Genetic Panel Testing for Malignant Hyperthermia in Japan: Discovery of Novel Variants and Clinical Implications
Abstract
1. Introduction
2. Materials and Methods
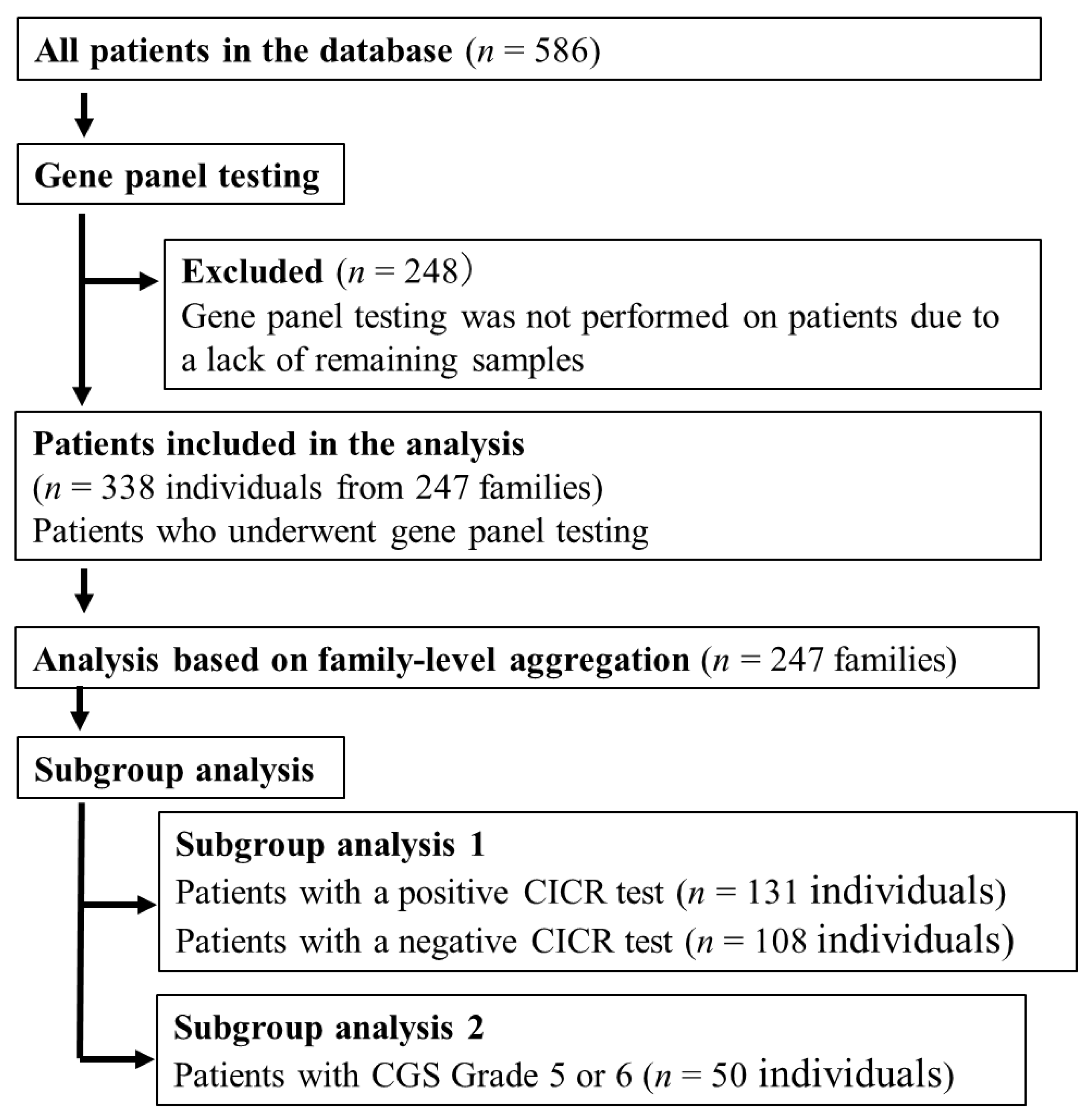
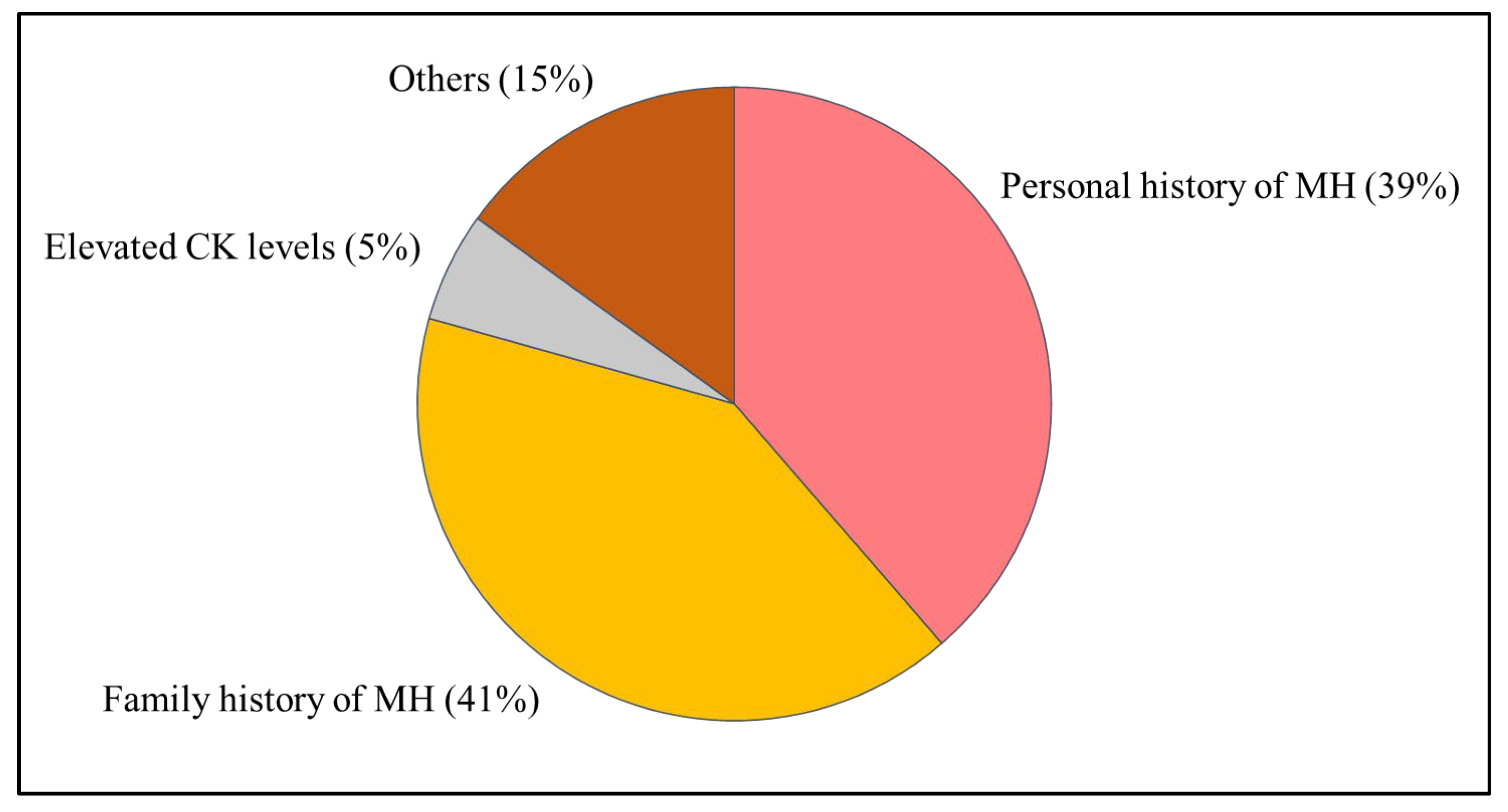
2.1. Study Design and Patient Selection
2.2. DNA Extraction
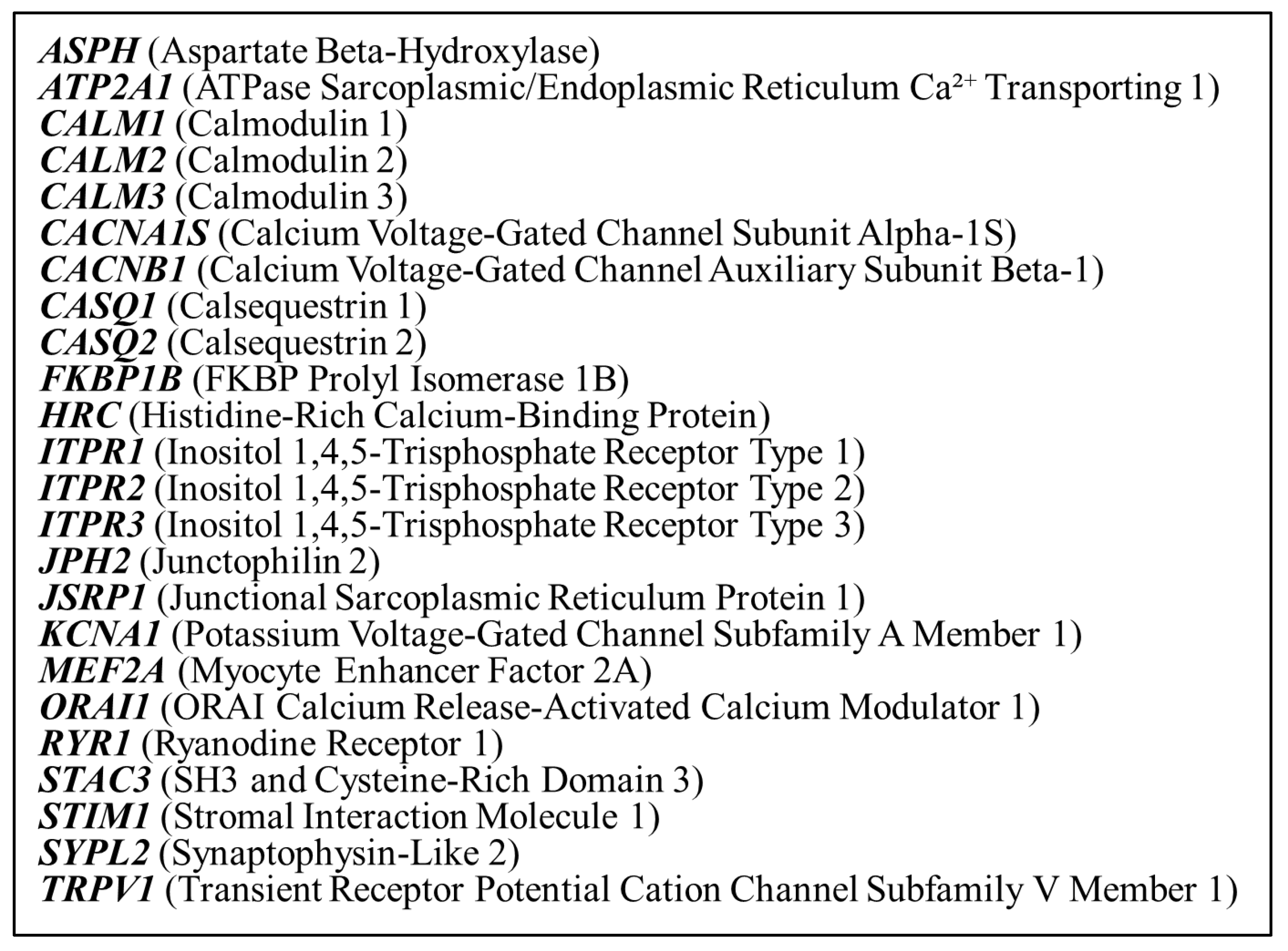
2.3. Genetic Panel Testing
2.4. Variant Calling and Filtration
2.5. CICR Rate Test
2.6. Clinical Grading Scale
2.7. Data Analysis
3. Results
3.1. Patient Characteristics
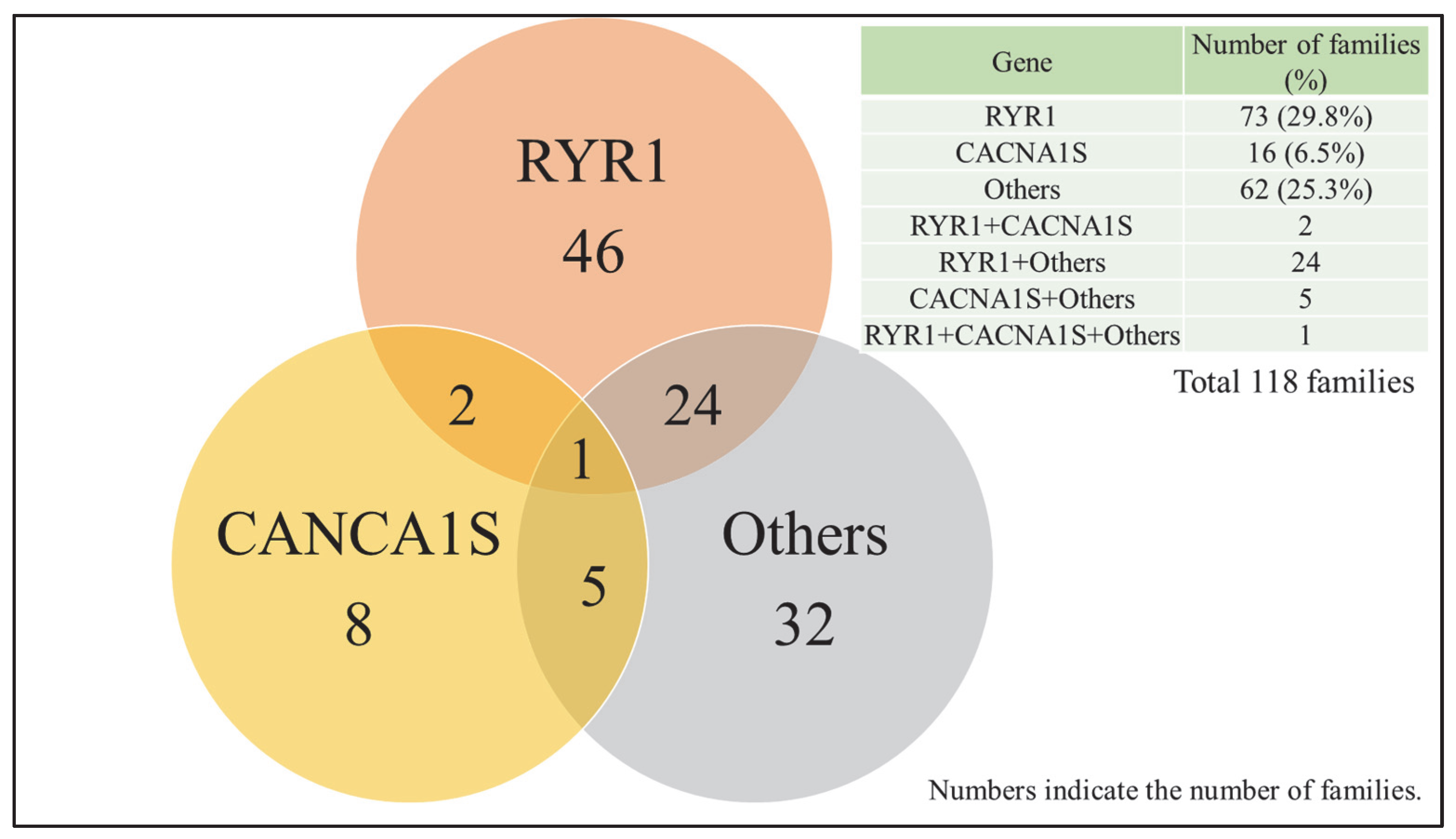
3.2. Genetic Testing Results
3.3. Correlation Between Genetic Findings and CICR Test Results
3.4. Clinical Grading Scale (CGS) and Genetic Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, H.; Pollock, N.; Schiemann, A.; Bulger, T.; Stowell, K. Malignant hyperthermia: A review. Orphanet J. Rare Dis. 2015, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Litman, R.S.; Rosenberg, H. Malignant hyperthermia: Update on susceptibility testing. JAMA 2005, 293, 2918–2924. [Google Scholar] [CrossRef]
- Rosero, E.B.; Adesanya, A.O.; Timaran, C.H.; Joshi, G.P. Trends and outcomes of malignant hyperthermia in the United States, 2000 to 2005. Anesthesiology 2009, 110, 89–94. [Google Scholar] [CrossRef]
- Ording, H. Incidence of malignant hyperthermia in Denmark. Anesth. Analg. 1985, 64, 700–704. [Google Scholar] [PubMed]
- Sumitani, M.; Uchida, K.; Yasunaga, H.; Horiguchi, H.; Kusakabe, Y.; Matsuda, S.; Yamada, Y. Prevalence of malignant hyperthermia and relationship with anesthetics in Japan: Data from the diagnosis procedure combination database. Anesthesiology 2011, 114, 84–90. [Google Scholar] [CrossRef]
