Genome-Wide Association Study and Meta-Analysis Uncovers Key Candidate Genes for Body Weight Traits in Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Populations and Phenotypic Data
2.2. Genetic Parameter Estimation
2.3. GWAS Analysis
2.4. Meta-Analysis
2.5. Definition of Significant Independent Loci
2.6. Gene Association Analysis
2.7. SNP QTL Annotation
2.8. Functional Annotation of Candidate Genes
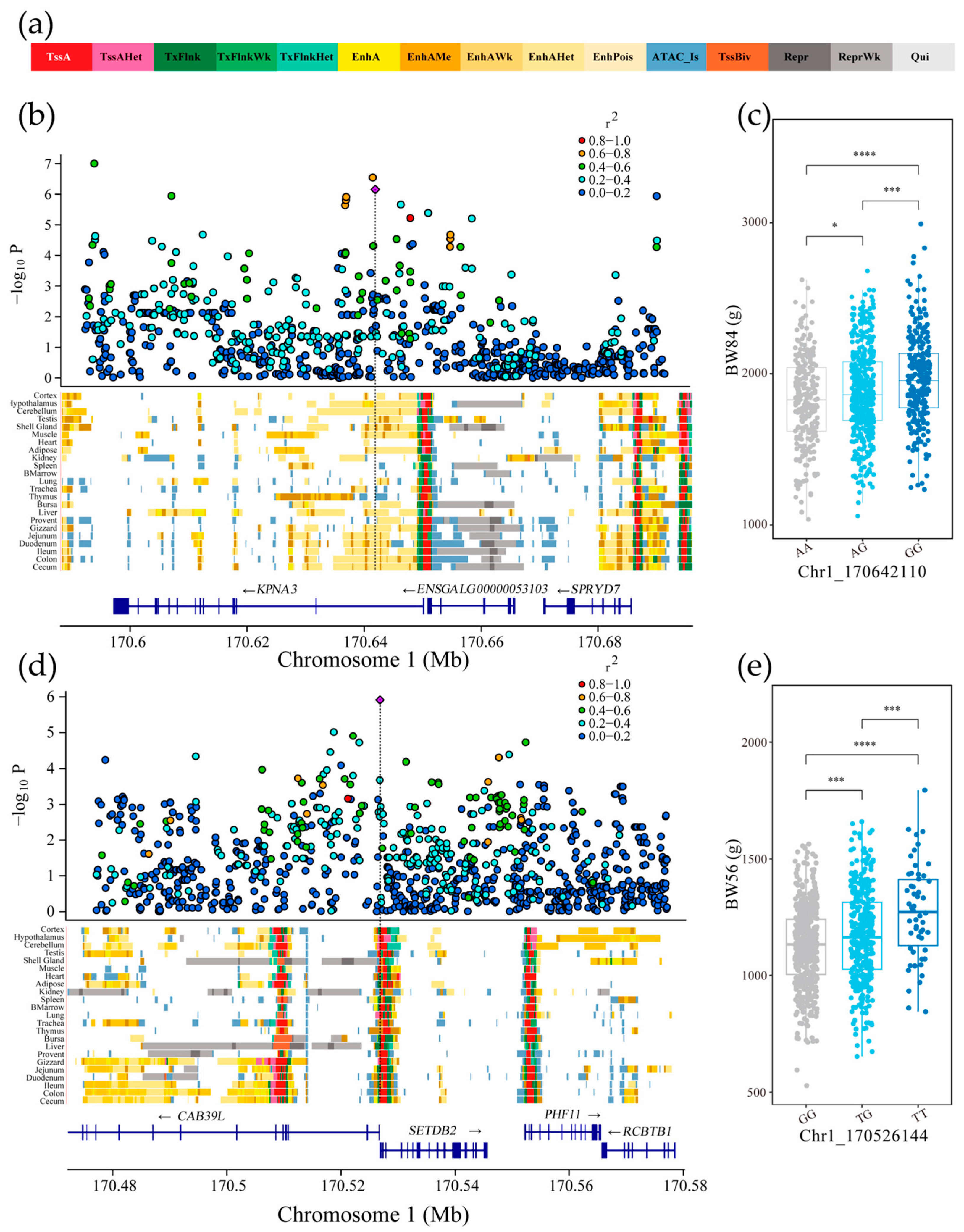
2.9. Linkage Disequilibrium and Chromatin State Annotation of Genome-Wide Significant Loci
3. Results
3.1. Phenotypic Statistics and Genetic Parameters for Growth Traits
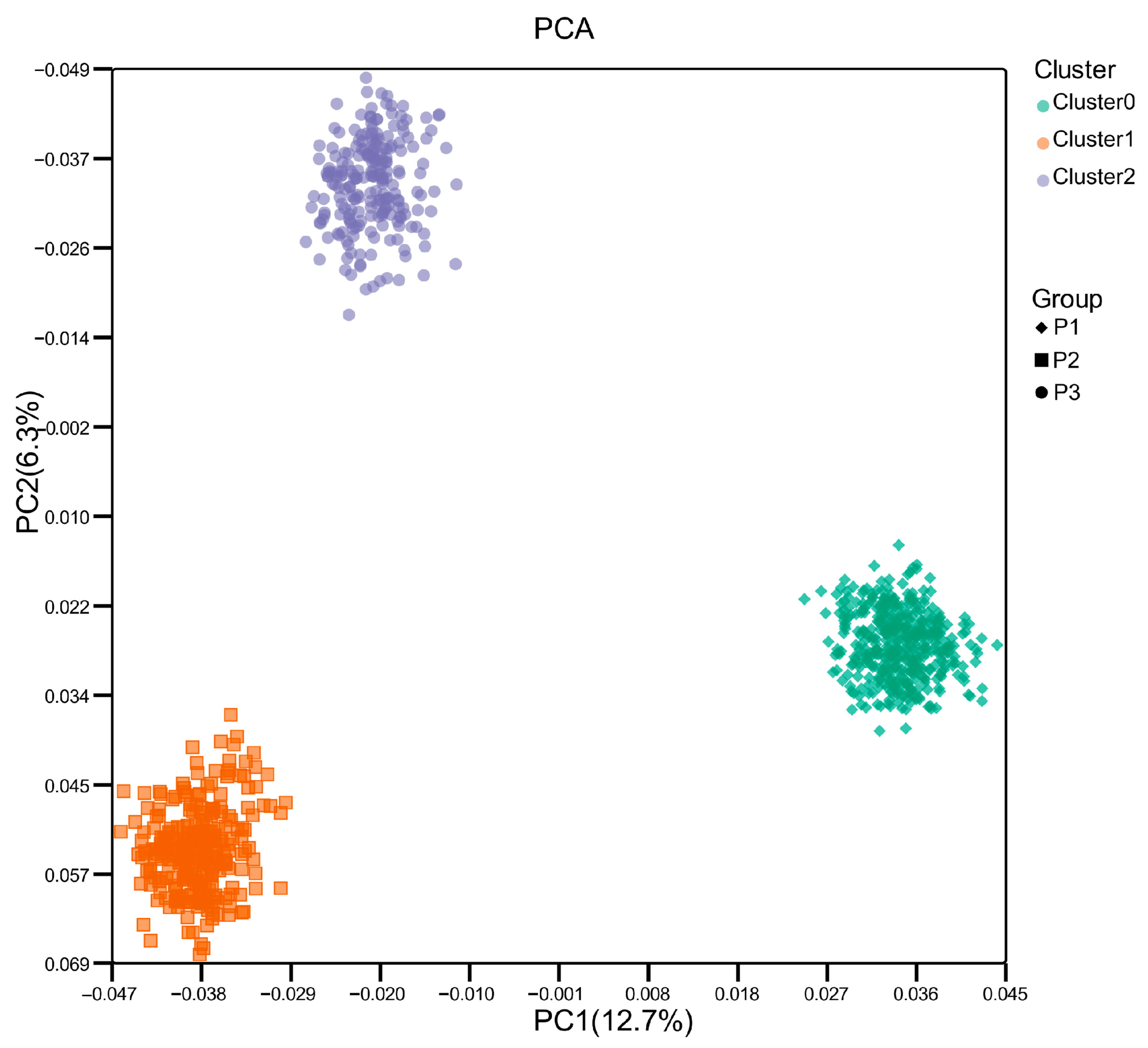
3.2. Population Structure
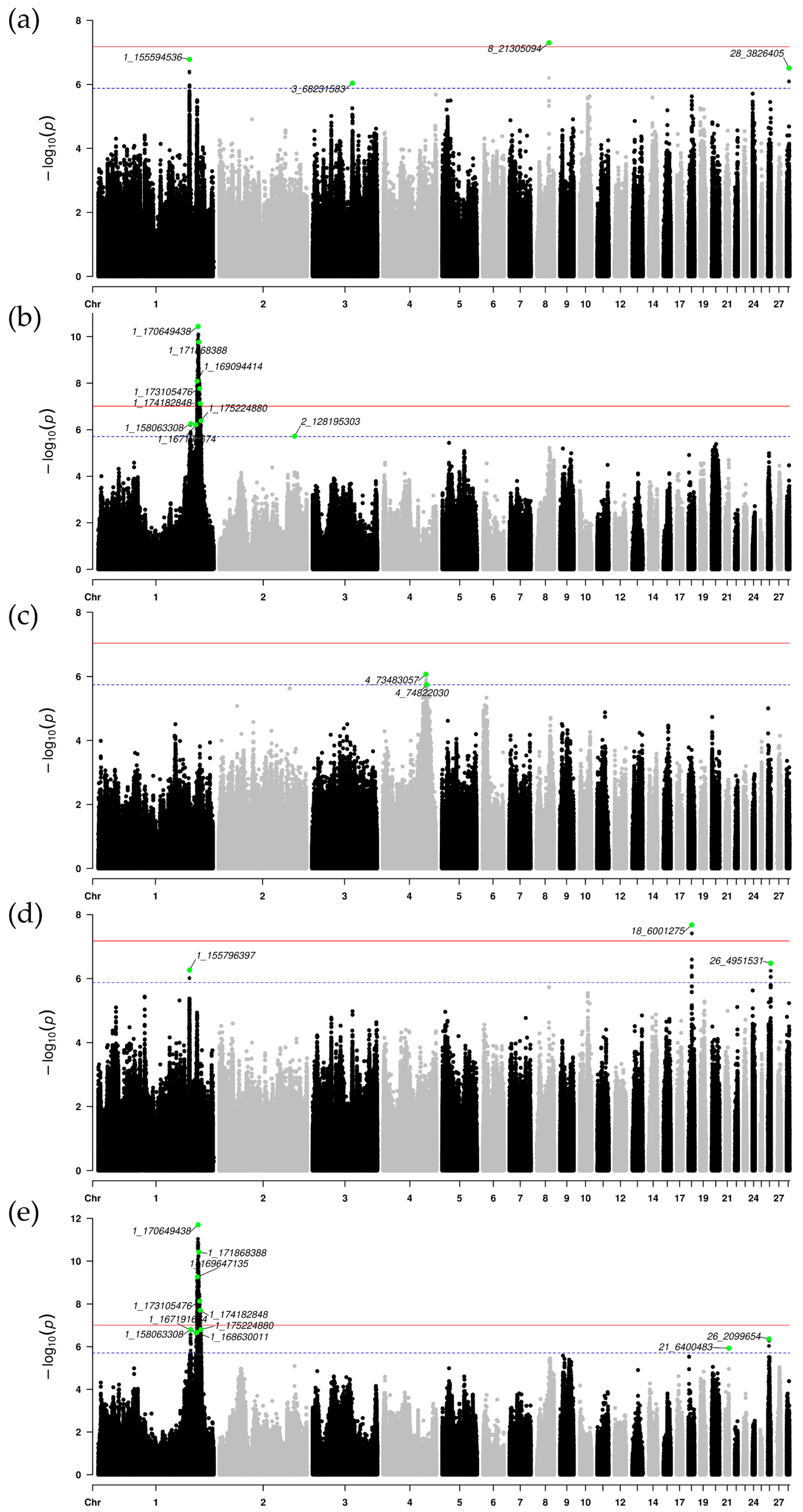
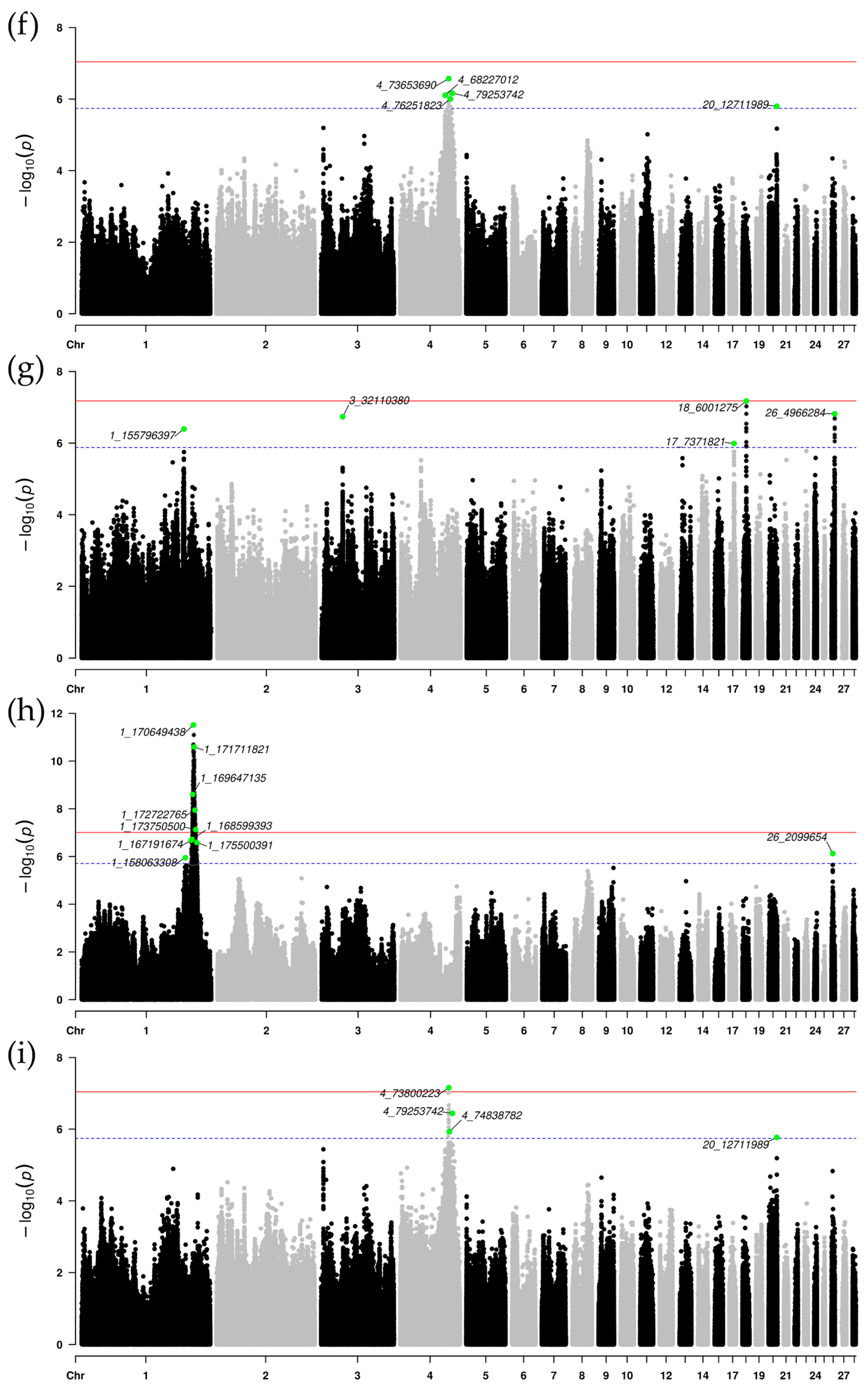
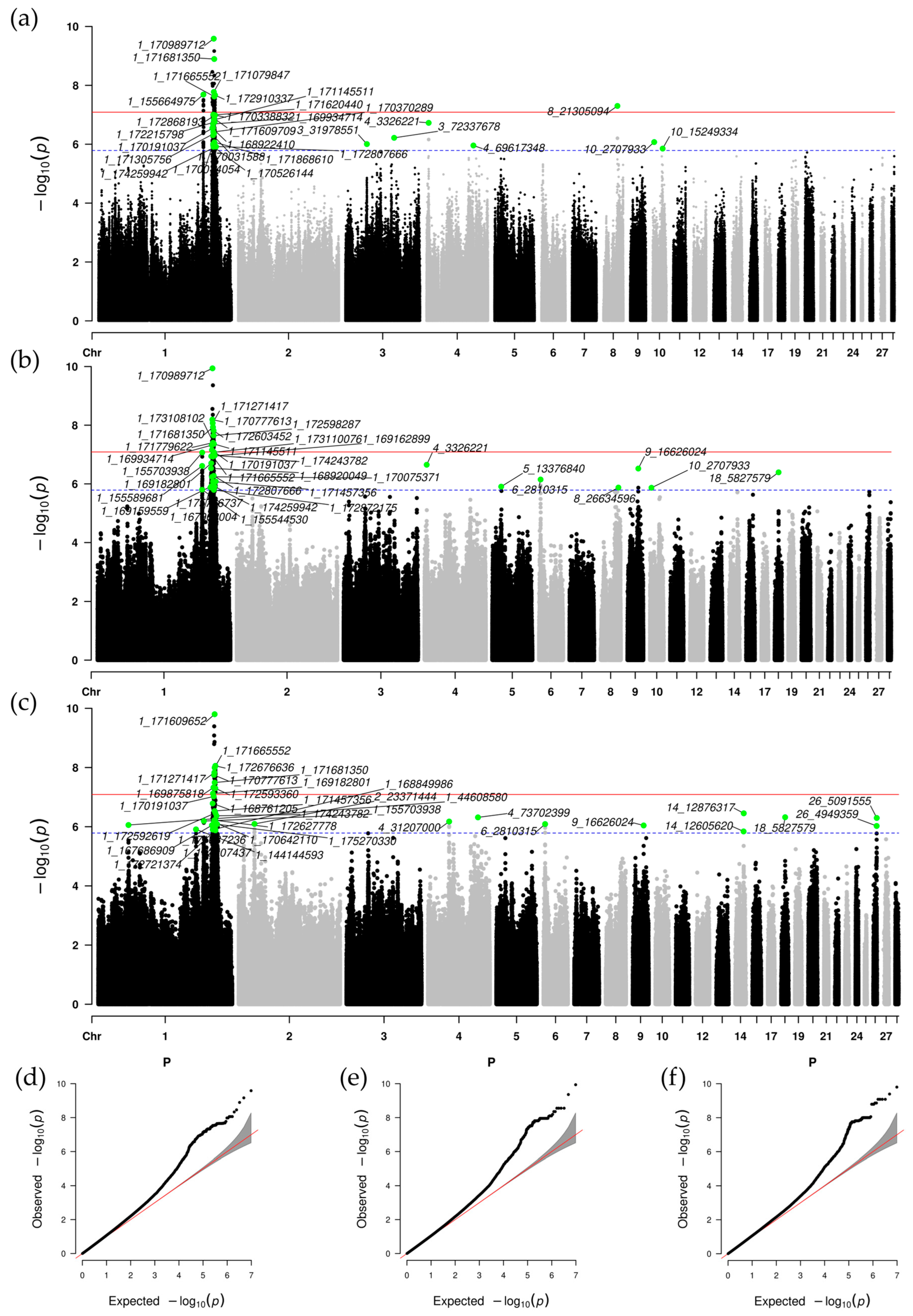
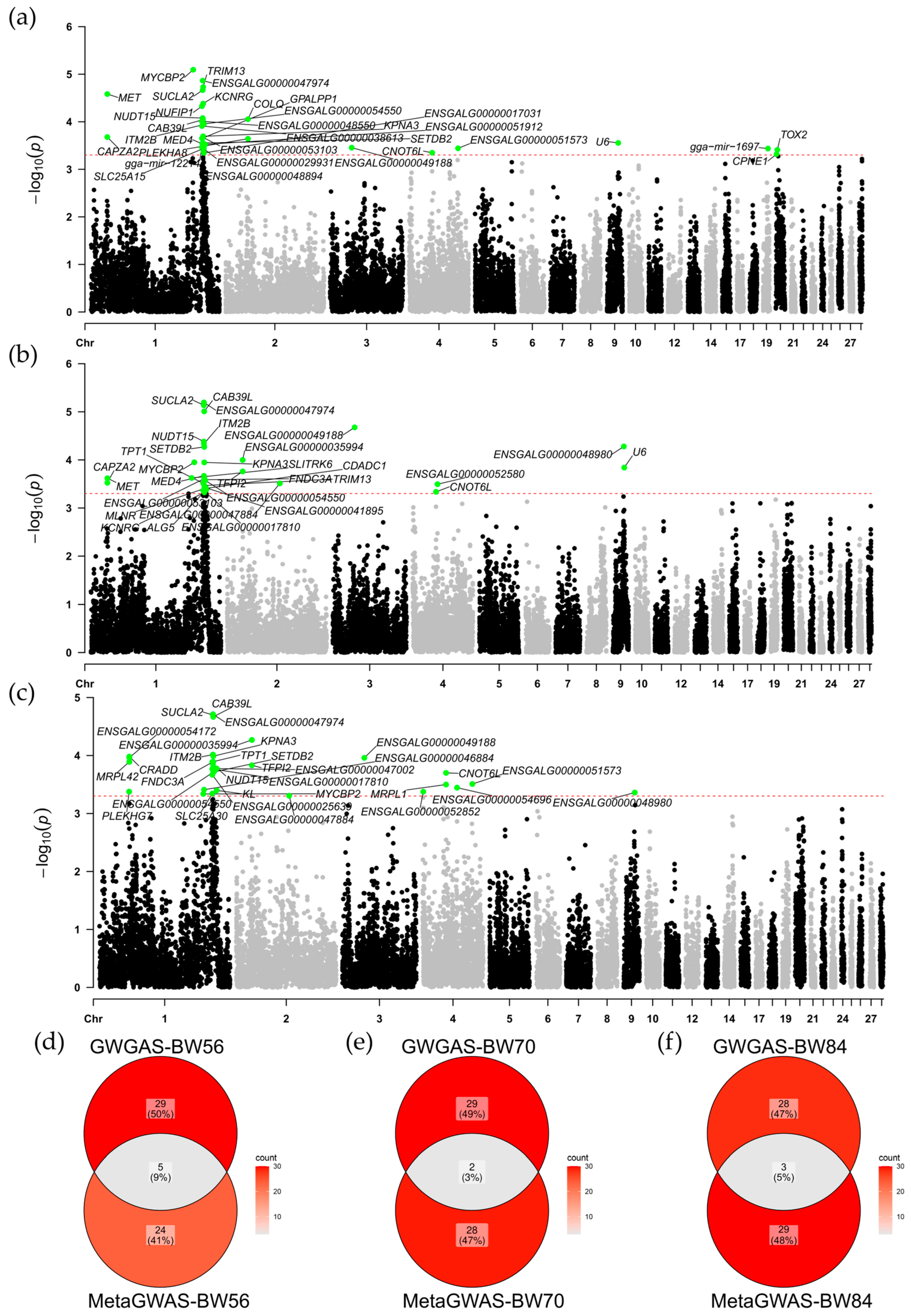
3.3. Single-Population GWAS Results
3.4. Meta-Analysis of GWAS Results
3.5. Gene Association Analysis
3.6. Chromatin State Annotation of Genome-Wide Significant Loci
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GWAS | Genome-wide association study |
| GWGAS | Genome-wide gene association analysis |
| PCA | Principal component analysis |
| SNPs | Single-nucleotide polymorphisms |
| BW56 | Weight at 56 days |
| BW70 | Weight at 70 days |
| BW84 | Weight at 84 days |
References
- Dzungwe, J.T.; Adjei-Mensah, B.; Chrysostome, C.; Tozo, K. Effect of crossbreeding on growth performance, meat quality, and the economics of production of the pure and reciprocal crosses between the Sasso and Wassachie chickens. Poult. Sci. 2024, 103, 103823. [Google Scholar] [CrossRef]
- Sulaiman, U.; Vaughan, R.; Siegel, P.; Liu, D.; Gilbert, E.; Cline, M. Dietary oleuropein supplementation affects lipolysis in broilers. Domest. Anim. Endocrinol. 2025, 92, 106942. [Google Scholar] [CrossRef]
- Tavárez, M.A.; Solis de los Santos, F. Impact of genetics and breeding on broiler production performance: A look into the past, present, and future of the industry. Anim. Front. 2016, 6, 37–41. [Google Scholar] [CrossRef]
