer, N.D.; Jenkins, S.A.; Bhattacharya, S.S.; Webster, A.R.; Holder, G.E.; Bird, A.C.; et al. RPGR mutation associated with retinitis pigmentosa, impaired hearing, and sinorespiratory infections. J. Med. Genet. 2003, 40, 609–615. [Google Scholar] [CrossRef]
- Iannaccone, A.; Breuer, D.K.; Wang, X.F.; Kuo, S.F.; Normando, E.M.; Filippova, E.; Baldi, A.; Hiriyanna, S.; MacDonald, C.B.; Baldi, F.; et al. Clinical and immunohistochemical evidence for an X linked retinitis pigmentosa syndrome with recurrent infections and hearing loss in association with an RPGR mutation. J. Med. Genet. 2003, 40, e118. [Google Scholar] [CrossRef] [PubMed]
- Demirci, F.Y.; Rigatti, B.W.; Wen, G.; Radak, A.L.; Mah, T.S.; Baic, C.L.; Traboulsi, E.I.; Alitalo, T.; Ramser, J.; Gorin, M.B. X-linked cone-rod dystrophy (locus COD1): Identification of mutations in RPGR exon ORF15. Am. J. Hum. Genet. 2002, 70, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, N.D.; Michaelides, M.; Jenkins, S.A.; Audo, I.; Webster, A.R.; Cheetham, M.E.; Stockman, A.; Maher, E.R.; Ainsworth, J.R.; Yates, J.R.; et al. Identification of novel RPGR ORF15 mutations in X-linked progressive cone-rod dystrophy (XLCORD) families. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.G.; Michaelides, M.; Luong, V.A.; Holder, G.E.; Bird, A.C.; Webster, A.R.; Moore, A.T.; Fitzke, F.W. Functional correlates of fundus autofluorescence abnormalities in patients with RPGR or RIMS1 mutations causing cone or cone rod dystrophy. Br. J. Ophthalmol. 2008, 92, 95–102. [Google Scholar] [CrossRef]
- Birtel, J.; Eisenberger, T.; Gliem, M.; Müller, P.L.; Herrmann, P.; Betz, C.; Zahnleiter, D.; Neuhaus, C.; Lenzner, S.; Holz, F.G.; et al. Clinical and genetic characteristics of 251 consecutive patients with macular and cone/cone-rod dystrophy. Sci. Rep. 2018, 8, 4824. [Google Scholar] [CrossRef]
- Wu, Y.; Tavares, E.; Liang, B.; Wee, W.; Mennella, V.; Feng, H.; Cao, J.; Zheng, J.; He, M.; Stephenson, K.; et al. RPGR regulates motile cilia by interfering with actin dynamics. bioRxiv 2025. [Google Scholar] [CrossRef]
- D’Esposito, F.; Gagliano, G.; Gagliano, C.; Maniaci, A.; Avitabile, A.; Giglio, R.; Reibaldi, M.; Cordeiro, M.F.; Zeppieri, M. Usher Syndrome: New Insights into Classification, Genotype-Phenotype Correlation, and Management. Genes 2025, 16, 332. [Google Scholar] [CrossRef]
- Kremer, H.; van Wijk, E.; Märker, T.; Wolfrum, U.; Roepman, R. Usher syndrome: Molecular links of pathogenesis, proteins and pathways. Hum. Mol. Genet. 2006, 15, R262–R270. [Google Scholar] [CrossRef]
- Smith, R.J.; Berlin, C.I.; Hejtmancik, J.F.; Keats, B.J.; Kimberling, W.J.; Lewis, R.A.; Möller, C.G.; Pelias, M.Z.; Tranebjaerg, L. Clinical diagnosis of the Usher syndromes. Usher Syndrome Consortium. Am. J. Med. Genet. 1994, 50, 32–38. [Google Scholar] [CrossRef]
- Delmaghani, S.; El-Amraoui, A. The genetic and phenotypic landscapes of Usher syndrome: From disease mechanisms to a new classification. Hum. Genet. 2022, 141, 709–735. [Google Scholar] [CrossRef]
- Mathur, P.; Yang, J. Usher syndrome: Hearing loss, retinal degeneration and associated abnormalities. Biochim. Biophys. Acta 2015, 1852, 406–420. [Google Scholar] [CrossRef]
- Weil, D.; Blanchard, S.; Kaplan, J.; Guilford, P.; Gibson, F.; Walsh, J.; Mburu, P.; Varela, A.; Levilliers, J.; Weston, M.D. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 1995, 374, 60–61. [Google Scholar] [CrossRef]
- Hasson, T.; Gillespie, P.G.; Garcia, J.A.; MacDonald, R.B.; Zhao, Y.; Yee, A.G.; Mooseker, M.S.; Corey, D.P. Unconventional myosins in inner-ear sensory epithelia. J. Cell Biol. 1997, 137, 1287–1307. [Google Scholar] [CrossRef]
- El-Amraoui, A.; Petit, C. Usher I syndrome: Unravelling the mechanisms that underlie the cohesion of the growing hair bundle in inner ear sensory cells. J. Cell Sci. 2005, 118, 4593–4603. [Google Scholar] [CrossRef]
- Xu, M.; Xie, Y.; Abouzeid, H.; Gordon, C.T.; Fiorentino, A.; Sun, Z.; Lehman, A.; Osman, I.S.; Dharmat, R.; Riveiro-Alvarez, R.; et al. Mutations in the Spliceosome Component CWC27 Cause Retinal Degeneration with or without Additional Developmental Anomalies. Am. J. Hum. Genet. 2017, 100, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Brea-Fernández, A.J.; Cabanas, P.; Dacruz-Álvarez, D.; Caamaño, P.; Limeres, J.; Loidi, L. Expanding the clinical and molecular spectrum of the CWC27-related spliceosomopathy. J. Hum. Genet. 2019, 64, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Busetto, V.; Barbosa, I.; Basquin, J.; Marquenet, É.; Hocq, R.; Hennion, M.; Paternina, J.A.; Namane, A.; Conti, E.; Bensaude, O.; et al. Structural and functional insights into CWC27/CWC22 heterodimer linking the exon junction complex to spliceosomes. Nucleic Acids Res. 2020, 48, 5670–5683. [Google Scholar] [CrossRef] [PubMed]
- Grainger, R.J.; Beggs, J.D. Prp8 protein: At the heart of the spliceosome. RNA 2005, 11, 533–557. [Google Scholar] [CrossRef]
- Mordes, D.; Luo, X.; Kar, A.; Kuo, D.; Xu, L.; Fushimi, K.; Yu, G.; Sternberg, P., Jr.; Wu, J.Y. Pre-mRNA splicing and retinitis pigmentosa. Mol. Vis. 2006, 12, 1259–1271. [Google Scholar]
- Mozaffari-Jovin, S.; Wandersleben, T.; Santos, K.F.; Will, C.L.; Lührmann, R.; Wahl, M.C. Inhibition of RNA helicase Brr2 by the C-terminal tail of the spliceosomal protein Prp8. Science 2013, 341, 80–84. [Google Scholar] [CrossRef]
