MicroRNAs as Potential Biomarkers for Alzheimer’s Disease in Women
Abstract
1. Introduction
2. Search Strategy and Inclusion Criteria
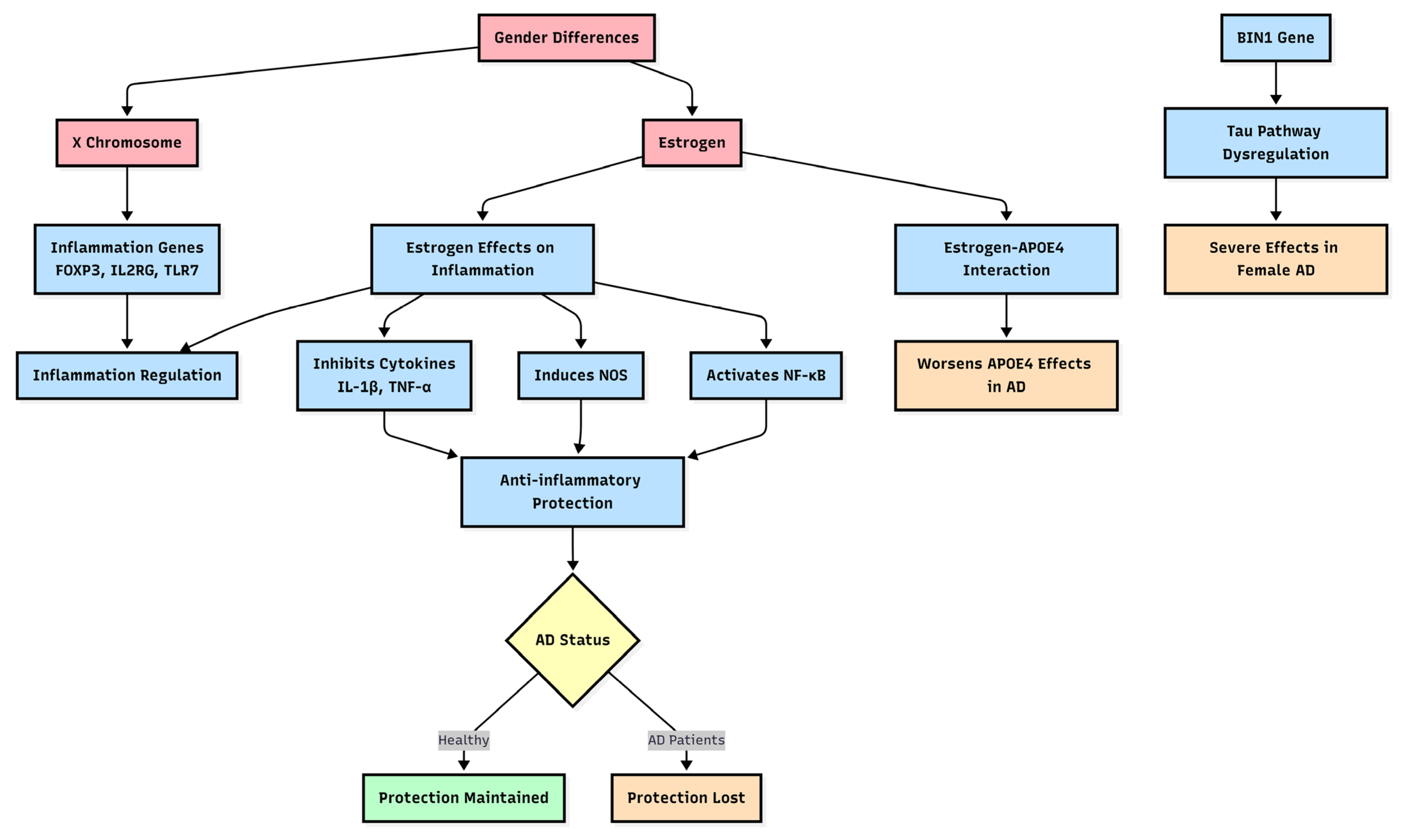
3. Gender Differences Lead to Biological Mechanical Differences in Diseases
3.1. Inflammation and Immune Cell Responses
3.2. Autophagy Difference
3.3. Metabolism Difference
4. Genetic Susceptibility to AD
5. Gender Differences in miRNA Expression
6. MiRNA Biomarker Candidates for AD in Women
6.1. miRAN Advantages as Biomarker Candidates for AD
6.2. Sex-Specific miRNA Biomarkers for AD in Females
| MiRNA Names | Up- or Down-Regulated | Tissue Types | Sample Size (AD/CON) | Reference |
|---|---|---|---|---|
| MiR-431-3P, -494, -653, and -668. MiR-105-3p | Downregulated Upregulated | Tissue Tissue | 15/12 | [52,57,58,59,60] |
| miR-296-5p, -766-3p, -1304-3p, -4326, -4685-3p, let-7d-3p, and -671-3p | Upregulated | Blood | 739/148 | [52,61,62] |
| MiR-146a, -34a, -125b, and -155-5p | Upregulated | Tissue | 18/18 | [56] |
| MiR-642-5p | Upregulated | Tissue | 460 f/188 m * | [63] |
| MiR-146b-5p, -150-5p, -342-3p | Upregulated | CSF | 28 f/28 m * | [64] |
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MAPK | Mitogen-activated protein kinase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| MAP3K1 | Mitogen-activated protein kinase kinase kinase 1 |
| ERK/p38 MAPK | Mitogen-activated protein kinase 1 |
| APP/PS1 | Amyloid beta precursor protein/Presenilin 1 |
| 5xFAD | Five familial Alzheimer’s disease mutations |
| ATP6AP2 | ATPase H+ transporting accessory protein 2 |
| LAMP2 | Lysosomal-associated membrane protein 2 |
| ADAM10 | Metallopeptidase domain 10 |
| LPS | Lipopolysaccharide |
| NMDAR2B | Glutamate Ionotropic receptor NMDA Type subunit 2B |
| SIRT1 | Sirtuin 1 |
| HTLV-1 | Human T-lymphotropic virus type 1 |
| CVB3 | Coxsackievirus B3 |
References
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016, 12, 459–509. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Valencia-Olvera, A.C.; Maldonado Weng, J.; Christensen, A.; LaDu, M.J.; Pike, C.J. Role of estrogen in women’s Alzheimer’s disease risk as modified by APOE. J. Neuroendocrinol. 2023, 35, e13209. [Google Scholar] [CrossRef]
- Kukurba, K.R.; Parsana, P.; Balliu, B.; Smith, K.S.; Zappala, Z.; Knowles, D.A.; Favé, M.J.; Davis, J.R.; Li, X.; Zhu, X.; et al. Impact of the X Chromosome and sex on regulatory variation. Genome Res. 2016, 26, 768–777. [Google Scholar] [CrossRef]
- Abidin, S.Z.; Mat Pauzi, N.A.; Mansor, N.I.; Mohd Isa, N.I.; Hamid, A.A. A new perspective on Alzheimer’s disease: MicroRNAs and circular RNAs. Front. Genet. 2023, 14, 1231486. [Google Scholar] [CrossRef]