- Yuan, Y.; Peng, D.; Gu, X.; Gong, Y.; Sheng, Z.; Hu, X. Polygenic Basis and Variable Genetic Architectures Contribute to the Complex Nature of Body Weight—A Genome-Wide Study in Four Chinese Indigenous Chicken Breeds. Front. Genet. 2018, 9, 229. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, Y.; Wu, J.; Wang, X.; Bian, C.; Tian, Y.; Sun, G.; Han, R.; Liu, X.; et al. Genome-wide association study reveals the genetic determinism of growth traits in a Gushi-Anka F2 chicken population. Heredity 2021, 126, 293–307. [Google Scholar] [CrossRef]
- Lyu, S.; Arends, D.; Nassar, M.K.; Weigend, A.; Weigend, S.; Wang, E.; Brockmann, G.A. High-density genotyping reveals candidate genomic regions for chicken body size in breeds of Asian origin. Poult. Sci. 2023, 102, 102303. [Google Scholar] [CrossRef]
- Zhong, C.; Li, X.; Guan, D.; The ChickenGTEx Consortium; Zhang, B.; Wang, X.; Qu, L.; Zhou, H.; Fang, L.; Sun, C.; et al. Age-dependent genetic architectures of chicken body weight explored by multidimensional GWAS and molQTL analyses. J. Genet. Genom. 2024, 51, 1423–1434. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Lei, Q.; Liu, Z.; Han, H.; Zhang, S.; Qi, C.; Liu, W.; Li, D.; Li, F.; et al. Elucidation of the genetic determination of body weight and size in Chinese local chicken breeds by large-scale genomic analyses. BMC Genom. 2024, 25, 296. [Google Scholar] [CrossRef]
- Evangelou, E.; Ioannidis, J.P.A. Meta-analysis methods for genome-wide association studies and beyond. Nat. Rev. Genet. 2013, 14, 379–389. [Google Scholar] [CrossRef]
- Sniekers, S.; Stringer, S.; Watanabe, K.; Jansen, P.R.; Coleman, J.R.I.; Krapohl, E.; Taskesen, E.; Hammerschlag, A.R.; Okbay, A.; Zabaneh, D.; et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat. Genet. 2017, 49, 1107–1112. [Google Scholar] [CrossRef]
- Bouwman, A.C.; Daetwyler, H.D.; Chamberlain, A.J.; Ponce, C.H.; Sargolzaei, M.; Schenkel, F.S.; Sahana, G.; Govignon-Gion, A.; Boitard, S.; Dolezal, M.; et al. Meta-analysis of genome-wide association studies for cattle stature identifies common genes that regulate body size in mammals. Nat. Genet. 2018, 50, 362–367. [Google Scholar] [CrossRef]