- den Hollander, A.I.; ten Brink, J.B.; de Kok, Y.J.; van Soest, S.; van den Born, L.I.; van Driel, M.A.; van de Pol, D.J.; Payne, A.M.; Bhattacharya, S.S.; Kellner, U.; et al. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat. Genet. 1999, 23, 217–221. [Google Scholar] [CrossRef] [PubMed]
- van de Pavert, S.A.; Kantardzhieva, A.; Malysheva, A.; Meuleman, J.; Versteeg, I.; Levelt, C.; Klooster, J.; Geiger, S.; Seeliger, M.W.; Rashbass, P.; et al. Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J. Cell Sci. 2004, 117, 4169–4177. [Google Scholar] [CrossRef]
- Bulgakova, N.A.; Knust, E. The Crumbs complex: From epithelial-cell polarity to retinal degeneration. J. Cell Sci. 2009, 122, 2587–2596. [Google Scholar] [CrossRef]
- Mehalow, A.K.; Kameya, S.; Smith, R.S.; Hawes, N.L.; Denegre, J.M.; Young, J.A.; Bechtold, L.; Haider, N.B.; Tepass, U.; Heckenlively, J.R.; et al. CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Hum. Mol. Genet. 2003, 12, 2179–2189. [Google Scholar] [CrossRef]
- Zacchigna, S.; Oh, H.; Wilsch-Bräuninger, M.; Missol-Kolka, E.; Jászai, J.; Jansen, S.; Tanimoto, N.; Tonagel, F.; Seeliger, M.; Huttner, W.B.; et al. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J. Neurosci. 2009, 29, 2297–2308. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Y.; Lillo, C.; Chien, J.; Yu, Z.; Michaelides, M.; Klein, M.; Howes, K.A.; Li, Y.; Kaminoh, Y.; et al. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J. Clin. Investig. 2009, 119, 1396. [Google Scholar] [CrossRef][Green Version]
- Maw, M.A.; Corbeil, D.; Koch, J.; Hellwig, A.; Wilson-Wheeler, J.C.; Bridges, R.J.; Kumaramanickavel, G.; John, S.; Nancarrow, D.; Röper, K.; et al. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum. Mol. Genet. 2000, 9, 27–34. [Google Scholar] [CrossRef]
- Zhang, Q.; Zulfiqar, F.; Xiao, X.; Riazuddin, S.A.; Ahmad, Z.; Caruso, R.; MacDonald, I.; Sieving, P.; Riazuddin, S.; Hejtmancik, J.F. Severe retinitis pigmentosa mapped to 4p15 and associated with a novel mutation in the PROM1 gene. Hum. Genet. 2007, 122, 293–299. [Google Scholar] [CrossRef]
- Michaelides, M.; Gaillard, M.C.; Escher, P.; Tiab, L.; Bedell, M.; Borruat, F.X.; Barthelmes, D.; Carmona, R.; Zhang, K.; White, E.; et al. The PROM1 mutation p.R373C causes an autosomal dominant bull’s eye maculopathy associated with rod, rod-cone, and macular dystrophy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4771–4780. [Google Scholar] [CrossRef]
- Rice, D.S.; Calandria, J.M.; Gordon, W.C.; Jun, B.; Zhou, Y.; Gelfman, C.M.; Li, S.; Jin, M.; Knott, E.J.; Chang, B.; et al. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat. Commun. 2015, 6, 6228. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Sluch, V.M.; Banks, A.; Li, H.; Crowley, M.A.; Davis, V.; Xiang, C.; Yang, J.; Demirs, J.T.; Vrouvlianis, J.; Leehy, B.; et al. ADIPOR1 is essential for vision and its RPE expression is lost in the Mfrprd6 mouse. Sci. Rep. 2018, 8, 14339. [Google Scholar] [CrossRef]
- Vasiliauskaité-Brooks, I.; Sounier, R.; Rochaix, P.; Bellot, G.; Fortier, M.; Hoh, F.; De Colibus, L.; Bechara, C.; Saied, E.M.; Arenz, C.; et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 2017, 544, 120–123. [Google Scholar] [CrossRef]
- Strettoi, E.; Gargini, C.; Novelli, E.; Sala, G.; Piano, I.; Gasco, P.; Ghidoni, R. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2010, 107, 18706–18711. [Google Scholar] [CrossRef]
- Chen, H.; Tran, J.T.; Brush, R.S.; Saadi, A.; Rahman, A.K.; Yu, M.; Yasumura, D.; Matthes, M.T.; Ahern, K.; Yang, H.; et al. Ceramide signaling in retinal degeneration. Adv. Exp. Med. Biol. 2012, 723, 553–558. [Google Scholar]
- Zhang, J.; Wang, C.; Shen, Y.; Chen, N.; Wang, L.; Liang, L.; Guo, T.; Yin, X.; Ma, Z.; Zhang, B.; et al. A mutation in ADIPOR1 causes nonsyndromic autosomal dominant retinitis pigmentosa. Hum. Genet. 2016, 135, 1375–1387. [Google Scholar] [CrossRef]
- Xu, M.; Eblimit, A.; Wang, J.; Li, J.; Wang, F.; Zhao, L.; Wang, X.; Xiao, N.; Li, Y.; Wong, L.J.; et al. ADIPOR1 Is Mutated in Syndromic Retinitis Pigmentosa. Hum. Mutat. 2016, 37, 246–249. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Paananen, J.; Nevalainen, T.; Sorri, I.; Seitsonen, S.; Immonen, I.; Salminen, A.; Pulkkinen, L.; Uusitupa, M. Adiponectin receptor 1 gene (ADIPOR1) variant is associated with advanced age-related macular degeneration in Finnish population. Neurosci. Lett. 2012, 513, 233–237. [Google Scholar] [CrossRef]
- Huang, C.H.; Yang, C.M.; Yang, C.H.; Hou, Y.C.; Chen, T.C. Leber’s Congenital Amaurosis: Current Concepts of Genotype-Phenotype Correlations. Genes 2021, 12, 1261. [Google Scholar] [CrossRef]
- Koenekoop, R.K. An overview of Leber congenital amaurosis: A model to understand human retinal development. Surv. Ophthalmol. 2004, 49, 379–398. [Google Scholar] [CrossRef]
- Sather, R., 3rd; Ihinger, J.; Simmons, M.; Lobo, G.P.; Montezuma, S.R. The Clinical Findings, Pathogenic Variants, and Gene Therapy Qualifications Found in a Leber Congenital Amaurosis Phenotypic Spectrum Patient Cohort. Int. J. Mol. Sci. 2024, 25, 1253. [Google Scholar] [CrossRef]
- Leroy, B.P.; Birch, D.G.; Duncan, J.L.; Lam, B.L.; Koenekoop, R.K.; Porto, F.B.O.; Russell, S.R.; Girach, A. Leber congenital amaurosis due to CEP290 mutations-severe vision impairment with a high unmet medical need: A review. Retina 2021, 41, 898–907. [Google Scholar] [CrossRef]
- den Hollander, A.I.; Koenekoop, R.K.; Yzer, S.; Lopez, I.; Arends, M.L.; Voesenek, K.E.J.; Zonneveld, M.N.; Strom, T.M.; Meitinger, T.; Brunner, H.G.; et al. Mutations in the CEP290 (NPHP6) Gene Are a Frequent Cause of Leber Congenital Amaurosis. Am. J. Hum. Genet. 2006, 79, 556–561. [Google Scholar] [CrossRef]
