Gut Dysbiosis Driven by CFTR Gene Mutations in Cystic Fibrosis Patients: From Genetic Disruption to Multisystem Consequences and Microbiota Modulation
Abstract
1. Introduction
2. The Composition of the Gut Microbiota of Adults with the CFTR Gene Mutation Is Dominated by Pathogenic Bacteria and a Reduced Amount of Short-Chain Fatty Acid Producers, in Comparison to Healthy Individuals
3. The Presence of Pathogenic Bacteria in the Intestines Leads to Changes in the Composition of Gastrointestinal Mucus in Adults with CFTR Gene Mutations
4. Biofilms Are a Consequence of Dysbiosis of the Intestinal Microbiota and Bacterial Defence Mechanisms in CFTR Gene Mutations
5. The Function of the Immune System Is Closely Linked to the Composition of the Gut Microbiome
6. CFTR Protein Modulators Make the Gut Microbiome of Cystic Fibrosis Patients Resemble a Healthy Gut Microbiome
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- López-Valdez, J.A.; Aguilar-Alonso, L.A.; Gándara-Quezada, V.; Ruiz-Rico, G.E.; Ávila-Soledad, J.M.; Reyes, A.A.; Pedroza-Jiménez, F.D. Cystic fibrosis: Current concepts. Boletín Médico Hosp. Infant. México 2021, 78, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.A.; Burgel, P.R.; Castellani, C.; Davies, J.C.; Salathe, M.; Taylor-Cousar, J.L. Cystic fibrosis. Nat. Rev. Dis. Primers 2024, 10, 53. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Durie, P.R. Cystic fibrosis from the gastroenterologist’s perspective. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Tam, R.Y.; van Dorst, J.M.; McKay, I.; Coffey, M.; Ooi, C.Y. Intestinal Inflammation and Alterations in the Gut Microbiota in Cystic Fibrosis: A Review of the Current Evidence, Pathophysiology and Future Directions. J. Clin. Med. 2022, 11, 649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, E.J.; Booth, C.; Fonseca, S.; Parker, A.; Cross, K.; Miquel-Clopés, A.; Hautefort, I.; Mayer, U.; Wileman, T.; Stentz, R.; et al. The Uptake, Trafficking, and Biodistribution of Bacteroides thetaiotaomicron Generated Outer Membrane Vesicles. Front. Microbiol. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, M.B.; Moreira, E.A.M.; Tomio, C.; Moreno, Y.M.F.; Daltoe, F.P.; Barbosa, E.; Ludwig Neto, N.; Buccigrossi, V.; Guarino, A. Altered intestinal microbiota composition, antibiotic therapy and intestinal inflammation in children and adolescents with cystic fibrosis. PLoS ONE 2018, 13, e0198457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Van-Wehle, T.; Vital, M. Investigating the response of the butyrate production potential to major fibers in dietary intervention studies. NPJ Biofilms Microbiomes 2024, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Mansuy-Aubert, V.; Ravussin, Y. Short chain fatty acids: The messengers from down below. Front. Neurosci. 2023, 17, 1197759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, D.; Jian, Y.P.; Zhang, Y.N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef] [PubMed]