- Guo, Y.; Hou, L.; Zhang, X.; Huang, M.; Mao, H.; Chen, H.; Ma, J.; Chen, C.; Ai, H.; Ren, J.; et al. A meta analysis of genome-wide association studies for limb bone lengths in four pig populations. BMC Genet. 2015, 16, 95. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, Q.; Cai, X.; Zhong, Z.; Teng, J.; Li, B.; Zeng, H.; Gao, Y.; Cai, Z.; Wang, X.; et al. Integrating large-scale meta-GWAS and PigGTEx resources to decipher the genetic basis of 232 complex traits in pigs. Natl. Sci. Rev. 2025, 12, nwaf048. [Google Scholar] [CrossRef]
- Ma, X.; Ying, F.; Li, Z.; Bai, L.; Wang, M.; Zhu, D.; Liu, D.; Wen, J.; Zhao, G.; Liu, R. New insights into the genetic loci related to egg weight and age at first egg traits in broiler breeder. Poult. Sci. 2024, 103, 103613. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Luo, N.; Liu, D.; Ying, F.; Zhu, D.; Wen, J.; Zhao, G.; An, B. Integrating GWAS and transcriptomics to identify candidate genes conferring relative growth rate trait in white-feathered broiler. Poult. Sci. 2024, 103, 104338. [Google Scholar] [CrossRef]
- Neale, B.M.; Sham, P.C. The future of association studies: Gene-based analysis and replication. Am. J. Hum. Genet. 2004, 75, 353–362. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Solomonson, M.; Chao, K.R.; Goodrich, J.K.; Tiao, G.; Lu, W.; Riley-Gillis, B.M.; Tsai, E.A.; Kim, H.I.; Zheng, X.; et al. Systematic single-variant and gene-based association testing of thousands of phenotypes in 394,841 UK Biobank exomes. Cell Genom. 2022, 2, 100168. [Google Scholar] [CrossRef]
- Lin, Q.; Feng, X.; Li, T.; Pan, X.; Diao, S.; Gao, Y.; Yuan, X.; Li, J.; Ding, X.; Zhang, Z. Integrating meta-analysis of genome-wide association study with Pig Genotype-Tissue Expression resources uncovers the genetic architecture for age at first farrowing in pigs. Anim. Res. One Health 2024, 2, 238–249. [Google Scholar] [CrossRef]
- Sun, J.; Yang, X.; Zhao, G.; He, Z.; Xing, W.; Chen, Y.; Tan, X.; Wang, M.; Li, W.; An, B.; et al. Protein phosphatase 1 catalytic subunit gamma is a causative gene for meat lightness and redness. PLoS Genet. 2024, 20, e1011467. [Google Scholar] [CrossRef]