- Kumaran, N.; Moore, A.T.; Weleber, R.G.; Michaelides, M. Leber congenital amaurosis/early-onset severe retinal dystrophy: Clinical features, molecular genetics and therapeutic interventions. Br. J. Ophthalmol. 2017, 101, 1147–1154. [Google Scholar] [CrossRef]
- den Hollander, A.I.; Roepman, R.; Koenekoop, R.K.; Cremers, F.P. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008, 27, 391–419. [Google Scholar] [CrossRef]
- Craige, B.; Tsao, C.C.; Diener, D.R.; Hou, Y.; Lechtreck, K.F.; Rosenbaum, J.L.; Witman, G.B. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 2010, 190, 927–940. [Google Scholar] [CrossRef]
- Bernardos, R.L.; Barthel, L.K.; Meyers, J.R.; Raymond, P.A. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J. Neurosci. 2007, 27, 7028–7040. [Google Scholar] [CrossRef]
- Conner, C.; Ackerman, K.M.; Lahne, M.; Hobgood, J.S.; Hyde, D.R. Repressing notch signaling and expressing TNFα are sufficient to mimic retinal regeneration by inducing Müller glial proliferation to generate committed progenitor cells. J. Neurosci. 2014, 34, 14403–14419. [Google Scholar] [CrossRef]
- Gorsuch, R.A.; Hyde, D.R. Regulation of Müller glial dependent neuronal regeneration in the damaged adult zebrafish retina. Exp. Eye Res. 2014, 123, 131–140. [Google Scholar] [CrossRef]
- Morimura, H.; Fishman, G.A.; Grover, S.A.; Fulton, A.B.; Berson, E.L.; Dryja, T.P. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc. Natl. Acad. Sci. USA 1998, 95, 3088–3093. [Google Scholar] [CrossRef]
- Lotery, A.J.; Namperumalsamy, P.; Jacobson, S.G.; Weleber, R.G.; Fishman, G.A.; Musarella, M.A.; Hoyt, C.S.; Héon, E.; Levin, A.; Jan, J.; et al. Mutation analysis of 3 genes in patients with Leber congenital amaurosis. Arch. Ophthalmol. 2000, 118, 538–543. [Google Scholar] [CrossRef]
- Hanein, S.; Perrault, I.; Gerber, S.; Tanguy, G.; Barbet, F.; Ducroq, D.; Calvas, P.; Dollfus, H.; Hamel, C.; Lopponen, T.; et al. Leber congenital amaurosis: Comprehensive survey of the genetic heterogeneity, refinement of the clinical definition, and genotype-phenotype correlations as a strategy for molecular diagnosis. Hum. Mutat. 2004, 23, 306–317. [Google Scholar] [CrossRef]
- Thompson, D.A.; Gyürüs, P.; Fleischer, L.L.; Bingham, E.L.; McHenry, C.L.; Apfelstedt-Sylla, E.; Zrenner, E.; Lorenz, B.; Richards, J.E.; Jacobson, S.G.; et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4293–4299. [Google Scholar]
- Moiseyev, G.; Chen, Y.; Takahashi, Y.; Wu, B.X.; Ma, J.X. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc. Natl. Acad. Sci. USA 2005, 102, 12413–12418. [Google Scholar] [CrossRef]
- Gu, S.M.; Thompson, D.A.; Srikumari, C.R.; Lorenz, B.; Finckh, U.; Nicoletti, A.; Murthy, K.R.; Rathmann, M.; Kumaramanickavel, G.; Denton, M.J.; et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat. Genet. 1997, 17, 194–197. [Google Scholar] [CrossRef]
- Redmond, T.M.; Yu, S.; Lee, E.; Bok, D.; Hamasaki, D.; Chen, N.; Goletz, P.; Ma, J.X.; Crouch, R.K.; Pfeifer, K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 1998, 20, 344–351. [Google Scholar] [CrossRef]
- Maguire, A.M.; Bennett, J.; Aleman, E.M.; Leroy, B.P.; Aleman, T.S. Clinical Perspective: Treating RPE65-Associated Retinal Dystrophy. Mol. Ther. 2021, 29, 442–463. [Google Scholar] [CrossRef]
- Cideciyan, A.V.; Jacobson, S.G.; Beltran, W.A.; Sumaroka, A.; Swider, M.; Iwabe, S.; Roman, A.J.; Olivares, M.B.; Schwartz, S.B.; Komáromy, A.M.; et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc. Natl. Acad. Sci. USA 2013, 110, E517–E525. [Google Scholar] [CrossRef]
- Suh, S.; Choi, E.H.; Leinonen, H.; Foik, A.T.; Newby, G.A.; Yeh, W.-H.; Dong, Z.; Kiser, P.D.; Lyon, D.C.; Liu, D.R.; et al. Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing. Nat. Biomed. Eng. 2021, 5, 169–178. [Google Scholar] [CrossRef]
- Freund, C.L.; Gregory-Evans, C.Y.; Furukawa, T.; Papaioannou, M.; Looser, J.; Ploder, L.; Bellingham, J.; Ng, D.; Herbrick, J.A.; Duncan, A.; et al. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell 1997, 91, 543–553. [Google Scholar] [CrossRef]
- Tran, N.M.; Zhang, A.; Zhang, X.; Huecker, J.B.; Hennig, A.K.; Chen, S. Mechanistically distinct mouse models for CRX-associated retinopathy. PLoS Genet. 2014, 10, e1004111. [Google Scholar]
- Silva, E.; Yang, J.M.; Li, Y.; Dharmaraj, S.; Sundin, O.H.; Maumenee, I.H. A CRX null mutation is associated with both Leber congenital amaurosis and a normal ocular phenotype. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2076–2079. [Google Scholar]
- Sohocki, M.M.; Sullivan, L.S.; Mintz-Hittner, H.A.; Birch, D.; Heckenlively, J.R.; Freund, C.L.; McInnes, R.R.; Daiger, S.P. A range of clinical phenotypes associated with mutations in CRX, a photoreceptor transcription-factor gene. Am. J. Hum. Genet. 1998, 63, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.B.; Jacobson, S.G.; Cideciyan, A.V.; Swiderski, R.; Streb, L.M.; Searby, C.; Beck, G.; Hockey, R.; Hanna, D.B.; Gorman, S.; et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat. Genet. 2000, 24, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.G.; Marmor, M.F.; Kemp, C.M.; Knighton, R.W. SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Investig. Ophthalmol. Vis. Sci. 1990, 31, 827–838. [Google Scholar]
- Marmor, M.F.; Jacobson, S.G.; Foerster, M.H.; Kellner, U.; Weleber, R.G. Diagnostic Clinical Findings of a New Syndrome with Night Blindness, Maculopathy, and Enhanced S Cone Sensitivity. Am. J. Ophthalmol. 1990, 110, 124–134. [Google Scholar]
