The Role of lncRNAs in Complicated Pregnancy: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Analysis
2.4. Methodological Quality Assessment
2.5. Outcome Measures
3. Results
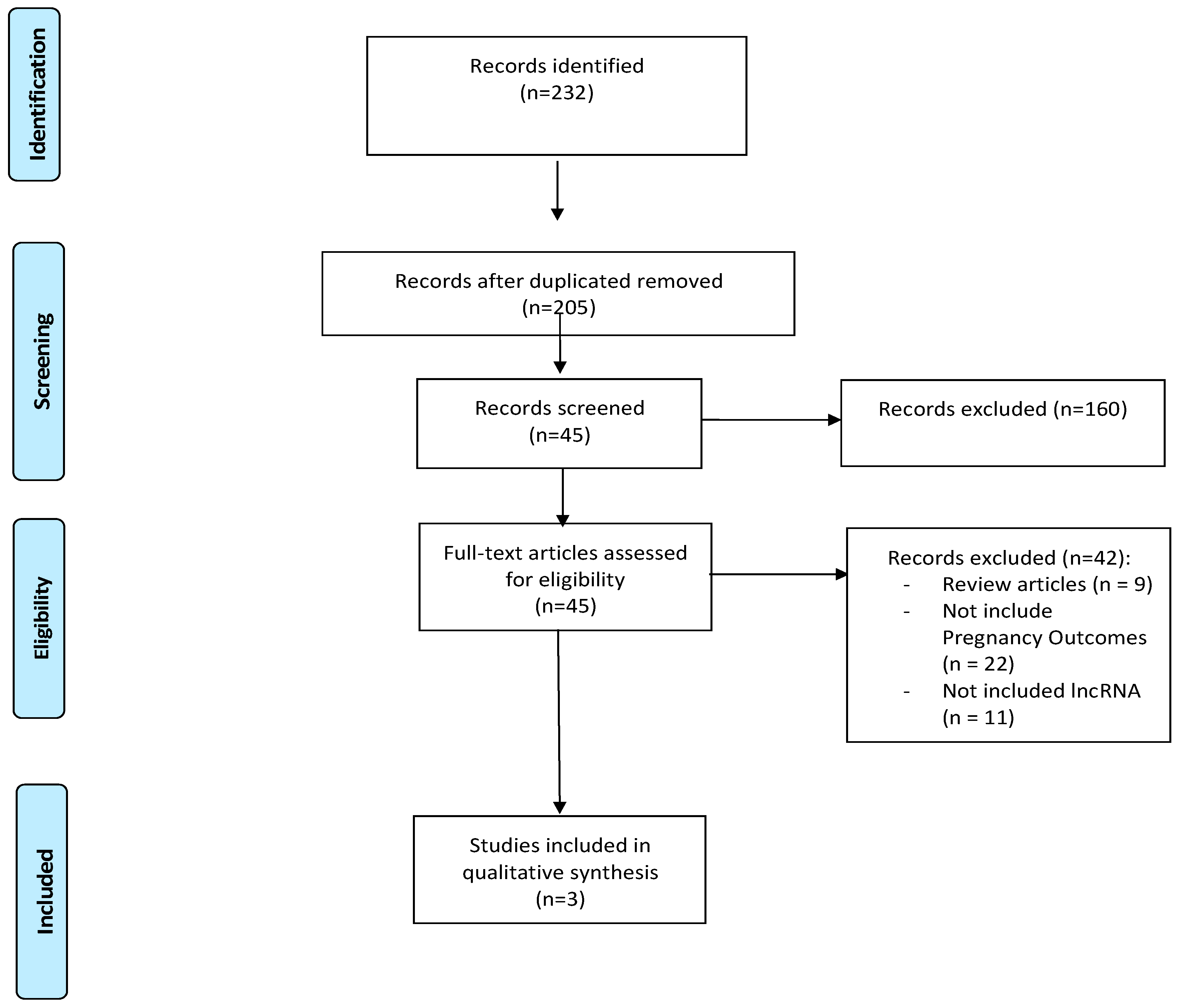
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias of Included Studies
3.4. Synthesis of Results
3.4.1. Hypertensive Disorders of Pregnancy
3.4.2. Intrahepatic Cholestasis of Pregnancy
3.4.3. Gestational Diabetes Mellitus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, M.; Kolluru, G.K.; Ahmed, A. Small Molecule, Big Prospects: MicroRNA in Pregnancy and Its Complications. J. Pregnancy 2017, 2017, 6972732. [Google Scholar] [CrossRef]
- Lihua, C.; Hua, S.; Wenzhan, W.; Standard, J.; Denghui, L. Expression and clinical significance of lncRNA PART1 in patients with unexplained recurrent pregnancy loss. Gynecol. Endocrinol. 2024, 40, 2375582. [Google Scholar] [CrossRef]
- Wang, K.C.; Chang, H.Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Kung, J.T.Y.; Colognori, D.; Lee, J.T. Long Noncoding RNAs: Past, Present, and Future. Genetics 2013, 193, 651–669. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Moran, V.A.; Perera, R.J.; Khalil, A.M. Emerging functional and mechanistic paradigms of mammalian long non-coding RNAs. Nucleic Acids Res. 2012, 40, 6391–6400. [Google Scholar] [CrossRef] [PubMed]
- Pisignano, G.; Ladomery, M. Post-Transcriptional Regulation through Long Non-Coding RNAs (lncRNAs). Noncoding RNA 2021, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, F.; Wu, P.; Chen, Y.; Jia, Y. Aberrant LncRNA Expression in Leukemia. J. Cancer 2020, 11, 4284–4296. [Google Scholar] [CrossRef]
- Ziats, M.N.; Rennert, O.M. Aberrant Expression of Long Noncoding RNAs in Autistic Brain. J. Mol. Neurosci. 2013, 49, 589–593. [Google Scholar] [CrossRef]
- Meng, H.; Han, L.; Hong, C.; Ding, J.; Huang, Q. Aberrant lncRNA Expression in Multiple Myeloma. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 26, 809–816. [Google Scholar] [CrossRef]
- Nakagawa, S.; Shimada, M.; Yanaka, K.; Mito, M.; Arai, T.; Takahashi, E.; Fujita, Y.; Fujimori, T.; Standaert, L.; Marine, J.-C.; et al. The lncRNA Neat1 is required for corpus luteum formation and the establishment of pregnancy in a subpopulation of mice. Development 2014, 141, 4618–4627. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. What is the placenta? Am J. Obstet. Gynecol. 2015, 213, S6.e1–S6.e4. [Google Scholar] [CrossRef]
- La Verde, M.; Torella, M.; Ronsini, C.; Riemma, G.; Cobellis, L.; Marrapodi, M.M.; Capristo, C.; Rapisarda, A.M.C.; Morlando, M.; De Franciscis, P. The association between fetal Doppler and uterine artery blood volume flow in term pregnancies: A pilot study. Ultraschall Der Med.—Eur. J. Ultrasound 2024, 45, 184–189. [Google Scholar] [CrossRef]