- Xu, Z.; Ji, C.; Zhang, Y.; Zhang, Z.; Nie, Q.; Xu, J.; Zhang, D.; Zhang, X. Combination analysis of genome-wide association and transcriptome sequencing of residual feed intake in quality chickens. BMC Genom. 2016, 17, 594. [Google Scholar] [CrossRef]
- Sheng, Z.; Pettersson, M.E.; Hu, X.; Luo, C.; Qu, H.; Shu, D.; Shen, X.; Carlborg, Ö.; Li, N. Genetic dissection of growth traits in a Chinese indigenous × commercial broiler chicken cross. BMC Genom. 2013, 14, 151. [Google Scholar] [CrossRef]
- Gu, X.; Feng, C.; Ma, L.; Song, C.; Wang, Y.; Da, Y.; Li, H.; Chen, K.; Ye, S.; Ge, C.; et al. Genome-wide association study of body weight in chicken F2 resource population. PLoS ONE 2011, 6, e21872. [Google Scholar] [CrossRef]
- Kranis, A.; Gheyas, A.A.; Boschiero, C.; Turner, F.; Yu, L.; Smith, S.; Talbot, R.; Pirani, A.; Brew, F.; Kaiser, P.; et al. Development of a high density 600K SNP genotyping array for chicken. BMC Genom. 2013, 14, 59. [Google Scholar] [CrossRef]
- Groenen, M.A.M.; Megens, H.-J.; Zare, Y.; Warren, W.C.; Hillier, L.W.; Crooijmans, R.P.M.A.; Vereijken, A.; Okimoto, R.; Muir, W.M.; Cheng, H.H. The development and characterization of a 60K SNP chip for chicken. BMC Genom. 2011, 12, 274. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Chang, C.L.; Villalun, M.; Geib, S.M.; Goodman, C.L.; Ringbauer, J.; Stanley, D. Pupal X-ray irradiation influences protein expression in adults of the oriental fruit fly, Bactrocera dorsalis. J. Insect Physiol. 2015, 76, 7–16. [Google Scholar] [CrossRef]
- Guan, D.; Bai, Z.; Zhu, X.; Zhong, C.; Hou, Y.; Zhu, D.; The ChickenGTEx Consortium; Li, H.; Lan, F.; Diao, S.; et al. Genetic regulation of gene expression across multiple tissues in chickens. Nat. Genet. 2025, 57, 1298–1308. [Google Scholar] [CrossRef]
- Browning, B.L.; Zhou, Y.; Browning, S.R. A One-Penny Imputed Genome from Next-Generation Reference Panels. Am. J. Hum. Genet. 2018, 103, 338–348. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Yin, D.; Fu, Y.; Yuan, X.; Li, X.; Liu, X.; Zhao, S. HIBLUP: An integration of statistical models on the BLUP framework for efficient genetic evaluation using big genomic data. Nucleic Acids Res. 2023, 51, 3501–3512. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A Tool for Genome-wide Complex Trait Analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef]