- Lam, B.L.; Goldberg, J.L.; Hartley, K.L.; Stone, E.M.; Liu, M. Atypical Mild Enhanced S-Cone Syndrome with Novel Compound Heterozygosity of the NR2E3 Gene. Am. J. Ophthalmol. 2007, 144, 157–159. [Google Scholar] [CrossRef]
- de Carvalho, E.R.; Robson, A.G.; Arno, G.; Boon, C.J.F.; Webster, A.A.; Michaelides, M. Enhanced S-Cone Syndrome: Spectrum of Clinical, Imaging, Electrophysiologic, and Genetic Findings in a Retrospective Case Series of 56 Patients. Ophthalmol. Retina 2021, 5, 195–214. [Google Scholar] [CrossRef]
- Schorderet, D.F.; Escher, P. NR2E3 mutations in enhanced S-cone sensitivity syndrome (ESCS), Goldmann-Favre syndrome (GFS), clumped pigmentary retinal degeneration (CPRD), and retinitis pigmentosa (RP). Hum. Mutat. 2009, 30, 1475–1485. [Google Scholar] [CrossRef]
- Oh, E.C.T.; Cheng, H.; Hao, H.; Jia, L.; Khan, N.W.; Swaroop, A. Rod differentiation factor NRL activates the expression of nuclear receptor NR2E3 to suppress the development of cone photoreceptors. Brain Res. 2008, 1236, 16–29. [Google Scholar] [CrossRef]
- Cheng, H.; Khanna, H.; Oh, E.C.T.; Hicks, D.; Mitton, K.P.; Swaroop, A. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum. Mol. Genet. 2004, 13, 1563–1575. [Google Scholar] [CrossRef]
- Cheng, H.; Khan, N.W.; Roger, J.E.; Swaroop, A. Excess cones in the retinal degeneration rd7 mouse, caused by the loss of function of orphan nuclear receptor Nr2e3, originate from early-born photoreceptor precursors. Hum. Mol. Genet. 2011, 20, 4102–4115. [Google Scholar] [CrossRef] [PubMed]
- Zaydon, Y.A.; Tsang, S.H. The ABCs of Stargardt disease: The latest advances in precision medicine. Cell Biosci. 2024, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Tanna, P.; Strauss, R.W.; Fujinami, K.; Michaelides, M. Stargardt disease: Clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 2017, 101, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef]
- Mata, N.L.; Tzekov, R.T.; Liu, X.; Weng, J.; Birch, D.G.; Travis, G.H. Delayed Dark-Adaptation and Lipofuscin Accumulation in abcr+/− Mice: Implications for Involvement of ABCR in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1685–1690. [Google Scholar]
- Haji Abdollahi, S.; Hirose, T. Stargardt-Fundus Flavimaculatus: Recent Advancements and Treatment. Semin. Ophthalmol. 2013, 28, 372–376. [Google Scholar] [CrossRef]
- Quazi, F.; Lenevich, S.; Molday, R.S. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat. Commun. 2012, 3, 925. [Google Scholar] [CrossRef]
- Molday, R.S.; Garces, F.A.; Scortecci, J.F.; Molday, L.L. Structure and function of ABCA4 and its role in the visual cycle and Stargardt macular degeneration. Prog. Retin. Eye Res. 2022, 89, 101036. [Google Scholar] [CrossRef]
- Zobor, D.; Zobor, G.; Kohl, S. Achromatopsia: On the Doorstep of a Possible Therapy. Ophthalmic Res. 2015, 54, 103–108. [Google Scholar] [CrossRef]
- Thiadens, A.A.H.J.; den Hollander, A.I.; Roosing, S.; Nabuurs, S.B.; Zekveld-Vroon, R.C.; Collin, R.W.J.; De Baere, E.; Koenekoop, R.K.; van Schooneveld, M.J.; Strom, T.M.; et al. Homozygosity Mapping Reveals PDE6C Mutations in Patients with Early-Onset Cone Photoreceptor Disorders. Am. J. Hum. Genet. 2009, 85, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, C.; Megaw, R. Molecular Mechanisms Governing Sight Loss in Inherited Cone Disorders. Genes 2024, 15, 727. [Google Scholar] [CrossRef] [PubMed]
- Weisschuh, N.; Stingl, K.; Audo, I.; Biskup, S.; Bocquet, B.; Branham, K.; Burstedt, M.S.; De Baere, E.; De Vries, M.J.; Golovleva, I.; et al. Mutations in the gene PDE6C encoding the catalytic subunit of the cone photoreceptor phosphodiesterase in patients with achromatopsia. Hum. Mutat. 2018, 39, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, S.; Gerhardt, M.; Rudolph, G.; Priglinger, S.; Priglinger, C. Achromatopsia: Genetics and Gene Therapy. Mol. Diagn. Ther. 2022, 26, 51–59. [Google Scholar] [CrossRef]
- Baxter, M.F.; Borchert, G.A. Gene Therapy for Achromatopsia. Int. J. Mol. Sci. 2024, 25, 9739. [Google Scholar] [CrossRef]
- Georgiou, M.; Robson, A.G.; Singh, N.; Pontikos, N.; Kane, T.; Hirji, N.; Ripamonti, C.; Rotsos, T.; Dubra, A.; Kalitzeos, A.; et al. Deep Phenotyping of PDE6C-Associated Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5112–5123. [Google Scholar] [CrossRef]
- Grau, T.; Artemyev, N.O.; Rosenberg, T.; Dollfus, H.; Haugen, O.H.; Cumhur Sener, E.; Jurklies, B.; Andreasson, S.; Kernstock, C.; Larsen, M.; et al. Decreased catalytic activity and altered activation properties of PDE6C mutants associated with autosomal recessive achromatopsia. Hum. Mol. Genet. 2011, 20, 719–730. [Google Scholar] [CrossRef]
- Ding, J.; Adiconis, X.; Simmons, S.K.; Kowalczyk, M.S.; Hession, C.C.; Marjanovic, N.D.; Hughes, T.K.; Wadsworth, M.H.; Burks, T.; Nguyen, L.T.; et al. Systematic comparison of single-cell and single-nucleus RNA-sequencing methods. Nat. Biotechnol. 2020, 38, 737–746. [Google Scholar] [CrossRef]
- Lähnemann, D.; Köster, J.; Szczurek, E.; McCarthy, D.J.; Hicks, S.C.; Robinson, M.D.; Vallejos, C.A.; Campbell, K.R.; Beerenwinkel, N.; Mahfouz, A.; et al. Eleven grand challenges in single-cell data science. Genome Biol. 2020, 21, 31. [Google Scholar] [CrossRef]
- 10x Genomics. Are There Considerations for Small Cells or Cells with Low RNA? Available online: https://kb.10xgenomics.com/hc/en-us/articles/360037656471-Are-there-considerations-for-small-cells-or-cells-with-low-RNA (accessed on 29 August 2025).