- Recharla, N.; Geesala, R.; Shi, X.-Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, K.; Nie, K.; Deng, M.; Luo, W.; Wu, X.; Huang, Y.; Wang, X. Assessment of the safety and probiotic properties of Roseburia intestinalis: A potential “Next Generation Probiotic”. Front. Microbiol. 2022, 13, 973046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Jesus, L.C.L.; Aburjaile, F.F.; De Jesus Sousa, T.; Felice, A.G.; De Castro Soares, S.; Alcantara, L.C.J.; De Carvalho Azevedo, V.A. Genomic Characterization of Lactobacillus delbrueckii Strains with Probiotics Properties. Front. Bioinform. 2022, 2, 912795. [Google Scholar] [CrossRef]
- Liu, X.-F.; Shao, J.-H.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.-J.; Liu, Z.-Z.; He, D.-D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, C.; Duan, H.; Narbad, A.; Zhao, J.; Chen, W.; Zhai, Q.; Yu, L.; Tian, F. Cross-feeding of bifidobacteria promotes intestinal homeostasis: A lifelong perspective on the host health. NPJ Biofilms Microbiomes 2024, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, C.; Kujawski, M.; Chu, H.; Li, L.; Mazmanian, S.K.; Cantin, E.M. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun. 2019, 10, 2153. [Google Scholar] [CrossRef]
- Durant, L.; Stentz, R.; Noble, A.; Brooks, J.; Gicheva, N.; Reddi, D.; O’Connor, M.J.; Hoyles, L.; McCartney, A.L.; Man, R.; et al. Bacteroides thetaiotaomicron-derived outer membrane vesicles promote regulatory dendritic cell responses in health but not in inflammatory bowel disease. Microbiome 2020, 8, 88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wrigley-Carr, H.E.; van Dorst, J.M.; Ooi, C.Y. Intestinal dysbiosis and inflammation in cystic fibrosis impacts gut and multi-organ axes. Med. Microecol. 2022, 13, 100057. [Google Scholar] [CrossRef]
- Doranga, S.; Krogfelt, K.A.; Cohen, P.S.; Conway, T. Nutrition of Escherichia coli within the intestinal microbiome. EcoSal Plus 2024, 12, eesp00062023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomes, T.A.; Elias, W.P.; Scaletsky, I.C.; Guth, B.E.; Rodrigues, J.F.; Piazza, R.M.; Ferreira, L.C.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47 (Suppl. 