- Witherspoon, J.W.; Meilleur, K.G. Review of RyR1 pathway and associated pathomechanisms. Acta Neuropathol. Commun. 2016, 4, 121. [Google Scholar] [CrossRef]
- Schartner, V.; Romero, N.B.; Donkervoort, S.; Treves, S.; Munot, P.; Pierson, T.M.; Dabaj, I.; Malfatti, E.; Zaharieva, I.T.; Zorzato, F.; et al. Dihydropyridine receptor (DHPR, CACNA1S) congenital myopathy. Acta Neuropathol. 2017, 133, 517–533. [Google Scholar] [CrossRef]
- Robinson, R.; Carpenter, D.; Shaw, M.A.; Halsall, J.; Hopkins, P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum. Mutat. 2006, 27, 977–989. [Google Scholar] [CrossRef]
- Riazi, S.; Kraeva, N.; Hopkins, P.M. Malignant Hyperthermia in the Post-Genomics Era: New Perspectives on an Old Concept. Anesthesiology 2018, 128, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, S.; Miyoshi, H.; Mukaida, K.; Yasuda, T.; Nakamura, R.; Tsutsumi, Y.M. Age-Specific Clinical Features of Pediatric Malignant Hyperthermia: A Review of 187 Cases Over 60 Years in Japan. Anesth. Analg. 2022, 135, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Larach, M.G.; Gronert, G.A.; Allen, G.C.; Brandom, B.W.; Lehman, E.B. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth. Analg. 2010, 110, 498–507. [Google Scholar] [CrossRef]
- Sambuughin, N.; Sei, Y.; Gallagher, K.L.; Wyre, H.W.; Madsen, D.; Nelson, T.E.; Fletcher, J.E.; Rosenberg, H.; Muldoon, S.M. North American malignant hyperthermia population: Screening of the ryanodine receptor gene and identification of novel mutations. Anesthesiology 2001, 95, 594–599. [Google Scholar] [CrossRef]