- Huang, S.W.; Ali, N.D.; Zhong, L.; Shi, J. MicroRNAs as biomarkers for human glioblastoma: Progress and potential. Acta Pharmacol. Sin. 2018, 39, 1405–1413. [Google Scholar] [CrossRef]
- Lopez-Lee, C.; Torres, E.R.S.; Carling, G.; Gan, L. Mechanisms of sex differences in Alzheimer’s disease. Neuron 2024, 112, 1208–1221. [Google Scholar] [CrossRef]
- Cunningham, M.A.; Wirth, J.R.; Naga, O.; Eudaly, J.; Gilkeson, G.S. Estrogen Receptor Alpha Binding to ERE is Required for Full Tlr7- and Tlr9-Induced Inflammation. SOJ Immunol. 2014, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.M.S.; da Silva Madureira, M.W.; de Castro, R.B.H.; Abreu, I.N.; da Silva Conde, S.R.S.; Demachki, S.; de Sousa, M.S.; Queiroz, M.A.F.; Rangel da Silva, A.N.M.; Lima, S.S.; et al. Sex and FOXP3 gene rs2232365 polymorphism may be associated with the clinical and pathological aspects of chronic viral diseases. BMC Immunol. 2020, 21, 60. [Google Scholar] [CrossRef]
- Puck, J.M.; Middelton, L.; Pepper, A.E. Carrier and prenatal diagnosis of X-linked severe combined immunodeficiency: Mutation detection methods and utilization. Hum. Genet. 1997, 99, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lee, C.; Kodama, L.; Fan, L.; Zhu, D.; Zhu, J.; Wong, M.Y.; Ye, P.; Norman, K.; Foxe, N.R.; Ijaz, L.; et al. Tlr7 drives sex differences in age- and Alzheimer’s disease-related demyelination. Science 2024, 386, eadk7844. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Horitani, K.; Ogawa, H.; Halvardson, J.; Chavkin, N.W.; Wang, Y.; Sano, M.; Mattisson, J.; Hata, A.; Danielsson, M.; et al. Hematopoietic loss of Y chromosome leads to cardiac fibrosis and heart failure mortality. Science 2022, 377, 292–297. [Google Scholar] [CrossRef]
- Mifflin, M.A.; Winslow, W.; Surendra, L.; Tallino, S.; Vural, A.; Velazquez, R. Sex differences in the IntelliCage and the Morris water maze in the APP/PS1 mouse model of amyloidosis. Neurobiol. Aging 2021, 101, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Guillot-Sestier, M.V.; Araiz, A.R.; Mela, V.; Gaban, A.S.; O’Neill, E.; Joshi, L.; Chouchani, E.T.; Mills, E.L.; Lynch, M.A. Microglial metabolism is a pivotal factor in sexual dimorphism in Alzheimer’s disease. Commun. Biol. 2021, 4, 711. [Google Scholar] [CrossRef]
- Liu, J.; Sato, Y.; Falcone-Juengert, J.; Kurisu, K.; Shi, J.; Yenari, M.A. Sexual dimorphism in immune cell responses following stroke. Neurobiol. Dis. 2022, 172, 105836. [Google Scholar] [CrossRef]
- Espeland, M.A.; Rapp, S.R.; Shumaker, S.A.; Brunner, R.; Manson, J.E.; Sherwin, B.B.; Hsia, J.; Margolis, K.L.; Hogan, P.E.; Wallace, R.; et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004, 291, 2959–2968. [Google Scholar] [CrossRef]