1), 3–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lorusso, A.B.; Carrara, J.A.; Barroso, C.D.N.; Tuon, F.F.; Faoro, H. Role of Efflux Pumps on Antimicrobial Resistance in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 15779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krysenko, S.; Wohlleben, W. Polyamine and Ethanolamine Metabolism in Bacteria as an Important Component of Nitrogen Assimilation for Survival and Pathogenicity. Med. Sci. 2022, 10, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lundgren, B.R.; Sarwar, Z.; Pinto, A.; Ganley, J.G.; Nomura, C.T. Ethanolamine Catabolism in Pseudomonas aeruginosa PAO1 Is Regulated by the Enhancer-Binding Protein EatR (PA4021) and the Alternative Sigma Factor RpoN. J. Bacteriol. 2016, 198, 2318–2329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaval, K.G.; Garsin, D.A. Ethanolamine Utilization in Bacteria. mBio 2018, 9, e00066-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reese, A.T.; Pereira, F.C.; Schintlmeister, A.; Berry, D.; Wagner, M.; Hale, L.P.; Wu, A.; Jiang, S.; Durand, H.K.; Zhou, X.; et al. Microbial nitrogen limitation in the mammalian large intestine. Nat. Microbiol. 2018, 3, 1441–1450. [Google Scholar] [CrossRef]
- Hammer, N.D.; Skaar, E.P. Molecular mechanisms of Staphylococcus aureus iron acquisition. Annu. Rev. Microbiol. 2011, 65, 129–147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghssein, G.; Ezzeddine, Z. A Review of Pseudomonas aeruginosa Metallophores: Pyoverdine, Pyochelin and Pseudopaline. Biology 2022, 11, 1711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alford, M.A.; Mann, S.; Akhoundsadegh, N.; Hancock, R.E.W. Competition between Pseudomonas aeruginosa and Staphylococcus aureus is dependent on intercellular signaling and regulated by the NtrBC two-component system. Sci. Rep. 2022, 12, 9027. [Google Scholar] [CrossRef]
- Alam, M.Z.; Madan, R. Clostridioides difficile Toxins: Host Cell Interactions and Their Role in Disease Pathogenesis. Toxins 2024, 16, 241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camus, L.; Briaud, P.; Bastien, S.; Elsen, S.; Doléans-Jordheim, A.; Vandenesch, F.; Moreau, K. Trophic cooperation promotes bacterial survival of Staphylococcus aureus and Pseudomonas aeruginosa. ISME J. 2020, 14, 3093–3105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pensinger, D.A.; Dobrila, H.A.; Stevenson, D.M.; Hryckowian, N.D.; Amador-Noguez, D.; Hryckowian, A.J. Exogenous butyrate inhibits butyrogenic metabolism and alters virulence phenotypes in Clostridioides difficile. mBio 2024, 15, e0253523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baldassare, M.A.; Bhattacharjee, D.; Coles, J.D.; Nelson, S.; McCollum, C.A.; Seekatz, A.M. Butyrate enhances Clostridioides difficile sporulation in vitro. J. Bacteriol. 2023, 205, e00138-23. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.L.; Pensinger, D.A.; Hryckowian, A.J. A short chain fatty acid-centric view of Clostridioides difficile pathogenesis. PLoS Pathog. 