- Willer, C.J.; Li, Y.; Abecasis, G.R. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010, 26, 2190–2191. [Google Scholar] [CrossRef]
- de Leeuw, C.A.; Neale, B.M.; Heskes, T.; Posthuma, D. The statistical properties of gene-set analysis. Nat. Rev. Genet. 2016, 17, 353–364. [Google Scholar] [CrossRef]
- McLaren, W.; Pritchard, B.; Rios, D.; Chen, Y.; Flicek, P.; Cunningham, F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 2010, 26, 2069–2070. [Google Scholar] [CrossRef]
- Fonseca, P.A.S.; Suárez-Vega, A.; Marras, G.; Cánovas, Á. GALLO: An R package for genomic annotation and integration of multiple data sources in livestock for positional candidate loci. GigaScience 2020, 9, giaa149. [Google Scholar] [CrossRef]
- Hu, Z.; Park, C.A.; Reecy, J.M. Bringing the Animal QTLdb and CorrDB into the future: Meeting new challenges and providing updated services. Nucleic Acids Res. 2022, 50, D956–D961. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Lewis, M.J.; Wang, S. locuszoomr: An R package for visualising publication-ready regional gene locus plots. Bioinform. Adv. 2025, 5, vbaf006. [Google Scholar] [CrossRef]
- Visscher, P.M.; Brown, M.A.; McCarthy, M.I.; Yang, J. Five years of GWAS discovery. Am. J. Hum. Genet. 2012, 90, 7–24. [Google Scholar] [CrossRef]
- Lake, J.A.; Dekkers, J.C.M.; Abasht, B. Genetic basis and identification of candidate genes for wooden breast and white striping in commercial broiler chickens. Sci. Rep. 2021, 11, 6785. [Google Scholar] [CrossRef]
- Yengo, L.; Sidorenko, J.; Kemper, K.E.; Zheng, Z.; Wood, A.R.; Weedon, M.N.; Frayling, T.M.; Hirschhorn, J.; Yang, J.; Visscher, P.M.; et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum. Mol. Genet. 2018, 27, 3641–3649. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Guo, Y.; Qiu, H.; Xiao, S.; Wu, Z.; Yang, M.; Yang, J.; Ren, J.; Huang, L. A genome-wide association study identifies genomic loci associated with backfat thickness, carcass weight, and body weight in two commercial pig populations. J. Appl. Genet. 2017, 58, 499–508. [Google Scholar] [CrossRef]