- Hopkins, P.M.; Rüffert, H.; Snoeck, M.M.; Girard, T.; Glahn, K.P.; Ellis, F.R.; Müller, C.R.; Urwyler, A.; European Malignant Hyperthermia Group. European Malignant Hyperthermia Group guidelines for investigation of malignant hyperthermia susceptibility. Br. J. Anaesth. 2015, 115, 531–539. [Google Scholar] [CrossRef]
- Miller, D.M.; Daly, C.; Aboelsaod, E.M.; Gardner, L.; Hobson, S.J.; Riasat, K.; Shepherd, S.; Robinson, R.L.; Bilmen, J.G.; Gupta, P.K.; et al. Genetic epidemiology of malignant hyperthermia in the UK. Br. J. Anaesth. 2018, 121, 944–952. [Google Scholar] [CrossRef]
- Robinson, R.L.; Anetseder, M.J.; Brancadoro, V.; van Broekhoven, C.; Carsana, A.; Censier, K.; Fortunato, G.; Girard, T.; Heytens, L.; Hopkins, P.M.; et al. Recent advances in the diagnosis of malignant hyperthermia susceptibility: How confident can we be of genetic testing? Eur. J. Hum. Genet. 2003, 11, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Monnier, N.; Kozak-Ribbens, G.; Krivosic-Horber, R.; Nivoche, Y.; Qi, D.; Kraev, N.; Loke, J.; Sharma, P.; Tegazzin, V.; Figarella-Branger, D.; et al. Correlations between genotype and pharmacological, histological, functional, and clinical phenotypes in malignant hyperthermia susceptibility. Hum. Mutat. 2005, 26, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Rueffert, H.; Olthoff, D.; Deutrich, C.; Meinecke, C.D.; Froster, U.G. Mutation screening in the ryanodine receptor 1 gene (RYR1) in patients susceptible to malignant hyperthermia who show definite IVCT results: Identification of three novel mutations. Acta Anaesthesiol. Scand. 