- Molitierno, R.; Imparato, A.; Iavazzo, N.; Salzillo, C.; Marzullo, A.; Laganà, A.S.; Etrusco, A.; Agrifoglio, V.; D’Amato, A.; Renata, E.; et al. Microscopic changes and gross morphology of placenta in women affected by gestational diabetes mellitus in dietary treatment: A systematic review. Open Med. 2025, 20, 20251142. [Google Scholar] [CrossRef] [PubMed]
- Maltepe, E.; Fisher, S.J. Placenta: The Forgotten Organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Ortega, J.; Saucedo, R.; Peña-Cano, M.I.; Hernández-Valencia, M.; Cruz-Durán, J.G. Immune tolerance at the maternal-placental interface in healthy pregnancy and pre-eclampsia. J. Obstet. Gynaecol. Res. 2020, 46, 1067–1076. [Google Scholar] [CrossRef]
- Ren, J.; Jin, H.; Zhu, Y. The Role of Placental Non-Coding RNAs in Adverse Pregnancy Outcomes. Int. J. Mol. Sci. 2023, 24, 5030. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, Z.; Zhang, E.; Zuo, Q.; Huang, S.; Yang, N.; Wu, D.; Zhang, Y.; Chen, Y.; Xu, H.; et al. The lncRNA TUG1 modulates proliferation in trophoblast cells via epigenetic suppression of RND3. Cell Death Dis. 2017, 8, e3104. [Google Scholar] [CrossRef]
- Wang, M.; Zhong, J.; Xiang, Y. LncRNA-GAS5 related to the processes of recurrent pregnancy loss by regulating Th1/Th2 balance. Kaohsiung J. Med. Sci. 2021, 37, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Virnicchi, G.; Tanigawa, A.; Naganuma, T.; Li, R.; Kimura, H.; Yokoi, T.; Nakagawa, S.; Bénard, M.; Fox, A.H.; et al. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol. Biol. Cell 2014, 25, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, H.; Kong, W.; Zhang, Y.; Cao, L.; Gao, L.; Zhou, R. Down-regulated long non-coding RNA-ATB in preeclampsia and its effect on suppressing migration, proliferation, and tube formation of trophoblast cells. Placenta 2017, 49, 80–87. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80. [Google Scholar] [CrossRef]
- Dai, C.; Zhao, C.; Xu, M.; Sui, X.; Sun, L.; Liu, Y.; Su, M.; Wang, H.; Yuan, Y.; Zhang, S.; et al. Serum lncRNAs in early pregnancy as potential biomarkers for the prediction of pregnancy-induced hypertension, including preeclampsia. Mol. Ther. Nucleic Acids 2021, 24, 416–425. [Google Scholar] [CrossRef]
- Zou, S.; Zhao, S.; Wang, J.; Dong, R.; Zou, P.; Liang, F.; Zhu, T.T.; Zhou, T.; Li, N.; Zhang, Y.; et al. Diagnostic and Prognostic Value of Long Noncoding RNAs as Potential Novel Biomarkers in Intrahepatic Cholestasis of Pregnancy. Biomed. Res. Int. 2021, 2021, 8858326. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Wang, F.; Ye, M.; Zhu, H.; Bu, S. Long non-coding RNA MALAT1 expression in patients with gestational diabetes mellitus. Int. J. Gynecol. Obstet. 2018, 140, 164–169. [Google Scholar] [CrossRef]