- Sant, P.; Rippe, K.; Mallm, J.-P. Approaches for single-cell RNA sequencing across tissues and cell types. Transcription 2023, 14, 127–145. [Google Scholar] [CrossRef]
- Bues, J.; Biočanin, M.; Pezoldt, J.; Dainese, R.; Chrisnandy, A.; Rezakhani, S.; Saelens, W.; Gardeux, V.; Gupta, R.; Sarkis, R.; et al. Deterministic scRNA-seq captures variation in intestinal crypt and organoid composition. Nat. Methods 2022, 19, 323–330. [Google Scholar] [CrossRef]
- De Rop, F.V.; Ismail, J.N.; Bravo González-Blas, C.; Hulselmans, G.J.; Flerin, C.C.; Janssens, J.; Theunis, K.; Christiaens, V.M.; Wouters, J.; Marcassa, G.; et al. Hydrop enables droplet-based single-cell ATAC-seq and single-cell RNA-seq using dissolvable hydrogel beads. eLife 2022, 11, e73971. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.; Gillis, J. Single cell RNA-sequencing: Replicability of cell types. Curr. Opin. Neurobiol. 2019, 56, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheung, F.; Shi, R.; Zhou, H.; Lu, W.; Candia, J.; Kotliarov, Y.; Stagliano, K.R.; Tsang, J.S.; CHI Consortium. PBMC fixation and processing for Chromium single-cell RNA sequencing. J. Transl. Med. 2018, 16, 198. [Google Scholar] [CrossRef] [PubMed]
- Fadl, B.R.; Brodie, S.A.; Malasky, M.; Boland, J.F.; Kelly, M.C.; Kelley, M.W.; Boger, E.; Fariss, R.; Swaroop, A.; Campello, L. An optimized protocol for retina single-cell RNA sequencing. Mol. Vis. 2020, 26, 705–717. [Google Scholar]
- Denisenko, E.; Guo, B.B.; Jones, M.; Hou, R.; de Kock, L.; Lassmann, T.; Poppe, D.; Clément, O.; Simmons, R.K.; Lister, R.; et al. Systematic assessment of tissue dissociation and storage biases in single-cell and single-nucleus RNA-seq workflows. Genome Biol. 2020, 21, 130. [Google Scholar] [CrossRef]
- Zimmerman, K.D.; Espeland, M.A.; Langefeld, C.D. A practical solution to pseudoreplication bias in single-cell studies. Nat. Commun. 2021, 12, 738. [Google Scholar] [CrossRef]
- Squair, J.W.; Gautier, M.; Kathe, C.; Anderson, M.A.; James, N.D.; Hutson, T.H.; Hudelle, R.; Qaiser, T.; Matson, K.J.E.; Barraud, Q.; et al. Confronting false discoveries in single-cell differential expression. Nat. Commun. 2021, 12, 5692. [Google Scholar] [CrossRef]
- Junttila, S.; Smolander, J.; Elo, L.L. Benchmarking methods for detecting differential states between conditions from multi-subject single-cell RNA-seq data. Brief. Bioinform. 2022, 23, bbac286. [Google Scholar] [CrossRef]
- Satija, R.; Farrell, J.A.; Gennert, D.; Schier, A.F.; Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015, 33, 495–502. [Google Scholar] [CrossRef]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [PubMed]
- Amezquita, R.A.; Lun, A.T.L.; Becht, E.; Carey, V.J.; Carpp, L.N.; Geistlinger, L.; Marini, F.; Rue-Albrecht, K.; Risso, D.; Soneson, C.; et al. Orchestrating single-cell analysis with Bioconductor. Nat. Methods 2020, 17, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.A.; Kim, J.K.; Svensson, V.; Marioni, J.C.; Teichmann, S.A. The Technology and Biology of Single-Cell RNA Sequencing. Mol. Cell 2015, 58, 610–620. [Google Scholar] [CrossRef]
- Haque, A.; Engel, J.; Teichmann, S.A.; Lönnberg, T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 2017, 9, 75. [Google Scholar] [CrossRef]
- Lyu, Y.; Zauhar, R.; Dana, N.; Strang, C.E.; Hu, J.; Wang, K.; Liu, S.; Pan, N.; Gamlin, P.; Kimble, J.A.; et al. Implication of specific retinal cell-type involvement and gene expression changes in AMD progression using integrative analysis of single-cell and bulk RNA-seq profiling. Sci. Rep. 2021, 11, 15612. [Google Scholar] [CrossRef]