2002, 46, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Galli, L.; Orrico, A.; Lorenzini, S.; Censini, S.; Falciani, M.; Covacci, A.; Tegazzin, V.; Sorrentino, V. Frequency and localization of mutations in the 106 exons of the RYR1 gene in 50 individuals with malignant hyperthermia. Hum. Mutat. 2006, 27, 830. [Google Scholar] [CrossRef] [PubMed]
- Kraeva, N.; Riazi, S.; Loke, J.; Frodis, W.; Crossan, M.L.; Nolan, K.; Kraev, A.; Maclennan, D.H. Ryanodine receptor type 1 gene mutations found in the Canadian malignant hyperthermia population. Can. J. Anaesth. 2011, 58, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Sambuughin, N.; Holley, H.; Muldoon, S.; Brandom, B.W.; de Bantel, A.M.; Tobin, J.R.; Nelson, T.E.; Goldfarb, L.G. Screening of the entire ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the north american population. Anesthesiology 2005, 102, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G.; O’Connell, K.M.; Flucher, B.E.; Allen, P.D.; Grabner, M.; Dirksen, R.T. Functional analysis of the R1086H malignant hyperthermia mutation in the DHPR reveals an unexpected influence of the III-IV loop on skeletal muscle EC coupling. Am. J. Physiol. Cell Physiol. 2004, 287, C1094–C1102. [Google Scholar] [CrossRef] [PubMed]
- Biesecker, L.G.; Dirksen, R.T.; Girard, T.; Hopkins, P.M.; Riazi, S.; Rosenberg, H.; Stowell, K.; Weber, J. Genomic Screening for Malignant Hyperthermia Susceptibility. Anesthesiology 2020, 133, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Parness, J.; Bandschapp, O.; Girard, T. The myotonias and susceptibility to malignant hyperthermia. Anesth. Analg. 2009, 109, 1054–1064. [Google Scholar] [CrossRef]