- Pang, Y.; Mao, C.; Liu, S. Encoding activities of non-coding RNAs. Theranostics 2018, 8, 2496–2507. [Google Scholar] [CrossRef]
- Žarković, M.; Hufsky, F.; Markert, U.R.; Marz, M. The Role of Non-Coding RNAs in the Human Placenta. Cells 2022, 11, 1588. [Google Scholar] [CrossRef]
- Chen, X.; Guo, D.-Y.; Yin, T.-L.; Yang, J. Non-Coding RNAs Regulate Placental Trophoblast Function and Participate in Recurrent Abortion. Front. Pharmacol. 2021, 12, 646521. [Google Scholar] [CrossRef]
- Majewska, M.; Lipka, A.; Paukszto, L.; Jastrzebski, J.P.; Gowkielewicz, M.; Jozwik, M.; Majewski, M.K. Preliminary RNA-Seq Analysis of Long Non-Coding RNAs Expressed in Human Term Placenta. Int. J. Mol. Sci. 2018, 19, 1894. [Google Scholar] [CrossRef]
- McAninch, D.; Roberts, C.; Bianco-Miotto, T. Mechanistic Insight into Long Noncoding RNAs and the Placenta. Int. J. Mol. Sci. 2017, 18, 1371. [Google Scholar] [CrossRef] [PubMed]
- Lipka, A.; Jastrzebski, J.P.; Paukszto, L.; Makowczenko, K.G.; Lopienska-Biernat, E.; Gowkielewicz, M.; Lepiarczyk, E.; Wiszpolska, M.; Majewski, M.K.; Majewska, M. Sex-Biased lncRNA Signature in Fetal Growth Restriction (FGR). Cells 2021, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.G.; Ibelli, A.M.G.; Cantão, M.E.; Oliveira, H.C.; Peixoto, J.O.; Ledur, M.C.; Guimarães, S.E.F. Long Non-Coding RNAs Differentially Expressed in Swine Fetuses. Animals 2024, 14, 1897. [Google Scholar] [CrossRef]
- Yan, H.; Liu, Q.; Jiang, J.; Shen, X.; Zhang, L.; Yuan, Z.; Wu, Y.; Liu, Y. Identification of sex differentiation-related microRNA and long non-coding RNA in Takifugu rubripes gonads. Sci. Rep. 2021, 11, 7459. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.M.; Goyal, R. LncRNA as a Therapeutic Target for Angiogenesis. Curr. Top. Med. Chem. 2017, 17, 1750–1757. [Google Scholar] [CrossRef]
- Hashemi, M.; Moosavi, M.S.; Abed, H.M.; Dehghani, M.; Aalipour, M.; Heydari, E.A.; Behroozaghdam, M.; Entezari, M.; Salimimoghadam, S.; Gunduz, E.S.; et al. Long non-coding RNA (lncRNA) H19 in human cancer: From proliferation and metastasis to therapy. Pharmacol. Res. 2022, 184, 106418. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, L.; Ma, S.; Lin, R.; Li, J.; Yang, S. Biogenesis and function of exosome lncRNAs and their role in female pathological pregnancy. Front. Endocrinol. 2023, 14, 1191721. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, Y.; Zhang, Z.; Wang, B.; Wei, R.; Chu, C.; Xu, K.; Li, L.; Liu, Y.; Li, X. Emerging role of miRNAs, lncRNAs, and circRNAs in pregnancy-associated diseases. Chin. Med. J. 2023, 136, 1300–1310. [Google Scholar] [CrossRef]