- Igoshin, A.V.; Yudin, N.S.; Belonogova, N.M.; Larkin, D.M. Genome-wide association study for body weight in cattle populations from Siberia. Anim. Genet. 2019, 50, 250–253. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, J.; Song, C.; Han, W.; Zhu, C.; Li, H.; Zhang, S.; Chen, K.; Li, N.; Carlborg, Ö.; et al. Mapping and Functional Dissection of the Rumpless Trait in Piao Chicken Identifies a Causal Loss of Function Mutation in the Novel Gene Rum. Mol. Biol. Evol. 2023, 40, msad273. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, M.; Cheng, H.; Fan, W.; Yuan, Z.; Gao, Q.; Xu, Y.; Guo, Z.; Zhang, Y.; Hu, J.; et al. An intercross population study reveals genes associated with body size and plumage color in ducks. Nat. Commun. 2018, 9, 2648. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Chen, Z.; Sun, J.; Cao, C.; Wu, F.; Xu, Z.; Zhao, W.; Sun, H.; Guo, L.; et al. PHARP: A pig haplotype reference panel for genotype imputation. Sci. Rep. 2022, 12, 12645. [Google Scholar] [CrossRef]
- Yang, R.; Guo, X.; Zhu, D.; Tan, C.; Bian, C.; Ren, J.; Huang, Z.; Zhao, Y.; Cai, G.; Liu, D.; et al. Accelerated deciphering of the genetic architecture of agricultural economic traits in pigs using a low-coverage whole-genome sequencing strategy. GigaScience 2021, 10, giab048. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Y.; Qu, H.; Feng, C.; Zhang, H.; Sheng, Z.; Jiang, Y.; Nie, Q.; Chu, S.; Shu, D.; et al. GCRP: Integrated Global Chicken Reference Panel from 11,951 Chicken Genomes. Genom. Proteom. Bioinform. 2025, qzaf032. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, G.; Yang, W.; Jin, W.; Gong, J.; Xu, X.; Niu, X. Animal-SNPAtlas: A comprehensive SNP database for multiple animals. Nucleic Acids Res. 2023, 51, D816–D826. [Google Scholar] [CrossRef]
- Chabris, C.F.; Lee, J.J.; Benjamin, D.J.; Beauchamp, J.P.; Glaeser, E.L.; Borst, G.; Pinker, S.; Laibson, D.I. Why it is hard to find genes associated with social science traits: Theoretical and empirical considerations. Am. J. Public Health 2013, 103 (Suppl. 1), S152–S166. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, W.; Gao, N.; Wei, C.; Wu, X.; Si, J.; Gao, Y.; Li, J.; Yin, T.; Zhang, Z. Integrating large-scale meta-analysis of genome-wide association studies improve the genomic prediction accuracy for combined pig populations. J. Anim. Breed. Genet. 2025, 142, 223–236. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, Y.; Wang, M.; Wang, Y.; Zhu, X.; Gu, S.; Zhong, C.; An, L.; Shan, M.; Damas, J.; et al. An atlas of regulatory elements in chicken: A resource for chicken genetics and genomics. Sci. Adv. 2023, 9, eade1204. [Google Scholar] [CrossRef]
- Xie, L.; Luo, C.; Zhang, C.; Zhang, R.; Tang, J.; Nie, Q.; Ma, L.; Hu, X.; Li, N.; Da, Y.; et al. Genome-wide association study identified a narrow chromosome 1 region associated with chicken growth traits. PLoS ONE 2012, 7, e30910. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Luo, C.; Sheng, Z.; Zhang, C.; Bian, C.; Feng, C.; Li, J.; Gao, F.; Zhao, Y.; et al. Multiple ancestral haplotypes harboring regulatory mutations cumulatively contribute to a QTL affecting chicken growth traits. Commun. Biol. 2020, 3, 472. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Lei, Q.; Han, H.; Liu, W.; Li, D.; Sun, Y.; Hao, D.; Li, F.; Cao, D.; et al. Identification of quantitative trait loci and candidate genes associated with growth curve parameters in chinese wenshang barred chickens. Poult. Sci. 2025, 104, 104767. [Google Scholar] [CrossRef]
- Kanlisi, R.A.; Amuzu-Aweh, E.N.; Naazie, A.; Otsyina, H.R.; Kelly, T.R.; Gallardo, R.A.; Lamont, S.J.; Zhou, H.; Dekkers, J.; Kayang, B.B. Genetic architecture of body weight, carcass, and internal organs traits of Ghanaian local chickens. Front. Genet. 2024, 15, 1297034. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Li, C.; Wu, H.; Zhang, R.; Hu, X. Chicken chromatin accessibility atlas accelerates epigenetic annotation of birds and gene fine-mapping associated with growth traits. Zool. Res. 2023, 44, 53–62. [Google Scholar] [CrossRef]
- Han, X.; Gharahkhani, P.; Hamel, A.R.; Ong, J.S.; Renteria, M.E.; Mehta, P.; Dong, X.; Pasutto, F.; Hammond, C.; Young, T.L.; et al. Large-scale multitrait genome-wide association analyses identify hundreds of glaucoma risk loci. Nat. Genet. 2023, 55, 1116–1125. [Google Scholar] [CrossRef]