- Ibarra Moreno, C.A.; Silva, H.C.A.; Voermans, N.C.; Jungbluth, H.; van den Bersselaar, L.R.; Rendu, J.; Cieniewicz, A.; Hopkins, P.M.; Riazi, S. Myopathic manifestations across the adult lifespan of patients with malignant hyperthermia susceptibility: A narrative review. Br. J. Anaesth. 2024, 133, 759–767. [Google Scholar] [CrossRef]
- Harwood, T.N.; Nelson, T.E. Massive postoperative rhabdomyolysis after uneventful surgery: A case report of subclinical malignant hyperthermia. Anesthesiology 1998, 88, 265–268. [Google Scholar] [CrossRef]
- Thomas, J.; Crowhurst, T. Exertional heat stroke, rhabdomyolysis and susceptibility to malignant hyperthermia. Intern. Med. J. 2013, 43, 1035–1038. [Google Scholar] [CrossRef]
- Santos, J.M.; Andrade, P.V.; Galleni, L.; Vainzof, M.; Sobreira, C.F.R.; Schmidt, B.; Oliveira, A.S.B.; Amaral, J.L.G.; Silva, H.C.A. Idiopathic hyperCKemia and malignant hyperthermia susceptibility. Can. J. Anaesth. 2017, 64, 1202–1210. [Google Scholar] [CrossRef]
- Hopkins, P.M. Is there a link between malignant hyperthermia and exertional heat illness? Br. J. Sports Med. 2007, 41, 283–284, discussion 284. [Google Scholar] [CrossRef] [PubMed]
- European Malignant Hyperthermia Group. Patient Referral Criteria [Internet]. 27 December 2017. Available online: https://www.emhg.org/testing-for-mh/2017/12/27/patient-referral-criteria (accessed on 29 June 2025).
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef]
- Ng, P.C.; Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Endo, M.; Iino, M. Measurement of Ca2+ release in skinned fibers from skeletal muscle. Methods Enzymol. 1988, 157, 12–26. [Google Scholar] [CrossRef]