- La Verde, M.; Molitierno, R.; Marrapodi, M.M.; Fordellone, M.; Laganà, A.S.; Palma, M.; Petillo, A.; Riemma, G.; Vastarella, M.G.; De Franciscis, P. Impact of the systemic inflammatory indices on birth weight: A prospective observational study. Gynecol. Obstet. Investig. 2025, 1–16. [Google Scholar] [CrossRef]
- La Verde, M.; Luciano, M.; Fordellone, M.; Sampogna, G.; Lettieri, D.; Palma, M.; Torella, D.; Marrapodi, M.M.; Di Vincenzo, M.; Torella, M. Postpartum Depression and Inflammatory Biomarkers of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, and Monocyte-Lymphocyte Ratio: A Prospective Observational Study. Gynecol. Obstet. Investig. 2024, 89, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, J.; Yang, W.; Xue, P.; Yin, Y.; Wang, Y.; Tian, P.; Peng, H.; Jiang, H.; Xu, W.; et al. Noninvasive preeclampsia prediction using plasma cell–free RNA signatures. Am. J. Obstet. Gynecol. 2023, 229, 553.e1–553.e16. [Google Scholar] [CrossRef]
- Ogoyama, M.; Takahashi, H.; Suzuki, H.; Ohkuchi, A.; Fujiwara, H.; Takizawa, T. Non-Coding RNAs and Prediction of Preeclampsia in the First Trimester of Pregnancy. Cells 2022, 11, 2428. [Google Scholar] [CrossRef]
| (1) Study design and sample representativeness: |
| 1 point: Study includes a sample size greater than or equal to 100. |
| 0 points: Sample size of less than 100 participants. |
| (2) Sampling technique: |
| 1 point: Patients recruited consecutively or randomly (randomization criteria clarified). |
| 0 points: Potential convenience sampling or unspecified sampling technique. |
| (3) Description of the lncRNA technique: |
| 1 point: The authors provided a comprehensive description of the equipment, setting, and adopted technique. |
| 0 points: The study did not report adequate information on the lncRNA technique. |
| (4) Quality of population description: |
| 1 point: The study reported a clear description of the population with proper measures of dispersion (e.g., mean, standard deviation). |
| 0 points: The study did not report a clear description of the population, incompletely reported descriptive statistics or did not report measures of dispersion. |
| (5) Incomplete outcome data: |
| 1 point: The study reported complete data about the pregnancy outcomes. |
| 0 points: Selective data reporting cannot be excluded. |
| Study (Year) | Country | Study Design/ Setting | Methodology | Population and Sample Size | Gestational Age (GA) | Maternal Age |
|---|---|---|---|---|---|---|
| Dai et al. (2021) [24] | China |
|
|
|
| 20–35 years |
| Zou et al. (2021) [25] | China |
|
|
|
| 26–29 years |
| Zhang et al. (2018) [26] | China |
|
|
| 36–40 weeks of gestation | 28–32 years |
| Author, Year | Study Design and Sample Representativeness | Sampling Technique | Description of the lncRNA Technique | Quality of Population Description | Incomplete Outcome Data | Total Score |