- Kolahchi, Z.; Henkel, N.; Eladawi, M.A.; Villarreal, E.C.; Kandimalla, P.; Lundh, A.; McCullumsmith, R.E.; Cuevas, E. Sex and Gender Differences in Alzheimer’s Disease: Genetic, Hormonal, and Inflammation Impacts. Int. J. Mol. Sci. 2024, 25, 8485. [Google Scholar] [CrossRef]
- Korvatska, O.; Strand, N.S.; Berndt, J.D.; Strovas, T.; Chen, D.H.; Leverenz, J.B.; Kiianitsa, K.; Mata, I.F.; Karakoc, E.; Greenup, J.L.; et al. Altered splicing of ATP6AP2 causes X-linked parkinsonism with spasticity (XPDS). Hum. Mol. Genet. 2013, 22, 3259–3268. [Google Scholar] [CrossRef]
- Addis, R.; Campesi, I.; Fois, M.; Capobianco, G.; Dessole, S.; Fenu, G.; Montella, A.; Cattaneo, M.G.; Vicentini, L.M.; Franconi, F. Human umbilical endothelial cells (HUVECs) have a sex: Characterisation of the phenotype of male and female cells. Biol. Sex Differ. 2014, 5, 18. [Google Scholar] [CrossRef]
- Türei, D.; Földvári-Nagy, L.; Fazekas, D.; Módos, D.; Kubisch, J.; Kadlecsik, T.; Demeter, A.; Lenti, K.; Csermely, P.; Vellai, T.; et al. Autophagy Regulatory Network—A systems-level bioinformatics resource for studying the mechanism and regulation of autophagy. Autophagy 2015, 11, 155–165. [Google Scholar] [CrossRef]
- Song, D.; He, H.; Indukuri, R.; Huang, Z.; Stepanauskaite, L.; Sinha, I.; Haldosén, L.A.; Zhao, C.; Williams, C. ERα and ERβ Homodimers in the Same Cellular Context Regulate Distinct Transcriptomes and Functions. Front. Endocrinol. 2022, 13, 930227. [Google Scholar] [CrossRef]
- Demetrius, L.A.; Eckert, A.; Grimm, A. Sex differences in Alzheimer’s disease: Metabolic reprogramming and therapeutic intervention. Trends Endocrinol. Metab. 2021, 32, 963–979. [Google Scholar] [CrossRef]
- Arbizu, J.; Festari, C.; Altomare, D.; Walker, Z.; Bouwman, F.; Rivolta, J.; Orini, S.; Barthel, H.; Agosta, F.; Drzezga, A.; et al. Clinical utility of FDG-PET for the clinical diagnosis in MCI. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1497–1508. [Google Scholar] [CrossRef]
- Tenkorang, M.A.; Snyder, B.; Cunningham, R.L. Sex-related differences in oxidative stress and neurodegeneration. Steroids 2018, 133, 21–27. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Maki, P.M.; Reddy, S.; Bondi, M.W.; Biegon, A.; Alzheimer’s Disease Neuroimaging Initiative. Women’s higher brain metabolic rate compensates for early Alzheimer’s pathology. Alzheimers Dement. 2020, 12, e12121. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.E.; Chen, K.; Pietrini, P.; Rapoport, S.I.; Reiman, E.M. Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer’s Disease Treatment Studies. Am. J. Psychiatry 2002, 159, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mao, Z.; Woody, S.K.; Brinton, R.D. Sex differences in metabolic aging of the brain: Insights into female susceptibility to Alzheimer’s disease. Neurobiol. Aging 2016, 42, 69–79. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.; Saha, O.; Soares Landeira, B.; Melo de Farias, A.R.; Hermant, X.; Carrier, A.; Pelletier, A.; Gadaut, J.; Davoine, L.; Dupont, C.; et al. The Alzheimer susceptibility gene BIN1 induces isoform-dependent neurotoxicity through early endosome defects. Acta Neuropathol. Commun. 2022, 10, 4. [Google Scholar] [CrossRef]
- Saha, O.; Melo de Farias, A.R.; Pelletier, A.; Siedlecki-Wullich, D.; Landeira, B.S.; Gadaut, J.; Carrier, A.; Vreulx, A.C.; Guyot, K.; Shen, Y.; et al. The Alzheimer’s disease risk gene BIN1 regulates activity-dependent gene expression in human-induced glutamatergic neurons. Mol. Psychiatry 2024, 29, 2634–2646. [Google Scholar] [CrossRef]
- Crotti, A.; Sait, H.R.; McAvoy, K.M.; Estrada, K.; Ergun, A.; Szak, S.; Marsh, G.; Jandreski, L.; Peterson, M.; Reynolds, T.L.; et al. BIN1 favors the spreading of Tau via extracellular vesicles. Sci. Rep. 2019, 9, 9477. [Google Scholar] [CrossRef] [PubMed]
- Stephen, T.L.; Cacciottolo, M.; Balu, D.; Morgan, T.E.; LaDu, M.J.; Finch, C.E.; Pike, C.J. APOE genotype and sex affect microglial interactions with plaques in Alzheimer’s disease mice. Acta Neuropathol. Commun. 2019, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Kotredes, K.P.; Pandey, R.S.; Persohn, S.; Elderidge, K.; Burton, C.P.; Miner, E.W.; Haynes, K.A.; Santos, D.F.S.; Williams, S.P.; Heaton, N.; et al. Characterizing molecular and synaptic signatures in mouse models of late-onset Alzheimer’s disease independent of amyloid and tau pathology. Alzheimers Dement. 2024, 20, 4126–4146. [Google Scholar] [CrossRef]
- Shi, J.; Huang, S. Comparative Insight into Microglia/Macrophages-Associated Pathways in Glioblastoma and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 25, 16. [Google Scholar] [CrossRef] [PubMed]
- Bajic, V.P.; Essack, M.; Zivkovic, L.; Stewart, A.; Zafirovic, S.; Bajic, V.B.; Gojobori, T.; Isenovic, E.; Spremo-Potparevic, B. The X Files: “The Mystery of X Chromosome Instability in Alzheimer’s Disease”. Front. Genet. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Gonçalves, T.F.; Piergiorge, R.M.; Dos Santos, J.M.; Gusmão, J.; Pimentel, M.M.G.; Santos-Rebouças, C.B. Network Profiling of Brain-Expressed X-Chromosomal MicroRNA Genes Implicates Shared Key MicroRNAs in Intellectual Disability. J. Mol. Neurosci. 2019, 67, 295–304. [Google Scholar] [CrossRef]
- Gantier, M.P. X-chromosome-encoded microRNA-19 and -18 are possible modulators of female immunity. Bioessays 2013, 35, 671. [Google Scholar] [CrossRef]