- Ohta, T.; Endo, M.; Nakano, T.; Morohoshi, Y.; Wanikawa, K.; Ohga, A. Ca-induced Ca release in malignant hyperthermia-susceptible pig skeletal muscle. Am. J. Physiol. 1989, 256, C358–C367. [Google Scholar] [CrossRef] [PubMed]
- Oku, S.; Mukaida, K.; Nosaka, S.; Sai, Y.; Maehara, Y.; Yuge, O. Comparison of the in vitro caffeine-halothane contracture test with the Ca-induced Ca release rate test in patients suspected of having malignant hyperthermia susceptibility. J. Anesth. 2000, 14, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Larach, M.G.; Localio, A.R.; Allen, G.C.; Denborough, M.A.; Ellis, F.R.; Gronert, G.A.; Kaplan, R.F.; Muldoon, S.M.; Nelson, T.E.; Ording, H.; et al. A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology 1994, 80, 771–779. [Google Scholar] [CrossRef]
- Carpenter, D.; Ringrose, C.; Leo, V.; Morris, A.; Robinson, R.L.; Halsall, P.J.; Hopkins, P.M.; Shaw, M.A. The role of CACNA1S in predisposition to malignant hyperthermia. BMC Med. Genet. 2009, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Sangkuhl, K.; Dirksen, R.T.; Alvarellos, M.L.; Altman, R.B.; Klein, T.E. PharmGKB summary: Very important pharmacogene information for CACNA1S. Pharmacogenet. Genomics 2020, 30, 34–44. [Google Scholar] [CrossRef]
- Malignant Hyperthermia Association of the United States. [Internet]. MHAUS: Sherburne, NY, USA. Available online: https://www.mhaus.org/ (accessed on 3 August 2025).
- Asian Malignant Hyperthermia Alliance. About AMHA [Internet]. Available online: https://amha.asia/ (accessed on 29 June 2025).