2021, 17, e1009959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheng, Y.H.; Hasnain, S.Z. Mucus and Mucins: The Underappreciated Host Defence System. Front. Cell. Infect. Microbiol. 2022, 12, 856962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suriano, F.; Nyström, E.E.L.; Sergi, D.; Gustafsson, J.K. Diet, microbiota, and the mucus layer: The guardians of our health. Front. Immunol. 2022, 13, 953196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damianos, J.; Abdelnaem, N.; Camilleri, M. Gut Goo: Physiology, Diet, and Therapy of Intestinal Mucus and Biofilms in Gastrointestinal Health and Disease. Clin. Gastroenterol. Hepatol. 2025, 23, 205–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joja, M.; Grant, E.T.; Desai, M.S. Living on the edge: Mucus-associated microbes in the colon. Mucosal Immunol. 2025, 18, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, J.; Narimatsu, Y.; de Haan, N.; Clausen, H.; de Vos, W.M.; Tytgat, H.L.P. Binding of Akkermansia muciniphila to mucin is O-glycan specific. Nat. Commun. 2024, 15, 4582. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luk, B.; Chang-Graham, A.L.; Hall, A.; Herrmann, B.; Ruan, W.; Endres, B.T.; Shi, Z.; Garey, K.W.; Hyser, J.M.; et al. Bifidobacterium dentium Fortifies the Intestinal Mucus Layer via Autophagy and Calcium Signaling Pathways. mBio 2019, 10, e01087-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen See, J.R.; Leister, J.; Wright, J.R.; Kruse, P.I.; Khedekar, M.V.; Besch, C.E.; Kumamoto, C.A.; Madden, G.R.; Stewart, D.B.; Lamendella, R. Clostridioides difficile infection is associated with differences in transcriptionally active microbial communities. Front. Microbiol. 2024, 15, 1398018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, Y.; Cai, D.; Zheng, Y.; Yan, S.; Wu, L.; Li, C.; Song, W.; Xin, T.; Lv, S.; Huang, R.; et al. Microscopic Mechanism of Carbon-Dopant Manipulating Device Performance in CGeSbTe-Based Phase Change Random Access Memory. ACS Appl. Mater. Interfaces 2020, 12, 23051–23059. [Google Scholar] [CrossRef] [PubMed]
- Fekete, E.; Buret, A.G. The role of mucin O-glycans in microbiota dysbiosis, intestinal homeostasis, and host-pathogen interactions. Am. J. Physiol.-Gastrointest. Liver Physiol. 2023, 324, G452–G465. [Google Scholar] [CrossRef] [PubMed]