- Rao, A.; Barkley, D.; França, G.S.; Yanai, I. Exploring tissue architecture using spatial transcriptomics. Nature 2021, 596, 211–220. [Google Scholar] [CrossRef]
- Choi, J.; Li, J.; Ferdous, S.; Liang, Q.; Moffitt, J.R.; Chen, R. Spatial organization of the mouse retina at single cell resolution by MERFISH. Nat. Commun. 2023, 14, 4929. [Google Scholar] [CrossRef]
- Schumann, U.; Liu, L.; Aggio-Bruce, R.; Cioanca, A.V.; Shariev, A.; Madigan, M.C.; Valter, K.; Wen, J.; Natoli, R. Spatial transcriptomics reveals regionally altered gene expression that drives retinal degeneration. Commun. Biol. 2025, 8, 629. [Google Scholar] [CrossRef]
- Longo, S.K.; Guo, M.G.; Ji, A.L.; Khavari, P.A. Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat. Rev. Genet. 2021, 22, 627–644. [Google Scholar] [CrossRef]
- Yan, C.; Zhu, Y.; Chen, M.; Yang, K.; Cui, F.; Zou, Q.; Zhang, Z. Integration tools for scRNA-seq data and spatial transcriptomics sequencing data. Brief. Funct. Genom. 2024, 23, 295–302. [Google Scholar] [CrossRef]
- Zhou, Y.; Sheng, Y.; Pan, M.; Tu, J.; Zhao, X.; Ge, Q.; Lu, Z. Spatial Transcriptomic Analysis Reveals Regional Transcript Changes in Early and Late Stages of rd1 Model Mice with Retinitis Pigmentosa. Int. J. Mol. Sci. 2023, 24, 14869. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhou, Q.; Chen, Z.; Zhuang, J.; Zhao, X.; Gan, Z.; Wang, Y.; Wang, C.; Molday, R.S.; et al. Spatiotemporally resolved transcriptomics reveals the cellular dynamics of human retinal development. Nat. Commun. 2025, 16, 2307. [Google Scholar] [CrossRef]
- Vickovic, S.; Eraslan, G.; Salmén, F.; Klughammer, J.; Stenbeck, L.; Schapiro, D.; Äijö, T.; Bonneau, R.; Bergenstråhle, L.; Navarro, J.F.; et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods 2019, 16, 987–990. [Google Scholar] [CrossRef] [PubMed]
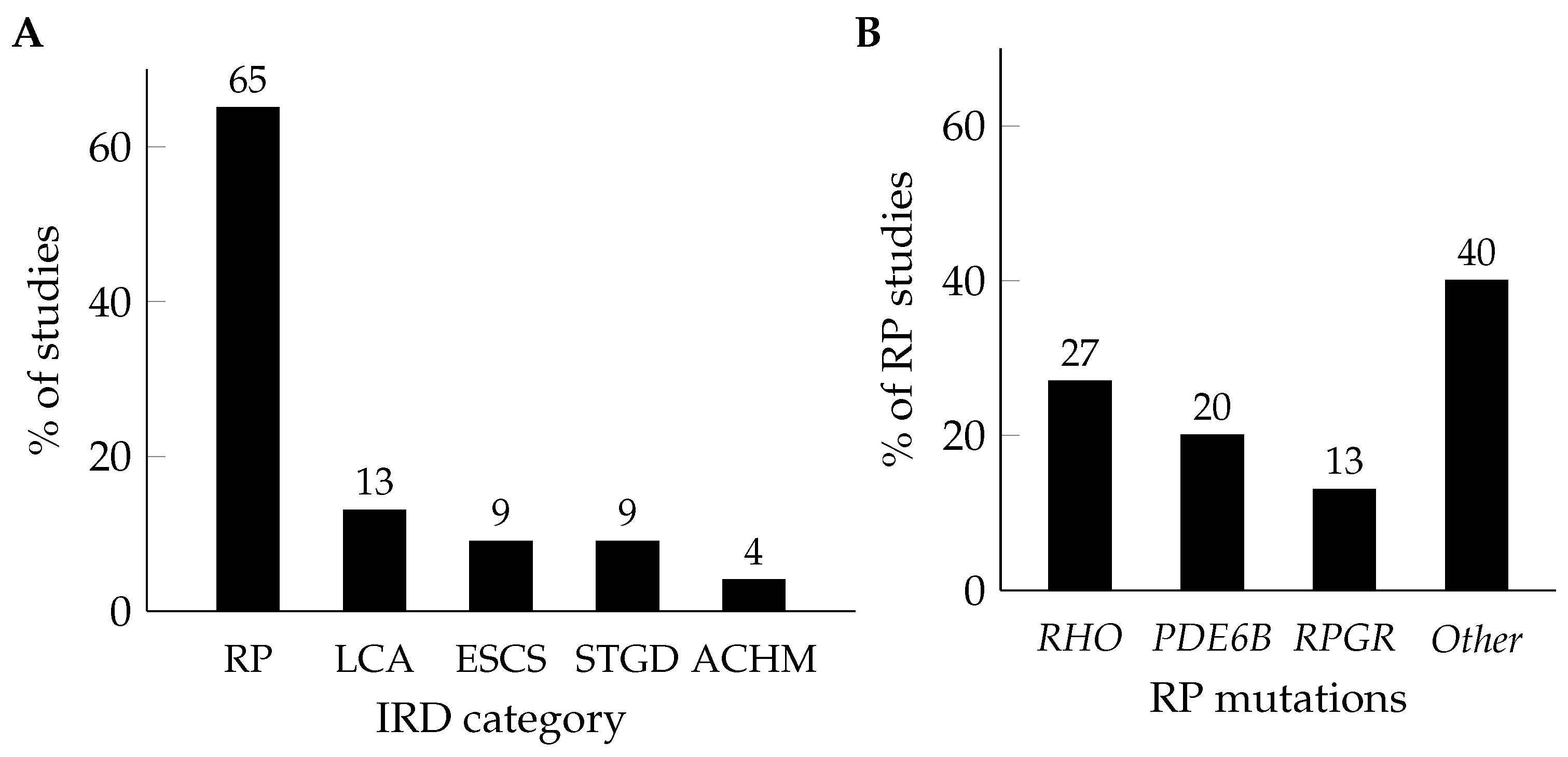
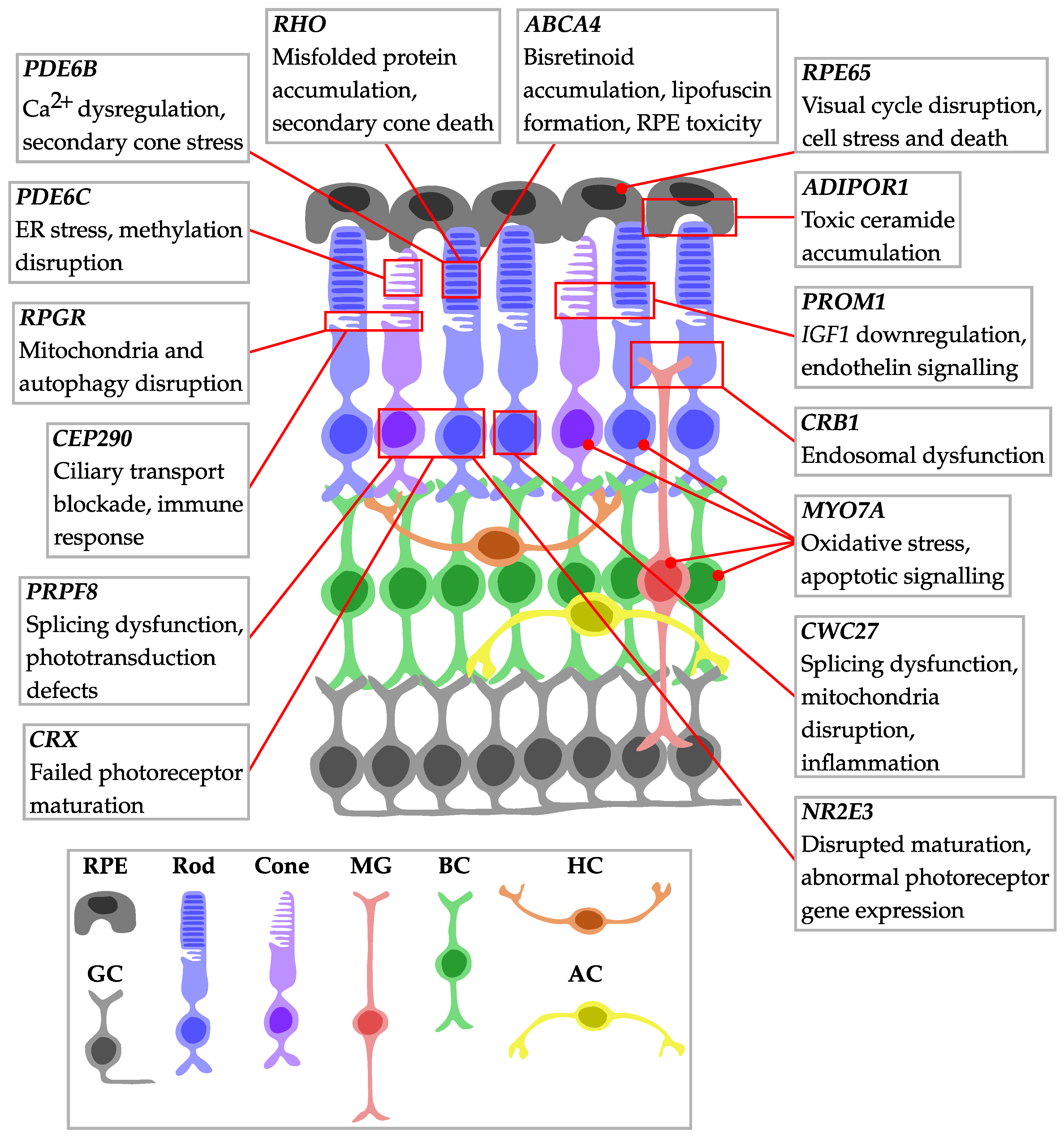
| IRD | Mutation | Study | Model | Biological Replicates | Disease Stage | Intervention | Platform | Main Dysregulated Pathways or Markers in Disease (or Treatment) Conditions |
|---|---|---|---|---|---|---|---|---|
| RP | RHO | Tomita et al. [16] | Mouse (RhoP23H) | 4 MUT and 4 WT | Active degeneration (PW7) | No | inDrop | ↓ in PRs: phototransduction, cilium, development, ATP processes, glycolysis; ↑ in Müller cells: PR maintenance, mitochondrial localisation and transport |
| RP | RHO | Fu et al. [17] | Mouse (RhoP23H) | 3 FGF21-treated and 3 vehicle-treated CTRL | Active degeneration (PW10) | FGF21 treatment | inDrop | FGF21-treated Müller cells and astrocytes: axon development↑, synapse formation↑, SRF signalling↑ |
| RP | RHO | Santhanam et al. [18] | Zebrafish | 1 MUT and 1 WT for V2 and V3 each; 3 fish pooled each | Active degeneration (age 6–10 months) | No | 10x | Oxidative stress, metabolic reprogramming, misfolded proteins, circadian rhythm disruption, synaptic remodelling, regenerative signalling |
| RP | RHO | Lin et al. [19] | Human iPSC-derived rOrgs | 1 patient and 1 healthy CTRL at D120 and D270 each; 5–6 rOrgs each | Immature (D120) and mature (D270) stage | No | 10x | Visual perception, PR development, phototransduction, rod maturation, immune and inflammatory signalling |
| RP | PDE6B | Chen et al. [20] 1 | Mouse (rd1) | MUT and WT at P11, P13, P17; 3 animals per time point | Early (P11), peak rod degeneration (P13), late rod degeneration (P17) | No | 10x | Visual perception, phototransduction, apoptosis, Ca2+ signalling, MAPK pathway, metabolic pathways |
| RP | PDE6B | Dong et al. [21] 1 | Mouse (rd1) | MUT and WT at P11, P13, P17; 3 animals per time point | Early (P11), peak rod degeneration (P13), late rod degeneration (P17) | No | 10x | Egr1↑ in PRs |