- Foo, C.T.Y.; To, Y.H.; Irwanto, A.; Ng, A.Y.; Yan, B.; Chew, S.T.H.; Liu, J.; Ti, L.K. Variant landscape of the RYR1 gene based on whole genome sequencing of the Singaporean population. Sci. Rep. 2022, 12, 5429. [Google Scholar] [CrossRef]
- Gillard, E.F.; Otsu, K.; Fujii, J.; Khanna, V.K.; de Leon, S.; Derdemezi, J.; Britt, B.A.; Duff, C.L.; Worton, R.G.; MacLennan, D.H. A substitution of cysteine for arginine 614 in the ryanodine receptor is potentially causative of human malignant hyperthermia. Genomics 1991, 11, 751–755. [Google Scholar] [CrossRef]
- Stewart, S.L.; Hogan, K.; Rosenberg, H.; Fletcher, J.E. Identification of the Arg1086His mutation in the alpha subunit of the voltage-dependent calcium channel (CACNA1S) in a North American family with malignant hyperthermia. Clin. Genet. 2001, 59, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Toppin, P.J.; Chandy, T.T.; Ghanekar, A.; Kraeva, N.; Beattie, W.S.; Riazi, S. A report of fulminant malignant hyperthermia in a patient with a novel mutation of the CACNA1S gene. Can. J. Anaesth. 2010, 57, 689–693. [Google Scholar] [CrossRef]
- Horstick, E.J.; Linsley, J.W.; Dowling, J.J.; Hauser, M.A.; McDonald, K.K.; Ashley-Koch, A.; Saint-Amant, L.; Satish, A.; Cui, W.W.; Zhou, W.; et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat. Commun. 2013, 4, 1952. [Google Scholar] [CrossRef] [PubMed]
- Bjorksten, A.R.; Gillies, R.L.; Hockey, B.M.; Du Sart, D. Sequencing of genes involved in the movement of calcium across human skeletal muscle sarcoplasmic reticulum: Continuing the search for genes associated with malignant hyperthermia. Anaesth. Intensive Care 2016, 44, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Mestre, T.A.; Manole, A.; MacDonald, H.; Riazi, S.; Kraeva, N.; Hanna, M.G.; Lang, A.E.; Männikkö, R.; Yoon, G. A novel KCNA1 mutation in a family with episodic ataxia and malignant hyperthermia. Neurogenetics 2016, 17, 245–249. [Google Scholar] [CrossRef]
- Hernández-Ochoa, E.O.; Schneider, M.F. Voltage sensing mechanism in skeletal muscle excitation-contraction coupling: Coming of age or midlife crisis? Skelet. Muscle 2018, 8, 22. [Google Scholar] [CrossRef]
- Rossi, D.; Gamberucci, A.; Pierantozzi, E.; Amato, C.; Migliore, L.; Sorrentino, V. Calsequestrin, a key protein in striated muscle health and disease. J. Muscle Res. Cell Motil. 2021, 42, 267–279. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Takahashi, T.; Nagano, M.; Koinuma, S.; Shigeyoshi, Y. The Effects of Aging on Sarcoplasmic Reticulum-Related Factors in the Skeletal Muscle of Mice. Int. J. Mol. Sci. 2024, 25, 2148. [Google Scholar] [CrossRef]
- Endo, Y.; Groom, L.; Celik, A.; Kraeva, N.; Lee, C.S.; Jung, S.Y.; Gardner, L.; Shaw, M.A.; Hamilton, S.L.; Hopkins, P.M.; et al. Variants in ASPH cause exertional heat illness and are associated with malignant hyperthermia susceptibility. Nat. Commun. 2022, 13, 3403. [Google Scholar] [CrossRef] [PubMed]
- Vanden Abeele, F.; Lotteau, S.; Ducreux, S.; Dubois, C.; Monnier, N.; Hanna, A.; Gkika, D.; Romestaing, C.; Noyer, L.; Flourakis, M.; et al. TRPV1 variants impair intracellular Ca2+ signaling and may confer susceptibility to malignant hyperthermia. Genet. Med. 2019, 21, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Yasuda, T.; Otsuki, S.; Kondo, T.; Haraki, T.; Mukaida, K.; Nakamura, R.; Hamada, H.; Kawamoto, M. Several Ryanodine Receptor Type 1 Gene Mutations of p.Arg2508 Are Potential Sources of Malignant Hyperthermia. Anesth. Analg. 2015, 121, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Miyoshi, H.; Benucci, S.; Gonzalez, A.; Bandschapp, O.; Girard, T.; Treves, S.; Zorzato, F. Functional characterization of RYR1 variants identified in malignant hyperthermia susceptible individuals. Neuromuscul. Disord. 2023, 33, 951–963. [Google Scholar] [CrossRef]