- Valiei, A.; Dickson, A.; Aminian-Dehkordi, J.; Mofrad, M.R.K. Metabolic interactions shape emergent biofilm structures in a conceptual model of gut mucosal bacterial communities. NPJ Biofilms Microbiomes 2024, 10, 99. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. NPJ Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Jandl, B.; Dighe, S.; Baumgartner, M.; Makristathis, A.; Gasche, C.; Muttenthaler, M. Gastrointestinal Biofilms: Endoscopic Detection, Disease Relevance, and Therapeutic Strategies. Gastroenterology 2024, 167, 1098–1112.e5. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.; Ghannoum, A.; Hager, C.; Retuerto, M.; Isham, N.; McCormick, T.S. The Probiotic BIOHM Improves Nutrient Absorption by Disrupting Gastrointestinal Biofilms. J. Probiotics Health 2019, 7, 1–5. [Google Scholar] [CrossRef]
- Molobela, I.; Cloete, T.; Beukes, M. Protease and amylase enzymes for biofilm removal and degradation of extracellular polymeric substances (EPS) produced by Pseudomonas fluorescens bacteria. Afr. J. Microbiol. Res. 2010, 4, 1515–1524. [Google Scholar]
- Tielen, P.; Kuhn, H.; Rosenau, F.; Jaeger, K.-E.; Flemming, H.-C.; Wingender, J. Interaction between extracellular lipase LipA and the polysaccharide alginate of Pseudomonas aeruginosa. BMC Microbiol. 2013, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Ruch, T.R.; Engel, J.N. Targeting the Mucosal Barrier: How Pathogens Modulate the Cellular Polarity Network. Cold Spring Harb. Perspect. Biol. 2017, 9, a027953. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amati, F.; Leonardi, G.; Contarini, M.; Morlacchi, L.C.; Stainer, A.; Pizzamiglio, G.; Aliberti, S.; Blasi, F.; Gramegna, A. Immunodeficiencies and CFTR dysfunction: Results from a systematic screening in a cohort of adults with cystic fibrosis and CFTR-related disorders. Ther. Adv. Respir. Dis. 2024, 18, 17534666241253945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Purushothaman, A.K.; Nelson, E.J.R. Role of innate immunity and systemic inflammation in cystic fibrosis disease progression. Heliyon 2023, 9, e17553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kristensen, M.; Prevaes, S.M.P.J.; Kalkman, G.; Tramper-Stranders, G.A.; Hasrat, R.; de Winter-de Groot, K.M.; Janssens, H.M.; Tiddens, H.A.; van Westreenen, M.; Sanders, E.A.M.; et al. Development of the gut microbiota in early life: The impact of cystic fibrosis and antibiotic treatment. J. Cyst. Fibros. 2020, 19, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baldwin-Hunter, B.L.; Rozenberg, F.D.; Annavajhala, M.K.; Park, H.; DiMango, E.A.; Keating, C.L.; Uhlemann, A.C.; Abrams, J.A. The gut microbiome, short chain fatty acids, and related metabolites in cystic fibrosis patients with and without colonic adenomas. J. Cyst. Fibros. 2023, 22, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Overby, H.B.; Ferguson, J.F. Gut Microbiota-Derived Short-Chain Fatty Acids Facilitate Microbiota: Host Cross talk and Modulate Obesity and Hypertension. Curr. Hypertens. Rep. 2021, 23, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borisova, D.; Paunova-Krasteva, T.; Strateva, T.; Stoitsova, S. Biofilm Formation of Pseudomonas aeruginosa in Cystic Fibrosis: Mechanisms of Persistence, Adaptation, and Pathogenesis. Microorganisms 2025, 13, 1527. [Google Scholar] [CrossRef] [PubMed]