- Estepa, M.; Niehues, M.H.; Vakhrusheva, O.; Haritonow, N.; Ladilov, Y.; Barcena, M.L.; Regitz-Zagrosek, V. Sex Differences in Expression of Pro-Inflammatory Markers and miRNAs in a Mouse Model of CVB3 Myocarditis. Int. J. Mol. Sci. 2024, 25, 9666. [Google Scholar] [CrossRef]
- Christoforidou, E.; Moody, L.; Joilin, G.; Simoes, F.A.; Gordon, D.; Talbot, K.; Hafezparast, M. An ALS-associated mutation dysregulates microglia-derived extracellular microRNAs in a sex-specific manner. Dis. Model Mech. 2024, 17, dmm050638. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Qiu, N.; Ni, A.; Xiong, T.; Xue, J.; Yin, K.J. Sex and Age-Dependent Effects of miR-15a/16-1 Antagomir on Ischemic Stroke Outcomes. Int. J. Mol. Sci. 2024, 25, 11765. [Google Scholar] [CrossRef]
- Sampath, D.; Branyan, T.E.; Markowsky, K.G.; Gunda, R.; Samiya, N.; Obenaus, A.; Sohrabji, F. Sex differences in cognitive impairment after focal ischemia in middle-aged rats and the effect of iv miR-20a-3p treatment. Neurobiol. Aging 2023, 129, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Engler-Chiurazzi, E.B.; Cavendish, J.Z.; Povroznik, J.M.; Russell, A.E.; Quintana, D.D.; Mathers, P.H.; Simpkins, J.W. Over-expression of miR-34a induces rapid cognitive impairment and Alzheimer’s disease-like pathology. Brain Res. 2019, 1721, 146327. [Google Scholar] [CrossRef]
- Vergallo, A.; Lista, S.; Zhao, Y.; Lemercier, P.; Teipel, S.J.; Potier, M.C.; Habert, M.O.; Dubois, B.; Lukiw, W.J.; Hampel, H.; et al. MiRNA-15b and miRNA-125b are associated with regional Aβ-PET and FDG-PET uptake in cognitively normal individuals with subjective memory complaints. Transl. Psychiatry 2021, 11, 78. [Google Scholar] [CrossRef]
- Wang, L.; Shui, X.; Diao, Y.; Chen, D.; Zhou, Y.; Lee, T.H. Potential Implications of miRNAs in the Pathogenesis, Diagnosis, and Therapeutics of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 16259. [Google Scholar] [CrossRef]
- Milà-Alomà, M.; Ashton, N.J.; Shekari, M.; Salvadó, G.; Ortiz-Romero, P.; Montoliu-Gaya, L.; Benedet, A.L.; Karikari, T.K.; Lantero-Rodriguez, J.; Vanmechelen, E.; et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nat. Med. 2022, 28, 1797–1801. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, F.; Kac, P.R.; Brum, W.S.; Zetterberg, H.; Blennow, K.; Karikari, T.K. Plasma phospho-tau in Alzheimer’s disease: Towards diagnostic and therapeutic trial applications. Mol. Neurodegener 2023, 18, 18. [Google Scholar] [CrossRef]
- Ma, C.; Ding, R.; Hao, K.; Du, W.; Xu, L.; Gao, Q.; Yu, C. Storage Stability of Blood Samples for miRNAs in Glycosylated Extracellular Vesicles. Molecules 2023, 29, 103. [Google Scholar] [CrossRef] [PubMed]