| No of Families | RYR1, n (%) | CACNA1S, n (%) | Others, n (%) | ||||
|---|---|---|---|---|---|---|---|
| Over All | 245 | 73 (29.8%) | 16 (6.5%) | 62 (25.3%) | |||
| No of families | RYR1 | CACNA1S | Others | p-value (RYR1) | p-value (CACNA1S) | p-value (Others) | |
| CGS Ranks 5 and 6 | 50 | 28 (56.0%) | 6 (12.0%) | 15 (30.0%) | 0.091 | 0.137 | 0.737 |
| CICR-Positive | 131 | 55 (42.0%) | 7 (5.3%) | 34 (27.5%) | |||
| CICR-Negative | 108 | 11 (10.2%) | 7 (6.5%) | 18 (15.7%) |
| RYR1 | CACNA1S | Others | |||
|---|---|---|---|---|---|
| R14W | P1592L | R3321S | N64D | E200K (ASPH) | R228Q (ITPR3) |
| D17N | W1625C | K3367R | R174W † | K350R (ASPH) | R527H (ITPR3) |
| T84M | R2163H | N3555I | L298I | R369C (ASPH) | T808I (ITPR3) |
| Q155K | V2168M | V3602M | G321V | D27G (ATP2A1) | F1750L (ITPR3) |
| R163C | T2206M | N3913D | A560T | I298S (ATP2A1) | V1867M (ITPR3) |
| D167G | M2208K | Q3964Rfs*105 | S879P | R134P (ATP2A1) | S2045G (ITPR3) |
| E176D | V2280I | L3984P | F1060V | R534W (ATP2A1) | V2386I (ITPR3) |
| E209K | R2336H | I4184M | F1161L † | P571L (ATP2A1) | R436C (JPH2) |
| R274H | S2345R | K4477N | D1382V | P571R (ATP2A1) | K249_E253del (JSRP1) |
| A291V | S2345T | L4769F | G1459S | R107H (CALM2) | Q420_P421insQQQ (MEF2A) |
| R316L | I2358T | L4838V | S279L (CACNB1) | R287C (ORAI1) | |
| G341R | P2366R | Y4852S | I164M (CASQ1) | S61Lfs* (ORAI1) | |
| K364R | D2431E | I4898T | A196V (CASQ1) | L30fs (STAC3) | |
| R367Q | D2431H | W5020G | G347* (CASQ1) | H89Lfs*20 (STAC3) | |
| R391C | R2454G | A5025G | D261_V262insDD (HRC) | G142S (STAC3) | |
| Y522C | R2508C | E376* (HRC) | T502dup (STIM1) | ||
| R530H | R2508H | H9Lfs*17 (HRC) | R522S (STIM1) | ||
| R533H | R2615H | T310A (ITPR2) | G586V (STIM1) | ||
| S604P | R2625C | D723N (ITPR2) | A115T (SYPL2) | ||
| R614L | D2769N | I1762T (ITPR2) | E211K (TRPV1) | ||
| R727L | E2824K | E975A (ITPR2) | D297N (TRPV1) | ||
| R870W | E3104K | T969I (ITPR2) | Q261P (TRPV1) | ||
| E887K | R3119H | A41V (ITPR3) | R772C (TRPV1) | ||
| N1164H | M3266I | A147T (ITPR3) | V508M (TRPV1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyoshi, H.; Mukaida, K.; Otsuki, S.; Kido, K.; Sumii, A.; Ikeda, T.; Xia, G.; Noda, Y.; Ishii, T.; Kamiya, S.; et al. Genetic Panel Testing for Malignant Hyperthermia in Japan: Discovery of Novel Variants and Clinical Implications. Genes 2025, 16, 944. https://doi.org/10.3390/genes16080944
Miyoshi H, Mukaida K, Otsuki S, Kido K, Sumii A, Ikeda T, Xia G, Noda Y, Ishii T, Kamiya S, et al. Genetic Panel Testing for Malignant Hyperthermia in Japan: Discovery of Novel Variants and Clinical Implications. Genes. 2025; 16(8):944. https://doi.org/10.3390/genes16080944
Chicago/Turabian StyleMiyoshi, Hirotsugu, Keiko Mukaida, Sachiko Otsuki, Kenshiro Kido, Ayako Sumii, Tsuyoshi Ikeda, Guoqiang Xia, Yuko Noda, Tomomi Ishii, Satoshi Kamiya, and et al. 2025. "Genetic Panel Testing for Malignant Hyperthermia in Japan: Discovery of Novel Variants and Clinical Implications" Genes 16, no. 8: 944. https://doi.org/10.3390/genes16080944
APA StyleMiyoshi, H., Mukaida, K., Otsuki, S., Kido, K., Sumii, A., Ikeda, T., Xia, G., Noda, Y., Ishii, T., Kamiya, S., Narasaki, S., Niinai, H., & Tsutsumi, Y. M. (2025). Genetic Panel Testing for Malignant Hyperthermia in Japan: Discovery of Novel Variants and Clinical Implications. Genes, 16(8), 944. https://doi.org/10.3390/genes16080944