- Jean-Pierre, V.; Boudet, A.; Sorlin, P.; Menetrey, Q.; Chiron, R.; Lavigne, J.P.; Marchandin, H. Biofilm Formation by Staphylococcus aureus in the Specific Context of Cystic Fibrosis. Int. J. Mol. Sci. 2022, 24, 597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thavamani, A.; Salem, I.; Sferra, T.J.; Sankararaman, S. Impact of Altered Gut Microbiota and Its Metabolites in Cystic Fibrosis. Metabolites 2021, 11, 123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Q.; Tian, X.; Maruyama, D.; Arjomandi, M.; Prakash, A. Lung immune tone via gut-lung axis: Gut-derived LPS and short-chain fatty acids’ immunometabolic regulation of lung IL-1β, FFAR2, and FFAR3 expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L65–L78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caley, L.R.; White, H.; de Goffau, M.C.; Floto, R.A.; Parkhill, J.; Marsland, B.; Peckham, D.G. Cystic Fibrosis-Related Gut Dysbiosis: A Systematic Review. Dig. Dis. Sci. 2023, 68, 1797–1814. [Google Scholar] [CrossRef] [PubMed]
- Suppakitjanusant, P.; Wang, Y.; Sivapiromrat, A.K.; Hu, C.; Binongo, J.; Hunt, W.R.; Weinstein, S.; Jathal, I.; Alvarez, J.A.; Chassaing, B.; et al. Impact of high-dose cholecalciferol (vitamin D3) and inulin prebiotic on intestinal and airway microbiota in adults with cystic fibrosis: A 2 × 2 randomized, placebo-controlled, double-blind pilot study. J. Clin. Transl. Endocrinol. 2024, 37, 100362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caverly, L.J.; Riquelme, S.A.; Hisert, K.B. The Impact of Highly Effective Modulator Therapy on Cystic Fibrosis Microbiology and Inflammation. Clin. Chest Med. 2022, 43, 647–665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marsh, R.; Santos, C.D.; Yule, A.; Dellschaft, N.S.; Hoad, C.L.; Ng, C.; Major, G.; Smyth, A.R.; Rivett, D.; van der Gast, C. Impact of extended Elexacaftor/Tezacaftor/Ivacaftor therapy on the gut microbiome in cystic fibrosis. J. Cyst. Fibros. 2024, 23, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.E.; Vo, A.T.; Hayden, H.S.; Weiss, E.J.; Durfey, S.; McNamara, S.; Ratjen, A.; Grogan, B.; Carter, S.; Nay, L.; et al. Changes in fecal microbiota with CFTR modulator therapy: A pilot study. J. Cyst. Fibros. 2021, 20, 742–746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.-A.; Cho, A.; Huang, E.; Xu, Y.; Quach, H.; Hu, W.J.; Wong, A. Gene therapy for cystic fibrosis: New tools for precision medicine. J. Transl. Med. 2021, 19, 452. [Google Scholar] [CrossRef] [PubMed]