- Kupec, T.; Bleilevens, A.; Iborra, S.; Najjari, L.; Wittenborn, J.; Maurer, J.; Stickeler, E. Stability of circulating microRNAs in serum. PLoS ONE 2022, 17, e0268958. [Google Scholar] [CrossRef]
- Xiao, Q.; Yan, X.; Sun, Y.; Tang, Y.; Hou, R.; Pan, X.; Zhu, X. Brain-Derived Exosomal miRNA Profiles upon Experimental SAE Rats and Their Comparison with Peripheral Exosomes. Mol. Neurobiol. 2024, 61, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Reho, P.; Kalia, V.; Jackson, G.L.; Wang, F.; Eiten, E.; Brennan, K.; Brickman, A.M.; Mayeux, R.; Miller, G.W.; Vardarajan, B.N.; et al. Preclinical Alzheimer’s disease shows alterations in circulating neuronal-derived extracellular vesicle microRNAs in a multiethnic cohort. Alzheimers Dement. 2025, 21, e70050. [Google Scholar] [CrossRef]
- Llera-Oyola, J.; Carceller, H.; Andreu, Z.; Hidalgo, M.R.; Soler-Sáez, I.; Gordillo, F.; Gómez-Cabañes, B.; Roson, B.; de la Iglesia-Vayá, M.; Mancuso, R.; et al. The role of microRNAs in understanding sex-based differences in Alzheimer’s disease. Biol. Sex Differ. 2024, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Visconte, C.; Fenoglio, C.; Serpente, M.; Muti, P.; Sacconi, A.; Rigoni, M.; Arighi, A.; Borracci, V.; Arcaro, M.; Arosio, B.; et al. Altered Extracellular Vesicle miRNA Profile in Prodromal Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14749. [Google Scholar] [CrossRef]
- Cheng, Q.; Yu, S.; Cui, Z.; Chen, H.; Fan, J.; Yu, Q.; Jin, Y.; Wang, Y.; Li, M.; Lu, Z. Non-invasive biomarkers for brain aging: The role of autophagy-related microRNAs in plasma exosomes. Front. Mol. Neurosci. 2025, 18, 1588007. [Google Scholar] [CrossRef]
- Tsamou, M.; Roggen, E.L. Sex-associated microRNAs potentially implicated in sporadic Alzheimer’s disease (sAD). Brain Res. 2024, 1829, 148791. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Martínez, M.; Carter, V.; Nessim, A.; Panchal, R.; John, D.; Dhawan, J.; Beach, T.G.; Serrano, G.E.; Sundermann, E.E.; Biegon, A. Sex- and region-dependent neuroinflammation in Alzheimer’s disease. Alzheimers Dement. 2025, 21, e14603. [Google Scholar] [CrossRef]
- Henriques, A.D.; Machado-Silva, W.; Leite, R.E.P.; Suemoto, C.K.; Leite, K.R.M.; Srougi, M.; Pereira, A.C.; Jacob-Filho, W.; Nóbrega, O.T.; Brazilian Aging Brain Study Group. Genome-wide profiling and predicted significance of post-mortem brain microRNA in Alzheimer’s disease. Mech. Ageing Dev. 2020, 191, 111352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Trebak, F.; Souza, L.A.C.; Shi, J.; Zhou, T.; Kehoe, P.G.; Chen, Q.; Earley, Y.F. Small RNA modifications in Alzheimer’s disease. Neurobiol. Dis. 2020, 145, 105058. [Google Scholar] [CrossRef]