| RP | PDE6B | Dong et al. [22] 1 | Mouse (rd1) | MUT and WT at P11, P13, P17; 3 animals per time point | Early (P11), peak rod degeneration (P13), late rod degeneration (P17) | No | 10x | Hdac1↑ in rods, Parp1↓ in cones |
| RP | PDE6B | Yan et al. [23] 1 | Mouse (rd1) | MUT and WT at P11, P13, P17; 3 animals per time point | Focus on peak rod degeneration (P13) | No | 10x | Ca2+ signalling in PRs |
| RP | PDE6B | Liao et al. [24] | Mouse (rd1) | 2 MUT and 2 WT | Peak rod degeneration (P15) | No | 10x | JNK signalling↑, Jun transcription factor↑ |
| RP | PDE6B | Karademir et al. [25] 2 | Mouse (rd10) | 2 MUT and 2 WT | Peak rod degeneration (P21) | No | 10x | Early phase: Ca2+ signalling, metabolic disruption, phototransduction, Egr1 activation; late phase: mitochondrial respiratory dysfunction, synaptic remodelling, structural changes, Cebpd activation |
| RP | PDE6B | Sigurdsson and Grimm [26] 2 | Mouse (rd10) | 2 MUT and 2 WT | Peak rod degeneration (P21) | No | 10x | Müller cell response: gliosis and metabolic markers, immune response, MHC components |
| RP, CRD, CD, MD | RPGR | Newton et al. [27] | Mouse (RpgrORFd5, RpgrEx3d8) | 1 MUT and 1 WT for RpgrORFd5 and RpgrEx3d8 each | Active photoreceptor degeneration (18 months) | No | 10x | ↓ in PRs: phototransduction; ↑ in PRs: PI3K/AKT pathway, autophagy, necroptosis, mitochondrial function, TNF-/NF-B signalling, lysosome biogenesis |
| RP | RPGR | Li et al. [28] | Human iPSC-derived rOrgs | 1 patient and 1 healthy CTRL at D40, D90, D150, D200 each; 2–3 rOrgs each | Multiple developmental time points (D40, D90, D150, D200) | No | 10x | ↑ of PR markers |
| RP | MYO7A | Leong et al. [29] | Human iPSC-derived rOrgs | 3 patients and 3 healthy CTRLs | Early/pre-degenerative stage (D245/35 weeks) | No | 10x | ↑ in rods: cellular and oxidative stress, hydrogen peroxide metabolism; no pathology detected for cones; ↑ in Müller cells: apoptosis |
| RP | CWC27 | Bertrand et al. [30] | Mouse (Cwc27K338fs) | 1 MUT and 1 WT | Early degenerative stage (4 months) | No | 10x | ↓ in rods: mitochondrial genes; ↑ in Müller cells: inflammation-related genes |
| RP | PRPF8 | Atkinson et al. [31] | Human iPSC-derived rOrgs | 4 patients and 4 isogenic CTRLs | Advanced degeneration (D210) | No | 10x | MALAT1↑ (degenerating rods), ↓ of cone (ARR3) and rod (NRL) markers, ↓ of phototransduction and mitochondrial genes, ciliary dysfunction |
| RP, LCA | CRB1 | Boon et al. [32] 3 | Human iPSC-derived rOrgs | 1 patient; 1st scRNA-seq: 1 patient and 1 healthy CTRL; 2nd scRNA-seq: 1 gene therapy-treated (gene 1) and 1 gene therapy-treated (gene 2) and 1 CTRL-treated; 5–6 rOrgs each | Active photoreceptor degeneration (D230) | AAV gene therapy | 10x | Untreated disease: endosomal system dysfunction in rods and Müller cells; phototransduction cascade activation in rods; cell adhesion, protein binding, cell death, iron ion transport in Müller cells; AAV-treated: partial restoration of endosomal system pathways toward control levels |
| RP, MD | PROM1 | Shigesada et al. [33] | Mouse (Prom1−/−) | 1 light-exposed MUT and 1 dark-reared MUT | Early/pre-symptomatic stage (P11) | No | 10x | Igf1↓ in rods and astrocytes, Edn2↑ in rods, glial activation in Müller glia and astrocytes, visual function dysfunction in PRs |
| RP | ADIPOR1 | Lewandowski et al. [34] | Mouse (AdiporR1−/−) | 2 MUT and 2 WT at P19, P25, P30 each | Early/pre-onset (P18), active/post-onset (P25), advanced (P30) degeneration | No | 10x | Pre-onset: translation↑, oxidative phosphorylation↑, mitochondrion organisation↑, RNA splicing↓, protein localisation↓; active and advanced phase: visual perception↓, translation↑; neurodegenerative pathways |