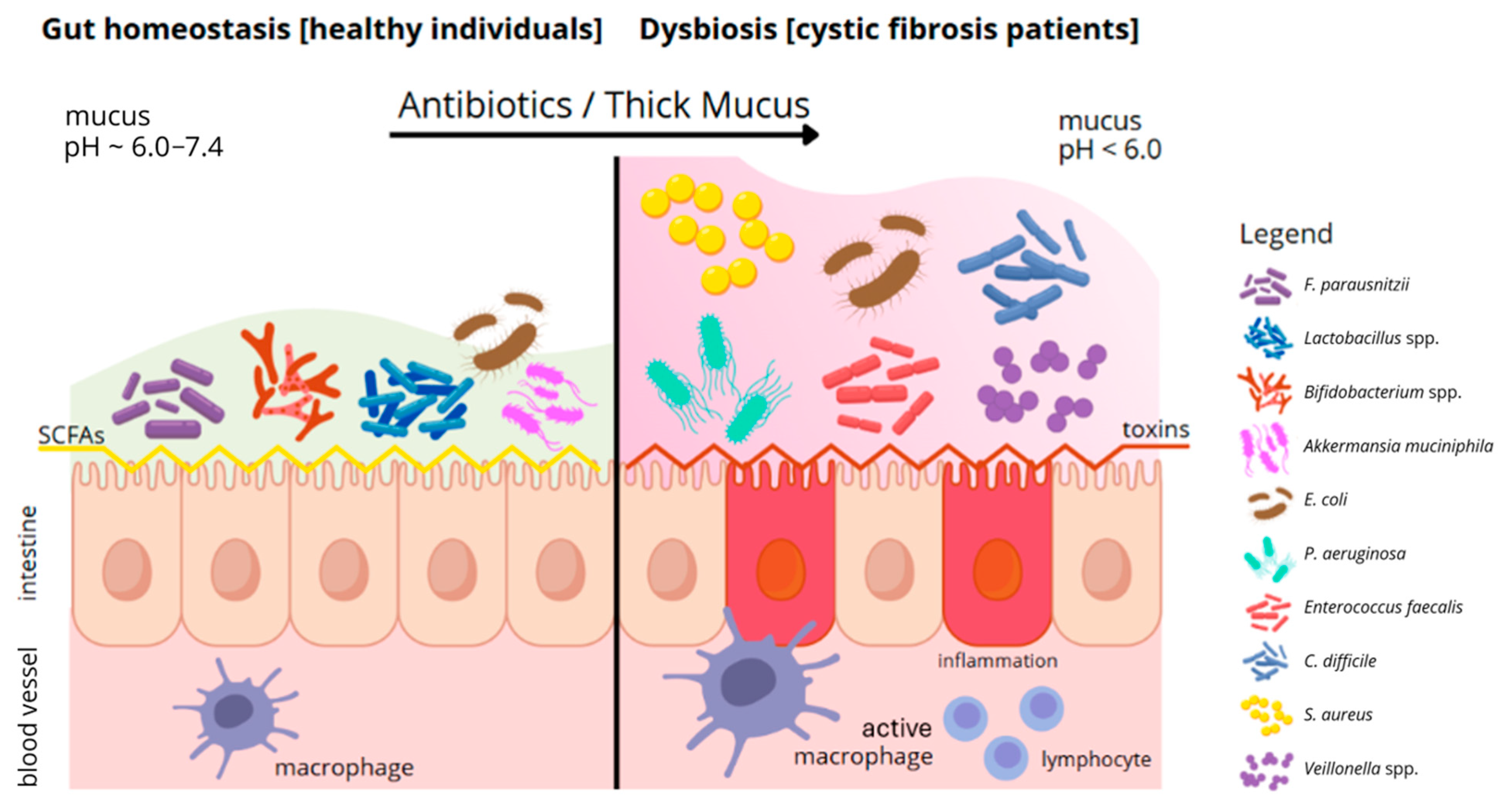
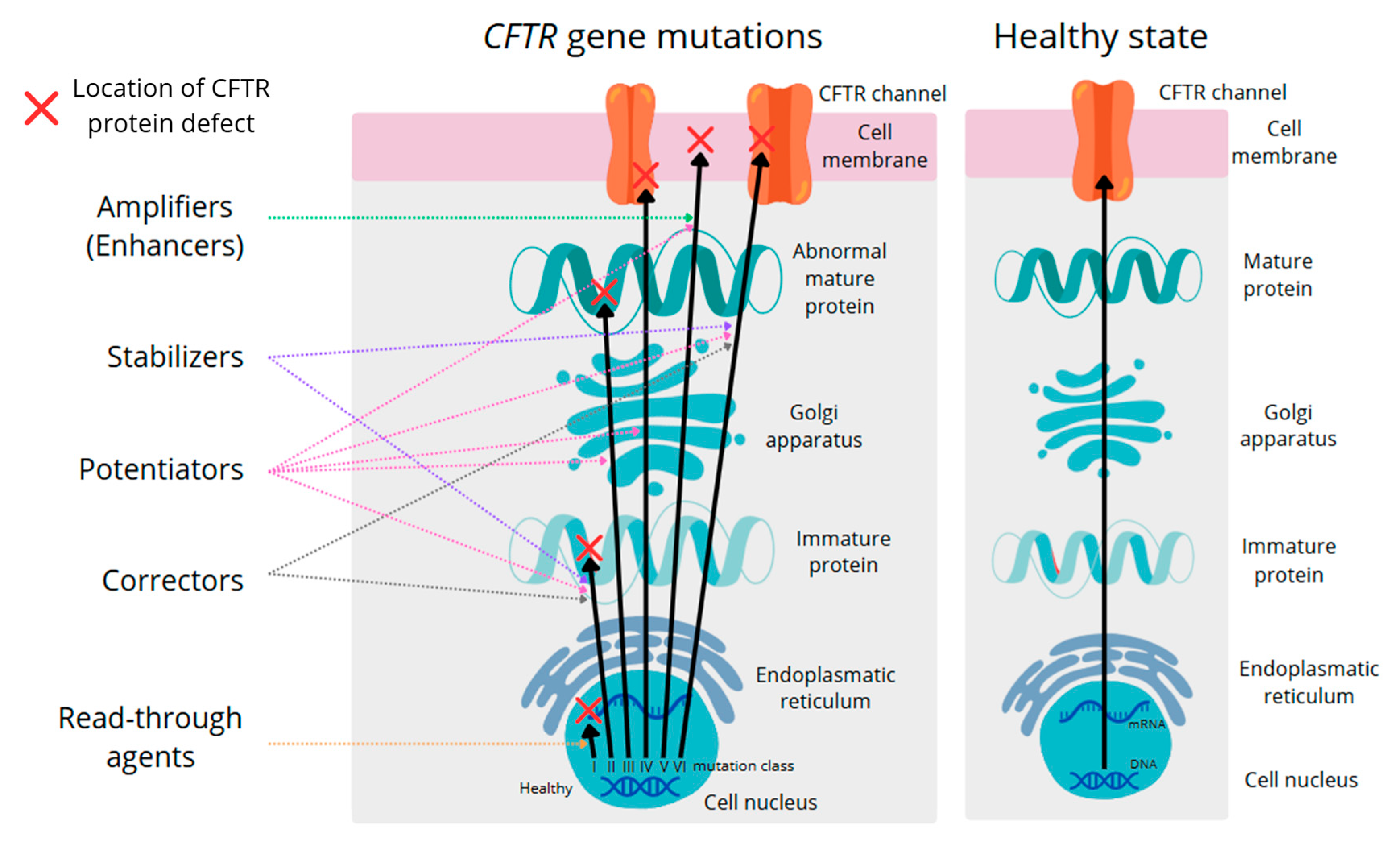
| Category | Healthy People | People with Cystic Fibrosis | References |
|---|---|---|---|
| Dominant bacteria | Bifidobacterium, Lactobacillus, Faecalibacterium, Roseburia, and Bacteroides | E. coli, S. aureus, P. aeruginosa, and C. difficile | [1,2,3,4,7,14,16,21,22,23] |
| Microbiome diversity | High: Enables symbiotic interactions and stabilises the intestinal environment | Low: Leads to dysbiosis and excessive growth of pathogens | [2,3,4,7,8,9,20] |
| SCFA production | High: Supports anti-inflammatory processes, regulates metabolism | Reduced: Low SCFA leads to inflammation | [4,10,11,12,13,16] |
| Effect on absorption | Increased absorption of minerals and vitamins; stabilisation of metabolism | Impaired absorption of nutrients due to inflammation and pathogens | [2,3,4,6,7] |
| Inflammation | Low: Beneficial microflora inhibits inflammation | High: The presence of pathogens leads to chronic inflammation | [2,3,4,7,8,20] |
| Bacterial interactions | Symbiosis between bacteria: Co-production of metabolites | Antagonism: Pathogenic bacteria dominate and compete with beneficial bacteria | [2,4,7,8,9,17] |
| Weight to achieve | It enables healthy development and weight maintenance | This often leads to malnutrition and weight loss | [2,4,6,7] |
| Toxin production | Low: No pathogenic strains | High: Presence of toxin-secreting pathogens | [2,3,4,7,22,23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowska, N.; Durda-Masny, M.; Cofta, S.; Springer, D.; Szwed, A. Gut Dysbiosis Driven by CFTR Gene Mutations in Cystic Fibrosis Patients: From Genetic Disruption to Multisystem Consequences and Microbiota Modulation. Genes 2025, 16, 1049. https://doi.org/10.3390/genes16091049
Pawłowska N, Durda-Masny M, Cofta S, Springer D, Szwed A. Gut Dysbiosis Driven by CFTR Gene Mutations in Cystic Fibrosis Patients: From Genetic Disruption to Multisystem Consequences and Microbiota Modulation. Genes. 2025; 16(9):1049. https://doi.org/10.3390/genes16091049
Chicago/Turabian StylePawłowska, Natalia, Magdalena Durda-Masny, Szczepan Cofta, Daria Springer, and Anita Szwed. 2025. "Gut Dysbiosis Driven by CFTR Gene Mutations in Cystic Fibrosis Patients: From Genetic Disruption to Multisystem Consequences and Microbiota Modulation" Genes 16, no. 9: 1049. https://doi.org/10.3390/genes16091049
APA StylePawłowska, N., Durda-Masny, M., Cofta, S., Springer, D., & Szwed, A. (2025). Gut Dysbiosis Driven by CFTR Gene Mutations in Cystic Fibrosis Patients: From Genetic Disruption to Multisystem Consequences and Microbiota Modulation. Genes, 16(9), 1049. https://doi.org/10.3390/genes16091049