- Nunez-Iglesias, J.; Liu, C.C.; Morgan, T.E.; Finch, C.E.; Zhou, X.J. Joint genome-wide profiling of miRNA and mRNA expression in Alzheimer’s disease cortex reveals altered miRNA regulation. PLoS ONE 2010, 5, e8898. [Google Scholar] [CrossRef]
- Lau, P.; Bossers, K.; Janky, R.; Salta, E.; Frigerio, C.S.; Barbash, S.; Rothman, R.; Sierksma, A.S.; Thathiah, A.; Greenberg, D.; et al. Alteration of the microRNA network during the progression of Alzheimer’s disease. EMBO Mol. Med. 2013, 5, 1613–1634. [Google Scholar] [CrossRef]
- Asanomi, Y.; Shigemizu, D.; Akiyama, S.; Sakurai, T.; Ozaki, K.; Ochiya, T.; Niida, S. Dementia subtype prediction models constructed by penalized regression methods for multiclass classification using serum microRNA expression data. Sci. Rep. 2021, 11, 20947. [Google Scholar] [CrossRef] [PubMed]
- Leidinger, P.; Backes, C.; Deutscher, S.; Schmitt, K.; Mueller, S.C.; Frese, K.; Haas, J.; Ruprecht, K.; Paul, F.; Stähler, C.; et al. A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biol. 2013, 14, R78. [Google Scholar] [CrossRef] [PubMed]
- Vattathil, S.M.; Tan, S.S.M.; Kim, P.J.; Bennett, D.A.; Schneider, J.A.; Wingo, A.P.; Wingo, T.S. Effects of brain microRNAs in cognitive trajectory and Alzheimer’s disease. Acta Neuropathol. 2024, 148, 59. [Google Scholar] [CrossRef] [PubMed]
- Sandau, U.S.; McFarland, T.J.; Smith, S.J.; Galasko, D.R.; Quinn, J.F.; Saugstad, J.A. Differential Effects of APOE Genotype on MicroRNA Cargo of Cerebrospinal Fluid Extracellular Vesicles in Females With Alzheimer’s Disease Compared to Males. Front. Cell Dev. Biol. 2022, 10, 864022. [Google Scholar] [CrossRef]
- Fang, F.; Chen, C. MiRNA let-7d-5p Alleviates Inflammatory Responses by Targeting Map3k1 and Inactivating ERK/p38 MAPK Signaling in Microglia. Crit. Rev. Immunol. 2024, 44, 13–25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Zhong, L.; Zheng, L.; Shi, J. MicroRNAs as Potential Biomarkers for Alzheimer’s Disease in Women. Genes 2025, 16, 943. https://doi.org/10.3390/genes16080943
Huang S, Zhong L, Zheng L, Shi J. MicroRNAs as Potential Biomarkers for Alzheimer’s Disease in Women. Genes. 2025; 16(8):943. https://doi.org/10.3390/genes16080943
Chicago/Turabian StyleHuang, Shiwei, Lily Zhong, Lilly Zheng, and Jian Shi. 2025. "MicroRNAs as Potential Biomarkers for Alzheimer’s Disease in Women" Genes 16, no. 8: 943. https://doi.org/10.3390/genes16080943
APA StyleHuang, S., Zhong, L., Zheng, L., & Shi, J. (2025). MicroRNAs as Potential Biomarkers for Alzheimer’s Disease in Women. Genes, 16(8), 943. https://doi.org/10.3390/genes16080943