| LCA | CEP290 | Fogerty et al. [35] | Zebrafish | 5 MUT and 5 HET CTRLs | Active degeneration (6 months) | No | SplitBio | ↑ in Müller cells: notch3, stat2, yap1, rest-associated genes |
| LCA | RPE65 | Choi et al. [36] | Mouse (rd12) | 4 treated MUT and 4 untreated MUT and 4 WT | Advanced degeneration (2 months) | Base editing therapy | 10x | Base editing-treated cones: phototransduction restoration (rescue of phototransduction genes), opsin recovery (Opn1sw), neuroprotection (Mt1↑), cell survival (cell death/stress genes↓) |
| LCA, CRD, MD | CRX | Kruczek et al. [37] | Human iPSC-derived rOrgs | 2 patients (treated and untreated) and 2 healthy CTRLs; 3–4 rOrgs each | Impaired photoreceptor maturation (D200) | AAV gene therapy | 10x | AAV-CRX-treated PRs: partial rescue of RHO, OPN1MW, CABP4 |
| ESCS | NR2E3 | Mullin et al. [38] | Human iPSC-derived rOrgs | 1 patient and 1 isogenic CTRL and 1 healthy CTRL at D40, D80, D120, D160, D260 each; 10 rOrgs each | Multiple time points (D40, D80, D120, D160, D260) | AAV gene therapy | 10x | Untreated PRs: phototransduction pathway disruption; “divergent rods”: RHO↓, cone genes↑ (ARR3, PDE6H, GNAT2), rod genes↑ (GNAT1) |
| ESCS | NR2E3 | Aísa-Marín et al. [39] | Mouse (Nr2e3 27, Nr2e3 E8) | P40: 2 E8 MUT and 2 WT; P80: 2 27 MUT and 1 WT | Early-onset, but stable retinal dysfunction (27 at P80); pre-symptomatic stage of progressive late-onset retinal degeneration (E8 at P40) | No | 10x | Formation of “hybrid photoreceptors”, phototransduction↑, autophagy and necrosis↑, stress response↑, homeostasis↓, mitochondria and synaptic function↓ |
| STGD | ABCA4 | Watson et al. [40] | Human iPSC-derived rOrgs | 3 patients and 2 healthy CTRLs; at least 25 rOrgs each | Impaired photoreceptor maturation (D200) | No | 10x | Stress response pathways (mTOR signalling, mitochondrial dysfunction, oxidative phosphorylation), phototransduction disruption, cell cycle dysregulation, and impaired maturation |
| STGD | ABCA4 | Zhao et al. [41] | Human iPSC-derived rOrgs | 1 patient and 1 healthy CTRL | Multiple time points (D40, D90, D150, D200, D260) | No | 10x | No specific pathways or markers reported |
| ACHM | PDE6C | Miller et al. [42] | Mouse (Pde6ccpfl1) | 1 treated MUT and 1 untreated MUT | Peak cone degeneration (P24) | H3K27 demethylase inhibitor treatment | SMART-seq2 / MARS-seq | GSK-J4-treated cones: oxidative phosphorylation↓, mitochondrial dysfunction↓, endoplasmic reticulum stress↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.; Vallejos, C.A.; Mill, P.; Megaw, R. Single-Cell Transcriptomics in Inherited Retinal Dystrophies: Current Findings and Emerging Perspectives. Genes 2025, 16, 1088. https://doi.org/10.3390/genes16091088
Nguyen L, Vallejos CA, Mill P, Megaw R. Single-Cell Transcriptomics in Inherited Retinal Dystrophies: Current Findings and Emerging Perspectives. Genes. 2025; 16(9):1088. https://doi.org/10.3390/genes16091088
Chicago/Turabian StyleNguyen, Linda, Catalina A. Vallejos, Pleasantine Mill, and Roly Megaw. 2025. "Single-Cell Transcriptomics in Inherited Retinal Dystrophies: Current Findings and Emerging Perspectives" Genes 16, no. 9: 1088. https://doi.org/10.3390/genes16091088
APA StyleNguyen, L., Vallejos, C. A., Mill, P., & Megaw, R. (2025). Single-Cell Transcriptomics in Inherited Retinal Dystrophies: Current Findings and Emerging Perspectives. Genes, 16(9), 1088. https://doi.org/10.3390/genes16091088