|---|---|---|---|---|---|---|
| Dai et al. (2021) [24] | ★ | ★ | ★ | ★ | ★ | ★★★★★ |
| Zou et al. (2021) [25] | ★ | - | ★ | ★ | ★ | ★★★★ |
| Zhang et al. (2018) [26] | - | - | ★ | ★ | ★ | ★★★ |
| Study | Pregnancy Complication Evaluated | Investigated lncRNAs | Expression/Changes | Correlation with Outcomes/Key Findings | Ref. |
|---|---|---|---|---|---|
| Dai et al. (2021) |
|
|
|
| [24] |
| Zou et al. (2021) |
|
|
|
| [25] |
| Zhang et al. (2018) | Gestational Diabetes Mellitus (GDM) |
|
|
| [26] |
| lncRNAs | Strengths | Limitations |
|---|---|---|
| Biological relevance | lncRNAs are involved in reproductive processes like trophoblast invasion, decidualization, and maternal immune tolerance. | The molecular lncRNAs’ pathways is incompletely mapped, and clinical adoption is limited. |
| Diagnostic and prognostic value | lncRNAs in maternal serum represent a promising, non-invasive way for early screening of pregnancy complications. | Actually, a robust diagnostic cutoff is lacking. |
| Clinical potential | Several lncRNAs show associations with adverse outcomes. | Validation is still lacking in large, multi-ethnic, prospective cohorts. |
| Mechanistic plausibility | lncRNAs interact with both mRNAs and miRNAs to regulate gene expression at transcriptional, epigenetic, and post-transcriptional levels. | The complexity and redundancy of lncRNAs make it difficult to isolate individual contributions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerillo, A.; Molitierno, R.; De Franciscis, P.; Nunziata, D.D.; Fordellone, M.; Capristo, C.; Marrapodi, M.M.; Etrusco, A.; Laganà, A.S.; La Verde, M. The Role of lncRNAs in Complicated Pregnancy: A Systematic Review. Genes 2025, 16, 959. https://doi.org/10.3390/genes16080959
Cerillo A, Molitierno R, De Franciscis P, Nunziata DD, Fordellone M, Capristo C, Marrapodi MM, Etrusco A, Laganà AS, La Verde M. The Role of lncRNAs in Complicated Pregnancy: A Systematic Review. Genes. 2025; 16(8):959. https://doi.org/10.3390/genes16080959
Chicago/Turabian StyleCerillo, Antonio, Rossella Molitierno, Pasquale De Franciscis, Debora Damiana Nunziata, Mario Fordellone, Carlo Capristo, Maria Maddalena Marrapodi, Andrea Etrusco, Antonio Simone Laganà, and Marco La Verde. 2025. "The Role of lncRNAs in Complicated Pregnancy: A Systematic Review" Genes 16, no. 8: 959. https://doi.org/10.3390/genes16080959
APA StyleCerillo, A., Molitierno, R., De Franciscis, P., Nunziata, D. D., Fordellone, M., Capristo, C., Marrapodi, M. M., Etrusco, A., Laganà, A. S., & La Verde, M. (2025). The Role of lncRNAs in Complicated Pregnancy: A Systematic Review. Genes, 16(8), 959. https://doi.org/10.3390/genes16080959