- Spencer, C.C.; Su, Z.; Donnelly, P.; Marchini, J. Designing genome-wide association studies: Sample size, power, imputation, and the choice of genotyping chip. PLoS Genet. 2009, 5, e1000477. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Jia, H.; Yu, M.; Ma, R.; Wang, L.; Cao, K.; Shen, Z.; Niu, L.; Tian, J.; et al. Peach genetic resources: Diversity, population structure and linkage disequilibrium. BMC Genet. 2013, 14, 84. [Google Scholar] [CrossRef]
- De Lillo, A.; D’Antona, S.; Pathak, G.A.; Wendt, F.R.; De Angelis, F.; Fuciarelli, M.; Polimanti, R. Cross-ancestry genome-wide association studies identified heterogeneous loci associated with differences of allele frequency and regulome tagging between participants of European descent and other ancestry groups from the UK Biobank. Hum. Mol. Genet. 2021, 30, 1457–1467. [Google Scholar] [CrossRef]
- Hsu, L.; Kooperberg, A.; Reiner, A.P.; Kooperberg, C. An empirical Bayes approach to improving population-specific genetic association estimation by leveraging cross-population data. Genet. Epidemiol. 2023, 47, 45–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Zheng, M.; Wang, Z.; Hu, X.; Li, Z. Genome-Wide Association Study and Meta-Analysis Uncovers Key Candidate Genes for Body Weight Traits in Chickens. Genes 2025, 16, 945. https://doi.org/10.3390/genes16080945
Wen J, Zheng M, Wang Z, Hu X, Li Z. Genome-Wide Association Study and Meta-Analysis Uncovers Key Candidate Genes for Body Weight Traits in Chickens. Genes. 2025; 16(8):945. https://doi.org/10.3390/genes16080945
Chicago/Turabian StyleWen, Jintian, Ming Zheng, Zhaochuan Wang, Xiaoxiang Hu, and Zhenhui Li. 2025. "Genome-Wide Association Study and Meta-Analysis Uncovers Key Candidate Genes for Body Weight Traits in Chickens" Genes 16, no. 8: 945. https://doi.org/10.3390/genes16080945
APA StyleWen, J., Zheng, M., Wang, Z., Hu, X., & Li, Z. (2025). Genome-Wide Association Study and Meta-Analysis Uncovers Key Candidate Genes for Body Weight Traits in Chickens. Genes, 16(8), 945. https://doi.org/10.3390/genes16080945




